A fast and easy-to-use liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the determination and quantification of a novel antifungal drug, olorofim (F901318), a member of the novel class of orotomides, in human plasma and serum was developed and validated. Sample preparation was based on protein precipitation with acetonitrile and subsequent centrifugation.
KEYWORDS: liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS), matrix effects, orotomide, antifungal drug, therapeutic drug monitoring, antifungal agents
ABSTRACT
A fast and easy-to-use liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the determination and quantification of a novel antifungal drug, olorofim (F901318), a member of the novel class of orotomides, in human plasma and serum was developed and validated. Sample preparation was based on protein precipitation with acetonitrile and subsequent centrifugation. An isotope-labeled analogue of F901318 was employed as an internal standard. Chromatographic separation was achieved using a 50-mm by 2.1-mm, 1.9-μm, polar Hypersil Gold C18 column and isocratic mobile phase consisting of 0.1% formic acid–acetonitrile (60%-40%, vol/vol) at a flow rate of 330 μl/min. The analyte was detected using a triple-stage quadrupole mass spectrometer operated in selected reaction monitoring (SRM) mode with positive heated electrospray ionization (HESI+) within a single runtime of 2.00 min. The present LC-MS/MS method was validated according to the international guidelines of the International Conference on Harmonisation (ICH) and the U.S. Food and Drug Administration (FDA). Linearity of F901318 concentration ranges was verified by the Mandel test. The calibration curve was tested linear across the range and fitted using least-squares regression with a weighting factor of the reciprocal concentration. The limit of detection was 0.0011 mg/liter, and the lower limit of quantitation was 0.0033 mg/liter. Intraday and interday precisions ranged from 1.17% to 3.23% for F901318, and intraday and interday accuracies (percent bias) ranged from 0.75% to 5.01%. In conclusion, a method was established for the rapid quantitation of F901318 concentrations in serum and plasma samples in patient trials, and it optimizes therapeutic drug monitoring in applying an easy-to-use single method.
INTRODUCTION
