This study characterizes the pharmacodynamics of antimicrobial prophylaxis and sternal wound infections following cardiac surgery. Duration of surgery and cefazolin plasma concentration during wound closure were independently associated with surgical site infection at 30 days.
KEYWORDS: surgical prophylaxis, cefazolin, pharmacodynamics, cardiac surgery, surgical site infection, wound infection
ABSTRACT
This study characterizes the pharmacodynamics of antimicrobial prophylaxis and sternal wound infections following cardiac surgery. Duration of surgery and cefazolin plasma concentration during wound closure were independently associated with surgical site infection at 30 days. Furthermore, a duration of surgery of >346 min and a total cefazolin closure concentration of <104 mg/liter were significant thresholds for an increased risk of infection. This study provides new data that informs dosing strategies for effective antimicrobial prophylaxis (AP) in patients undergoing cardiac surgery with cardiopulmonary bypass.
TEXT
Interventions such as antimicrobial prophylaxis (AP) that prevent surgical site infection (SSI) are associated with significant reductions in patient morbidity, mortality, and health care costs (1, 2). The goal of AP is to maintain effective plasma and tissue concentrations from surgical incision to closure, thereby reducing the risk of postoperative wound infection (3). Although antimicrobial pharmacodynamics that relate drug concentrations to clinical outcome are well established for the treatment of infectious diseases (4, 5), the study of antimicrobial activity and target concentrations for effective AP are limited (6, 7). Current clinical practice guidelines emphasize that “clarification is needed regarding targeted antimicrobial concentrations and intraoperative monitoring…to optimize efficacy” (3). One previous study characterized the pharmacodynamics of gentamicin (plus metronidazole) prophylaxis in patients undergoing colorectal surgery (8). Notably, gentamicin plasma concentration during wound closure was one of the strongest independent risk factors for SSI.
AP is particularly beneficial in cardiac surgery, where serious and potentially life-threatening infections, such as mediastinitis, can be prevented (1, 2, 9). In this population, AP must also account for complex and prolonged procedures, as well as for altered pharmacokinetics with cardiopulmonary bypass (CPB) (10). In the absence of defined targets, there are various approaches that adjust AP for cardiac surgery by using higher doses, more frequent redosing, or continuous infusions (11). Although the benefits versus risks have not been established, it is also common practice to extend “prophylaxis” for 24 to 48 h postoperatively. Our goal was to conduct the first pharmacodynamic study of AP in cardiac surgery with the hypothesis that low intraoperative cefazolin concentrations increase the risk of postoperative wound infection. The objective was to identify clinically relevant targets for effective AP in patients undergoing cardiac surgery with CPB.
A secondary pharmacodynamic analysis was conducted using data from a published pharmacokinetic study of cefazolin prophylaxis in elective cardiac surgery with CPB (August 2014 to May 2015) (11). The study received approval from the University of Manitoba Health Research Ethics Board (approval no. H2014:142). Patients (creatinine clearance of ≥50 ml/min/72 kg) received cefazolin prophylaxis according to our institutional protocol (i.e., administered within 60 min prior to incision, every 4 h during surgery, and every 8 h for 48 h postoperatively). Blood samples were collected 30 min after the preoperative dose, prior to redosing during surgery, and within 15 min of wound closure (closure concentration). Cefazolin concentrations in plasma (total) and ultrafiltrate (free) were determined by liquid chromatography-tandem mass spectrometry (Shimadzu 8040), using a stable isotope as the internal standard (i.e., 13C215N cefazolin) (12, 13).
Patient demographics, medical history, relevant clinical and laboratory data, and surgery details were obtained from the patients' medical records. Patients were monitored for SSI during hospitalization, (14) and were contacted 30 days postsurgery to document wound infections requiring systemic antimicrobial therapy that presented after discharge. Potential risk factors for SSI were examined using univariate analysis (Table 1), and significant variables (P < 0.1) were included in multivariate logistic regression (MVLR) analysis to test conditional associations with infection. MVLR models were evaluated by examining area under receiver operating curves (AUROC) and the Hosmer-Lemeshow goodness of fit test. For continuous variables, significant thresholds for increased risk of SSI were identified using classification and regression tree (CART) analysis. (SYSTAT 13; Systat Software Inc., San Jose, CA).
