The magnitude of azole resistance in Aspergillus flavus and its underlying mechanism is obscure. We evaluated the frequency of azole resistance in a collection of clinical (n = 121) and environmental isolates (n = 68) of A. flavus by the broth microdilution method.
KEYWORDS: aspergillosis, antifungal resistance, efflux pumps, mechanism of resistance, Aspergillus flavus, mutational studies
ABSTRACT
The magnitude of azole resistance in Aspergillus flavus and its underlying mechanism is obscure. We evaluated the frequency of azole resistance in a collection of clinical (n = 121) and environmental isolates (n = 68) of A. flavus by the broth microdilution method. Six (5%) clinical isolates displayed voriconazole MIC greater than the epidemiological cutoff value. Two of these isolates with non-wild-type MIC were isolated from same patient and were genetically distinct, which was confirmed by amplified fragment length polymorphism analysis. Mutations associated with azole resistance were not present in the lanosterol 14-α demethylase coding genes (cyp51A, cyp51B, and cyp51C). Basal and voriconazole-induced expression of cyp51A homologs and various efflux pump genes was analyzed in three each of non-wild-type and wild-type isolates. All of the efflux pump genes screened showed low basal expression irrespective of the azole susceptibility of the isolate. However, the non-wild-type isolates demonstrated heterogeneous overexpression of many efflux pumps and the target enzyme coding genes in response to induction with voriconazole (1 μg/ml). The most distinctive observation was approximately 8- to 9-fold voriconazole-induced overexpression of an ortholog of the Candida albicans ATP binding cassette (ABC) multidrug efflux transporter, Cdr1, in two non-wild-type isolates compared to those in the reference strain A. flavus ATCC 204304 and other wild-type strains. Although the dominant marker of azole resistance in A. flavus is still elusive, the current study proposes the possible role of multidrug efflux pumps, especially that of Cdr1B overexpression, in contributing azole resistance in A. flavus.
INTRODUCTION
Our current knowledge about antifungal resistance mechanisms largely emerges from studies on Candida species and Aspergillus fumigatus. Azoles are the cornerstone of antifungal therapy due to their broad spectrum of activity and ease of administration (1). Azoles are also used in agriculture against phytopathogenic fungi and for material preservation (2). Azole resistance in A. fumigatus arises via two routes, namely, (i) the in vivo or in-host acquisition that occurs after prolonged azole therapy in chronic pulmonary aspergillosis (CPA) or allergic bronchopulmonary aspergillosis (ABPA) and (ii) acquisition in the environment due to use of azole fungicides (3, 4). Mutations in cyp51A are the most common underlying mechanism of resistance in either of these pathways. However, the role of multidrug efflux pumps (EPs) in antifungal drug resistance in mycelial fungi in general and in A. fumigatus in particular is not widely reported (5–10).
ATP binding cassettes (ABC) and major facilitator superfamily (MFS) transporters are the two major groups of efflux pumps (EPs) implicated in resistance (11). Although the most prevalent mechanism of azole resistance is alterations in cyp51A, many resistant isolates with no mutations or alterations in cyp51A or its promoter have been reported (12). A study identified a putative homolog of the Candida albicans ABC multidrug efflux transporter, Cdr1, as playing a role in mediating itraconazole resistance in 80% of the non-cyp51A-resistant clinical isolates of A. fumigatus (9). Furthermore, multidrug EPs contribute to voriconazole resistance in A. fumigatus biofilms. Rajendran et al. found voriconazole could induce the expression of AfuMdr4 in A. fumigatus biofilm under both in vitro and in vivo conditions, and the overexpression of the efflux pump correlated with azole resistance in biofilms of A. fumigatus (10).