Treating invasive fungal infections is a challenge with regard to antifungal (azole and echinocandin) resistance. Azole-resistant Aspergillus species, including isolates of Aspergillus fumigatus, cause invasive fungal infections with substantial mortality in immunocompromised patients (1, 2). A variety of new investigational antifungals are currently in clinical development to overcome resistance problems, including olorofim (F901318).
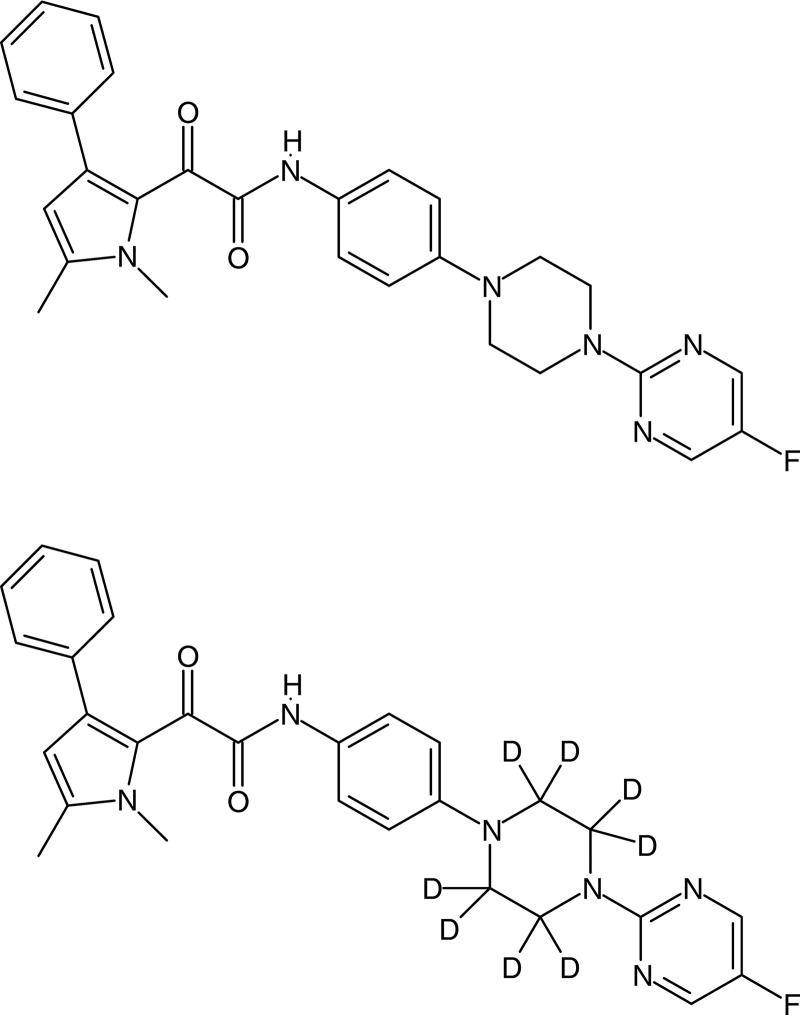
F901318 (F2G, Ltd., Manchester, United Kingdom) (Fig. 1) is a novel investigational agent that belongs to the orotomide class of compounds. It is a highly specific, competitive inhibitor of the oxidoreductase enzyme dihydroorotate dehydrogenase (DHODH), which is important for pyrimidine biosynthesis (3, 4). The activity of F901318 is fungus specific, as its activity against A. fumigatus DHODH is significantly more potent than that of the human enzyme (50% inhibitory concentration [IC50], 0.044 μM versus >90 μM, respectively) (1, 3). Potent in vitro activity against other Aspergillus species, including azole-resistant A. fumigatus, has also been observed (1). F901318 has demonstrated potent in vitro activity against Aspergillus spp., Scedosporium spp., and Lomentospora prolificans (5–7). Other rare molds considered uniformly susceptible include Penicillium spp., e.g., Penicillium (Talaromyces) marneffii, Rasamsonia spp., Acremonium spp., Scopulariopsis brevicaulis, Microascus spp., Trichoderma spp., Paecilomyces spp., Pleurosomophora spp., Phaeoacremonium spp., Sporothrix schenckii, Acrophialaphora spp., and Chaetomium spp. F901318 is also active against the agents of endemic mycoses, Blastomyces dermatitidis, Coccidioides immitis, Coccidioides posadasii, and Histoplasma capsulatum, and dermatophytic fungi, Epidermophyton floccosum, Microsporum spp., and Trichophyton spp. All Candida species, Cryptococcus spp., and, among the clinically relevant molds, the Mucorales group (Zygomycetes) are considered resistant (2).
FIG 1.
Structural formulas of F901318 and deuterated internal standard.
To expand the knowledge regarding the pharmacokinetics of F901318 in patients, an easy-to-use method has been developed to analyze serum and plasma samples in small sample volumes. The present method sets the stage for pharmacokinetic studies with patients of all ages with invasive fungal infections.
RESULTS
Liquid chromatography and mass spectrometry (selectivity).
