There is a critical need for new antibacterial strategies to counter the growing problem of antibiotic resistance. In Gram-negative bacteria, the outer membrane (OM) provides a protective barrier against antibiotics and other environmental insults.
KEYWORDS: ABC transporters, Enterobacteriaceae, Escherichia coli, MsbA, lipopolysaccharide, outer membrane
ABSTRACT
There is a critical need for new antibacterial strategies to counter the growing problem of antibiotic resistance. In Gram-negative bacteria, the outer membrane (OM) provides a protective barrier against antibiotics and other environmental insults. The outer leaflet of the outer membrane is primarily composed of lipopolysaccharide (LPS). Outer membrane biogenesis presents many potentially compelling drug targets as this pathway is absent in higher eukaryotes. Most proteins involved in LPS biosynthesis and transport are essential; however, few compounds have been identified that inhibit these proteins. The inner membrane ABC transporter MsbA carries out the first essential step in the trafficking of LPS to the outer membrane. We conducted a biochemical screen for inhibitors of MsbA and identified a series of quinoline compounds that kill Escherichia coli through inhibition of its ATPase and transport activity, with no loss of activity against clinical multidrug-resistant strains. Identification of these selective inhibitors indicates that MsbA is a viable target for new antibiotics, and the compounds we identified serve as useful tools to further probe the LPS transport pathway in Gram-negative bacteria.
INTRODUCTION
The spread of antibiotic-resistant bacterial strains has exceeded the pace of new antibiotic discovery for the past several decades, leading to concerns that we are on the verge of a “postantibiotic era” (1). To meet this challenge, it is critically important to discover and develop antibiotics with novel targets and mechanisms of action that are able to evade existing resistance mechanisms. One of the most urgent threats is the rise of carbapenem-resistant Enterobacteriaceae (2), a group of Gram-negative bacteria that includes Escherichia coli, Klebsiella pneumoniae, and Enterobacter species.
As with all Gram-negative species, the Enterobacteriaceae have two membrane barriers: a phospholipid inner membrane and an asymmetric outer membrane (OM). The outer leaflet of the outer membrane is composed primarily of lipopolysaccharide (LPS), whereas the inner leaflet is composed of phospholipids (3–5). The presence of two membranes with vastly different physical properties constitutes a significant accessibility barrier, particularly for inhibitors of cytoplasmic proteins, and contributes to the scarcity of new antibiotic discoveries (6, 7). Small polar molecules that are able to cross the outer membrane through porin channels are typically unable to passively diffuse across the inner membrane. In contrast, hydrophobic molecules that can cross the inner membrane are readily blocked by the strong lateral interactions between LPS monomers, which dramatically slow passive diffusion across the OM. Since very few surface-exposed outer membrane proteins (OMPs) are essential for bacterial viability, identifying inhibitors of proteins with domains exposed to the periplasm is an attractive option, as such inhibitors would only need to cross a single membrane barrier.
One such target that is essential for OM biogenesis, has a domain in the periplasm, and is highly conserved among the Enterobacteriaceae is MsbA. MsbA is a member of the ABC transporter superfamily and carries out the first step of LPS transport, flipping core LPS from its site of synthesis on the inner leaflet of the inner membrane to the outer leaflet of the inner membrane (8–10). LPS is then further modified and subsequently transported to the outer leaflet of the outer membrane by the Lpt transport machinery (11). A temperature-sensitive msbA mutant accumulates core LPS on the inner leaflet of the inner membrane and ceases growth at the restrictive temperature (9).
Consistent with its role in flipping LPS across the cytoplasmic membrane, structural studies indicate that MsbA transitions through large conformational changes during the ATP-driven transport cycle (12). In the prevailing model, MsbA uses the power of ATP binding and hydrolysis to transition through open inward (cytoplasmic) and open outward (periplasmic) conformations during the LPS transport cycle (13, 14). A mutation in the transmembrane domain that prevents the conformational changes impairs ATP hydrolysis (15), suggesting that a small molecule that blocks the conformational changes required for transport activity of MsbA is likely to also block ATPase activity.
We recently reported on the results of a screen of nearly three million compounds to identify molecules that block the ATPase activity of purified full-length MsbA (14). Hits from this biochemical screen were tested for their ability to inhibit the growth of E. coli, leading to the identification of a series of quinoline compounds with bactericidal activity. In this study, we characterize the biological activity of this family of quinoline compounds and demonstrate that their antibacterial activity arises through selective inhibition of MsbA. Our results demonstrate the ability to target this essential protein and also suggest challenges and opportunities for future efforts to discover clinically useful MsbA inhibitors.
RESULTS
Construction of an MsbA conditional deletion strain.
We constructed an arabinose-inducible conditional knockout (cKO) of msbA in the uropathogenic E. coli strain CFT073 (CFT073 msbA-cKO) and E. coli K-12 strain MG1655 (K-12 msbA-cKO) as control strains for analyzing the phenotypes expected upon MsbA inhibition. In these strains, the endogenous msbA open reading frame (ORF) was deleted while the remainder of the operon was left intact, and an arabinose-inducible copy of msbA was integrated at the λ att site on the chromosome to allow for complementation of the deletion in the presence of arabinose.
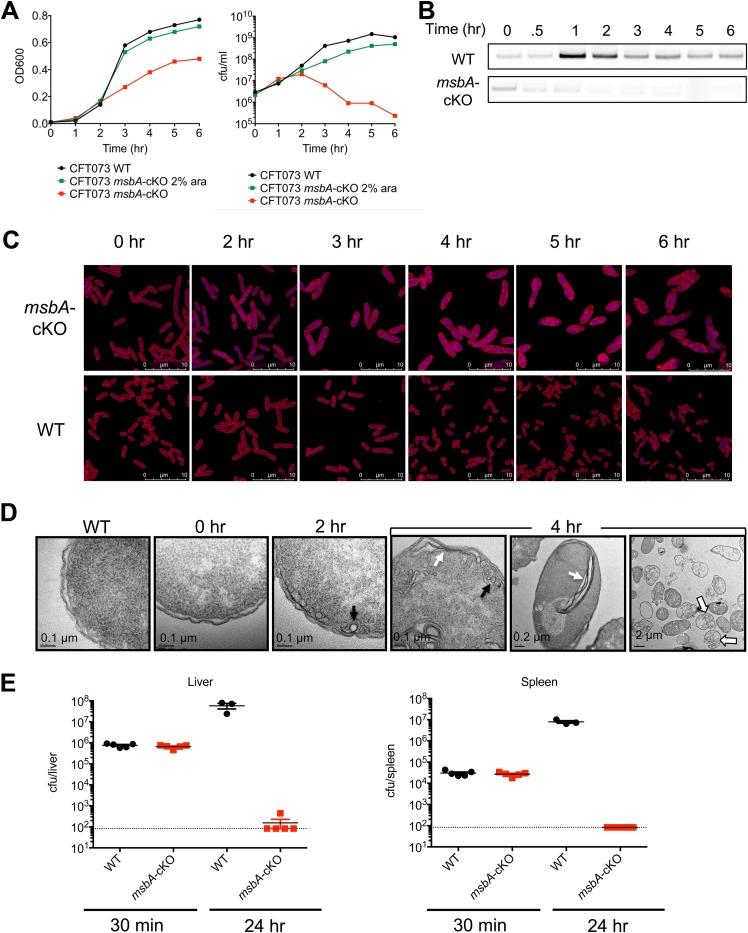
Growth of the CFT073 msbA-cKO and MG1655 msbA-cKO mutants was dependent on the presence of arabinose in the growth medium (Fig. 1A). LB medium supplemented with 2% (wt/vol) arabinose was sufficient to produce a nearly wild-type growth rate as measured by the optical density at 600 nm (OD600) and CFU count (Fig. 1A). MsbA protein dropped to a level undetectable by Western blotting within 2 h of removing arabinose from CFT073 msbA-cKO (Fig. 1B). In the absence of arabinose, the OD600 of CFT073 msbA-cKO increased slowly over the first 5 h of growth. The number of viable cells began to drop after 2 h of MsbA depletion, and this was accompanied by a dramatic change in cell morphology, as revealed by confocal microscopy analysis of cells depleted of MsbA. As shown in Fig. 1C, cells increased in size in a time-dependent manner upon MsbA depletion, consistent with the increase in the ratio of OD to CFU count.
FIG 1.