In tropical regions, besides A. fumigatus, Aspergillus flavus also plays a key role in human infections (13–17). The burden of azole resistance in A. flavus and the mechanism deployed by this species have been reported in a few studies (8, 18–22). Natesan et al. found that 60% of resistant isolates of A. flavus selected in vitro under voriconazole pressure had no alteration in either cyp51A or cyp51B (23). A study also noted a heterogeneous upregulation of several multidrug EPs in A. flavus under both voriconazole-inducible and noninducible conditions (8). Recently, a study reported upregulation of Mdr1, Mdr2, and atrF in a non-wild-type A. flavus isolate, but overexpression of those pumps was not found in two other non-wild-type isolates (18).
The above observations prompted us to evaluate the role of efflux pump gene expression in azole-resistant A. flavus isolates lacking any mutation in azole target enzyme-coding genes. A collection of clinical and environmental isolates of A. flavus was screened for azole resistance. The mechanism of resistance of the isolates was investigated by sequencing cyp51A and its putative homologs, cyp51B and cyp51C. The isolates were further screened for expression of cyp51A homologs and various multidrug efflux transporters.
(A portion of this work was presented at the 8th Advances Against Aspergillosis conference, held in Lisbon, Portugal, 2018.)
RESULTS
In vitro antifungal susceptibility testing of A. flavus isolates.
The results of antifungal susceptibility testing of clinical and environmental isolates of A. flavus are presented in Table 1. Among the triazoles, itraconazole and posaconazole had better in vitro activity than voriconazole, as geometric mean MIC of clinical and environmental isolates (respectively) were 0.14 μg/ml and 0.06 μg/ml (itraconazole), 0.05 μg/ml and 0.02 μg/ml (posaconazole), and 0.43 μg/ml and 0.50 μg/ml (voriconazole). Six (5%) clinical isolates displayed voriconazole MIC above the epidemiological cutoff value (ECV), of which three had MIC of 2 μg/ml, two of 4 μg/ml, and one of 8 μg/ml. Two of those isolates (NCCPF761476 and NCCPF761488) were isolated from the same patient, who was on prolonged azole therapy for allergic bronchopulmonary aspergillosis, but the isolates appeared to be nonclonal by amplified fragment length polymorphism (AFLP) study (see Fig. S2 in the supplemental material). The clinical details of the patient are provided in the supplemental material. Besides these two isolates, one A. flavus isolate (NCCPF761157), which was previously demonstrated to harbor a mutation (Y319H) in the cyp51C gene, was also included as the third non-wild-type isolate in the study (21).
TABLE 1.
In vitro antifungal susceptibility of 189 clinical and environmental isolates of A. flavus to azoles
| Drug | Source | Susceptibilitya |
|||||
|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | GM | ECV | % ≤ECV | ||
| Itraconazole | Clinical | 0.03–16.0 | 0.12 | 0.5 | 0.14 | 1 | 98.3 |
| Environmental | 0.03–0.125 | 0.06 | 0.125 | 0.06 | 100 | ||
| Voriconazole | Clinical | 0.03–8.0 | 0.5 | 1.0 | 0.43 | 1 | 95.04 |
| Environmental | 0.15–1.0 | 0.5 | 1.0 | 0.50 | 100 | ||
| Posaconazole | Clinical | 0.015–0.5 | 0.06 | 0.25 | 0.05 | 0.25 | 98.95 |
| Environmental | 0.015–0.06 | 0.03 | 0.03 | 0.022 | 100 | ||
All values are in μg/ml. GM, geometric mean; ECV, epidemiological cut off value (34).
Constitutive (basal) and inducible expression of cyp51A and its homologs.
The cyp51A, cyp51B, and cyp51C genes were expressed at ≤10% of the levels of the comparator β-tubulin gene in all six isolates. cyp51C expression was at the minimal detectable level in only one non-wild-type and one wild-type isolate and was not at a detectable level in rest of the isolates (Fig. 1).
FIG 1.
Basal expression of cyp51A and its orthologs relative to that of the β-tubulin comparator gene. The scale shown is logarithmic. Results represent mean of biological replicates, and error bars illustrate standard deviation. The fold expression relative to β-tubulin was derived using the 2−ΔCT method.
