The manufacturer-recommended aztreonam dosing for patients with creatinine clearance values of <10 ml/min/1.73 m2 is complex. It is not known whether simpler posthemodialysis dosing administered once daily or thrice weekly can reliably achieve pharmacodynamic goals.
KEYWORDS: aztreonam, pharmacodynamics, kidney dysfunction, end-stage renal disease, Monte Carlo simulation, renal failure
ABSTRACT
The manufacturer-recommended aztreonam dosing for patients with creatinine clearance values of <10 ml/min/1.73 m2 is complex. It is not known whether simpler posthemodialysis dosing administered once daily or thrice weekly can reliably achieve pharmacodynamic goals. We found that 1 or 2 g administered once daily after hemodialysis had >90% probability of target attainment up to MICs of 4 or 8 mg/liter, respectively. Thrice-weekly dosing should generally be avoided, except in nonsevere infections with MICs of ≤0.5 mg/liter.
TEXT
For more than 3 decades, aztreonam has continued to serve an important role in the treatment of Enterobacteriaceae and Pseudomonas infections in patients with significant β-lactam allergies (1). In addition, aztreonam may be a therapeutic option for increasingly common multidrug-resistant Gram-negative bacilli, given its unique activity against Ambler class B metallo-β-lactamases (2). To improve patient outcomes, dosing should be optimized based on pharmacodynamic parameters; updates to the interpretative breakpoints for aztreonam by the Clinical and Laboratory Standards Institute (CLSI) are based on the likelihood of achieving ≥50% free-drug time above the MIC (fTMIC) (3–5). Furthermore, the manufacturer's recommendations for aztreonam dosing in patients with creatinine clearance (CLCR) values of <10 ml/min/1.73 m2 state that the dose should be 25% of the normal dose administered with the same interval; this typically results in a dose of 250 mg or 500 mg administered every 8 h, with a supplemental dose of 125 mg or 250 mg administered after hemodialysis (HD) in severe infections (1, 6). However, it is not known whether simpler, post-HD dosing once daily or thrice weekly can reliably achieve pharmacodynamic goals, given that the half-life of aztreonam is prolonged in renal dysfunction (1, 6). Therefore, we conducted a Monte Carlo simulation (MCS) using a population pharmacokinetic model developed with data from patients with normal or impaired renal function, including patients with CLCR values of <10 ml/min/1.73 m2, to determine the probability of target attainment (PTA) with simplified aztreonam dosing (once daily or thrice weekly) (6).
The aztreonam population pharmacokinetic model involving 11 male volunteers with CLCR values of <10 ml/min/1.73 m2, based on the Cockcroft-Gault formula, was described previously (6–8). In those studies, aztreonam serum concentrations were determined at standard times after a single 1-g dose; patients did not receive dialysis during the sampling period (7, 8). MCS was conducted as described previously (6). Briefly, 1,000 concentration-time profiles for patients with CLCR values of <10 ml/min/1.73 m2 were simulated from the aztreonam population pharmacokinetic model using nonlinear mixed-effects modeling (NONMEM) (version 7.3; ICON Development Solution, Hanover, MD) (6). Body weight was a significant covariate in the population pharmacokinetic model. In the MCS, the body weight for each of the 1,000 patients was sampled based on the body weight distribution (mean, 71.4 kg; standard deviation [SD], 11.7 kg) for patients with CLCR values of <10 ml/min/1.73 m2 (7, 8). Three dosing strategies (1 g or 2 g once daily or 2 g thrice weekly [days 1, 3, and 5], using a 30-min intravenous infusion for all regimens) were used in the simulation. Aztreonam concentrations were assumed to be zero following HD. The fTMIC values on day 1 for 1-g or 2-g once-daily dosing and on days 1 to 7 for 2-g thrice-weekly dosing were determined for MICs ranging from 0.03 mg/liter to 32 mg/liter, and pharmacodynamic targets of 50% and 60% fTMIC were evaluated. The PTAs for each dosing regimen at each MIC were then calculated as the fractions of 1,000 subjects achieving 50% and 60% fTMIC. The 50% fTMIC was used for the primary analysis, given that this is the target used by the CLSI for interpretive breakpoints and in vitro data found this target to be associated with a 1-log10 unit decrease in bacterial counts at 24 h (3–5, 9). Given the limited underlying data, however, we also assessed the target of 60% fTMIC as the most conservative sensitivity analysis; this target has been associated with a 2-log10 unit reduction in bacterial counts at 24 h (9). To evaluate safety, the daily mean maximum concentration (Cmax) and the area under the concentration-time curve from 0 to 24 h (AUC0–24) for the regimen with the highest dose (2 g once daily) were evaluated. A plasma protein-binding value of 42.1% was used for all simulations and was presumed to be dose independent (10, 11).