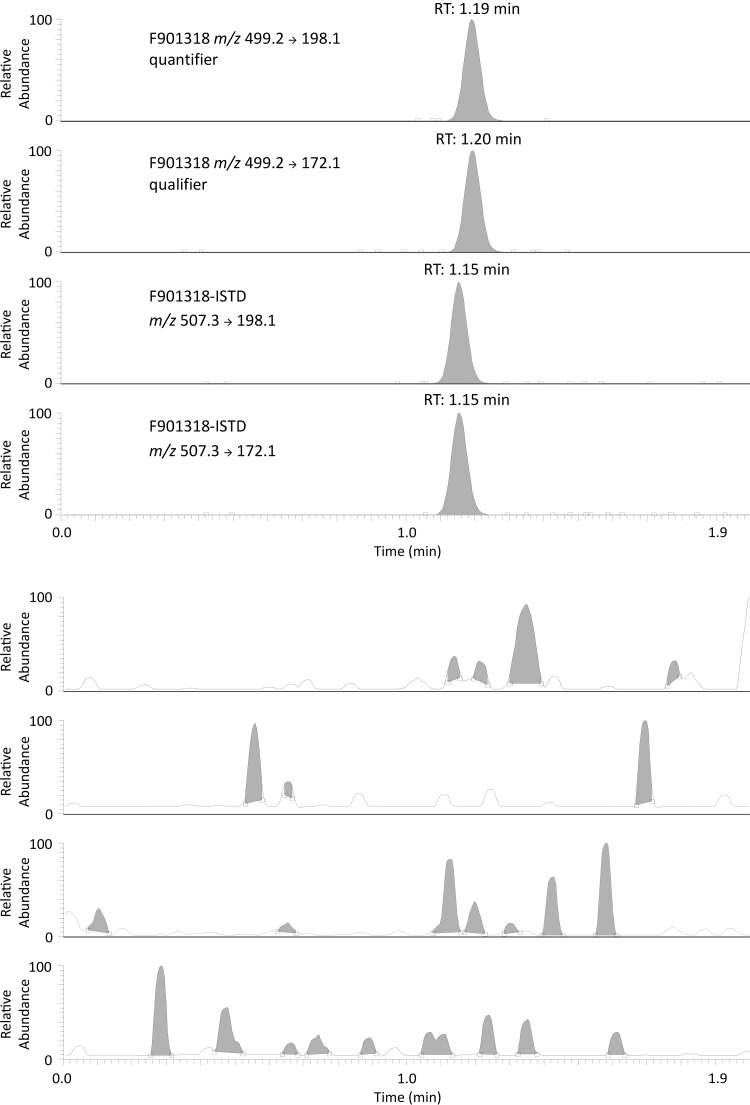
Representative chromatograms of the lowest calibration standard and blank plasma sample are presented in Fig. 2. The extraction procedure consisted of protein precipitation with acetonitrile and subsequent centrifugation. Within a total runtime of 2.00 min, the expected retention times of F901318 and its deuterated internal standard were 1.19 min and 1.15 min, respectively. No significant difference was detectable between analyses in serum and plasma (8).
FIG 2.
Representative chromatograms with quantifier and qualifier traces of an extracted blank plasma sample and F901318 with its deuterated internal standard of the lowest calibration standard, upper four traces (c = 0.05 mg/liter).
The following selected reaction monitoring (SRM) transitions were found during method optimization (quantifier/qualifier): F901318 m/z 499.2 → 198.1/172.1 and isotopically labeled internal standard m/z 507.3 → 198.1/172.1 (Table 1).
TABLE 1.
Mass spectrometer parameter settings
| Analyte | Parent (m/z) | Product (m/z) | CE (eV) | S-lens (V) |
|---|---|---|---|---|
| F901318a | 499.2 | 172.1 | 24 | 114 |
| F901318b | 499.2 | 198.1 | 19 | 114 |
| [2H8]F901318a | 507.3 | 172.1 | 26 | 115 |
| [2H8]F901318b | 507.3 | 198.1 | 20 | 115 |
Qualifier.
Quantifier.
Linearity.
Validation of linearity was tested with the Mandel test according to the method of Kromidas (8). The calibration curve was fitted by using least-squares regression with a weighting factor of the reciprocal concentration (1/x). The correlation coefficients of these curves were 0.999 or better. Table 2 shows the concentration range, the Mandel test values (Fcalc), and the Ftab values to prove linearity.
TABLE 2.
Validation of linearity tested with Mandel test according to the method of Kromidas (8)
| Analyte | Concn range (mg/liter) | Test value (Fcalc = Ds2/SyQ2)a | Ftab value | Proof of linearity |
|---|---|---|---|---|
| F901318 | 0.05–12.00 | 9.977 | 21.1977 | Yes: y = 0.34986x + 0.038507; R2 = 0.99955 |
Ds2, variance difference; SyQ2, variance of quadratic regression fit.
Precision and accuracy.