TABLE 1.
Univariate analysis of variables in patients with and in those without surgical site infection
| Patient variablea | SSIc |
P value | |
|---|---|---|---|
| With (n = 8) | Without (n = 32) | ||
| Male | 5 (62.5) | 20 (62.5) | 1.00 |
| Age (yrs) | 63 ± 12 | 66 ± 9 | 0.47 |
| Weight (kg) | 95.7 ± 21.8 | 86.2 ± 14.4 | 0.28 |
| Body mass index ≥35 kg/m2 | 3 (37.5) | 3 (9.4) | 0.082 |
| CLCR (ml/min/72 kg)b | 86.5 ± 25.0 | 78.7 ± 16.5 | 0.42 |
| Diabetes mellitus | 4 (50.0) | 9 (28.1) | 0.40 |
| Charlson comorbidity index | 3.0 (2.5–3.0) | 3.0 (2.0–4.0) | 0.75 |
| CABG with or without other procedure | 6 (75.0) | 22 (68.8) | 1.00 |
| Duration of surgery (min) | 324 ± 100 | 266 ± 60 | 0.045 |
| Albumin at end of surgery (g/liter) | 30.9 ± 1.7 | 31.5 ± 3.0 | 0.45 |
| Glucose at end of surgery (mmol/liter) | 8.7 ± 1.5 | 8.2 ± 1.5 | 0.39 |
| Fluid balance (ml) | 4,169 ± 1,207 | 3,422 ± 1,188 | 0.15 |
| Surgery complications | 1 (12.5) | 3 (9.4) | 1.00 |
| Hospital stay following surgery (days) | 4.5 (4.0–7.3) | 5.0 (4.0–6.3) | 0.58 |
| Preoperative cefazolin dose (mg/kg) | 22.9 ± 6.1 | 23.6 ± 5.3 | 0.77 |
| Timing of preoperative cefazolin dose (min prior to incision) | 36.3 ± 6.1 | 34.3 ± 13.9 | 0.55 |
| Redosing of cefazolin during surgery | 7 (87.5) | 23 (71.9) | 0.65 |
| Total cefazolin dose, including preoperative and redosing during surgery (mg/kg for every h of surgery) | 8.6 ± 2.5 | 9.0 ± 2.3 | 0.64 |
| Cefazolin closure concn (mg/liter) | |||
| Total | 70.4 ± 35.6 | 105.9 ± 57.8 | 0.042 |
| Free | 21.6 ± 14.8 | 35.5 ± 27.9 | 0.066 |
Data are presented as no. (%), mean ± standard deviation, or median (interquartile range).
See Ariano et al. (18).
SSI, surgical site infection.
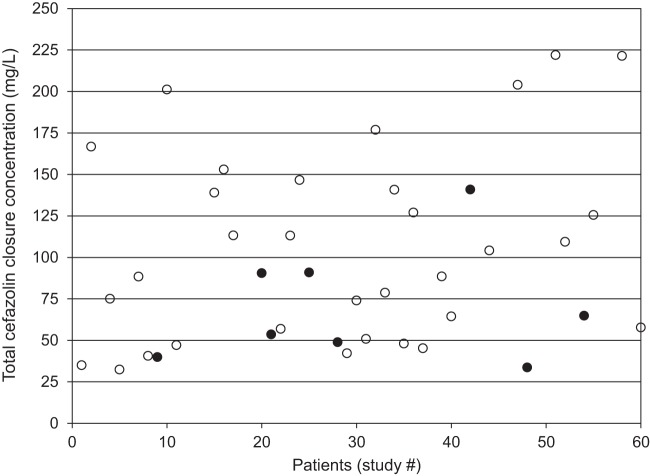
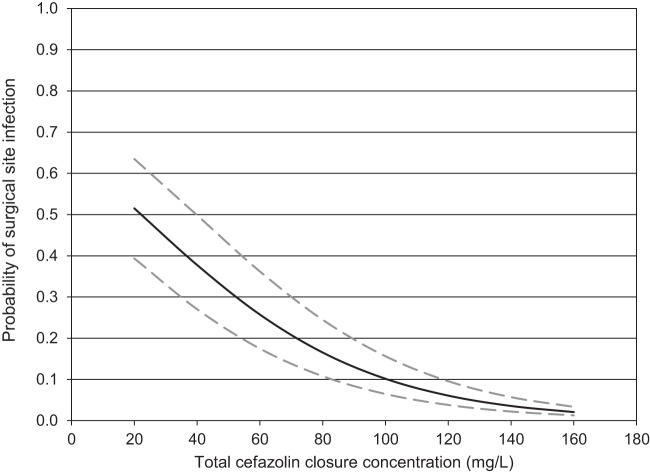
Forty patients (62.5% male; mean age 65 ± 10 years; mean weight, 88.1 ± 16.3 kg) were included in the pharmacodynamic study. Seventy percent (28/40) of surgeries involved coronary artery bypass grafting with or without another procedure, and 30% (12/40) were an isolated valve replacement/repair. The mean preoperative cefazolin dose was 23.5 ± 5.4 mg/kg administered 35 ± 13 min prior to incision, and the duration of surgery was 278 ± 74 min. The mean total cefazolin concentration during wound