Table 1 and Fig. 1 include the results of the MCS for the PTAs for aztreonam at 1 g or 2 g administered once daily or 2 g administered thrice weekly, stratified by bacterial MIC. Aztreonam at 2 g or 1 g once daily (timed after HD for patients receiving HD) provided the best PTAs, with 96.2% chances of achieving 50% fTMIC for pathogens with MICs of 8 mg/liter or 4 mg/liter, respectively. Aztreonam at 2 g administered thrice weekly performed the worst; 90% PTA was achieved only with a MIC of ≤0.5 mg/liter. The daily mean Cmax and AUC0–24 values for the highest dose (2 g once daily) were 82.7 mg/liter and 1,138 mg · h/liter, respectively.
TABLE 1.
Probability of target attainment with simplified aztreonam dosing in patients with CLCR values of <10 ml/min/1.73 m2
| Aztreonam dose and MIC (mg/liter)a | PTA (%) |
|
|---|---|---|
| 50% fTMIC | 60% fTMIC | |
| 1 g once daily | ||
| 0.03 | 100 | 100 |
| 0.06 | 100 | 100 |
| 0.125 | 100 | 100 |
| 0.25 | 100 | 100 |
| 0.5 | 100 | 99.9 |
| 1 | 99.9 | 99.4 |
| 2 | 99.4 | 97.2 |
| 4 | 96.2 | 87.4 |
| 8 | 67 | 44.7 |
| 16 | 3.6 | 0.8 |
| 32 | 0 | 0 |
| 2 g once daily | ||
| 0.03 | 100 | 100 |
| 0.06 | 100 | 100 |
| 0.125 | 100 | 100 |
| 0.25 | 100 | 100 |
| 0.5 | 100 | 100 |
| 1 | 100 | 99.6 |
| 2 | 99.7 | 99.2 |
| 4 | 99.2 | 97.4 |
| 8 | 96.2 | 85.7 |
| 16 | 69.8 | 43.7 |
| 32 | 2.9 | 0.9 |
| 2 g thrice weekly (days 1, 3, and 5) | ||
| 0.03 | 99.7 | 98.4 |
| 0.06 | 99.4 | 96.5 |
| 0.125 | 98.3 | 93.4 |
| 0.25 | 95.8 | 87.7 |
| 0.5 | 90.9 | 78.8 |
| 1 | 82.1 | 66 |
| 2 | 66.9 | 46.5 |
| 4 | 43.1 | 24.2 |
| 8 | 14.3 | 5.6 |
| 16 | 0.7 | 0.1 |
| 32 | 0 | 0 |
It is assumed that, for patients requiring HD, all doses (including daily doses) are administered immediately after HD.
FIG 1.
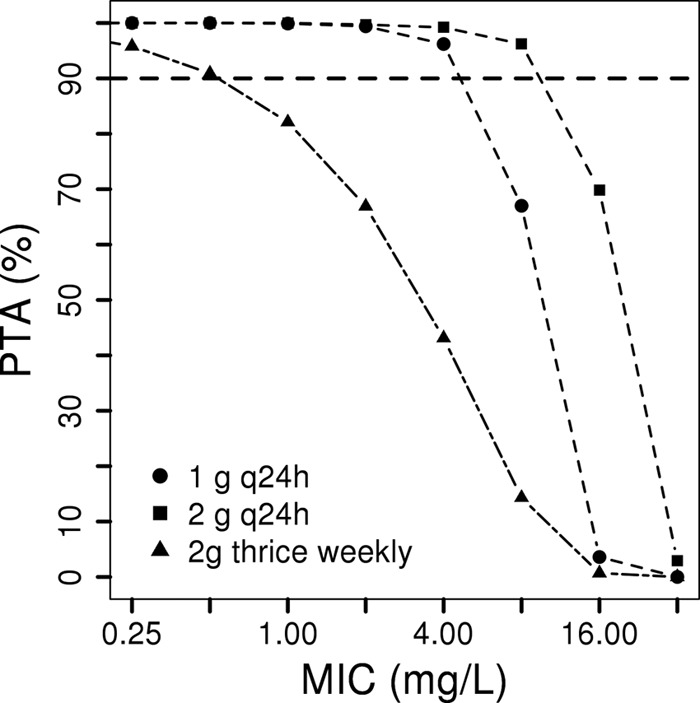
Probability of target attainment (50% fTMIC) with simplified aztreonam dosing in patients with CLCR values of <10 ml/min/1.73 m2. The dosing scheme was 1 g every 24 h, 2 g every 24 h, or 2 g thrice weekly (days 1, 3, and 5). For patients requiring HD, it was assumed that all doses (including daily doses) were administered immediately after HD.