Precisions and accuracies were evaluated over the entire concentration range (Table 3). Seven individual samples were measured on the same day for the intraday assay, and two individual samples were measured on 7 days for the interday assay. The accuracies were within ±5.1% for F901318. The intraday precisions (relative standard deviations [RSDs]) were within 3.2% for F901318, while the interday assays showed a variability of up to 3.3%. Table 3 shows the RSDs and the systematic error/bias for the three quality control (QC) levels.
TABLE 3.
Precision and accuracy at three IQC levels for the intraday and interday assays
| Analyte | QC level | Specified concn (mg/liter) | Intraday (n = 7) |
Interday (n = 7) |
||
|---|---|---|---|---|---|---|
| Precision, RSDa (%) | Accuracy, bias (%) | Precision, RSD (%) | Accuracy, bias (%) | |||
| F901318 | QC1 | 0.150 | 3.13 | 5.01 | 1.94 | 4.90 |
| QC2 | 4.000 | 1.88 | 0.85 | 3.23 | 0.59 | |
| QC3 | 11.000 | 1.17 | 0.75 | 2.37 | 0.92 | |
RSD, relative standard deviation.
Serum-plasma comparison.
No significant difference was obtained after extraction and analysis (Student's t test) of a total of 20 samples of the spiked serum and spiked plasma, respectively. Therefore, the present method is suitable for serum and plasma samples.
Limit of detection (LOD), lower limit of quantification (LLOQ), and carryover.
Calculated limits of detection and lower limits of quantification were 0.0011 mg/liter and 0.0033 mg/liter, respectively. The LLOQ is significantly lower than the lowest calibration standard concentration; thus, the method is perfectly suited for measuring even very low trough concentrations. Carryover results—expressed as a percentage of the analyte peak area ratio of blank sample and the preceding calibration standard sample—showed no relevant interfering peak signal ratios (<20% of LLOQ concentrations).
Stability.
Short-term stability was demonstrated for different conditions, and results for F901318 remained within the prespecified acceptance criterion of ±15%. Thus, no significant decrease in drug concentration was substantiated; i.e., mean changes in concentration of the analyte were within the variability of the method.
Recovery.
Mean extraction recoveries after protein precipitation with acetonitrile were 89.6%, 92.8%, and 94.0% for QC1, QC2, and QC3, respectively.
Matrix effects and carryover.
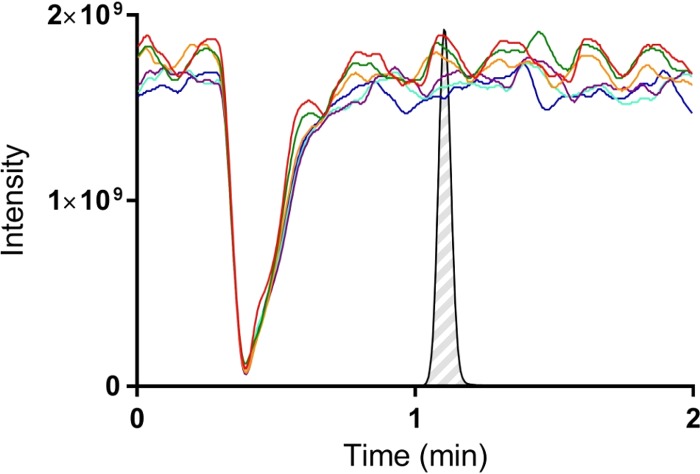
Post-column-infusion experiments revealed a distinct suppression of the MS/MS signal of all mass transitions between 0.31 and 0.63 min and nearly complete ion suppression at 0.38 min. Therefore, quantification of F901318 is not affected by the ion suppression phenomenon (Fig. 3). No relative matrix effects (RME) were observed for F901318 at all QC levels, indicating that the isotopically labeled orotomide analogue used for internal standardization fully compensated for these effects. No carryover was observed (<20% of LOQ concentrations).
FIG 3.
Suppression-enhancement-ionization (SEI) experiment (post-column infusion). Drifts and shifts of MS/MS signal intensities of F901318 (during analysis of six extracted blank plasma samples [indicated by colored lines]). MS/MS signal intensities were plotted against time. A representative chromatogram of F901318 is shown.