MsbA conditional deletion strain. (A) Growth of CFT073 wild type (WT) versus CFT073 msbA-cKO with or without arabinose (ara) to induce MsbA expression, as measured by OD600 (left) and CFU count (right). Data are shown from a representative experiment. (B) Western blots of MsbA protein expression over time. Loading was normalized by the OD600 value, and MsbA protein was detected using a rabbit polyclonal antibody generated in-house. (C) Confocal images of the CFT073 wild type versus the CFT073 msbA-cKO strain grown in the absence of arabinose for the indicated amount of time. Membrane is stained with Nile red, and DNA is stained with DAPI (blue). (D) TEM images of the CFT073 wild type versus the CFT073 msbA-cKO strain grown in the absence of arabinose for the indicated amount of time. Examples of vesicle-like membrane accumulation are indicated with black arrows, and parallel membrane stacks are indicated with white arrows. Lysed cells at the 4-h time point are indicated by open arrows. (E) CFT073 wild-type versus CFT073 msbA-cKO CFU counts isolated from mouse livers and spleens 30 min or 24 h following intravenous injection with 106 CFU in neutropenic mice. Ten mice were injected with each strain, and five were sacrificed at each time point. Two mice injected with the wild-type CFT073 died prior to the 24-h time point, so CFU counts are plotted for the remaining three mice.
Other morphological changes in MsbA-depleted cells were detected by confocal microscopy (Fig. 1C). The enlarged, misshapen cells with internal lipid staining have a very distinct phenotype compared to cells treated with antibiotics that work on other pathways (16). To further assess the impact of MsbA inhibition on subcellular morphological changes, MsbA-depleted cells were also examined by electron microscopy (EM). These studies revealed that the bacteria undergo dramatic changes at the inner membrane, where membrane elaborations and invaginations appear within 2 h after removal of arabinose. The invaginated membranes accumulate, culminating in bacterial lysis (Fig. 1D). The exact appearance of the excess membrane varies, ranging from vesicle- or tubule-like shapes to stacks of parallel membranes (Fig. 1D). The observed membrane accumulation is similar to that previously observed in a temperature-sensitive MsbA mutant, in which excess membrane is visible throughout the cytoplasm while the outer membrane appears morphologically unchanged (9). This phenotype is distinct from the accumulation of membrane in the periplasmic space that is observed upon depletion of the Lpt proteins, in which the excess membrane tends to accumulate in one location near the outer edge of the cell and is often accompanied by blebbing of the outer membrane (11, 17, 18).
To evaluate the essentiality of MsbA during a mouse infection, we infected neutropenic mice with an equivalent number of wild-type or CFT073 msbA-cKO bacteria. Wild-type CFT073 injected intravenously proliferated and disseminated to the liver and spleen (Fig. 1E), whereas the growth of CFT073 msbA-cKO cells was significantly attenuated (Fig. 1E). These results confirmed that MsbA is essential for E. coli growth in vitro and in vivo.
Identification of small-molecule inhibitors of MsbA.
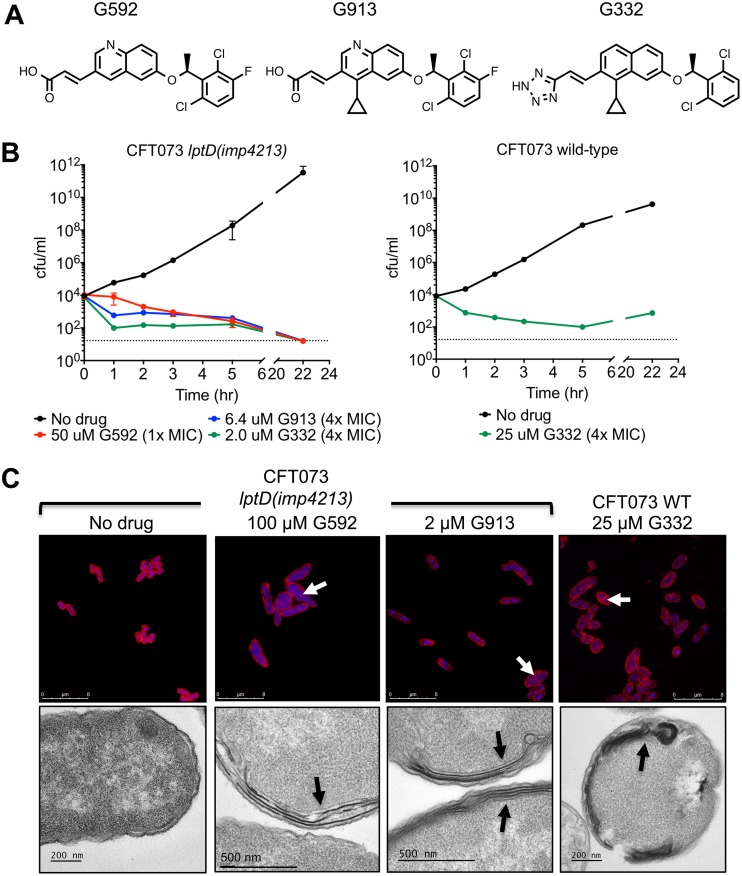
We conducted a high-throughput screen of an ∼3-million-compound small-molecule library (a combined Roche/Genentech collection) to identify compounds that inhibited the ATPase activity of purified, nanodisc-incorporated E. coli MsbA (14). We further characterized the subset of compounds that were able to inhibit growth of E. coli CFT073 lptD(imp4213) (an lptD mutation that produces a hyperpermeable outer membrane) and also displayed decreased potency (>2-fold increase in the 50% effective concentration [EC50]) on cells overexpressing MsbA as a first indication that the cellular growth inhibition was on target. Among the hits with the most potent antagonist activity was a quinoline-containing compound, G592, which inhibited growth of E. coli CFT073 lptD(imp4213) (Fig. 2A and B and Table 1). Medicinal chemistry efforts aimed at increasing the biochemical and cellular potency of G592 led to the discovery of compounds G913 and G332 (Fig. 2A) (14). Both displayed increased potency in the biochemical and cellular assays (Fig. 2B and Table 1). Similar to genetic depletion of MsbA, chemical inhibition of MsbA resulted in cell death, reducing the number of viable E. coli CFT073 lptD(imp4213) cells by more than 400-fold following a 22-h exposure to 4× the MIC of compound (Fig. 2B). The most potent of these compounds, G332, was bacteriostatic against wild-type CFT073, with a slight increase in the CFU count after overnight growth, likely due to degradation or poor solubility of the compound following prolonged incubation (Fig. 2B). In addition, G332 had MICs of 2.8 μg/ml against both wild-type K. pneumoniae and Enterobacter cloacae (Table 1). All of the quinoline compounds were significantly more potent against lptD(imp4213) cells with a compromised outer membrane, as well as against cells in which the TolC efflux pump was deleted (Table 1), indicating that the efficacy of these compounds is limited by both the outer membrane barrier and efflux.
FIG 2.
Small-molecule inhibitors of E. coli MsbA. (A) Chemical structures of the three quinoline inhibitors described in this paper. (B) Time-kill assay of CFT073 lptD(imp4213) and CFT073 with MsbA inhibitors. Viable cells were measured by plating on LB agar at 0, 1, 2, 3, 5, and 22 h after addition of compound. (C) Confocal microscopy and TEM of CFT073 and CFT073 lptD(imp4213) treated with the indicated concentration of inhibitor for 3 h. For confocal microscopy, membranes were stained with Nile red, and DNA was stained with DAPI (blue). Arrows in confocal and TEM images indicate sites of excess membrane accumulation. Untreated CFT073 cells looked identical to untreated CFT073 lptD(imp4213) cells (data not shown).
TABLE 1.
