The endotracheal tube (ETT) is an essential interface between the patient and ventilator in mechanically ventilated patients. However, a microbial biofilm is formed gradually on this tube and is associated with the development of ventilator-associated pneumonia.
KEYWORDS: Pseudomonas aeruginosa, biofilm, ventilator-associated pneumonia, endotracheal tube, poly-l-lysine, antibiofilm agent
ABSTRACT
The endotracheal tube (ETT) is an essential interface between the patient and ventilator in mechanically ventilated patients. However, a microbial biofilm is formed gradually on this tube and is associated with the development of ventilator-associated pneumonia. The bacteria present in the biofilm are more resistant to antibiotics, and current medical practices do not make it possible to eliminate. Pseudomonas aeruginosa is one of the leading pathogens that cause biofilm infections and ventilator-associated pneumonia. Poly-l-lysine (pLK) is a cationic polypeptide possessing antibacterial properties and mucolytic activity by compacting DNA. Here, we explored the antibiofilm activity of pLK to treat P. aeruginosa biofilms on ETTs while taking into consideration the necessary constraints for clinical translation in our experimental designs. First, we showed that pLK eradicates a P. aeruginosa biofilm formed in vitro on 96-well microplates. We further demonstrated that pLK alters bacterial membrane integrity, as revealed by scanning electron microscopy, and eventually eradicates biofilm formed either by reference or clinical strains of P. aeruginosa biofilms generated in vitro on ETTs. Second, we collected the ETT from patients with P. aeruginosa ventilator-associated pneumonia. We observed that a single dose of pLK is able to immediately disrupt the biofilm structure and kills more than 90% of bacteria present in the biofilm. Additionally, we did not observe any lung tolerance issue when the pLK solution was instilled into the ETT of ventilated pigs, an animal model particularly relevant to mimic invasive mechanical ventilation in humans. In conclusion, pLK appears as an innovative antibiofilm molecule, which could be applied in the ETT of mechanically ventilated patients.
INTRODUCTION
Ventilator-associated pneumonia (VAP) is the most common hospital-acquired infection in patients requiring mechanical ventilation, and its estimated incidence is 15% (1). In addition to being an independent factor for mortality, VAP is associated with longer intensive care unit and hospital stays, prolonged mechanical ventilation, and higher costs (1, 2). Pseudomonas aeruginosa is the principal pathogen of nosocomial respiratory infections (3). The presence of an endotracheal tube (ETT) in ventilated patients is a key component in the pathophysiology of VAP. First, it impairs mucociliary clearance, thus promoting the accumulation of tracheobronchial secretions. Second, the formation of biofilms on the ETT surface has been suggested to play a critical role in the development of nosocomial lung infections. Biofilms are multicellular, three-dimensional aggregates that form on the surface of ETTs. They are a complex structure comprised of pathogens enclosed within a self-produced polymeric matrix and respiratory secretions. This adaptive mode of growth is highly resistant to environmental conditions, such as physical disruption and host immune clearance mechanisms. P. aeruginosa is one of the leading pathogens that causes biofilm infections (4).
Biofilms are found in 95% of the ETTs of patients mechanically ventilated for more than 24 h, and their accumulation progressively obstructs the lumen (5). An association between the pathogens cultured from ETT biofilms and the lower respiratory tract has been observed for most patients who develop VAP (6). A laboratory animal study clearly demonstrated that healthy pigs intubated with ETTs containing P. aeruginosa biofilms developed airway infections by the translocation of pathogens from the biofilm (7). The inspiratory flow interacts with the biofilm surface which becomes unstable and can result in the dissemination of particles into the airways (8). Furthermore, bacteria causing VAP persist in ETT biofilms in half the cases, despite appropriate antibiotic treatment (5). Indeed, biofilms are an adaptive survival mechanism for bacteria, as they increase bacterial resistance to antimicrobials (9). It has been estimated that biofilm cells are up to 1,000 times more resistant to most antimicrobial agents than planktonic cells (10). Furthermore, antibiotics are not detectable or are found at concentrations far below the MIC in ETT biofilms during systemic treatment of VAP (11, 12). Thus, VAP may reoccur due to bacterial dissemination from the ETT biofilm toward the lower respiratory tract, giving rise to reinfection.
Hence, new therapeutic options are urgently needed to eradicate P. aeruginosa biofilm-related infections, and considerations for clinical translation should be anticipated earlier in the process of preclinical model assessment. The clinical practice guidelines advise treatment of an ETT without disconnecting the artificial airways to avoid desaturation and recommended that endotracheal suctioning be performed several times per day in a mechanically ventilated patient (13). Endotracheal suctioning involves the aspiration of pulmonary secretions from a patient under mechanical ventilation. The instillation of sterile normal saline in the ETT precedes the suctioning for thick secretions (13). Taking into account these practical constrains, innovative antibiofilm drugs should be administered directly into the ETT during the endotracheal suctioning of ventilated patients. An ideal antibiofilm candidate should exhibit strong and rapid antibacterial activity against P. aeruginosa, together with an antimucolytic property to disrupt the biofilm.
Our previous studies showed that poly-l-lysine (pLK), a cationic polypeptide, possesses an antibacterial property by killing P. aeruginosa but also a mucolytic activity by compacting DNA (14). Here, we explored the antibiofilm activity of pLK, taking into consideration the necessary constraints for clinical translation in our experimental design. We studied the effect of pLK on experimental biofilms made in vitro from different P. aeruginosa strains as well as ex vivo biofilms present in ETTs collected from mechanically ventilated patients. We also verified the lung tolerance of pLK instillations in mechanically ventilated pigs.