Discussion and conclusion.
F901318 is currently in clinical development. Therefore, only limited data have been published about instrumental analytics, method development, and determination of plasma and/or serum concentrations of this new antifungal drug.
Hope et al. describe a method to determine F901318 in murine plasma using high-performance liquid chromatography (HPLC; online extraction procedure; 40-μl sample volume) and UV detection within a concentration range of 0.01 to 20.00 mg/liter. The limit of detection was 0.01 mg/liter, and F901351—an orotomide derivative—was used as an internal standard (4). All relevant validation parameters were within the expected specification limits, although the analytes were eluted comparatively slowly (4.9 min for F901318 and 1.9 min for F901351).
Our method was performed based on a runtime of 2 min (F901318 was eluted at retention time of 1.19 min), and we provided evidence that the present assay is linear within a therapeutically relevant concentration range of 0.05 to 12 mg/liter of F901318.
Negri et al. (reference 9 and related supplemental material) used a mass spectrometric assay, although data about monitored transitions (m/z) and retention times were not specified. The extraction procedure consisted of precipitation of 100 μl of matrix with acetonitrile (containing phenacetin as an internal standard). Chromatographic separation was achieved for F901318 using a gradient method. The standard curve for F901318 encompassed a concentration range of 0.001 to 20.0 mg/liter; however, no information about accuracies and precisions was provided.
Our method was optimized with a smaller sample volume (50 μl), which is advantageous in the patient setting. With regard to the use of an internal standard, we chose an isotopically labeled version of F901318 since extraction recovery, ionization response, and chromatographic retention time are similar to those of the parent molecule, which provided ideal properties for internal standardization, especially for mass spectrometric analyses with electrospray ionization. For therapeutic drug monitoring purposes and pharmacokinetic studies, one needs to keep in mind that when performing analyses with citrate plasma, dilution of the sample by 10% will occur due to the presence of liquid citrate in clinical sample tubes. Quantification of F901318 in human plasma and serum is conveniently feasible. When using other plasma matrices, such as EDTA plasma, validity of the method needs to be verified in alternative matrices prior to its application.
Conclusion.
The present LC-MS/MS method for the determination of F901318 in human plasma and serum met all required validation parameters and sets the stage for the rapid collection of pharmacokinetic data in patient trials. This method exhibited good interday assay and intraday assay precision and accuracy over the F901318 calibration range of 0.05 mg/liter to 12 mg/liter. Protein precipitation with acetonitrile is a simple, rapid, sensitive, and selective method that requires no additional extraction procedures.
MATERIALS AND METHODS
Chemicals and reagents.
F901318 and the deuterated F901318 (D8) analogue were kindly provided by F2G Ltd. (Manchester, United Kingdom). Acetonitrile was purchased from Merck (Darmstadt, Germany) in LC-MS standard quality, and methanol was purchased from Roth (Karlsruhe, Germany). Distilled water was purified with a Milli-Q Plus ultrapure water system (Millipore Corporation, Bedford, MA). Blood plasma samples and pooled serum samples were obtained from the Department of Transfusion Medicine of the University Hospital of Cologne.
Instrumentation and LC-MS/MS conditions.
Experiments were carried out on a calibrated TSQ Vantage triple-stage quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, CA), which was operated in selected reaction monitoring (SRM) mode using heated positive electrospray ionization (HESI+). The mass spectrometric parameters were as follows: MS runtime (tstop), 2.00 min; Q2 gas pressure, 1.5 mTorr (argon); nitrogen was the sheath and auxiliary gas; spray voltage (U), 4.0 kV; evaporation temperature (ϑHESI), 350°C; and capillary temperature (ϑCT), 300°C. Chromatographic separation was performed on an Accela system (Thermo Fisher Scientific) equipped with an Accela 1250 pump and an Accela autosampler. A Hypersil Gold C18 column (50 by 2.1 mm; 1.9-μm particle size) was used, and the column oven was maintained at 45°C. The mobile phase was composed of 0.1% formic acid–acetonitrile (60%-40%, vol/vol), and the flow rate was set to 330 μl/min.