Susceptibility of bacterial strains to MsbA inhibitors
| Parametera | Strainb | Value for the parameter with:c |
||
|---|---|---|---|---|
| G592 | G913 | G332 | ||
| Biochemical IC50 (nM) | Purified E. coli MsbA | 150 ± 48 | 3.8 ± 0.98 | 2.8 ± 0.75 |
| EC50 (μM) | CFT073 lptD(imp4213) msbAlow | 4.5 ± 2.9 | 0.15 ± 0.045 | 0.11 ± 0.032 |
| CFT073 lptD(imp4213) msbAWT | 8.3 ± 2.7 | 0.27 ± 0.075 | 0.19 ± 0.04 | |
| CFT073 lptD(imp4213) msbAhigh | 40 ± 23 | 1.1 ± 0.40 | 0.46 ± 0.16 | |
| MIC (μg/ml) | CFT073 lptD(imp4213) | 21 ± 16 | 0.24 ± 0.13 | 0.22 ± 0.11 |
| CFT073 ΔtolC | 18 ± 5 | 0.22 ± 0.15 | 0.31 ± 0.22 | |
| CFT073 WT | >41 | 39 ± 11 | 2.8 ± 0 | |
| K. pneumoniae ATCC 700721 | ND | >45 | 2.8 ± 0 | |
| E. cloacae ATCC 13047 | ND | >45 | 2.8 ± 0 | |
IC50, 50% inhibitory concentration.
Low and high levels of MsbA expression are indicated by msbAlow and msbAhigh, respectively; wild-type expression is indicated as msbAWT.
For all samples, n ≥ 3. ND, not determined.
Nonspecific binding to serum proteins can significantly reduce the activity of antibiotics (19, 20). To evaluate the effect of serum on the activity of the quinoline inhibitors, we measured MICs of G592, G913, and G332 for the CFT073 ΔtolC strain in medium with or without 10% mouse serum (Table 2). As shown in Table 2, the addition of 10% mouse serum to cultures increased the MICs of G913 and G332 at least 10-fold. Since activity is completely lost in the presence of 50% serum (data not shown), it is not currently feasible to test these compounds in an animal model or to further develop them as therapeutics.
TABLE 2.
Potency of quinoline inhibitors in the presence of seruma
| Compound | MIC against E. coli CFT073 ΔtolC (μg/ml) |
|
|---|---|---|
| No serum | 10% mouse serum | |
| G592 | 18 ± 5 | >41 |
| G913 | <0.22 ± 0.15 | 5.6 ± 3.9 |
| G332 | 0.31 ± 0.22 | 4.2 ± 1.6 |
For all samples, n ≥ 3. The plasma protein binding level for all compounds was 99%.
Quinoline series compounds inhibit cell growth through on-target inhibition of MsbA.
To determine if the whole-cell activity of the quinoline compounds resulted primarily from inhibition of MsbA, we measured the growth inhibition of a panel of cells expressing various levels of MsbA (Table 1; see also Fig. S1 in the supplemental material), reasoning that if the compounds directly inhibited MsbA activity, decreasing or increasing MsbA expression should result in a concomitant shift in the EC50. EC50 rather than MIC values were used because the shorter growth period (6 h rather than overnight) allowed greater sensitivity for detecting compounds that inhibited growth but had limited solubility or stability that would prevent determination of an MIC value. By titrating the level of arabinose in the culture medium, we were able to vary the level of MsbA expression from ∼3-fold below to ∼50-fold above the wild-type level (Fig. S2). As shown in Table 1, MsbA expression levels qualitatively correlated with changes in the EC50, consistent with on-target activity. Confocal and electron microscopy further supported the conclusion that G592 and its potent analogs were on target for MsbA inhibition, as each demonstrated membrane defects similar to those observed in the msbA-cKO strain (Fig. 2C).
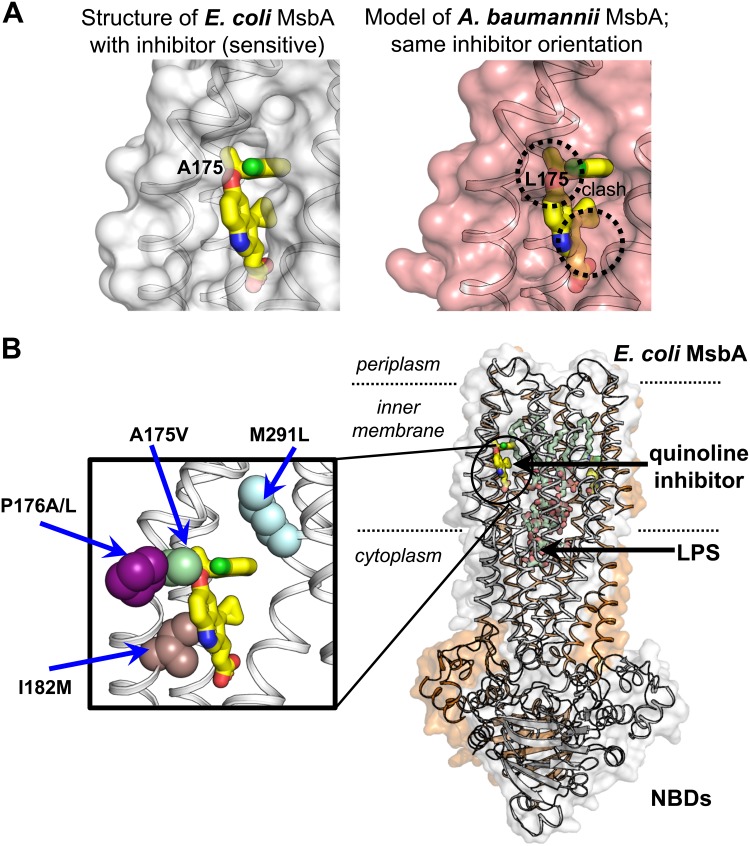
High-resolution structural studies of MsbA conducted by our group (14) revealed a lack of conservation in the MsbA inhibitor binding site between Acinetobacter baumannii and bacteria in the Enterobacteriaceae family. The degree of nonconservation suggested that the quinoline compounds would be unable to bind to A. baumannii MsbA due to steric clash with the inhibitor molecule (Fig. 3A). We reasoned that these differences could be exploited to establish an additional phenotypic assay that would report on the specificity of the quinoline compounds. We first determined that expression of either A. baumannii or E. coli MsbA from a plasmid in the MG1655 lptD(imp4213) msbA-cKO background was sufficient to rescue growth of the strain when it was grown in the absence of arabinose (Fig. S3). While G592 and G913 potently inhibited growth of cells expressing E. coli MsbA, no growth inhibition was detected in cells expressing A. baumannii MsbA (Table 3), consistent with on-target inhibition of MsbA in cells and lack of inhibition of the A. baumannii MsbA homolog. In contrast, control antibiotics such as streptomycin and polymyxin B had similar MICs in both strains. These data demonstrate that the inhibition of bacterial growth by G592 and G913 is primarily dependent on MsbA rather than on nonspecific mechanisms. Notably, G332 inhibited growth of cells expressing A. baumannii MsbA, albeit at a >100-fold-higher MIC (Table 3), suggesting that, while this compound inhibits bacterial growth primarily through inhibition of MsbA, it also displays some off-target activity at much higher concentrations.
FIG 3.
Binding site of MsbA inhibitors. (A) Comparison of inhibitor binding sites in E. coli MsbA (from crystal structure) and A. baumannii MsbA (modeled). (B) Location of inhibitor binding site in the full-length MsbA crystal structure (right) and location of quinoline-resistant mutations within the binding site. NBD, nucleotide binding domains.
TABLE 3.
Susceptibility of E. coli expressing E. coli or A. baumannii MsbA
| Compounda | MIC (μg/ml)b |
|
|---|---|---|
| pEcMsbA | pAbMsbA | |
| G592 | 20 ± 0 | >41 |
| G913 | 0.94 ± 0.41 | >45 |
| G332 | 0.27 ± 0.10 | 32 ± 23 |
| CIP | 0.006 ± 0 | 0.012 ± 0 |
| PMB | 0.11 ± 0.038 | 0.16 ± 0.080 |
| TET | 0.69 ± 0 | 0.69 ± 0 |
| ERM | 0.05 ± 0 | 0.10 ± 0 |
CIP, ciprofloxacin; PMB, polymyxin B; TET, tetracycline; ERM, erythromycin.
For all samples, n ≥ 3. MICs were determined for E. coli MG1655 msbA-cKO lptD(imp4213) carrying a plasmid expressing either E. coli MsbA (pEcMsbA) or A. baumannii MsbA (pAbMsbA).