RESULTS
pLK eliminates P. aeruginosa (strain PAK-Lux) biofilms from 96-well microplates.
In vitro biofilms were generated using a luminescent P. aeruginosa strain (PAK-Lux) in 96-well microplates and visualized after treatment with 0, 10, or 100 μM pLK. In the absence of pLK, we observed a strong and homogeneous luminescent signal (mean of 535.02 light units/area). After a treatment with 10 μM pLK, the luminescence decreased, indicating degradation of the P. aeruginosa biofilm (mean of 198.30 light units/area; P < 0.001). Moreover, treatment with 100 μM pLK resulted in the total absence of luminescence (mean of 113.92 light units/area; P < 0.001), corresponding to the elimination of the biofilm (Fig. 1).
FIG 1.
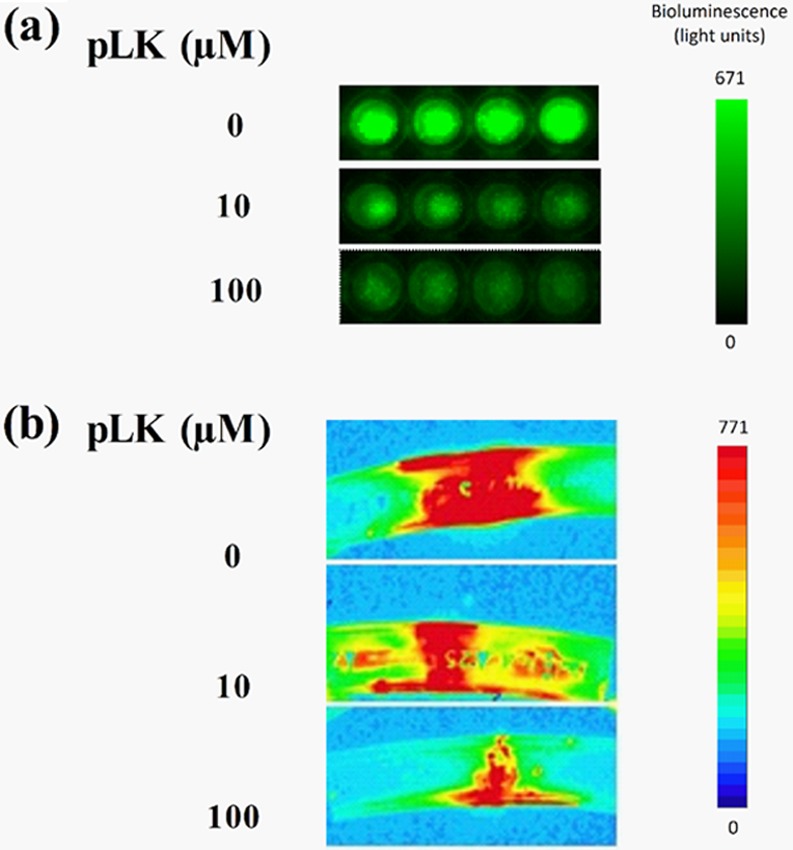
Antibiofilm activity of pLK against the PAK-LUX strain of P. aeruginosa. Visualization of P. aeruginosa (PAK-Lux) biofilms (by bioluminescence measurement) after treatment with 0, 10, or 100 μM pLK. (a) Treatment for 24 h on biofilms formed in vitro in 96-well plates (4 wells by condition). (b) Treatment for 2 min on biofilms formed in vitro in EETs.
pLK eliminates P. aeruginosa biofilms generated in vitro on ETTs.
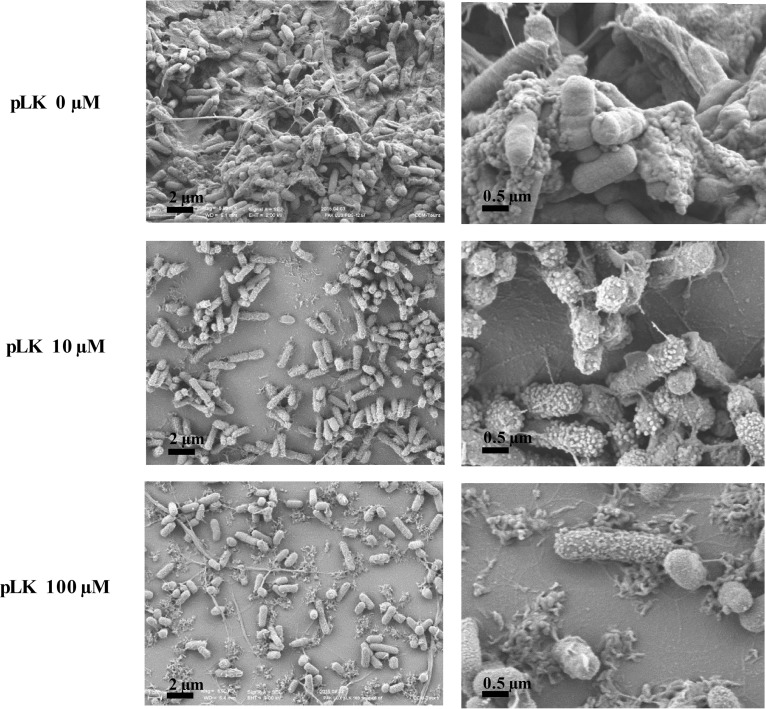
In vitro biofilms were generated using the luminescent PAK-Lux strain in ETTs for 24 h. To mimic the clinical process of ETT instillation, we treated the experimental ETT biofilm for 2 min with 0, 10, or 100 μM pLK. A strong and homogeneous signal of luminescence was observed in the absence of pLK (mean of 410.02 light units/area), whereas it decreased markedly after incubation with 10 (mean of 203.57 light units/area; P < 0.01) or 100 μM pLK (mean of 100.83 light units/area; P < 0.001), highlighting the destruction of the biofilm. These results were dose dependent (Fig. 1B). The biofilm structure was examined by scanning electron microscopy to visualize the bacterial morphology in the biofilm. We obtained high-resolution images of P. aeruginosa biofilms (Fig. 2; magnification ×5,000 and ×20,000). In the absence of pLK, the bacteria surface was smooth and bacteria were interconnected by fiber-like structures. After a 2-min incubation with 10 μM or 100 μM pLK, the bacterial surface changed dramatically; microvesicles were visible and the number of fiber-like structures greatly decreased (Fig. 2). We also evaluated the bactericidal action of pLK against P. aeruginosa bacteria present in the biofilms. ETT biofilm can be divided in two parts or layers depending on its resistance properties. Indeed, a first part of the ETT biofilm named “outer layer” is easily removable, corresponding to the bacteria and the associated matrix of the outer part of the biofilm (one-half or two-thirds of the biofilm thickness). The second part, or “inner layer,” corresponds to the bacteria and the associated matrix which are