Modeling and simulation have been widely utilized in drug development to support dose selection, development decisions, and regulatory submission and labeling (12, 13). Our results indicate that aztreonam dosing for patients with CLCR values of <10 ml/min/1.73 m2 can be greatly simplified while still achieving pharmacodynamic goals for susceptible isolates. Aztreonam breakpoints from the CLSI recommend that Enterobacteriaceae strains with MICs of ≤4 mg/liter and Pseudomonas strains with MICs of ≤8 mg/liter be considered susceptible, based on dosing of 1 g every 8 h or 2 g every 8 h, respectively (4). The European Committee on Antimicrobial Susceptibility Testing considers strains with MICs of ≤1 mg/liter susceptible for both Enterobacteriaceae and Pseudomonas, regardless of aztreonam dosing (14). Thrice-weekly post-HD dosing in patients with end-stage renal disease has been shown to achieve pharmacodynamic goals, based on population pharmacokinetic studies and MCS, for cefazolin, ceftazidime, and cefepime (15–18). Relative to those agents, however, there is a greater proportion of nonrenal elimination of aztreonam and thus thrice-weekly dosing of aztreonam is unlikely to be effective unless the pathogen MIC is ≤0.5 mg/liter (1). Notably, in an international database containing data from multiple sites and time periods, only 79 of 3,191 P. aeruginosa isolates had aztreonam MICs of ≤0.5 mg/liter; therefore, it is unlikely that thrice-weekly dosing of aztreonam would be appropriate for P. aeruginosa (19). We also suggest avoiding thrice-weekly dosing for neutropenic patients and patients with severe or high-inoculum infections due to Enterobacteriaceae, even when the MIC is confirmed to be ≤0.5 mg/liter. A dose of 2 g daily should be preferred when possible because of the greater PTAs, especially for P. aeruginosa isolates, given higher CLSI breakpoints and higher MIC distributions (4, 19). A dose of 1 g daily may be adequate for susceptible Enterobacteriaceae strains, based on current CLSI breakpoints (4).
In addition to the pharmacodynamics, the potential toxicity of the studied regimens should be considered. Aztreonam is generally well tolerated, with the most common adverse effects typically being injection site reactions, gastrointestinal adverse effects, and rash (20). The mean daily Cmax and AUC0–24 for the most intensive dosing regimen we evaluated (2 g once daily) were similar to exposures with FDA-approved regimens (i.e., 204 mg/liter and 1,336 mg · h/liter, respectively) (1, 21). Therefore, the tested regimens are not expected to be associated with toxicity risks greater than normal.
This study has some limitations. It was based on a small population of otherwise healthy male volunteers, and elimination of the drug during HD was not determined in the source pharmacokinetic studies (7, 8). To mitigate this limitation and to provide the most conservative PTA, we assumed that no drug would remain at the end of HD. With the use of 1 or 2 g daily for organisms with MICs of 4 or 8 mg/liter, respectively, the PTA for the sensitivity analysis (60% fTMIC) fell just below 90%. Thus, we suggest that 2 g once daily be used for most patients and that clinicians exercise caution if the MIC is equivalent to 8 mg/liter and the patient either is neutropenic or is being treated for a severe or high-inoculum infection.
In conclusion, we found that aztreonam dosing can be greatly simplified for patients with CLCR values of <10 ml/min/1.73 m2 by using 1 to 2 g administered once daily (timed after HD on HD days). Thrice-weekly dosing of aztreonam after HD should generally be avoided, with the exception of nonsevere infections due to Enterobacteriaceae strains with MICs of ≤0.5 mg/liter.
ACKNOWLEDGMENTS
We received no specific funding for this work.
H.X., D.Z., and N.A. are employees of AstraZeneca. A.E.G. has no conflicts to disclose.