The structural differences between E. coli and A. baumannii MsbA proteins highlighted the possibility that selective pressure could yield fully functional MsbA proteins that are resistant to our inhibitors. To assess whether similar resistance can evolve spontaneously in E. coli, we performed selection experiments in our wild-type and OM-permeable E. coli strains. Spontaneous mutants that resulted in resistance to the inhibitors were generated and characterized (Fig. 3B). When E. coli cells were plated in the presence of inhibitor at 4× MIC, the frequency of resistance (FOR) was less than 1.8 × 10−9 for all quinoline compounds tested (Table S2). While we did not isolate any resistant mutants following selection at 4× MIC, we were able to isolate five unique mutants resistant to a selection concentration of 2× MIC in MG1655 lptD(imp4213). The MICs of these mutants ranged from 3-fold to 26-fold higher than the MIC of the original MG1655 lptD(imp4213) strain (Table 4). Sequencing of the msbA gene identified five unique point mutations in MsbA that conferred overlapping resistance to all the quinolone series inhibitors: A175V, P176A, P176L, I182M, and M291L (Table 4). All of these mutations localized to the binding site of the quinoline inhibitors, as identified in a cocrystal structure (Fig. 3B) (14).
TABLE 4.
MICs of quinoline-resistant mutants in MG1655 lptD(imp4213)
| msbA mutationa | MIC (μg/ml)b |
||||||
|---|---|---|---|---|---|---|---|
| G592 | G913 | G332 | CIP | PMB | TET | ERM | |
| None | 10 ± 0 | 0.15 ± 0.17 | 0.023 ± 0 | 0.009 ± 0.003 | 0.23 ± 0.15 | 1.2 ± 0.35 | 0.18 ± 0.18 |
| A175V | >41 | 1.9 ± 0.80 | 0.20 ± 0.11 | 0.009 ± 0.003 | 0.17 ± 0.058 | 1.2 ± 0.35 | 0.18 ± 0.18 |
| P176A | >41 | 3.7 ± 1.6 | 0.29 ± 0.10 | 0.011 ± 0.002 | 0.23 ± 0.15 | 1.0 ± 0.40 | 0.20 ± 0.17 |
| P176L | >41 | 3.7 ± 1.6 | 0.29 ± 0.10 | 0.011 ± 0.002 | 0.23 ± 0.15 | 1.2 ± 0.35 | 0.18 ± 0.18 |
| I182M | 41 ± 0 | 2.1 ± 1.2 | 0.59 ± 0.59 | 0.009 ± 0.003 | 0.23 ± 0.15 | 1.2 ± 0.35 | 0.18 ± 0.18 |
| M291L | 34 ± 12 | 1.6 ± 1.1 | 0.18 ± 0.12 | 0.011 ± 0.002 | 0.29 ± 0.17 | 1.2 ± 0.35 | 0.18 ± 0.18 |
The amino acid change encoded by mutation of msbA is given.
For all samples, n ≥ 3. CIP, ciprofloxacin; PMB, polymyxin B; TET, tetracycline; ERM, erythromycin.
Outer membrane stress responses induced by MsbA inhibition.
Disruption of LPS transport by the Lpt proteins activates multiple envelope stress response pathways in E. coli (21). As a further characterization of the MsbA inhibitors, we sought to determine if depletion or pharmacological inhibition of MsbA likewise induced envelope stress responses. We measured changes in the transcription of several genes induced by multiple stress response pathways using quantitative reverse transcription-PCR (qRT-PCR) on RNA isolated from CFT073 lptD(imp4213) treated with G913 for 2 h or CFT073 msbA-cKO depleted of MsbA for 2 h or 4 h. All of the envelope stress responses tested were induced in both MsbA-depleted cells and cells treated with quinoline inhibitors; this included the sigma E pathway as well as the Rcs and Bae responses (Fig. 4A and B).
FIG 4.
E. coli stress response following treatment with MsbA inhibitors. (A) qRT-PCR of RNA isolated from E. coli CFT073 lptD(imp4213) treated for 2 h with 1.2 μM G913. Corresponding stress response pathways are indicated below gene names. (B) qRT-PCR of RNA isolated from CFT073 msbA-cKO grown in the absence of arabinose for 2 h or 4 h. (C) LPS gel showing colanic acid modification of LPS following MsbA depletion. WT, MG1655; msbA-cKO, MG1655 msbA-cKO; imp, MG1655 lptD(imp4213); RQ, relative quantification.
One downstream effect of the Rcs response is upregulation of colanic acid biosynthesis genes, and colanic acid modification of LPS has been previously described when components of the Lpt pathway are depleted (11). We observed modification of the LPS in MsbA-depleted cells and cells treated with the MsbA inhibitor G332 (Fig. 4C); this modification was dependent on the presence of WcaJ, which catalyzes the first step of colanic acid biosynthesis (22).
MsbA inhibitors evade known antibiotic resistance mechanisms.
Because no known antibiotics target MsbA, we hypothesized that selective MsbA inhibitors would have no overlapping resistance with existing antibiotic resistance mechanisms. To test this hypothesis, we measured the MIC of G332 for eight multidrug-resistant (MDR) strains of E. coli, K. pneumoniae, and E. cloacae selected from a panel of MDR clinical isolates (Tables 5 and S1). All of the MICs were within 2-fold of the MICs on antibiotic-sensitive strains (Tables 1 and 5). These results are consistent with the expectation that an MsbA inhibitor should not be susceptible to existing mechanisms of antibiotic resistance.
TABLE 5.
MICs of G332 on multidrug-resistant strainsa
| Species | Strain | Known acquired resistanceb | G332 MIC (μg/ml) |
|---|---|---|---|
| E. coli | CDC 0006 | KPC+, NDM− | 2.8 ± 0 |
| CDC 0055 | KPC−, NDM− | 2.8 ± 0 | |
| CDC 0061 | NDM | 4.5 ± 1.6 | |
| CDC 0001 | KPC-3, TEM-1 | 5.9 ± 0 | |
| K. pneumoniae | CDC 0079 | NDM | 2.8 ± 0 |
| CDC 0106 | KPC-3, TEM-1 | 2.8 ± 0 | |
| E. cloacae | CDC 0053 | CTX-M14, DHA-1, OmpF/OmpK35 | 5.9 ± 0 |
| CDC 0038 | NDM | 5.9 ± 0 |
For all samples, n ≥ 3.
KPC+, strain contains a Klebsiella pneumoniae carbapenemase (KPC); KPC−, strain does not contain a KPC; NDM−, strain does not contain a New Delhi metallo-β-lactamase (NDM).
DISCUSSION
Over the past several decades, elegant genetic and biochemical studies have established the pathway for LPS biosynthesis on the cytoplasmic face of the inner membrane (23–31). More recently, the mechanisms by which newly synthesized LPS is transported from its site of synthesis in the cytoplasm to the outer membrane have also been elucidated. MsbA (9) and the Lpt proteins (11, 17, 18, 32, 33) were identified through genetic experiments as being required for LPS transport. Further, cryo-EM studies and high-resolution crystal structures of MsbA bound to LPS now provide a detailed molecular mechanism of LPS flipping from the inner leaflet to the outer leaflet of the inner membrane (13, 14). In combination with crystal structures of the seven Lpt proteins (34–41), in vitro reconstitution of the Lpt pathway supports a model in which the Lpt proteins form a physical bridge that shuttles LPS from the inner to the outer membrane (42). A growing understanding of the mechanisms underlying outer membrane biogenesis has led to a new focus on efforts to discover novel antibacterial compounds that target the LPS transport pathway. For example, inhibitors of LpxC (43–45) and LptD (46) have been identified although only the LptD inhibitor has so far progressed beyond phase 1 clinical trials.
Several features of MsbA make it an ideal candidate for identifying novel inhibitors of LPS biogenesis and transport. Like other genes in the LPS transport pathway, MsbA is essential in the Enterobacteriaceae, making it an attractive target for new antibiotics. Of the other LPS transport proteins, only LptD has the potential to be inhibited by a compound that does not need to cross the outer membrane and would, hence, not be subject to the permeability or efflux barriers that make antibiotic discovery challenging. However, the lack of biochemical assays suitable for high-throughput screening has made the identification of small-molecule LptD inhibitors challenging (46). The majority of the LPS transport pathway proteins are exposed, to a greater or lesser extent, to the periplasm; in fact, only LptB is entirely cytoplasmic. This is important because targets that are accessible from the periplasm have the advantage of potentially requiring a drug to cross only a single (outer) membrane although efflux is still likely to be an issue (47). Indeed, we observed that our MsbA inhibitors were more effective against a strain lacking the TolC efflux pump although it was possible to kill cells despite the efflux barrier if the inhibitors were sufficiently potent, as was the case with G332 (Table 1).