On induction with voriconazole, the expression of cyp51A of two voriconazole non-wild-type isolates (NCCPF761476 and NCCPF761488) was 10-fold (10.3 ± 2.15) and 12-fold (11.9 ± 1.3) higher than that of their corresponding voriconazole-untreated controls. The third non-wild-type isolate (NCCPF761157) showed around 3-fold (3.2 ± 0.1) upregulation of cyp51A relative to the control. The three wild-type isolates exhibited various degrees of upregulation of cyp51A (NCCPF760690 [11.7 ± 3.1], NCCPF761267 [6.5 ± 6.1], and ATCC 204304 [6.9 ± 0.8]) after induction with voriconazole (Fig. 2A). The induced expression of cyp51B in two of the three non-wild-type isolates was 3-fold and 1.8-fold, respectively, whereas, none of the wild-type strains exhibited upregulation of cyp51B on exposure to voriconazole (Fig. 2B). Except for low-level expression of cyp51C in NCCPF761157 and NCCPF760690, the induced expression of this gene was undetectable in other isolates (data not shown).
FIG 2.
Voriconazole-inducible expression of cyp51A, cyp51B, and efflux transporters represented as fold change relative to that of untreated controls. The scale bar shown is linear. Results represent mean of biological replicates, and error bars illustrate standard deviation. The fold expression was derived using the 2−ΔΔCT method. P values were derived using multiple-comparison analysis of variance in panels A through G and panel I and by Student's t test in panel H. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001.
Constitutive (basal) and inducible expression of multidrug EPs.
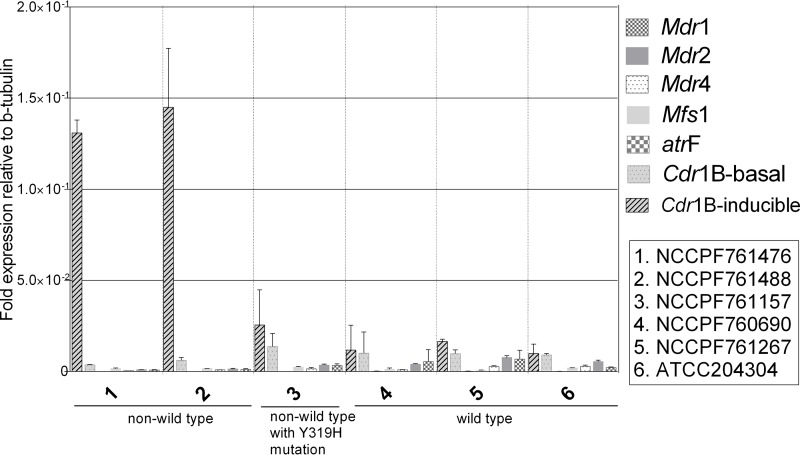
The genes evaluated for the expression of efflux pumps under the basal conditions (without any voriconazole pressure) showed poor expression (<5%) compared to the expression of the comparator gene, β-tubulin. While the constitutive expression of Cdr1B was largely similar in all of the strains, its expression was upregulated after voriconazole exposure in two non-wild-type strains (NCCPF761476 and NCCPF761488) (Fig. 3).
FIG 3.
Basal expression of multidrug efflux transporters represented as fold change relative to β-tubulin. Also shown is the voriconazole-inducible expression of the Cdr1B efflux pump relative to that of the β-tubulin reference gene. Results represent mean of biological replicates, and error bars illustrate standard deviation.