still present and persist on the ETT, even after several washes. A two-step protocol was developed to estimate viable bacteria in each layer. The first count of CFU reflected viable bacteria present in the outer layer of biofilm and the second one, viable bacteria present in the inner layer of biofilm. CFU obtained with 0 μM pLK corresponded to 100% survival. After treatment with a 10 μM or 100 μM pLK solution, an almost complete bacterial killing was observed, with less than 1% of viable bacteria in both layers (Table 1, line 1) (P < 0.05 and P < 0.01, respectively). In vitro biofilms were also generated using different clinical strains of P. aeruginosa in ETTs, and we observed similar results with those of the PAK-Lux strain. Less than 0.5% of viable bacteria remained when the ETTs were treated with a 10 or 100 μM pLK solution (Table 1, line 2) (P < 0.01). Altogether, these results suggest that pLK possesses an antibiofilm effect characterized by the degradation of the biofilm structure, an alteration of the bacterial membrane integrity, and a bactericidal effect.
FIG 2.
Action of pLK against strain PAK-LUX biofilms formed in ETTs. Scanning electron micrographs of PAK-LUX biofilms formed in vitro in ETTs after treatment with 0, 10, or 100 μM pLK.
TABLE 1.
pLK activity against P. aeruginosa biofilms in ETTsa
| Type of P. aeruginosa biofilms | Mean (± SEM) CFU (%) in biofilm outer layer with pLK treatment of: |
Mean (± SEM) CFU (%) in biofilm inner layer with pLK treatment of: |
||||
|---|---|---|---|---|---|---|
| 0 µM | 10 µM | 100 µM | 0 µM | 10 µM | 100 µM | |
| In vitro biofilms from PAK strain (n = 6) | 100 | 0.80 ± 0.53 | 0.24 ± 0.17 | 100 | 0.21 ± 0.16 | 0.21 ± 0.15 |
| In vitro biofilms from clinical strains (6 strains; n = 1) | 100 | 0.25 ± 0.17 | 0.03 ± 0.02 | 100 | 0.26 ± 0.19 | 0.05 ± 0.04 |
| Ex vivo biofilms from patient ETTs (7 ETTs) | 100 | 0.86 ± 0.74 | 0.38 ± 0.37 | 100 | 7.72 ± 7.06 | 0.61 ± 0.60 |
Biofilms were treated with pLK for 2 min. A two-step protocol determined the percentage of CFU in the ETT biofilm layers.
To compare the pLK antibacterial effect to its antibiofilm effect, MIC and minimal bactericidal concentration (MBC) of pLK were evaluated using a reference strain (PAK-lux) and the 6 tested clinical strains, under their planktonic form. The results determined an MIC of 4 μM pLK for PAK-lux and the 6 clinical strains. The MBC was of 4 μM pLK for PAK-lux and between 4 and 16 μM pLK for the 6 clinical strains. These data showed that the pLK concentrations able to eliminate P. aeruginosa biofilm were very close to those determined for the MBC, keeping in mind that incubation time was very different (2 min for antibiofilm activity compared with 24 h for that of MBC determination).
pLK degrades the biofilm matrix and eliminates P. aeruginosa from the ETT of infected patients.