In this study, we focused on MsbA because measurement of its ATPase activity is suitable for high-throughput screening assays. Further, MsbA is accessible from the periplasm to various degrees during the transport cycle, and we reasoned that we might identify inhibitors that bound to the periplasmic face. While we chose MsbA as a target because of the potential for identifying periplasmic binders, the quinoline inhibitors bound to a site buried in the inner membrane (Fig. 3) (14). The transmembrane location of the inhibitor binding site likely contributes to some of the challenges we encountered when we tried to improve the properties of these compounds. For example, biochemically potent compounds with increased polarity and reduced serum binding could be identified; however, these lost phenotypic activity (data not shown), possibly due to a reduced ability to access the binding site in the inner membrane. Unfortunately, the requisite hydrophobic nature of the phenotypically active quinoline compounds always correlated with unacceptably high levels of plasma protein binding and significant loss of bacterial growth inhibition in the presence of serum (unpublished data). To date, we have been unable to sufficiently improve the physiochemical and serum binding properties of the quinoline compounds without complete loss of whole-cell growth inhibition, suggesting that inhibitors that bind the membrane region of MsbA may not be suitable for new antibiotic development.
The identification of highly hydrophobic compounds in screens for novel antibiotics has been an ongoing problem in the field due to the fact that most corporate compound libraries were originally assembled for screening against mammalian targets (48, 49). Despite an effort to increase the number of compounds in our library with lipophilicity and molecular weights in the ranges more typical of known antibiotics (50), most hits from the initial screen were still very hydrophobic, contributing to the observed loss of activity in the presence of serum. Nevertheless, our studies validate MsbA as a potential drug target, and further efforts to identify alternative inhibitor binding sites are warranted. Perhaps screening of libraries with different chemical properties, such as natural product libraries, may identify compounds that bind to less hydrophobic regions of MsbA and therefore possess more drug-like properties. Structural studies of MsbA in distinct transport states reveal that a portion of the transmembrane LPS binding site becomes exposed to the periplasm during the transport cycle, particularly as MsbA adopts an open-outward conformation (12–14). This is thought to promote LPS flipping whereby exposure of the acyl tails to solvent drives hydrophobic partitioning of LPS into the membrane outer leaflet while simultaneously exposing a concave and polar surface in the transmembrane region to the periplasm. Given our aforementioned challenges in optimizing small-molecule inhibitors that target a site on MsbA that is buried in the inner membrane, strategies aimed at targeting the exposed periplasmic cleft of the transporter may identify compounds with properties better suited to antibiotic development. For example, in the presence of the nonspecific ATPase inhibitor vanadate, MsbA can be stably trapped in a conformation that exposes the polar periplasmic cleft (12). A screening effort aimed at identifying compounds that bind in the cleft may identify compounds that stabilize an outward open conformation and thereby block transport. In theory, such inhibitors need not traverse the inner membrane. Also, a recently reported inhibitor of A. baumannii MsbA likely binds to a different site than the quinoline inhibitors, given the lack of overlap among the identified resistant mutants. However, the actual binding site of the A. baumannii inhibitor remains to be determined (51).
In summary, this study describes a novel class of antibacterial compounds that target the LPS transport system of Gram-negative bacteria. Because MsbA is not a target of existing antibiotics, inhibitors of MsbA are able to evade the preexisting resistance mechanisms of other antibiotic classes. While the particular inhibitors described here are not viable lead candidates due to a lack of activity in the presence of serum, they provide a proof of concept for MsbA as a novel target for Gram-negative pathogens and can serve as useful tool compounds for investigation of the LPS transport pathway.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli strains were constructed in MG1655, an E. coli K-12 strain, and CFT073, a uropathogenic E. coli strain (52, 53) (Table 6). For MsbA overexpression, the MsbA ORF was cloned into the EcoRI/XbaI sites of the arabinose-inducible, high-copy-number expression vector pBAD24 using PCR primers msbA-f2 and msbA-r2 (Table 7). Overexpression of the MsbA protein was confirmed by Western blotting (see Fig. S1B in the supplemental material) and quantitated using ImageJ (NIH).
TABLE 6.
Strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | E. coli K-12 F− lambda− ilvG negative, rfb-50 rph-1 | ATCC 700926 |
| CFT073 | Uropathogenic E. coli isolated from a woman with acute pyelonephritis, Baltimore, MD | ATCC 700928 |
| MG1655 msbA-cKO | E. coli K-12 with arabinose-inducible conditional knockout of MsbA | This study |
| CFT073 msbA-cKO | Uropathogenic E. coli with arabinose-inducible conditional knockout of MsbA | 14 |
| MG1655 lptD(imp4213) | E. coli K-12 with permeable outer membrane | This study |
| CFT073 lptD(imp4213) | Uropathogenic E. coli with permeable outer membrane | 14 |
| MG1655 msbA-cKO lptD(imp4213) | E. coli K-12 with arabinose-inducible conditional knockout of MsbA and permeable outer membrane | This study |
| CFT073 msbA-cKO lptD(imp4213) | Uropathogenic E. coli with arabinose-inducible conditional knockout of MsbA and permeable outer membrane | 14 |
| K. pneumoniae 700721 | MGH78578, isolated from sputum from a 66 year-old man, 1994 | ATCC 700721 |
| E. cloacae 13047 | Strain isolated from spinal fluid | ATCC 13047 |
| MG1655 msbA-cKO ΔwcaJ | E. coli with transduced wcaJ deletion from JW2032 into MG1655 msbA-cKO | This study |
| MG1655 ΔwcaJ | E. coliwith transduced wcaJ deletion from JW2032 into MG1655 | This study |
| E. coli JW2032 | wcaJ::kanR deletion from Keio collection | 58 |
| MG1655 ΔtolC | E. coli with transduced tolC deletion from JW5503 into MG1655 | 14 |
| E. coli JW5503 | tolC::kanR deletion from Keio collection | 58 |
| CDC 0006 | MDR E. coli; KPC+, NDM− | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0055 | MDR E. coli; KPC−, NDM− | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0061 | MDR E. coli; NDM | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0001 | MDR E. coli; KPC-3, TEM-1 | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0079 | MDR E. cloacae; NDM | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0106 | MDR E. cloacae; KPC-3, TEM-1 | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0053 | MDR K. pneumoniae; CTX-M14, DHA-1 OmpF/OmpK35 | CDC and FDA Antibiotic Resistance Isolate Bank |
| CDC 0038 | MDR K. pneumoniae; NDM | CDC and FDA Antibiotic Resistance Isolate Bank |
| Plasmids | ||
| pBAD24 | Arabinose-inducible expression vector | ATCC 87399 |
| pBAD24-MsbA | Plasmid for arabinose-inducible MsbA overexpression | This study |
| pLDR9 | Lambda att site integration vector | ATCC 77358 |
| pLDR8 | Lambda integrase expression vector | ATCC 77357 |
| pCDF-1b | IPTG-inducible expression vector with streptomycin resistance marker | Novagen |
| pKD46 | Temperature-sensitive plasmid for Red recombinase expression | Coli Genetic Stock Center 7669 |
| pKD4 | Template plasmid with kanamycin marker flanked by FRT sites | Coli Genetic Stock Center 7632 |
| pKD46-Sm | pKD46 with streptomycin resistance marker replacing ampicillin resistance marker | This study |
| pET28b | IPTG-inducible expression vector | Novagen |
| pLMG18 | Low-copy-number IPTG-inducible expression vector with chloramphenicol resistance marker | 57 |
| pLMG18-EcMsbA | Low-copy-number IPTG-inducible expression vector for E. coli MsbA | This study |
| pLMG18-AbMsbA | Low-copy IPTG-inducible expression vector for A. baumannii MsbA | This study |
KPC+, strain contains a Klebsiella pneumoniae carbapenemase (KPC); KPC−, strain does not contain a KPC; NDM−, strain does not contain a New Delhi metallo-β-lactamase (NDM).
TABLE 7.