The induced expression of the Mdr1 efflux pump was relatively higher in two non-wild-type isolates (NCCPF761157 and NCCPF761488) compared to that in wild-type isolates. The transcript levels of Mdr1 increased by 16-fold in NCCPF761476 on voriconazole exposure. Similarly, the other non-wild-type isolate (NCCPF761488) showed 11-fold higher expression than that of the nonexposed control. The third non-wild-type isolate (NCCPF761157) demonstrated only 2-fold induction of Mdr1. The expression of Mdr1 in this isolate was lower than that of the wild-type isolates (Fig. 2C). In the case of the Mdr2 efflux pump, NCCPF761476 and NCCPF761488 showed 3-fold and 2-fold induction, respectively (P value < 0.0001). All of the wild-type isolates and one non-wild-type isolate (NCCPF761157) demonstrated no change in the levels of Mdr2 (Fig. 2D). The Mdr4 levels were elevated in only one non-wild-type isolate, NCCPF761476 (Fig. 2E). None of the isolates showed induction of MFS1 upon voriconazole treatment. (Fig. 2F). Only one non-wild-type isolate (NCCPF761476) showed 3-fold higher expression of atrF on exposure to voriconazole compared to that of other isolates (P < 0.05) (Fig. 2G).
Inducible expression of the Cdr1B homolog of A. flavus.
The homolog of the Cdr1B efflux pump was determined by the BLAST reciprocal best hits method. BLAST analysis identified a putative homolog transporter (XM_002377627.1) in the A. flavus NRRL3357 genome. For convenience, we retained the nomenclature Cdr1B for this EP of A. flavus. Upon induction with voriconazole, the expression of Cdr1B was highest of all the EPs evaluated, and Cdr1B was upregulated by 8-fold relative to the reference strain (ATCC 204304) in one non-wild-type isolate (P value < 0.01) and by 8.8-fold (P value < 0.05) in another non-wild-type isolate (Fig. 2H). Compared to their corresponding untreated controls, NCCPF761476 showed 35-fold induction, whereas the other non-wild-type isolate, NCCPF761488, demonstrated a 24-fold increase (P value < 0.0001) (Fig. 2I). Interestingly, the Cdr1B induction was very low in the third non-wild-type isolate (NCCPF761157) and in all of the wild-type isolates.
DISCUSSION
In the present study, two non-wild-type strains were isolated 1 month apart from an ABPA patient (see Fig. S1 in the supplemental material). Upon AFLP analysis, these two isolates segregated into two different clusters with only 93% fingerprint similarity index (Fig. S2). As the discriminatory power of AFLP for Aspergillus species has been reported to be comparable to that of microsatellite typing, these two isolates were considered nonclonal (24). In our previous study, we demonstrated a novel mutation, Y319H associated with non-wild-type voriconazole MIC, in cyp51C of an A. flavus isolate (NCCPF761157) (21). However, in the present study, neither of the two non-wild-type isolates (NCCPF761476 and NCCPF761488) had any mutation in either cyp51A or its homologs, cyp51B and cyp51C. We therefore evaluated the expression of cyp51A and its homologs and various efflux pump genes in these three isolates along with three wild-type comparator isolates.
Among the three homologs, the basal expressions of cyp51A and cyp51B were greater than that of cyp51C, indicating that lanosterol 14α-demethylase activity was largely contributed by cyp51A and cyp51B. cyp51C demonstrated extremely low but strain-dependent expression. cyp51C expression was not detectable in two non-wild-type isolates, two wild-type strains, and the reference strain A. flavus ATCC 204304, either at basal level or upon voriconazole induction. Very low expression of cyp51C was noted in the Y319H cyp51C mutant and in one wild-type isolate. Although cyp51A and cyp51B are two orthologous genes that catalyze two parallel pathways of ergosterol biosynthesis in A. fumigatus, many fungi possess multiple paralogs of the lanosterol 14α-demethylase gene (25, 26). Given the role played by cyp51C in contributing to voriconazole resistance in A. flavus, it is quite possible that a small fraction of lanosterol 14α-demethylase activity might be contributed by cyp51C in a few strains (20, 21).