closure was 98.8 ± 55.6 mg/liter (Fig. 1), whereas the free closure concentration was 32.8 ± 26.2 mg/liter. Eight cases of superficial sternal SSI were documented, including six that presented after discharge. Only lower total cefazolin closure concentration (P = 0.038; odds ratio [OR] = 1.3 per 10% decrease) and longer duration of surgery (P = 0.027; OR = 2.9 per hour increase) were independently associated with SSI (AUROC = 0.789; 95% confidence interval [CI] = 0.583 to 0.996; Hosmer-Lemeshow P = 0.21) (Fig. 2). Duration of surgery of >346 min (60.0% versus 14.3% SSI) and total cefazolin closure concentration of <104 mg/liter (30.4% versus 5.9% SSI) were significant thresholds for an increased risk of infection.
FIG 1.
Total cefazolin closure concentrations in patients undergoing cardiac surgery with cardiopulmonary bypass (n = 40), where solid circles are patients with surgical site infection (SSI) and open circles are those without SSI.
FIG 2.
Probability of surgical site infection based on the logistic model of total cefazolin closure concentration stratified for duration of surgery, where the solid line represents the median duration of surgery of 230 min, the lower hatched line is the 25th percentile (200 min), and the upper hatched line is the 75th percentile (260 min).
The importance of AP pharmacodynamics during cardiac surgery was first suggested by Goldmann et al., who found that patients with longer procedures and lower cephalothin concentrations during valve replacement surgery were more likely to develop wound infection (15). In a more recent study of cefazolin prophylaxis, Kosaka et al. noted a higher incidence of SSI in patients with better renal function, a potential surrogate for lower cefazolin concentrations during surgery (16). Our study provides the first direct evidence of a relationship between intraoperative antimicrobial concentrations during cardiac surgery and postoperative wound infection. The finding was particularly notable where the relevance of closure concentration was evident even though “prophylaxis” was continued for 48 h postoperatively. Furthermore, the lack of correlation between cefazolin closure concentration and duration of surgery (r2 = 0.08) in our analysis supported the independence of these risk factors.