PCR primers
| Primer name | Primer sequencea |
|---|---|
| msbA-f2 | AAACGTCTCGAATTCATGCATAACGACAAAGATCTCTC |
| msbA-r2 | AAAATCTAGAtCATTGGCCAAACTGCATT |
| PBAD-msbA-f | AAAAGAGCTCGATGCATAATGTGCCTGTC |
| PBAD-msbA-r | AAAAGAGCTCGTTCACCGACAAACAACAG |
| msbA-KO-noPkan-f | TGGATAACGGGTAGAATATGCGGCTATTTCAACAAATGCTGGTTTTTTGAGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCAGGAGGAATTCATGATTGAACAAGATGGATTGC |
| msbA-KO-kan-r | GGCAGCAATAGCCGCCACAAAGGGGATTCACCAGACCAGATTTTTTCGATCATATGAATATCCTCCTTAGTTCCTATTC |
| SmR-f | AAAAAAGACTCCCCGTCAGAAGCACACGGTCACACTG |
| SmR-r | AAAAAAAAATCGAGACCCCTGCTTATCCACGGAATCT |
| msbA-f | ATGCTGGTTTTTCATATGCATAACGAC |
| msbA-r | CGGGATCCTCGAGTCATTGGCCAAACTGCATTTTG |
Bolded sequence at 3' ends is homologous to template sequence. Additional bolded sequence in msbA-KO-noPkan-f primer introduces an FRT recombinase site.
Conditional MsbA knockout strains were constructed by subcloning the arabinose-inducible MsbA from pBAD24 into the SacI site of the integration vector pLDR9 (54) using PCR primers PBAD-msbA-f and PBAD-msbA-r (Table 7). The NotI-digested and religated construct was integrated into the lambda att site upon expression of the lambda recombinase from pLDR8 according to a previously described protocol (54). The endogenous msbA gene was then replaced with a promoterless kanamycin marker flanked by Flp recognition target (FRT) sites, amplified from pKD4 using primers msbA-KO-noPkan-f and msbA-KO-kan-r (Table 7), and integrated into MG1655 and CFT073 containing the arabinose-inducible MsbA construct by Red recombinase-mediated homologous recombination (55). Because the arabinose-inducible copy of MsbA at the lambda att site was marked with an ampicillin resistance gene, the ampicillin resistance marker on the Red recombinase was replaced with a streptomycin resistance gene amplified from pCDF-1b using primers SmR-f and SmR-r. The PCR product was digested with AhdI and BsaI and ligated into AhdI- and BspMI-digested pKD46.
To make strains with a permeabilized outer membrane, the endogenous lptD gene of CFT073, CFT073 msbA-cKO, MG1655, or MG1655 msbA-cKO was replaced with an lptD(imp4213) mutant allele. A construct was synthesized (GenScript) with 200 bp of the lptD ORF upstream of the lptD(imp4213) deletion followed by the remaining 1,299 bp of the lptD ORF with codon changes throughout the sequence to keep the same amino acid sequence but prevent recombination during integration, a promoterless kanamycin resistance marker flanked by FRT sites, and 200 bp homologous to the chromosomal sequence downstream of the lptD ORF (Fig. S3). The construct was integrated onto the chromosome by Red recombinase-mediated homologous recombination (55).
To compare strains complemented with either E. coli or A. baumannii MsbA, the MsbA genes from E. coli MG1655 and A. baumannii 17978 were cloned into the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible expression vector pLMG18. The E. coli homolog was PCR amplified from MG1655 genomic DNA using primers msbA-f and msbA-r and inserted into the NdeI and BamHI sites of pET28b; the MsbA gene plus an N-terminal six-His tag was then subcloned into the XbaI and HindIII sites of pLMG18. A. baumannii MsbA was codon optimized for E. coli expression, synthesized, and inserted into pLMG18. The MsbA-expressing plasmids were introduced into the MG1655 lptD(imp4213) msbA-cKO strain. Leaky expression from the lac promoter was sufficient to give a wild-type growth rate without addition of inducer.
Time course of MsbA depletion in CFT073 msbA-cKO.
Overnight cultures of wild-type CFT073 and CFT073-msbA-cKO in LB medium plus 2% arabinose were diluted 1:100 in fresh LB medium with 2% arabinose and grown for 2.5 h into log phase. Cells were pelleted, washed with phosphate-buffered saline (PBS), and resuspended in LB medium at an OD600 of 1. These cultures were then diluted to and OD600 of 0.01 in LB medium or LB medium with 2% arabinose and incubated at 37°C with shaking. Each hour, the OD600 was measured, the equivalent of 1 ml culture at an OD600 of 1 was pelleted and frozen for later Western blotting, 1.5 ml was pelleted and fixed for microscopy as described below, and dilutions were plated on LB medium plus 2% arabinose to determine CFU counts.
Mouse sepsis model.
Mouse infections were performed in 7-week-old female A/J mice (Jackson). CFT073 and CFT073 msbA-cKO cultures for infection were prepared as follows. Overnight cultures grown in M9 medium plus 2% arabinose were diluted 1:100 in fresh M9 medium with 2% arabinose and grown for 3 h into mid-log phase. Cells were pelleted, washed with PBS, and resuspended in PBS–20% glycerol at an OD600 of 10, and aliquots were frozen at −80°C. One aliquot was thawed the next day, and dilutions were plated on LB medium plus 2% arabinose to determine the number of CFU per milliliter. The day of the mouse infection, aliquots were thawed and diluted to a final concentration of 106 CFU per 100 μl of PBS. Neutropenia was induced by intraperitoneal injection of 3 mg of cyclophosphamide 4 days prior to infection and of 2 mg at 1 day prior to infection. A total of 106 CFU of CFT073 or CFT073 msbA-cKO was injected into the tail vein of each mouse. Five mice infected with each strain were sacrificed at 30 min postinfection and at 24 h postinfection (two mice infected with the wild-type strain died before the 24-h time point, so only three mice were available for that group). Each liver and spleen were homogenized in 5 ml of PBS, and dilutions were plated on LB agar plates. Colonies were counted after overnight growth at 37°C. All mouse experiments followed a protocol approved by the Genentech IACUC.
Phenotypic EC50 assays.
Aliquots (200 nl) of eight 2-fold dilutions of each compound were dispensed in duplicate into 384-well plates using an Echo Liquid Handler (Labcyte). Strains to be tested were grown in M9 medium supplemented with 1% maltose and, for the overexpression strains, 0.2% arabinose overnight for 16 h, washed with PBS, resuspended at an OD600 of 1, and frozen in 100-μl aliquots for use in screening. For each experiment, aliquots were thawed on ice and diluted 1:1,000 into M9 medium and 1% maltose plus 0.2% arabinose for overexpression strains. Ten microliters of cells/well was dispensed into 384-well plates containing compound using a Multidrop Combi Reagent Dispenser (Thermo). Plates were incubated for 6 h at 37°C; then 10 μl of BacTiter-Glo reagent (Promega) was added to each well, and luminescence was measured. EC50s were calculated using Genedata Screener software.
MIC assays.
Broth MICs were determined in cation-adjusted Mueller-Hinton broth according to a standard Clinical and Laboratory Standards Institute protocol (56). To measure MIC shifts due to serum binding, 10% sterile-filtered mouse serum (catalog no. 103219-618; VWR) was added to the medium.
Time-kill assays.
For time-kill assays, overnight cultures in LB broth were diluted 1:100 in 5 ml of LB broth and grown to log phase for 2.5 h at 37°C. These cultures were then diluted 1:10,000 from an OD of 0.2 in LB broth for a starting inoculum of 104 CFU/ml. Either compound or dimethyl sulfoxide (DMSO)-only control was added to 2-ml aliquots, and cultures were grown at 37°C. At each time point, dilutions were plated on LB agar and incubated overnight at 37°C; colonies were counted after overnight growth to determine the number of viable CFU per milliliter.
Microscopy.
To prepare cells for microscopy, overnight cultures of CFT073 and CFT073 lptD(imp4213) grown in LB medium were diluted 1:100 in LB medium, grown to log phase for 2.5 h at 37°C, pelleted, and resuspended at an OD600 of 0.1 in LB medium. Sixty microliters of 10 mM G592, 1.2 μl of 10 mM G913, or 60 μl of DMSO (no compound control) was added to 6 ml of CFT073 lptD(imp4213). Fifteen microliters of 10 mM G332 or DMSO (no compound control) was added to 6 ml of CFT073. All cultures were incubated for 3 h at 37°C. Cells were pelleted, washed with PBS, and fixed with 300 μl of Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, ph7.2).