Stock solutions, calibration standards, and quality controls.
Known amounts of F901318 were dissolved in a solution of acetonitrile-methanol (33%-67%, vol/vol). Two solutions were prepared which served as the primary stocks for preparation of calibration standards (CS) and quality controls (QCs), respectively. Working stock solutions were arranged by diluting the primary stock solutions in acetonitrile-methanol solution. In order to reduce the potential matrix effects resulting from different compositions of solvents and biological matrices, the ratios of working stock solutions and blank plasma volumes used for preparation of CS and QC samples were kept constant at 2%. CS were prepared at different concentrations from one stock solution (cF901318 = 819.06 mg/liter). CS concentrations (CS1 to CS7) were 0.05, 0.5, 1.5, 3.0, 5.0, 8.0, and 12.0 mg/liter. QC were prepared at low, medium, and high concentrations from the other stock solution (cF901318 = 616.28 mg/liter). QC concentrations (QC1 to QC3) were 0.15, 4.0, and 11.0 mg/liter. CS and QC samples were aliquoted and stored at −80°C until use. D8-F901318 was dissolved in acetonitrile-methanol solution (cD8-F901318 = 34.72 mg/liter).
Sample preparation.
Samples were thawed and briefly vortexed. Fifty-microliter volumes of CS and QC samples were admixed with 50 μl of internal standard solution and 200 μl of acetonitrile. After vortexing for 30 s, the mixture was centrifuged at 4°C and 15,000 × g for 10 min. The clear supernatant was transferred to LC-MS vials (Macherey-Nagel, Düren, Germany) and subjected to LC-MS/MS analysis.
Method of validation.
Validation of the method was performed by means of linearity testing across the calibration range, assessment of inter- and intraday variabilities, determination of the limit of detection (LOD) and lower limit of quantification (LLOQ), assessment of recovery and matrix effects, and stability testing based on recommendations of International Conference on Harmonisation guideline Q2(R1) (Validation of Analytical Procedures) and the U.S. Food and Drug Administration (FDA) (10, 11).
Linearity.
A linearity experiment was conducted by repeated measurements of seven CS concentrations (seven times each) over the concentration range of 0.05 to 12.0 mg/liter. Peak area ratios of analytes and internal standard were plotted against concentrations of the analyte to obtain calibration curves. Linearity was then evaluated according to the method of Mandel, and the confidence intervals as well as the ranges for all the seven CS were calculated. Calculated F values (Fcalc) found at the 99% confidence interval were compared to the tabulated limit (Ftab). Fcalc values lower than those reported in the tabulated limit indicate a significantly better fit for linear regression.
Accuracy and precision.
To exclude any significant interfering signals at baseline, blank plasma samples (with and without internal standard) were analyzed before each calibration. Intraday variability was assessed by measuring seven individually extracted QC samples at each QC level once on the same day. Interday variability was assessed by measuring the QC samples at each QC level in duplicates on seven different days. Precision was then calculated as ratio of the standard deviation of the observed concentration and the mean concentration, expressed as relative standard deviation (RSD). Meanwhile, accuracy was calculated as the ratio of mean and specified concentration, expressed as bias (percent).
Serum-plasma comparison.
Pooled human serum (n = 6) and pooled citrate plasma (n = 6) were spiked with stock solution containing F901318 to obtain concentrations within the working range of the CS samples (c = 3.0 mg/liter). A total of 10 samples of the spiked serum and of the spiked plasma each were extracted and analyzed. The results were then evaluated using Student's t test (differences were calculated based on mean t test). If the calculated t was larger than the critical one, the difference was considered to be significant.
Limit of detection and lower limit of quantification.
Six individual blank plasma samples were extracted in duplicate (total, 12), and the standard deviation (SD) of the blank responses was evaluated. A calibration curve was generated to determine the slopes of the regression line. LOD was calculated using the equation LOD = 3.3 σ/S ′, where σ is the SD of the blank response and S′ is the slope of the calibration curve. Similarly, the LLOQ was calculated using the equation LLOQ = 10 σ/S′.