Absence of any target site mutation in two non-wild-type isolates prompted us to study the role of EPs in conferring resistance. We measured the transcript levels of different ABC and MFS multidrug EPs pre- and post-voriconazole exposure. The basal expression of both ABC and MFS EPs was very low compared to that of the β-tubulin comparator gene. This suggests that in the absence of any antifungal stress, multidrug EPs may play a limited role. However, we observed a heterogeneous expression of one or more EPs in both non-wild-type and wild-type isolates after exposure to voriconazole. Natesan et al. evaluated the role of multidrug EPs in voriconazole non-wild-type A. flavus isolates selected under in vitro conditions and in two non-wild-type clinical isolates (8). They observed similar upregulation of more than one EP in non-wild-type strains. Recently, Fraczek et al. demonstrated the role of Cdr1B, an ortholog of the Candida albicans Cdr1 efflux pump, in conferring itraconazole resistance to A. fumigatus (9). The non-wild-type isolates analyzed in that study did not carry any change in cyp51A. They identified the putative homolog of C. albicans Cdr1 in A. fumigatus by performing a BLAST search using the best reciprocal hits method and named this putative ortholog Cdr1B (AFUA_1G14330). Taking a cue from that study, we investigated the presence of an ortholog of Cdr1B in A. flavus. By using the best reciprocal hits method of BLAST analysis with 1E−20 cutoff values, we identified a putative transporter (XM_ 002377627.1) in the A. flavus genome. The induced expressions of the Cdr1B transporter were 35-fold and 24-fold higher in two of our non-wild-type strains, NCCPF761476 and NCCPF761488, respectively, while a modest increase (1.4- to 3-fold) was noted in all of the wild-type strains tested. This was the most distinguishable feature in two of our non-wild-type isolates. However, in addition to Cdr1B upregulation, one of the two sequential non-wild-type isolates from the same patient also demonstrated greater overexpression of Mdr1, Mdr2, Mdr4, and atrF compared to that in the other isolate.
Therefore, it can be reasonably argued that efflux transporters, including Cdr1B, may play a role in mediating voriconazole resistance in A. flavus isolates, which lack target site mutations. Nevertheless, these findings need to be verified by testing a greater number of non-wild-type isolates and by checking the azole susceptibility profile of a Cdr1B deletion mutant.
The main limitation of our study was the difficulty in obtaining serial, isogenic, matched-pair isolates from aspergillosis patients on prolonged azole therapy. The ideal candidate for evaluating the mechanism of resistance would be an isogenic pair of isolates in which the non-wild-type isolate is genetically identical to the wild-type isolate (27–29). Isogeneity nullifies the background genetic differences between the strains. As a result, any small genetic change during the evolution of high MIC can be correlated with the resistance. Another limitation was the small number of non-wild-type strains evaluated in this study. Also, further functional characterization of Cdr1B is warranted. Although these results indicate the likely contribution of EPs to voriconazole resistance in A. flavus, the role of other unknown supplementary mechanisms cannot be ruled out in these strains. As heterogeneous upregulation of efflux pumps was observed in certain strains of A. flavus, a global transcriptomics approach may be helpful to further dissect the mechanism of resistance in the non-wild-type strains.
In conclusion, the frequency of azole non-wild-type isolates was low in our collection of clinical A. flavus isolates, whereas, in spite of exposure to azole-analogue fungicides in the environment (40%), none of our environmental isolates could be categorized as azole non-wild type. The current study proposes the possible role of multidrug EPs, especially that of Cdr1B overexpression, in contributing to azole resistance in A. flavus.
MATERIALS AND METHODS
Isolates.
A collection of A. flavus isolates (n = 121) from respiratory, sinonasal, and corneal samples collected during 2012 through 2014 at the Postgraduate Institute of Medical Education and Research, Chandigarh, India, were used in the study. Simultaneously, A. flavus environmental isolates (n = 68) from air, soil, and crops from a field in Mohali, Punjab, India (n = 27) and from different indoor areas of our hospital (n = 41) were also included. The isolates were identified phenotypically and by sequencing partial genes of β-tubulin and calmodulin, as described in our previous study (21).
Antifungal drug susceptibility testing.