For confocal microscopy, 50 μl of fixed cells was pelleted, washed with PBS, and resuspended in 25 μl (compound-treated) or 200 μl (DMSO control) of PBS with 10 μg/ml Nile Red (72485; Sigma). Cells were incubated for 5 min at room temperature and then diluted 1:30 in 30 μl of water on a number 1.5 coverslip and allowed to dry. Coverslips were mounted on slides using ProLong Diamond with DAPI (P36962; ThermoFisher) and cured overnight at room temperature prior to imaging with a Leica SP8 stimulated emission depletion (STED) 3× confocal microscope using a 100× oil immersion lens. Deconvolution was performed using Huygens Pro software (Scientific Volume Imaging).
For transmission electron microscopy (TEM), the remaining 250 μl of cells was postfixed in 1% reduced osmium tetroxide (EM Sciences, Hatfield, PA) for 1 h, followed by incubation in 0.5% uranyl acetate for 1 h. The samples were then dehydrated through an ascending series of ethanol (50%, 70%, 90%, and 100%), followed by a propylene oxide wash, and finally embedded in Eponate 12 (Ted Pella, Redding, CA). Ultrathin sections (80 nm) were cut with an Ultracut microtome (Leica), stained with 0.2% lead citrate, and examined in a JEOL JEM-1400 transmission electron microscope at 80 kV. Digital images were captured with a Gatan Ultrascan 1000 charge-coupled-device (CCD) camera.
Frequency of resistance and sequencing of resistant mutants.
To determine frequencies of resistance, 3 to 4 colonies of the strain to be tested were picked from a fresh plate into 1 ml of cation-adjusted Mueller-Hinton broth (MHBII) and diluted to an OD600 of 0.00001 in 10 ml of broth. Ten independent 3-ml cultures in MHBII were inoculated with 3 μl of each of the diluted colonies for an initial inoculum of about 100 CFU per tube. Cultures were grown overnight at 37°C. One milliliter of each overnight culture was pelleted and resuspended in 600 μl of MHBII. Due to limited compound availability, 40 μl of cells was plated on 2 ml of agar with 4× MIC of inhibitor in six-well dishes, with one culture per well. Any colonies that grew in the first 48 h were counted as resistant CFU. Due to low frequencies of resistance, resistant mutants were isolated following growth on 2× or 3× MIC of inhibitor. Genomic DNA was isolated from 2 ml of overnight culture of the resistant mutants using a DNeasy blood and tissue kit (Qiagen). The msbA gene was PCR amplified and sequenced.
qRT-PCR for stress response genes.
Overnight cultures in LB medium (or LB medium plus 2% arabinose for the CFT073 msbA-cKO strain) were diluted 1:200 in 50 ml of the same medium and grown to log phase for 2.5 h at 37°C. Cells were diluted to an OD600 of 0.05, and 10 ml per tube was aliquoted into 50-ml conical tubes. For MsbA depletion, the CFT073 msbA-cKO strain was grown in LB medium with no arabinose for 2 h or 4 h at 37°C. For compound-treated samples, CFT073 lptD(imp4213) was treated with 1.2 μM G913 or an equivalent volume of DMSO as a no-compound control, and cultures were incubated for 2 h at 37°C. For both MsbA depletion and compound inhibition, the equivalent of 1 ml at an OD600 of 1 was harvested by addition of 2 volumes of RNA Protect (Qiagen), followed by vortexing, incubation for 5 min at room temperature, pelleting, and freezing at −20°C. RNA was isolated using an RNeasy kit (Qiagen), and qRT-PCR was performed on 1 ng of genomic DNA per reaction volume using ae SuperScript III Platinum One-Step Quantitative RT-PCR system (11732020; ThermoFisher) and custom TaqMan primers (ThermoFisher).
LPS gels.
Samples for LPS analysis were prepared as follows. Overnight cultures were diluted 1:100 in LB medium with 2% arabinose and incubated with shaking for 2.5 h at 37°C. Cells were washed twice with PBS, resuspended at an OD600 of 1 in LB medium, and diluted 1:20 in LB medium with the relevant sugar or MsbA inhibitor. These cultures were incubated with shaking for 4 h at 37°C. A volume equivalent to 1 ml at an OD600 of 1 was pelleted, washed with 1 ml of PBS, and resuspended in 100 μl of lithium dodecyl sulfate (LDS) sample buffer (NP0008; ThermoFisher) plus 4 μl of beta-mercaptoethanol (161-0710; Bio-Rad). Samples were heated for 10 min at 98°C. Proteins were digested by addition of 0.62 μl/sample Proteinase K (Qiagen 19133) and incubation overnight at 55°C. The Proteinase K was heat inactivated by incubating the samples for 10 min at 98°C. Five microliters of each sample was run on a 12-well 12% Bolt Bis-Tris Plus gel (NW00122; ThermoFisher) in 1× morpholineethanesulfonic acid (MES) buffer (NP0002) for 40 min at 150 V. Gels were stained for LPS using a Pro-Q Emerald 300 lipopolysaccharide gel stain kit (P20495; ThermoFisher).
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues for support and feedback, particularly P. Smith, S. Kapadia, and S. Rutherford. We thank M. Beresini for printing many plates for the initial phenotypic screens.
All funding for this project came from Genentech, Inc. Reagents are available under a materials transfer agreement with Genentech.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01142-18.
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland: http://www.who.int/drugresistance/documents/surveillancereport/en/. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 3.Kamio Y, Nikaido H. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- 4.Osborn MJ, Gander JE, Parisi E. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem 247:3973–3986. [PubMed] [Google Scholar]
- 5.Osborn MJ, Gander JE, Parisi E, Carson J. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem 247:3962–3972. [PubMed] [Google Scholar]
- 6.Zgurskaya HI, Lopez CA, Gnanakaran S. 2015. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect Dis 1:512–522. doi: 10.1021/acsinfecdis.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikaido H. 1988. Bacterial resistance to antibiotics as a function of outer membrane permeability. J Antimicrob Chemother 22(Suppl A):17–22. doi: 10.1093/jac/22.Supplement_A.17. [DOI] [PubMed] [Google Scholar]
- 8.Doerrler WT, Gibbons HS, Raetz CR. 2004. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J Biol Chem 279:45102–45109. doi: 10.1074/jbc.M408106200. [DOI] [PubMed] [Google Scholar]
- 9.Doerrler WT, Reedy MC, Raetz CR. 2001. An Escherichia coli mutant defective in lipid export. J Biol Chem 276:11461–11464. doi: 10.1074/jbc.C100091200. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, White KA, Polissi A, Georgopoulos C, Raetz CR. 1998. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J Biol Chem 273:12466–12475. doi: 10.1074/jbc.273.20.12466. [DOI] [PubMed] [Google Scholar]
- 11.Sperandeo P, Lau FK, Carpentieri A, De Castro C, Molinaro A, Deho G, Silhavy TJ, Polissi A. 2008. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J Bacteriol 190:4460–4469. doi: 10.1128/JB.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward A, Reyes CL, Yu J, Roth CB, Chang G. 2007. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci U S A 104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi W, Li Y, Yoon SH, Ernst RK, Walz T, Liao M. 2017. Structural basis of MsbA-mediated lipopolysaccharide transport. Nature 549:233–237. doi: 10.1038/nature23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho H, Miu A, Alexander MK, Garcia NK, Oh A, Zilberleyb I, Reichelt M, Austin CD, Tam C, Shriver S, Hu H, Labadie SS, Liang J, Wang L, Wang J, Lu Y, Purkey HE, Quinn J, Franke Y, Clark K, Beresini MH, Tan MW, Sellers BD, Maurer T, Koehler MFT, Wecksler AT, Kiefer JR, Verma V, Xu Y, Nishiyama M, Payandeh J, Koth CM. 2018. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 557:196–201. doi: 10.1038/s41586-018-0083-5. [DOI] [PubMed] [Google Scholar]