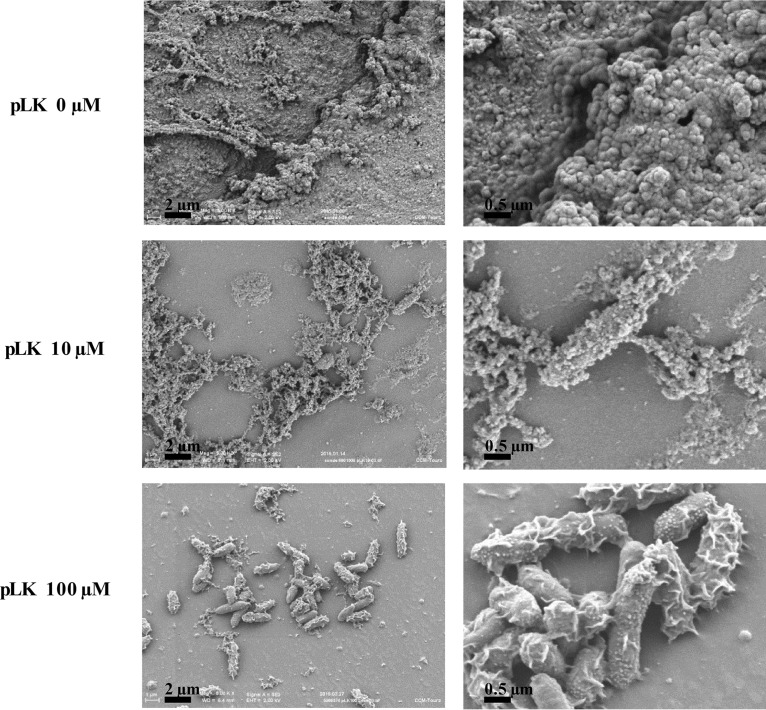
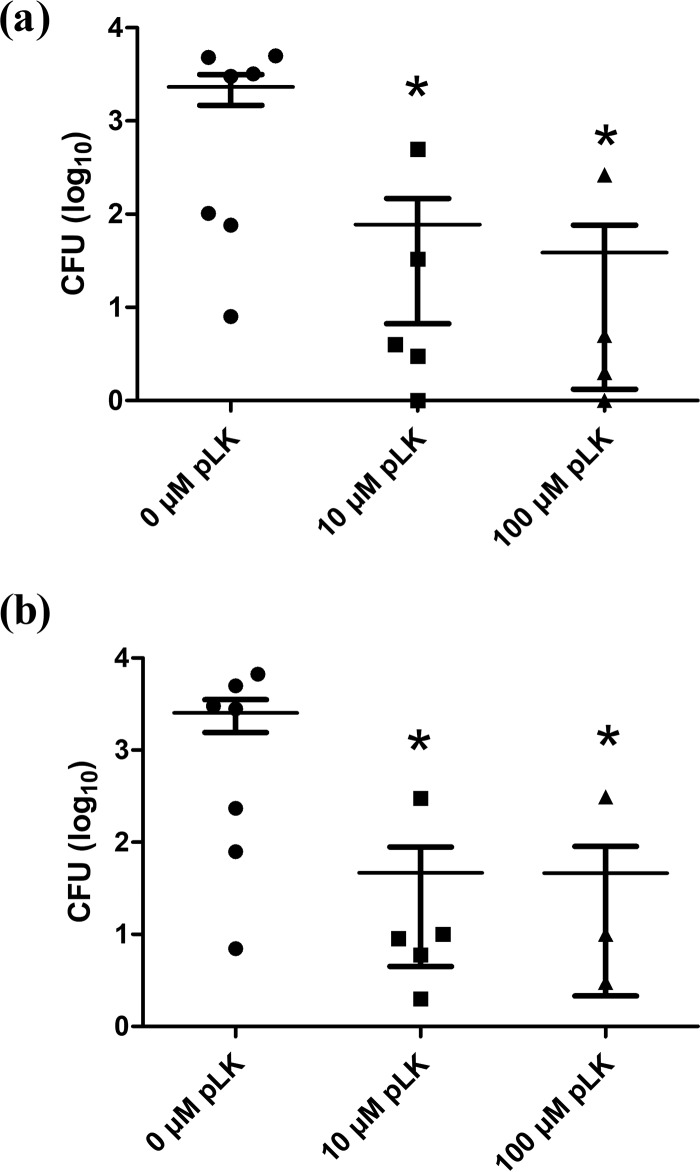
ETTs were collected from mechanically ventilated patients colonized by P. aeruginosa (Table 2). Without pLK treatment, there was an abundant biofilm with complex matrices in the inner surface of the ETTs, with no observable bacteria on the matrix surface. As expected, these ex vivo biofilms were different from the in vitro ones (Fig. 2 and 3). This is probably due to the presence of patient respiratory secretions, which play an important role in biofilm formation. After pLK treatment, we observed compaction of the biofilm matrix structure, rendering the ETT wall visible and bacteria unmasked (Fig. 3). The bactericidal action of pLK eliminated more than 99% of bacteria with 10 μM or 100 μM pLK (Table 1, line 3) (P < 0.05). Taken individually, we observed less than 1% of viable bacteria for five out of seven patient ETT biofilms (Fig. 4). Among them, two patients were colonized by multidrug-resistant (MDR) P. aeruginosa strains, demonstrating the ability of pLK to eradicate these MDR strains (Table 3). For two patients out of seven, approximately 90% and 50% of P. aeruginosa bacteria were eliminated, respectively. In these two cases, the biofilms were thicker than that of the other patients. Of note, these results were obtained only 2 min after a single pLK administration. Altogether, these results demonstrate the double action of pLK: (i) its property to condense and degrade the biofilm matrix leading to bacterial unmasking and (ii) its antibacterial effect.
TABLE 2.
Baseline characteristics of patientsa
| Patient characteristics at the time of ETT collectionb | Values |
|---|---|
| Age (yrs), mean ± SEM | 62 ± 5 |
| Sex, female, n (%) | 5/7 (71) |
| SAPS II, mean ± SEM | 44 ± 7 |
| Cause of intubation, n (%) | |
| Neurologic disorder | 5/7 (71) |
| Acute heart failure | 2/7 (29) |
| Duration of mechanical ventilation (days), mean ± SEM | 19 ± 5 |
| Mortality in ICU, n (%) | 2/7 (29) |
The total number of patients was 7.
SAPS, simplified acute physiologic score; ICU, intensive care unit.
FIG 3.