In vitro antifungal susceptibility testing for azoles, including voriconazole, itraconazole, and posaconazole, was performed by the reference broth microdilution method as per the CLSI M38 document (30). The epidemiological cutoff values defined for Aspergillus spp., including A. flavus, were used to distinguish between the non-wild-type and wild-type isolates (31).
Amplified fragment length polymorphism (AFLP) analysis.
To assess the clonality of two sequential non-wild-type isolates obtained from the same patient, NCCPF761476 and NCCPF761488, AFLP was performed as per a previously reported method (32).
Sequencing of cyp51A and its homologs.
PCR and sequencing analysis of lanosterol 14α demethylase-coding genes were performed as described in our previous study (21). The primer sequences used for real-time quantification of cyp51A, cyp51B and cyp51C, and efflux pump genes are mentioned in Table S1 in the supplemental material.
Quantification of expression of azole target genes and multidrug transporter genes.
The experimental design of Fraczek et al. (9) was followed in the present study. Both basal and voriconazole-induced expressions were evaluated. The basal, or constitutive, expression was defined as the expression levels of different target genes under normal growth conditions in a basal or defined medium (Aspergillus minimal medium [AMM] in the present study) in the absence of antifungal exposure. The induced expression is the transcript levels of different target genes when the mycelia were challenged with 1 μg/ml voriconazole. A total of six isolates were selected. These included three voriconazole non-wild-type and three voriconazole wild-type isolates (Table 2). All of the isolates were first subcultured on potato dextrose agar (PDA) and incubated at 37°C for 3 to 5 days. The conidia were harvested by rolling a sterile wet cotton swab over the slant surface and were dislodged in sterile normal saline tube. Approximately 1 × 105 conidia/ml (measured by hemocytometer) were inoculated in 40 ml of AMM (33). The flasks were incubated under shaking conditions (150 rpm) at 37°C. To evaluate azole-inducible expression, 1 μg/ml voriconazole dissolved in dimethyl sulfoxide (DMS)O was added to each flask after 18 h of initial incubation. The control flasks received an equal volume of DMSO without the antifungal drug. The cultures were then allowed to grow for another 6 h under the same conditions.
TABLE 2.
In vitro azole susceptibility profile and polymorphism in cyp51A and its orthologs of six isolates included in the expression study
| Isolate | MIC fora: |
Mutation(s) in gene target: |
||||
|---|---|---|---|---|---|---|
| VOR | ITR | POS | cyp51A | cyp51B | cyp51C | |
| NCCPF761476 | 8 | 16 | 0.125 | A205T | M54T and S240A | |
| NCCPF761488 | 4 | 0.5 | 0.125 | A205T | M54T and S240A | |
| NCCPF761157 | 4 | 16 | 0.25 | A205T | M54T, S240A, D254N, I285V, and Y319H | |
| NCCPF761267 | 0.5 | 0.12 | 0.06 | A205T | M54T and S240A | |
| NCCPF760690 | 0.125 | 0.06 | 0.03 | A205T | M54T and S240A | |
| ATCC 204304 | 1 | 0.12 | 0.03 | A205T | NAb | |
VOR, voriconazole; ITR, itraconazole; and POS, posaconazole.
NA, not applicable.
Total RNA was extracted from the fungal isolates using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. In short, fungal balls were harvested from the cultures, and 20 to 30 mg of mycelial balls were crushed in liquid nitrogen using a sterile mortar and pestle. The crushed material was transferred to 1 ml of Trizol reagent (Life Technologies, India). Roughly 0.2 ml of molecular grade chloroform was added to each lysate and centrifuged at 12,000 rpm for 15 min. The upper layer was transferred to a sterile microcentrifuge tube, and an equal volume of 70% ethanol was added. Further steps, including DNase (Thermo Scientific, India) treatment, were performed as per the column manufacturer's guidelines. A 1-μg aliquot of total RNA was used to synthesize cDNA, utilizing a high-capacity cDNA synthesis kit (Thermo Scientific, India) as per the manufacture's guidelines.