REFERENCES
- 1.Bristol-Myers Squibb. 2013. AZACTAM (aztreonam for injection). Bristol-Myers Squibb, Princeton, NJ: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050580s042lbl.pdf. [Google Scholar]
- 2.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN. 2013. Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa. I. Cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 5.Ramsey CBK, Noel AR, Attwood MLG, Tomaselli ST, MacGowan AP. 2015. Pharmacodynamics of aztreonam against E. coli studied in an in vitro model of infection. Abstr 55th Intersci Conf Antimicrob Agents Chemother, abstr A471. [Google Scholar]
- 6.Xu H, Zhou W, Zhou D, Li J, Al-Huniti N. 2017. Evaluation of aztreonam dosing regimens in patients with normal and impaired renal function: a population pharmacokinetic modeling and Monte Carlo simulation analysis. J Clin Pharmacol 57:336–344. doi: 10.1002/jcph.810. [DOI] [PubMed] [Google Scholar]
- 7.el Guinaidy MA, Nawishy S, Abd el Bary M, Sabbour MS. 1989. Single-dose pharmacokinetics of aztreonam in healthy volunteers and renal failure patients. J Chemother 1:164–169. doi: 10.1080/1120009X.1989.11738886. [DOI] [PubMed] [Google Scholar]
- 8.Mihindu JC, Scheld WM, Bolton ND, Spyker DA, Swabb EA, Bolton WK. 1983. Pharmacokinetics of aztreonam in patients with various degrees of renal dysfunction. Antimicrob Agents Chemother 24:252–261. doi: 10.1128/AAC.24.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S, O'Donnell JP, Bradford PA, Eakin AE. 2015. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. doi: 10.1093/jac/dkv132. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson EN, Karlsson MO. 1999. Xpose: an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. doi: 10.1016/S0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW. 2007. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using Monte Carlo simulation. Antimicrob Agents Chemother 51:3049–3055. doi: 10.1128/AAC.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Huniti N, Zhou D, Xu H, Aksenov S, Bui KH, Fox R, Helmlinger G, Stanski D. 2017. Pharmacometric modeling of naloxegol efficacy and safety: impact on dose and label. Clin Pharmacol Ther 102:741–744. doi: 10.1002/cpt.719. [DOI] [PubMed] [Google Scholar]
- 13.Trang M, Dudley MN, Bhavnani SM. 2017. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr Opin Pharmacol 36:107–113. doi: 10.1016/j.coph.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0. http://www.eucast.org/clinical_breakpoints.
- 15.Loo AS, Neely M, Anderson EJ, Ghossein C, McLaughlin MM, Scheetz MH. 2013. Pharmacodynamic target attainment for various ceftazidime dosing schemes in high-flux hemodialysis. Antimicrob Agents Chemother 57:5854–5859. doi: 10.1128/AAC.00474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx MA, Frye RF, Matzke GR, Golper TA. 1998. Cefazolin as empiric therapy in hemodialysis-related infections: efficacy and blood concentrations. Am J Kidney Dis 32:410–414. doi: 10.1053/ajkd.1998.v32.pm9740156. [DOI] [PubMed] [Google Scholar]
- 17.Schmaldienst S, Traunmuller F, Burgmann H, Rosenkranz AR, Thalhammer-Scherrer R, Horl WH, Thalhammer F. 2000. Multiple-dose pharmacokinetics of cefepime in long-term hemodialysis with high-flux membranes. Eur J Clin Pharmacol 56:61–64. doi: 10.1007/s002280050721. [DOI] [PubMed] [Google Scholar]
- 18.Sowinski KM, Mueller BA, Grabe DW, Manley HJ, Frye RF, Bailie GR, Marx MA. 2001. Cefazolin dialytic clearance by high-efficiency and high-flux hemodialyzers. Am J Kidney Dis 37:766–776. doi: 10.1016/S0272-6386(01)80126-8. [DOI] [PubMed] [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing. 2018. Aztreonam/Pseudomonas aeruginosa international MIC distribution: reference database. http://mic.eucast.org/Eucast2/. Accessed 28 April 2018.
- 20.Newman TJ, Dreslinski GR, Tadros SS. 1985. Safety profile of aztreonam in clinical trials. Rev Infect Dis 7(Suppl 4):S648–S655. doi: 10.1093/clinids/7.Supplement_4.S648. [DOI] [PubMed] [Google Scholar]
- 21.Smith PF, Ballow CH, Booker BM, Forrest A, Schentag JJ. 2001. Pharmacokinetics and pharmacodynamics of aztreonam and tobramycin in hospitalized patients. Clin Ther 23:1231–1244. doi: 10.1016/S0149-2918(01)80103-X. [DOI] [PubMed] [Google Scholar]