Action of pLK against ex vivo biofilm from patient ETT. Scanning electron micrographs of ex vivo ETT biofilms (collected from mechanically ventilated patients) after treatment with 0, 10, or 100 μM pLK.
FIG 4.
Antibiofilm activity of pLK against ETT biofilm from mechanically ventilated patients. Ex vivo patient ETT biofilms (n = 7) were treated with 0, 10, or 100 μM pLK for 2 min. The CFU number (expressed as log10) was determined after treatment in the outer layer (a) and inner layer (b) of a patient ETT biofilm (*, P < 0.05).
TABLE 3.
Characteristics of P. aeruginosa strains isolated from ex vivo ETT biofilm collected from mechanically ventilated patients
| Patient no. | Antibiotic treatment | P. aeruginosa susceptibility | P. aeruginosa serotype |
|---|---|---|---|
| 1 | Yes | Susceptible | Nontypeable |
| 2 | No | Susceptible | O6 |
| 3 | Yes | MDRa | O3 |
| 4 | Yes | Susceptible | O10 |
| 5 | No | Susceptible | O3 |
| 6 | Yes | MDR | O9 |
| 7 | No | Susceptible | O11 |
MDR, multidrug resistant.
Lung tolerance assessment of repeated instillations of pLK solution in ventilated pigs.
Altogether, our results show that instillation of pLK in solution is a highly efficient and simple way to eliminate P. aeruginosa biofilms in ETTs. We previously demonstrated the absence of lung toxicity induced by intratracheal administration of pLK in mice (14). However, it was necessary to demonstrate that repeated instillations of pLK solution have no side effects on the trachea and lungs in a model relevant for transposition in the clinics. Four pigs were mechanically ventilated, and ETTs were instilled every 2 h for 6 h, as performed in the intensive care unit, either with physiological serum or a 10-μM pLK solution. Bronchoalveolar lavage fluid (BAL) analysis revealed no proinflammatory cytokine production. The mean (SEM) concentration of interleukin-6 (IL-6) was 146 pg/ml (26 pg/ml) for the control group and 155 pg/ml (155 pg/ml) for the pLK-treated group, and the mean concentration of interleukin-8 (IL-8) was 271 pg/ml (11 pg/ml) for the control group and 85 pg/ml (85 pg/ml) for the pLK-treated group. Histological studies showed no tracheal or lung lesions, nor local inflammation in pLK-treated pigs relative to those of controls by histological analysis, suggesting that repeated ETT instillation with a 10-μM pLK solution was well tolerated.
DISCUSSION
The main finding of our work is the potential role of pLK as a fast-acting antibiofilm agent. We demonstrate the ability of pLK to eliminate a P. aeruginosa biofilm, experimentally or naturally formed in ETTs, using an original protocol that mimics the clinical practice for ETT instillation. A single administration of pLK immediately leads to the elimination of 99% of bacteria from experimental biofilms and more than 90% from ETT biofilms from infected patients. Observations by scanning electron microscopy showed matrix degradation by pLK treatment as well as an alteration of the membrane integrity of the bacteria. However, the most remarkable result was the disruption of patient ETT biofilms with the condensation of biofilm matrix, uncovering and killing the bacteria.
The primary therapeutic strategy against microbial biofilms in ETTs has historically been to remove and replace the infected device. This practice is currently not recommended due to the high morbidity associated with ETT replacement. Preventing or treating the formation of microbial biofilms in ETTs appears to be the best alternative solution for reducing the incidence of VAP. Multiple approaches have been investigated to prevent the formation of P. aeruginosa biofilms by coating medical surfaces with antibiotics or silver particles (15–18), but the use of this approach for all patients has raised the issue of cost-effectiveness. More importantly, this method is useless for treating already established biofilms.
The rates of antibiotic resistance by P. aeruginosa are increasing worldwide, and the overuse of antibiotics in intensive care units (ICUs) is a recognized risk factor for the emergence of multidrug-resistant bacteria (19). Thus, the use of antibiotic prophylaxis for VAP remains debatable. Recent research has focused on developing novel therapeutics based on cationic host defense peptides, as they may have potential anti-infective agents. Synthetic peptides have been generated from these natural peptides to treat P. aeruginosa biofilms (4, 20). Indeed, the human host defense peptide LL-37 prevents P. aeruginosa biofilm formation and dissolves pre-existing biofilms (21). Screening of small peptides has also identified promising anti-infective agents against bacterial biofilms, including peptide 1018, a potential therapeutic agent against biofilms formed by P. aeruginosa (22). Two aspects must be taken into consideration before the transfer of these antibiofilm agents to the clinic. First, the ETT is within the trachea, and the biofilm develops mainly on the internal surface of the tube. Pharmacokinetic analysis has demonstrated that systemically administered drugs fail to reach the biofilm due to its localization (i.e., not in contact with the vascularized tissue). Direct administration of drugs into the ETT in situ is probably necessary, but it is not possible to expose the biofilm to the anti-infective drugs for several hours, as performed in the majority of the studies (21–23). Thus, ideal antibiofilm therapeutics should be rapidly efficient. Remarkably, pLK disrupted biofilms in 2 min following a single administration. We considered 2 min to be the approximate estimated time of contact during instillation in the ETT. The second aspect to consider is the biofilm composition. Biofilm models grown experimentally (in vitro) are very different from patient ETT biofilms (ex vivo), as observed in the scanning electron micrographs of ETT biofilms from patients mechanically ventilated for several days. Indeed, experimental P. aeruginosa biofilms consist of a homogeneous layer containing visible bacteria interconnected by fiber-like structures. By contrast, patient ETT biofilms are more heterogeneous without visible bacteria on the surface, due to the matrix composition which results from an accumulation of respiratory secretions containing microorganisms, immune cells and cell fragments, polysaccharides, and proteins (24). In these natural biofilms from ETTs of patients, we showed that the bacteria were hidden by the other matrix constituents and that pLK could disrupt this matrix and unmask and kill the bacteria.