Reverse transcription-PCR (RT-PCR) was performed in Roche Light Cycler 480 system using hot-start 2× SYBR green I master mix (Roche Diagnostics, India). Briefly, 5 μl of SYBR green master mix, 0.25 μl of each 10-pmol primer, 3.5 μl of nuclease-free water, and 1 μl of cDNA template were used per reaction. Nontemplate controls were included in each experiment to check for nonspecific amplification. Each sample was put in three biological replicates and two technical replicates. The cycling conditions were as follows: initial heat activation of SYBR green master mix at 95°C for 10 min, followed by 40 cycles of annealing at 56°C for 15 s and extension at 72°C for 20 min. Basal or constitutive transcription levels of each gene were calculated as fold change relative to β-tubulin as the internal control, using the threshold cycle (ΔCT) method. Inducible levels were calculated as fold change relative to the untreated control, using the ΔΔCT method of Livak (34). The expression data were analyzed by unpaired t test with Welch correction factor and by multiple-comparison analyses of variance in GraphPad Prism 6.0.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the financial support by Indian Council of Medical Research, New Delhi.
We declare no potential conflicts of interest. The authors alone are responsible for the contents and writing of the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01022-18.
REFERENCES
- 1.Sheehan DJ, Hitchcock CA, Sibley CM. 1999. Current and emerging azole antifungal agents. Clin Microbiol Rev 12:40–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 4.Howard SJ, Pasqualotto AC, Denning DW. 2010. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin Microbiol Infect 16:683–688. doi: 10.1111/j.1469-0691.2009.02911.x. [DOI] [PubMed] [Google Scholar]
- 5.Tobin MB, Peery RB, Skatrud PL. 1997. Genes encoding multiple drug resistance-like proteins in Aspergillus fumigatus and Aspergillus flavus. Gene 200:11–23. doi: 10.1016/S0378-1119(97)00281-3. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento AM, Goldman GH, Park S, Marras SAE, Delmas G, Oza U, Lolans K, Dudley MN, Mann PA, Perlin DS. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother 47:1719–1726. doi: 10.1128/AAC.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva Ferreira ME, Malavazi I, Savoldi M, Brakhage AA, Goldman MHS, Kim HS, Nierman WC, Goldman GH. 2006. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr Genet 50:32–44. doi: 10.1007/s00294-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 8.Natesan SK, Lamichchane AK, Swaminathan S, Wu W. 2013. Differential expression of ATP-binding cassette and/or major facilitator superfamily class efflux pumps contributes to voriconazole resistance in Aspergillus flavus. Diagn Microbiol Infect Dis 76:458–463. doi: 10.1016/j.diagmicrobio.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, Ramage G, Denning DW, Bowyer P. 2013. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother 68:1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 10.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. 2011. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother 55:2092–2097. doi: 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman JJ, Mylonakis E. 2009. Efflux in fungi: la pièce de résistance. PLoS Pathog 5:e1000486. doi: 10.1371/journal.ppat.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 13.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan S, Manavathu EK, Chandrasekar PH. 2009. Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses 52:206–222. doi: 10.1111/j.1439-0507.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti A, Rudramurthy SM, Panda N, Das A, Singh A. 2015. Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses 58:294–302. doi: 10.1111/myc.12314. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK, Gupta A, Rudramurthy SM, Paul S, Hallur VK, Chakrabarti A. 2016. Fungal keratitis in North India: spectrum of agents, risk factors and treatment. Mycopathologia 181:843–850. doi: 10.1007/s11046-016-0042-3. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA, Gupta A. 2008. Fungal endophthalmitis: fourteen years' experience from a center in India. Retina 28:1400–1407. doi: 10.1097/IAE.0b013e318185e943. [DOI] [PubMed] [Google Scholar]