The pharmacodynamics of AP in our study were best described by the total as opposed to the free (pharmacologically active) cefazolin concentrations. Given the significant variability in protein binding during cardiac surgery with CPB, it is likely that total concentration was a more stable predictive variable for SSI. In the absence of established targets, AP regimens are evaluated indirectly, typically based on their ability to cover the most common “potential” pathogens. For example, the suggested target for cefazolin prophylaxis is usually based on maintaining concentrations at or above the susceptible MIC breakpoint of 8 mg/liter, equivalent to a total concentration of 40 mg/liter (estimated protein binding of 80% in patients) (11, 17). Notably, our study supports a significantly higher threshold of ≥104 mg/liter, or free concentration of 29 mg/liter, using our measured protein binding of 72% in patients undergoing cardiac surgery. First, it is important to consider that CART analysis is dependent on the distribution of data and does not represent the only definitive target. However, our finding may also indicate the need for higher cefazolin concentrations during cardiac surgery where sternal wound closures are more complex and prolonged than others. It may also signify the need for higher concentrations with bactericidal, as opposed to bacteriostatic, activity against the contamination of sternal wounds. Finally, effective AP targets may be influenced by local distributions of SSI pathogens and antimicrobial susceptibilities. Since most SSIs presented after discharge, microbiology data were not available for our analysis of cefazolin prophylaxis. For context, however, most sternal infections are associated with Staphylococcus spp. In those documented from 2014 to 2016 in our institution, coagulase-negative staphylococcus and Staphylococcus aureus strains accounted for 54% and 18% of SSI pathogens, respectively. Compared to our threshold of 104 mg/liter (29 mg/liter free), MIC data (equivalent to free concentrations) from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) showed that 87% (1,296/1,498) of methicillin-susceptible Staphylococcus epidermidis isolates had MICs of ≤16 mg/liter, and all but one isolate had MICs of ≤32 mg/liter (see https://mic.eucast.org/Eucast2/SearchController/search.jsp?action=init). Ninety-two percent (17,708/19,252) of methicillin-susceptible S. aureus isolates had MICs of ≤16 mg/liter and 99% had MICs of ≤32 mg/liter (see https://mic.eucast.org/Eucast2/SearchController/search.jsp?action=init).
Although our study was limited by a small sample size, it still detected the significance of antimicrobial pharmacodynamics (i.e., closure concentration) in patients undergoing cardiac surgery. Our retrospective design was also a limitation, where the concentration data were the consequence of an institutional protocol for cefazolin prophylaxis, as opposed to those of dosing regimens designed to achieve a certain distribution of concentrations. Finally, our primary clinical outcome (i.e., SSI) was prospectively monitored for 30 days postsurgery; however, infections that presented after discharge were documented through phone interviews.
In conclusion, this study supports the important role of antimicrobial pharmacodynamics, particularly plasma concentration during wound closure, for effective AP. It further informs dosing strategies for cefazolin prophylaxis in patients undergoing cardiac surgery with CPB.
ACKNOWLEDGMENTS
We appreciate the support of the Cardiac Science Program at the St. Boniface Hospital, including the surgeons, anesthesiologists, and, in particular, Brett Hiebert (programmer and statistical analyst).
The original pharmacokinetic study was supported by a Canadian Society of Hospital Pharmacists foundation grant (UM#317380), Leslie F. Buggey professorship (S.Z.), and a University of Manitoba graduate fellowship (D.C.). The Pharmaceutical Analysis Laboratory (TML) was also supported by grants from the Dr. Paul H.T. Thorlakson Foundation, the University of Manitoba Research Program, and the Natural Sciences and Engineering Research Council of Canada.
We have no conflicts of interest to declare.