We previously reported the mucolytic activity of pLK on cystic fibrosis sputum. It exerts its action by compacting DNA in the sputum and then liquefying the secretions (14). This new antibiofilm property is crucial because it allows access to the bacteria. Then, pLK can kill the unmasked bacteria due to its antimicrobial properties against P. aeruginosa (14). Hyldgaard et al. (25) proposed that pLK interacts with bacterial membranes through a carpet-like mechanism that forms vesicles or micelles by imposing negative curvature through its interaction with the phospholipid headgroups of the bacterial membrane. Conversely, other studies have suggested that pLK is relatively nontoxic for mammalian cells because it interacts more readily with negatively charged headgroups, and differences in susceptibility among microorganisms may be caused by differences in membrane composition (26). This is consistent with the absence of tracheal or lung lesions that we observed after repeated instillation of pLK in mechanically ventilated pigs. The animal model used herein has a high translational potential for clinical applications; ventilator, circuit components, ventilator settings, and the endotracheal suctioning process were all identical with those used for patients under mechanical ventilation. The overall design of this experiment allows us to be confident in the potential translation into the clinics (27).
In conclusion, we demonstrated that pLK efficiently and rapidly disrupts P. aeruginosa biofilms in ETTs from ventilated ICU patients. These antibiofilm properties of pLK are consistent with its potential use by direct administration into the ETT during endotracheal suctioning, as recommended for ventilated patients. Instillation with pLK could be integrated with endotracheal suctioning as an add-on therapy to enhance the effectiveness of systemic antibiotics during VAP treatment. This combination treatment (i.e., systemic antibiotics plus pLK instillations into the ETTs) could efficiently eradicate the pathogen from both the lungs and ETT and potentially reduce relapse originating from persistent bacteria in ETT biofilms.
MATERIALS AND METHODS
Chemical agent.
Poly-l-lysine (pLK) (PubChem compound identifier 5962) was purchased from Sigma (Saint-Quentin-Fallavier, France) unless otherwise stated. pLK was diluted in phosphate-buffered saline (PBS, pH 7.4; PubChem compound identifier 24978514; Gibco, Invitrogen, Life Technologies, Saint-Aubin, France), and a fresh stock was made for each experiment. pLK was used at 10 and 100 μM.
ETT collection.
ETTs were purchased from Covidien (TaperGuard Tracheal Tube; Mallinckrodt, Mansfield, MA, USA). We collected ETTs from mechanically ventilated patients with current or former P. aeruginosa respiratory infections that were extubated due to clinical improvement, a change in the ETT for technical reasons, or patient death. This study was approved by the French bioethics authorities (L'Espace de Réflexion Ethique Région Centre) and was conducted in accordance with the ethical standards of the Helsinki Declaration. All patients (or their relatives) included in this study were personally informed by a written document about the collection of used ETTs, as well as their right to object to the study and obtain access to the data, according to articles L.1121-1 and R1121-2 of the French Public Health Code.
Strains of P. aeruginosa.
Two reference strains of P. aeruginosa were used to establish proof of concept, PAK and PAK-Lux, a luminescent strain, kindly supplied by Reuben Ramphal (USA). Clinical strains of P. aeruginosa were collected from ETTs.
Evaluation of pLK minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) against strains of P. aeruginosa.
The antibacterial effect of pLK was determined by a standardized microdilution test in 96-well polystyrene plates. Different concentrations of pLK were prepared and tested through serial dilution (1 to 32 μM) in Mueller-Hinton (MH) medium and incubated for 24 h with different strains of P. aeruginosa in exponential phase culture (105 bacteria/ml). The MIC was considered as the lowest concentration of pLK that resulted in the absence of visible bacterial growth, and MBC was determined by using 50 μl of bacterial suspension from the wells corresponding to each concentration tested and plating out several serial dilutions onto Cetrimide plates. After an 18-h incubation at 37°C, the number of CFUs corresponding to the number of viable bacteria in the original sample was counted. The MBC was the lowest concentration that killed 99.99% of bacteria. Each strain was tested three times.
In vitro biofilm formation and susceptibility assay in 96-well microplates.
P. aeruginosa (PAK-Lux) was used to form a biofilm in 96-well microplates. The wells of a 96-well plate were inoculated with 100 μl of a 1/100 dilution of an exponential phase culture (0.01 optical density). The microplate was incubated for 48 h at 37°C. Then, the 96-well microplates were rinsed with PBS and placed in contact with various concentrations of pLK (0, 10, and 100 μM) for 24 h at 37°C (4 wells by condition). Finally, the luminescence was measured using an Infinite M200 plate reader (Tecan, Lyon, France). This experiment was performed three times.
Susceptibility assay of P. aeruginosa biofilm in ETTs.
To evaluate pLK antibiofilm activity, we decided to mimic the clinical process of ETT washes. Different strains of P. aeruginosa were used to form in vitro biofilms in ETTs. Three milliliters of a 1/100 dilution of an exponential phase culture (0.01 optical density) was incubated in the ETT for 24 h at 37°C with shaking (200 rpm). After incubation, the ETT was rinsed with PBS, placed in a 15-ml tube containing a 5-ml solution of various concentrations of pLK (0, 10, and 100 μM), and incubated for 2 min at room temperature. For each condition, a segment of the ETT with a homogeneous biofilm was selected for electron microscopy and to evaluate the bactericidal action of pLK.