- 18.Sharma C, Kumar R, Kumar N, Masih A, Gupta D, Chowdhary A. 2018. Investigation of multiple resistance mechanisms in voriconazole resistant Aspergillus flavus clinical isolates from a chest hospital surveillance in Delhi, India. Antimicrob Agents Chemother 62:e01928-17. doi: 10.1128/AAC.01928-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudramurthy SM, Chakrabarti A, Geertsen E, Mouton JW, Meis JF. 2011. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn Microbiol Infect Dis 71:370–377. doi: 10.1016/j.diagmicrobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Sun Y, Chen W, Liu W, Wan Z, Bu D, Li R. 2012. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob Agents Chemother 56:2598–2603. doi: 10.1128/AAC.05477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul RA, Rudramurthy SM, Meis JF, Mouton JW, Chakrabarti A. 2015. A novel Y319H substitution in CYP51C associated with azole resistance in Aspergillus flavus. Antimicrob Agents Chemother 59:6615–6619. doi: 10.1128/AAC.00637-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nami S, Baradaran B, Mansoori B, Kordbacheh P, Rezaie S, Falahati M, Khosroshahi LM, Safara M, Zaini F. 2017. The utilization of RNA silencing technology to mitigate the voriconazole resistance of aspergillus flavus; lipofectamine-based delivery. Adv Pharm Bull 7:53–59. doi: 10.15171/apb.2017.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan-Natesan S, Chandrasekar PH, Alangaden GJ, Manavathu EK. 2008. Molecular characterisation of cyp51A and cyp51B genes coding for P450 14α-lanosterol demethylases A (CYP51Ap) and B (CYP51Bp) from voriconazole-resistant laboratory isolates of Aspergillus flavus. Int J Antimicrob Agents 32:519–524. doi: 10.1016/j.ijantimicag.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 24.de Valk HA, Klaassen CH, Meis JF. 2008. Molecular typing of Aspergillus species. Mycoses 51:463–476. doi: 10.1111/j.1439-0507.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 25.Alcazar-Fuoli L, Mellado E, Garcia-Effron G, Lopez JF, Grimalt JO, Cuenca-Estrella JM, Rodriguez-Tudela JL. 2008. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 73:339–347. doi: 10.1016/j.steroids.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Cools HJ, Fraaije BA. 2008. Are azole fungicides losing ground against Septoria wheat disease? Resistance mechanisms in Mycosphaerella graminicola Pest Manag Sci. 64:681–684. doi: 10.1002/ps.1568. [DOI] [PubMed] [Google Scholar]
- 27.Camps SMT, Dutilh BE, Arendrup MC, Rijs AJMM, Snelders E, Huynen MA, Verweij PE, Melchers World J Gastroenterol . 2012. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7:e50034. doi: 10.1371/journal.pone.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losada L, Sugui JA, Eckhaus MA, Chang YC, Mounaud S, Figat A, Joardar V, Pakala SB, Pakala S, Venepally P, Fedorova N, Nierman WC, Kwon-Chung KJ. 2015. Genetic analysis using an isogenic mating pair of Aspergillus fumigatus identifies azole resistance genes and lack of MAT locus's role in virulence. PLoS Pathog 11:e1004834. doi: 10.1371/journal.ppat.1004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posteraro B, Sanguinetti M, Sanglard D, La Sorda M, Boccia S, Romano L, Morace G, Fadda G. 2003. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol Microbiol 47:357–371. doi: 10.1046/j.1365-2958.2003.03281.x. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—2nd ed. CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Canton E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J Clin Microbiol 48:3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudramurthy SM, de Valk HA, Chakrabarti A, Meis JF, Klaassen CH. 2011. High resolution genotyping of clinical Aspergillus flavus isolates from India using microsatellites. PLoS One 6:e16086. doi: 10.1371/journal.pone.0016086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gsaller F, Hortschansky P, Furukawa T, Carr PD, Rash B, Capilla J, Müller C, Bracher F, Bowyer P, Haas H, Brakhage AA, Bromley MJ. 2016. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog 12:e1006106. doi: 10.1371/journal.ppat.1006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.