- 15.Doshi R, Ali A, Shi W, Freeman EV, Fagg LA, van Veen HW. 2013. Molecular disruption of the power stroke in the ATP-binding cassette transport protein MsbA. J Biol Chem 288:6801–6813. doi: 10.1074/jbc.M112.430074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonejuie P, Burkart M, Pogliano K, Pogliano J. 2013. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc Natl Acad Sci U S A 110:16169–16174. doi: 10.1073/pnas.1311066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperandeo P, Cescutti R, Villa R, Di Benedetto C, Candia D, Deho G, Polissi A. 2007. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J Bacteriol 189:244–253. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, McCandlish AC, Gronenberg LS, Chng SS, Silhavy TJ, Kahne D. 2006. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A 103:11754–11759. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolinson GN, Sutherland R. 1965. The binding of antibiotics to serum proteins. Br J Pharmacol Chemother 25:638–650. doi: 10.1111/j.1476-5381.1965.tb01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RN, Barry AL. 1987. Antimicrobial activity of ceftriaxone, cefotaxime, desacetylcefotaxime, and cefotaxime-desacetylcefotaxime in the presence of human serum. Antimicrob Agents Chemother 31:818–820. doi: 10.1128/AAC.31.5.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martorana AM, Motta S, Di Silvestre D, Falchi F, Deho G, Mauri P, Sperandeo P, Polissi A. 2014. Dissecting Escherichia coli outer membrane biogenesis using differential proteomics. PLoS One 9:e100941. doi: 10.1371/journal.pone.0100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KB, Toh E, Fernandez XB, Hanuszkiewicz A, Hardy GG, Brun YV, Bernards MA, Valvano MA. 2012. Functional characterization of UDP-glucose:undecaprenyl-phosphate glucose-1-phosphate transferases of Escherichia coli and Caulobacter crescentus. J Bacteriol 194:2646–2657. doi: 10.1128/JB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galloway SM, Raetz CR. 1990. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem 265:6394–6402. [PubMed] [Google Scholar]
- 24.Young K, Silver LL, Bramhill D, Cameron P, Eveland SS, Raetz CR, Hyland SA, Anderson MS. 1995. The envA permeability/cell division gene of Escherichia coli encodes the second enzyme of lipid A biosynthesis. UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase. J Biol Chem 270:30384–30391. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TM, Stachula SA, Raetz CR, Anderson MS. 1993. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase. The third step of endotoxin biosynthesis. J Biol Chem 268:19866–19874. [PubMed] [Google Scholar]
- 26.Babinski KJ, Ribeiro AA, Raetz CR. 2002. The Escherichia coli gene encoding the UDP-2,3-diacylglucosamine pyrophosphatase of lipid A biosynthesis. J Biol Chem 277:25937–25946. doi: 10.1074/jbc.M204067200. [DOI] [PubMed] [Google Scholar]
- 27.Crowell DN, Anderson MS, Raetz CR. 1986. Molecular cloning of the genes for lipid A disaccharide synthase and UDP-N-acetylglucosamine acyltransferase in Escherichia coli. J Bacteriol 168:152–159. doi: 10.1128/jb.168.1.152-159.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrett TA, Kadrmas JL, Raetz CR. 1997. Identification of the gene encoding the Escherichia coli lipid A 4′-kinase. Facile phosphorylation of endotoxin analogs with recombinant LpxK. J Biol Chem 272:21855–21864. [DOI] [PubMed] [Google Scholar]
- 29.Belunis CJ, Raetz CR. 1992. Biosynthesis of endotoxins. Purification and catalytic properties of 3-deoxy-d-manno-octulosonic acid transferase from Escherichia coli. J Biol Chem 267:9988–9997. [PubMed] [Google Scholar]
- 30.Clementz T, Bednarski JJ, Raetz CR. 1996. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem 271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 31.Clementz T, Zhou Z, Raetz CR. 1997. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem 272:10353–10360. [DOI] [PubMed] [Google Scholar]
- 32.Braun M, Silhavy TJ. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol 45:1289–1302. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. 2008. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A 105:5537–5542. doi: 10.1073/pnas.0801196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suits MD, Sperandeo P, Deho G, Polissi A, Jia Z. 2008. Novel structure of the conserved Gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J Mol Biol 380:476–488. doi: 10.1016/j.jmb.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 35.Dong H, Xiang Q, Gu Y, Wang Z, Paterson NG, Stansfeld PJ, He C, Zhang Y, Wang W, Dong C. 2014. Structural basis for outer membrane lipopolysaccharide insertion. Nature 511:52–56. doi: 10.1038/nature13464. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Xiang Q, Zhu X, Dong H, He C, Wang H, Zhang Y, Wang W, Dong C. 2014. Structural and functional studies of conserved nucleotide-binding protein LptB in lipopolysaccharide transport. Biochem Biophys Res Commun 452:443–449. doi: 10.1016/j.bbrc.2014.08.094. [DOI] [PubMed] [Google Scholar]
- 37.Sherman DJ, Lazarus MB, Murphy L, Liu C, Walker S, Ruiz N, Kahne D. 2014. Decoupling catalytic activity from biological function of the ATPase that powers lipopolysaccharide transport. Proc Natl Acad Sci U S A 111:4982–4987. doi: 10.1073/pnas.1323516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran AX, Dong C, Whitfield C. 2010. Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli. J Biol Chem 285:33529–33539. doi: 10.1074/jbc.M110.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao S, Luo Q, Zhao Y, Zhang XC, Huang Y. 2014. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature 511:108–111. doi: 10.1038/nature13484. [DOI] [PubMed] [Google Scholar]
- 40.Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, Buchanan SK. 2016. Structural and functional characterization of the LPS transporter LptDE from Gram-negative pathogens. Structure 24:965–976. doi: 10.1016/j.str.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malojcic G, Andres D, Grabowicz M, George AH, Ruiz N, Silhavy TJ, Kahne D. 2014. LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc Natl Acad Sci U S A 111:9467–9472. doi: 10.1073/pnas.1402746111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman DJ, Xie R, Taylor RJ, George AH, Okuda S, Foster PJ, Needleman DJ, Kahne D. 2018. Lipopolysaccharide is transported to the cell surface by a membrane-to-membrane protein bridge. Science 359:798–801. doi: 10.1126/science.aar1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barb AW, McClerren AL, Snehelatha K, Reynolds CM, Zhou P, Raetz CR. 2007. Inhibition of lipid A biosynthesis as the primary mechanism of CHIR-090 antibiotic activity in Escherichia coli. Biochemistry 46:3793–3802. doi: 10.1021/bi6025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onishi HR, Pelak BA, Gerckens LS, Silver LL, Kahan FM, Chen MH, Patchett AA, Galloway SM, Hyland SA, Anderson MS, Raetz CR. 1996. Antibacterial agents that inhibit lipid A biosynthesis. Science 274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 45.Liang X, Lee CJ, Chen X, Chung HS, Zeng D, Raetz CR, Li Y, Zhou P, Toone EJ. 2011. Syntheses, structures and antibiotic activities of LpxC inhibitors based on the diacetylene scaffold. Bioorg Med Chem 19:852–860. doi: 10.1016/j.bmc.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RL, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Kach A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. 2010. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 47.Lomovskaya O, Totrov M. 2005. Vacuuming the periplasm. J Bacteriol 187:1879–1883. doi: 10.1128/JB.187.6.1879-1883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macielag MJ. 2012. Chemical properties of antimicrobials and their uniqueness, p 793–820. In Dougherty TJ, Pucci MJ (ed), Antibiotic discovery and development. Springer US, Boston, MA. [Google Scholar]
- 49.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 50.O'Shea R, Moser HE. 2008. Physicochemical properties of antibacterial compounds: implications for drug discovery. J Med Chem 51:2871–2878. doi: 10.1021/jm700967e. [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Baidin V, Pahil KS, Moison E, Tomasek D, Ramadoss NS, Chatterjee AK, McNamara CW, Young TS, Schultz PG, Meredith TC, Kahne D. 2018. Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. Proc Natl Acad Sci U S A 115:6834–6839. doi: 10.1073/pnas.1804670115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 54.Diederich L, Rasmussen LJ, Messer W. 1992. New cloning vectors for integration in the lambda attachment site attB of the Escherichia coli chromosome. Plasmid 28:14–24. doi: 10.1016/0147-619X(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 55.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. CLSI document M07-A10. CLSI, Wayne, PA. [Google Scholar]
- 57.Storek KM, Auerbach MR, Shi H, Garcia NK, Sun D, Nickerson NN, Vij R, Lin Z, Chiang N, Schneider K, Wecksler AT, Skippington E, Nakamura G, Seshasayee D, Koerber JT, Payandeh J, Smith PA, Rutherford ST. 2018. Monoclonal antibody targeting the beta-barrel assembly machine of Escherichia coli is bactericidal. Proc Natl Acad Sci U S A 115:3692–3697. doi: 10.1073/pnas.1800043115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.