ETT biofilm can be divided in two parts or layers depending on their resistance properties. Indeed, a part of the ETT biofilm is easily removable, corresponding to the bacteria and the associated matrix, forming the outer part of the biofilm (one-half or two-thirds of the biofilm thickness). We called this part the “outer layer.” The second part corresponds to the bacteria and the associated matrix which are still present on the ETT and persist even after several washes, forming the inner part of the biofilm and named the “inner layer.” We developed a two-step protocol to determine the bactericidal effect of pLK on each layer. The first step determined the number of viable bacteria in the biofilm outer layer; after treatment, the ETT section was placed in a Falcon tube containing 5 ml PBS, vortexed for 30 s, sonicated for 5 min, and vortexed again for 30 s. The PBS solution was removed and plated out in several serial dilutions onto Cetrimide plates. After an 18-h incubation at 37°C, the number of CFUs corresponding to the number of viable bacteria in the original sample were counted. After this first step, the remaining biofilm on the ETT section was entirely removed with a 10-μl loop and diluted in PBS, and the number of viable bacteria in the original sample were counted as previously described. This second step allowed counting viable bacteria in the inner layer of the biofilm. All results were expressed as the percentage (mean ± SEM) of CFUs. The CFU number obtained without pLK treatment corresponded to 100% survival. This protocol was used for in vitro biofilm, either with the PAK-lux strain (n = 4 for bioluminescence measurement and n = 6 for bacterial viability) or with clinical strains (6 different strains, n = 1). The same protocol was also used on “ex vivo”-collected patient EET biofilms, the results of which were expressed as a percentage (100% corresponds to the CFU number obtained without pLK treatment) or log of CFU.
Scanning electron microscopy.
For scanning electron microscopy, the section of the ETT was fixed with 1.3% glutaraldehyde and 0.05% ruthenium red in 0.07 M cacodylate buffer, pH 7.4, postfixed in 1% (vol/vol) osmium tetroxide, dehydrated in a graded ethanol series, dried with hexamethyldisilazane, and sputter coated with platinum. The sections were examined with a Zeiss Ultra Plus scanning electron microscope.
Tolerance of pLK in ventilated pigs.
Experiments were performed on four piglets (Large White, 2 to 3 months of age, weight 30 ± 1 kg) according to the guidelines of Council Directive no. 86/609 of the European Economic Community. The protocol was approved by the Comité d'Ethique en Expérimentation Animale Val de Loire (protocol no. 00028.01). The animals were sedated and ventilated as previously described (28, 29). After tracheal intubation with a 7.0-mm-internal-diameter ETT, the lungs of the animals were mechanically ventilated. We assessed the tolerance to pLK by mimicking the endotracheal suctioning procedure in the pig model and by replacing the sterile normal saline used for suctioning with a 10 μM pLK solution. The suctioning event consisted of the instillation of 3 ml of solution (sterile normal saline or 10-μM pLK solution) in the ETT; at the same time, we inserted a suction catheter through the ETT and applied a negative pressure as the catheter was being withdrawn. The instillation/suctioning events were repeated 4 times during 6 h with either normal saline or the pLK solution. At the end of the experiment, the pigs were euthanized with an intravenous injection of sodium pentobarbital. Bronchoalveolar lavage fluid (BAL) was obtained for each pig by instillation of 2 × 50 ml of PBS. BAL cytokine levels of interleukin-6 (IL-6) and IL-8 were assessed using ELISA kits containing pig-specific monoclonal antibodies (R&D Systems, Minneapolis, MN, USA). Histological studies were performed on eight collected samples per pig, including trachea, bronchial ramification, right and left bronchus, and different areas of the lung (right and left cranial and medial lobes). The samples were fixed in a 4% formaldehyde solution and embedded in paraffin, and sections were stained with hematoxylin and eosin. A pathologist who was blinded to the study groups performed the histological analyses.
Statistical analysis.
For statistical analysis and representation of the results, GraphPad version Prism 5 software was used. Bioluminescence results were analyzed with repeated-measures analysis of variance and the post hoc Bonferroni test (α = 0.05). For bacterial viability in ETTs, results were analyzed by using Kruskal-Wallis and Dunn's multiple-comparisons post hoc tests (α = 0.05).
ACKNOWLEDGMENTS
We thank all the patients who volunteered for this study. We also thank Veronique Siméon, Christine Mabilat, Aurélie Aubrey, and all the physicians of the Tours intensive care department for collecting the samples. We kindly thank Jérémy Pezant and Céline Barc for their contributions to the in vivo pig experimentation.
This work was supported in part by a grant from the Association Vaincre la Mucoviscidose (grant number RF20140501135).
We declare no competing financial interests.