REFERENCES
- 1.Berríos-Torres S, Umscheid C, Bratzler W, Leas B, Stone E, Kelz R, Reinke C, Morgan S, Solomkin J, Mazuski JE, Dellinger P, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP, for the Healthcare Infection Control Practices Advisory Committee . 2017. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection. JAMA Surg 152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2016. Global guidelines for the prevention of surgical site infection. World Health Organization, Geneva, Switzerland: http://www.who.int/gpsc/ssi-prevention-guidelines/en/. [Google Scholar]
- 3.Bratzler D, Dellinger E, Olsen K, Perl T, Auwaerter P, Bolon M, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 4.Tängdén T, Ramos Martín V, Felton TW, Nielsen EI, Marchand S, Brüggemann RJ, Bulitta JB, Bassetti M, Theuretzbacher U, Tsuji BT, Wareham DW, Friberg LE, De Waele JJ, Tam VH, Roberts JA, Infection Section for the European Society of Intensive Care Medicine the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases the International Society of Anti-Infective Pharmacology and the Critically Ill Patients Study Group of European Society of Clinical Microbiology and Infectious Diseases . 2017. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med 43:1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- 5.Labreche M, Graber C, Nguyen H. 2015. Recent updates on the role of pharmacokinetics-pharmacodynamics in antimicrobial susceptibility testing as applied to clinical practice. Clin Infect Dis 61:1446–1452. doi: 10.1093/cid/civ498. [DOI] [PubMed] [Google Scholar]
- 6.Zelenitsky S, Lawson C, Calic D, Ariano R, Roberts J, Lipman J, Zhanel GG. 2016. Integrated pharmacokinetic-pharmacodynamic modelling to evaluate antimicrobial prophylaxis in abdominal surgery. J Antimicrob Chemother 71:2902–2908. doi: 10.1093/jac/dkw247. [DOI] [PubMed] [Google Scholar]
- 7.Moine P, Fish D. 2013. Pharmacodynamic modelling of intravenous antibiotic prophylaxis in elective colorectal surgery. Int J Antimicrob Agents 41:167–173. doi: 10.1016/j.ijantimicag.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Zelenitsky S, Ariano R, Harding G, Silverman R. 2002. Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob Agents Chemother 46:3026–3030. doi: 10.1128/AAC.46.9.3026-3030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2009. WHO guidelines for safe surgery: safe surgery saves lives. World Health Organization, Geneva, Switzerland: http://www.who.int/patientsafety/safesurgery/tools_resources/9789241598552/en/. [PubMed] [Google Scholar]
- 10.Paruk F, Sime F, Lipman J, Roberts J. 2017. Dosing antibiotic prophylaxis during cardiopulmonary bypass-a higher level of complexity? A structured review. Int J Antimicrob Agents 49:395–402. doi: 10.1016/j.ijantimicag.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Calic D, Ariano R, Arora R, Grocott H, Lakowski T, Lillico R, Zelenitsky S. 2018. Evaluation of cefazolin antimicrobial prophylaxis during cardiac surgery with cardiopulmonary bypass. J Antimicrob Chemother 73:768–771. doi: 10.1093/jac/dkx439. [DOI] [PubMed] [Google Scholar]
- 12.Lillico R, Sayre C, Sitar D, Davies NM, Baron CM, Lakowski TM. 2016. Quantification of cefazolin in serum and adipose tissue by ultra high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS): application to a pilot study of obese women undergoing cesarean delivery. J Chromatogr B Analyt Technol Biomed Life Sci 1031:94–98. doi: 10.1016/j.jchromb.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration (FDA). 2001. Bioanalytical method validation: guidance for industry. Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD. http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- 14.Centers for Disease Control and Prevention. 2014. Surgical site infection (SSI) event. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. [Google Scholar]
- 15.Goldmann DA, Hopkins CC, Karchmer AW, Abel RM, McEnany MT, Akins C, Buckley MJ, Moellering RC. 1977. Cephalothin prophylaxis in cardiac valve surgery: a prospective, double-blind comparison of two-day and six-day regimens. J Thorac Cardiovasc Surg 73:470–479. [PubMed] [Google Scholar]
- 16.Kosaka T, Hosokawa K, Shime N, Taniguchi F, Kokufu T, Hashimoto S, Fujiwara H, Yaku H, Sugioka N, Okada K, Fujita N. 2012. Effects of renal function on the pharmacokinetics and pharmacodynamics of prophylactic cefazolin in cardiothoracic surgery. Eur J Clin Microbiol Infect Dis 31:193–199. doi: 10.1007/s10096-011-1293-z. [DOI] [PubMed] [Google Scholar]
- 17.Aalbers M, ter Horst PGJ, Hospes W, Hijmering ML, Spanjersberg AJ. 2015. Targeting cefuroxime plasma concentrations during coronary artery bypass graft surgery with cardiopulmonary bypass. Int J Clin Pharm 37:592–598. doi: 10.1007/s11096-015-0101-8. [DOI] [PubMed] [Google Scholar]
- 18.Ariano RE, Zelenitsky SA, Poncsak KR, Davis JC, Vercaigne LM. 2017. No role for patient body weight on renal function assessment for drug dosing. J Antimicrob Chemother 72:1802–1811. doi: 10.1093/jac/dkx036. [DOI] [PubMed] [Google Scholar]