Recovery, matrix effects, and carryover.
Extraction recovery was tested using six individual blank plasma samples for all three QC levels. One set of the blank plasma samples was spiked with the working solutions to the final QC levels, extracted, and analyzed, while another set was extracted first and subsequently spiked with the working solutions to obtain final QC levels as references. Extraction recovery was then calculated as the ratio of the MS analyte response areas obtained in both sets of matrices. Suppression-enhancement-ionization (SEI) phenomena were visualized by continuous postcolumn infusion of F901318 during the analysis of 6 different lots of extracted blank plasma samples (Fig. 3). Relative matrix effects (RME) were assessed by comparing MS/MS responses obtained in the 6 lots of blank plasma spiked with F901318 to final QC levels. RME were expressed as variability of obtained MS/MS responses (coefficient of variation). Carryover was assessed by alternately injecting the highest calibration standard (c = 40 mg/liter) and six different lots of blank plasma samples. Carryover was calculated as the analyte peak area ratio of blank sample and the preceding calibration standard sample.
Stability.
Short-term stability was tested under different conditions. Bench stability was evaluated by thawing five individual QC samples at all three QC levels and keeping them at room temperature for 12 and 24 h. For the postpreparative stability (autosampler) evaluation, five individually extracted QC samples at the low and high QC levels were measured after storage in the autosampler at 23°C for 24 h. Freeze-thaw stability was assessed after 3 consecutive freeze-thaw cycles of three individually extracted QCs at each QC level. Each thaw process lasted 1 h, and the samples were then frozen again overnight at −18°C. All measured samples were then compared to freshly prepared QC samples at the various levels. QC samples were considered stable if the relative error was within ±15% for the different conditions.
REFERENCES
- 1.Wiederhold NP. 2017. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist 10:249–259. doi: 10.2147/IDR.S124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiederhold NP. 2018. The antifungal arsenal: alternative drugs and future targets. Int J Antimicrob Agents 51:333–339. doi: 10.1016/j.ijantimicag.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Oliver JD, Sibley GE, Beckmann N, Dobb KS, Slater MJ, McEntee L, du Pre S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 113:12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope WW, McEntee L, Livermore J, Whalley S, Johnson A, Farrington N, Kolamunnage-Dona R, Schwartz J, Kennedy A, Law D, Birch M, Rex JH. 2017. Pharmacodynamics of the orotomides against Aspergillus fumigatus: new opportunities for treatment of multidrug-resistant fungal disease. mBio 8:e01157-17. doi: 10.1128/mBio.01157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas C, Law D, Birch M, Halliday C, Sorrell TC, Rex J, Slavin M, Chen SC. 2018. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol doi: 10.1093/mmy/myx161. [DOI] [PubMed] [Google Scholar]
- 6.Buil JB, Rijs A, Meis JF, Birch M, Law D, Melchers WJG, Verweij PE. 2017. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 72:2548–2552. doi: 10.1093/jac/dkx177. [DOI] [PubMed] [Google Scholar]
- 7.Wiederhold NP, Law D, Birch M. 2017. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 72:1977–1980. doi: 10.1093/jac/dkx065. [DOI] [PubMed] [Google Scholar]
- 8.Kromidas S. 2011. Validierung in der Analytik. John Wiley & Sons, Weinheim, Germany. [Google Scholar]
- 9.Negri CE, Johnson A, McEntee L, Box H, Whalley S, Schwartz JA, Ramos-Martin V, Livermore J, Kolamunnage-Dona R, Colombo AL, Hope WW. 2018. Pharmacodynamics of the novel antifungal agent F901318 for acute sinopulmonary aspergillosis caused by Aspergillus flavus. J Infect Dis 217:1118–1127. doi: 10.1093/infdis/jix479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA. 2018. Guidance for industry: bioanalytical method validation (2018). FDA, Washington, DC: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf. [Google Scholar]
- 11.ICH. 2011. Validation of analytical procedures: text and methodology, Q2(R1) Geneva, 2005. ICH, Geneva, Switzerland. [Google Scholar]