REFERENCES
- 1.Bekaert M, Timsit J-F, Vansteelandt S, Depuydt P, Vésin A, Garrouste-Orgeas M, Decruyenaere J, Clec'h C, Azoulay E, Benoit D, Outcomerea Study Group . 2011. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med 184:1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon J-Y. 2002. Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PK, Yeung ATY, Hancock REW. 2014. Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J Biotechnol 191:121–130. doi: 10.1016/j.jbiotec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, Menendez R, Bonastre J. 2012. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care 16:R93. doi: 10.1186/cc11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis TJ, Millar MR, Jones JG, Robinson DA. 1989. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol 27:2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Bassi G, Fernandez-Barat L, Saucedo L, Giunta V, Marti JD, Tavares Ranzani O, Aguilera Xiol E, Rigol M, Roca I, Muñoz L, Luque N, Esperatti M, Saco MA, Ramirez J, Vila J, Ferrer M, Torres A. 2015. Endotracheal tube biofilm translocation in the lateral Trendelenburg position. Crit Care 19:59. doi: 10.1186/s13054-015-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, McCarthy GJ, Milligan KR. 1999. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med 25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 10.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 11.Adair CG, Gorman SP, Byers LM, Jones DS, Feron B, Crowe M, Webb HC, McCarthy GJ, Milligan KR. 2002. Eradication of endotracheal tube biofilm by nebulised gentamicin. Intensive Care Med 28:426–431. doi: 10.1007/s00134-002-1223-8. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Barat L, Ferrer M, Sierra JM, Soy D, Guerrero L, Vila J, Li Bassi G, Cortadellas N, Martínez-Olondris P, Rigol M, Esperatti M, Luque N, Saucedo LM, Agustí C, Torres A. 2012. Linezolid limits burden of methicillin-resistant Staphylococcus aureus in biofilm of tracheal tubes. Crit Care Med 40:2385–2389. doi: 10.1097/CCM.0b013e31825332fc. [DOI] [PubMed] [Google Scholar]
- 13.American Association for Respiratory Care. 2010. AARC clinical practice guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir Care 55:758–764. [PubMed] [Google Scholar]
- 14.Dubois AV, Midoux P, Gras D, Si-Tahar M, Bréa D, Attucci S, Khelloufi M-K, Ramphal R, Diot P, Gauthier F, Hervé V. 2013. Poly-l-lysine compacts DNA, kills bacteria, and improves protease inhibition in cystic fibrosis sputum. Am J Respir Crit Care Med 188:703–709. doi: 10.1164/rccm.201305-0912OC. [DOI] [PubMed] [Google Scholar]
- 15.Darouiche RO. 1999. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis 29:1371–1377. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 16.Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, Craven DE, Roberts PR, Arroliga AC, Hubmayr RD, Restrepo MI, Auger WR, Schinner R, Investigation Group NASCENT . 2008. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA 300:805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 17.Oga M, Arizono T, Sugioka Y, Naylor PT, Myrvik QN, Gristina AG. 1992. The inhibition of bacterial adhesion to a tobramycin-impregnated polymethylmethacrylate substratum. J Long Term Eff Med Implants 1:321–328. [PubMed] [Google Scholar]
- 18.Mijnendonckx K, Leys N, Mahillon J, Silver S, Van Houdt R. 2013. Antimicrobial silver: uses, toxicity and potential for resistance. Biometals 26:609–621. doi: 10.1007/s10534-013-9645-z. [DOI] [PubMed] [Google Scholar]
- 19.Nseir S, Di Pompeo C, Diarra M, Brisson H, Tissier S, Boulo M, Durocher A. 2007. Relationship between immunosuppression and intensive care unit-acquired multidrug-resistant bacteria: a case-control study. Crit Care Med 35:1318–1323. doi: 10.1097/01.CCM.0000261885.50604.20. [DOI] [PubMed] [Google Scholar]
- 20.Pletzer D, Hancock REW. 2016. Antibiofilm peptides: potential as broad-spectrum agents. J Bacteriol 198:2572–2578. doi: 10.1128/JB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overhage J, Campisano A, Bains M, Torfs ECW, Rehm BHA, Hancock REW. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Fuente-Núñez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernández D, Brackman G, Coenye T, Hancock REW. 2015. d-Enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol 22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pintucci JP, Corno S, Garotta M. 2010. Biofilms and infections of the upper respiratory tract. Eur Rev Med Pharmacol Sci 14:683–690. [PubMed] [Google Scholar]
- 25.Hyldgaard M, Mygind T, Vad BS, Stenvang M, Otzen DE, Meyer RL. 2014. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl Environ Microbiol 80:7758–7770. doi: 10.1128/AEM.02204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida T, Nagasawa T. 2003. Epsilon-poly-l-lysine: microbial production, biodegradation and application potential. Appl Microbiol Biotechnol 62:21–26. doi: 10.1007/s00253-003-1312-9. [DOI] [PubMed] [Google Scholar]
- 27.Guillon A, Sécher T, Dailey LA, Vecellio L, de Monte M, Si-Tahar M, Diot P, Page CP, Heuzé-Vourc'h N. 2018. Insights on animal models to investigate inhalation therapy: relevance for biotherapeutics. Int J Pharm 536:116–126. doi: 10.1016/j.ijpharm.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Guillon A, Mercier E, Lanotte P, Haguenoer E, Darrouzain F, Barc C, Sarradin P, Si-Tahar M, Heuzé-Vourc'h N, Diot P, Vecellio L. 2015. Aerosol route to administer teicoplanin in mechanical ventilation: in vitro study, lung deposition and pharmacokinetic analyses in pigs. J Aerosol Med Pulm Drug Deliv 28:290–298. doi: 10.1089/jamp.2014.1164. [DOI] [PubMed] [Google Scholar]
- 29.Mankikian J, Ehrmann S, Guilleminault L, Le Fol T, Barc C, Ferrandière M, Boulain T, Dequin PF, Guillon A. 2014. An evaluation of a new single-use flexible bronchoscope with a large suction channel: reliability of bronchoalveolar lavage in ventilated piglets and initial clinical experience. Anaesthesia 69:701–706. doi: 10.1111/anae.12641. [DOI] [PubMed] [Google Scholar]