Rezafungin (CD101) is a novel echinocandin under development for once-weekly intravenous (i.v.) dosing. We evaluated the pharmacodynamics (PD) of rezafungin against 4 Candida auris strains, using the neutropenic mouse invasive candidiasis model.
KEYWORDS: Candida auris, pharmacodynamics, antifungal therapy, rezafungin
ABSTRACT
Rezafungin (CD101) is a novel echinocandin under development for once-weekly intravenous (i.v.) dosing. We evaluated the pharmacodynamics (PD) of rezafungin against 4 Candida auris strains, using the neutropenic mouse invasive candidiasis model. The area under the concentration-time curve (AUC)/MIC was a robust predictor of efficacy (R2 = 0.76). The stasis free-drug 24-h AUC/MIC target exposure for the group was 1.88, whereas the 1-log-kill free-drug 24-h AUC/MIC target exposure was 5.77. These values are very similar to those in previous rezafungin PD studies with other Candida spp. Based on recent surveillance susceptibility data, AUC/MIC targets are likely to be exceeded for >90% of C. auris isolates with the previously studied human dose of 400 mg administered i.v. once weekly.
TEXT
Rezafungin (CD101) is a novel echinocandin with intravenous (i.v.) administration that is undergoing clinical study for the treatment and prophylaxis of fungal infections, including invasive candidiasis. Pharmacologically, rezafungin has a terminal half-life of approximately 130 h in humans, which allows extended-interval dosing, such as the once-weekly regimens that were studied in phase 1 and phase 2 trials (1). Rezafungin exhibits broad in vitro potency against fungal pathogens that is comparable to that of other echinocandins (2–4), and previous in vivo pharmacodynamic (PD) studies demonstrated robust efficacy against Candida albicans, Candida glabrata, and Candida parapsilosis (5). In those studies, the area under the concentration-time curve (AUC)/MIC was strongly associated with efficacy, and rezafungin demonstrated favorable AUC/MIC targets (e.g., free-drug AUC/MIC values of <3 for net stasis) for each species group.
Candida auris is an established global threat to human health that demonstrates unique epidemiological and clinical characteristics, including environmental persistence, enhanced interhuman transmission, multidrug resistance to available antifungal agents, and mortality rates between 40 and 60% (6–8). Previous studies demonstrated that echinocandins exhibited greater efficacy than triazoles or amphotericin B against a collection of C. auris isolates in the mouse neutropenic invasive candidiasis model (9). Furthermore, a recent in vitro evaluation of rezafungin against 100 C. auris isolates performed by Berkow and Lockhart demonstrated potent activity, including activity against some strains that exhibited elevated MICs with other echinocandins (10). The goal of the current studies was to define the pharmacokinetic (PK)/PD target of rezafungin against 4 C. auris strains, using the neutropenic mouse invasive candidiasis model.
C. auris strains were chosen based on different susceptibilities to rezafungin and fitness in the mouse disseminated candidiasis model (Table 1). Susceptibility testing was performed according to CLSI guidelines (11). The MICs for rezafungin varied 32-fold (range, 0.06 to 2 μg/ml), and a single strain (B11211) contained an FKS hot spot mutation (FKS1_HS1_S639F) conferring elevated echinocandin MICs. The neutropenic mouse disseminated candidiasis model was used for all experiments. Mice were rendered neutropenic by subcutaneous cyclophosphamide injections (150 mg/kg on day −4 and 100 mg/kg on day −1, day +2, and day +4), to ensure neutropenia throughout the experimental period. Three mice were included in each treatment or control group. Mice were inoculated with each of the 4 strains at 5.99 ± 0.29 log10 CFU/ml, via the lateral tail vein. Antifungal treatment began 2 h after inoculation. Rezafungin dosing regimens were selected to mimic the human once-weekly dosing regimens evaluated in clinical trials. Specifically, because the half-life of rezafungin in mice is 30 to 40 h (5), doses were administered once every 3 days over a 7-day experimental period (i.e., doses on days 0, 3, and 6). Rezafungin doses were 1, 4, 16, and 64 mg/kg by the intraperitoneal route. After 7 days, mice were euthanized for determination of fungal CFU in the kidneys. Organism burdens in mouse kidneys after 7 days of therapy were compared to the quantity at the start of therapy. The treatment results were analyzed using a sigmoidal maximum effect (Emax) model (12). PK exposures in this mouse model were obtained in our laboratory, and protein binding of 99.2% was used for determination of free-drug concentrations (5). The PK exposures were plotted relative to MICs and the previously defined PK/PD driver AUC/MIC (13). The magnitudes of the PK/PD index (AUC/MIC) associated with net stasis and 1-log kill (when achieved) for each strain were calculated with the equation: log10 D = log10 [E/(Emax − E)]/(N + log10 ED50), where E is the control growth for the stasis dose (D), E + 1 is the control growth for the 1-log-kill dose (D), and ED50 is the 50% effective dose.
TABLE 1.
Characteristics of clinical C. auris strains used in the in vivo studies
| Strain | Country of origin | Growth in untreated controls (log10 CFU/kidneys) |
In vitro MIC (mg/liter) |
|||
|---|---|---|---|---|---|---|
| Rezafungin | Fluconazole | Micafungin | Amphotericin B | |||
| B11220 | Japan | 3.06 | 0.06 | 4 | 0.125 | 0.38 |
| B11785 | Colombia | 3.43 | 0.125 | 8 | 0.5 | 1.5 |
| B11799 | Colombia | 3.67 | 0.25 | 16 | 2 | 0.5 |
| B11211a | India | 3.17 | 2 | 256 | 4 | 1.5 |
With FKS1_HS1_S639F.
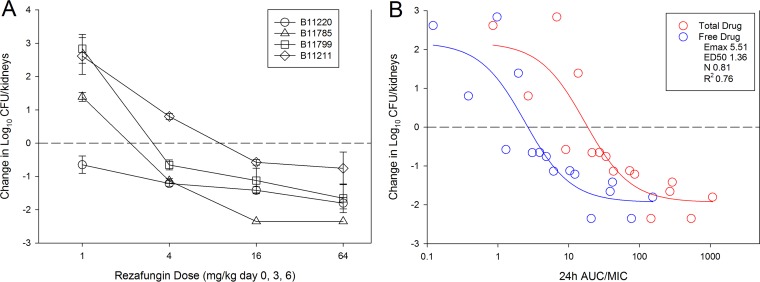
The results of rezafungin dose-ranging studies with the 4 C. auris isolates are shown in Fig. 1A. Dose-dependent activity was observed with each strain, and a net killing effect was achieved against all strains, compared to the burden at the start of therapy. A >1-log kill was achieved for 3 of 4 strains; only the most resistant strain (B11211) did not achieve a 1-log-kill endpoint over the dose range. The treatment data modeled relative to the AUC/MIC index (using both total and free-drug concentrations) are shown in Fig. 1B. As expected, there was a strong relationship between AUC/MIC and treatment effect, based on nonlinear regression analysis using the Hill equation (R2 = 0.76). The stasis doses, 1-log-kill doses (when achieved), and associated AUC/MIC exposures for each strain are shown in Table 2. For the aggregate isolate group, the stasis and 1-log-kill free-drug 24-h AUC/MIC targets were 1.88 and 5.77, respectively. These PK/PD targets are very similar to those against other Candida species in this in vivo model. In our previous study, for example, the stasis free-drug 24-h AUC/MIC target for C. albicans was 2.89, and 1-log kill was observed at a target of 5.14. Our efficacy results are also congruent with recent data obtained in the mouse fungal infection model by Hager and colleagues (14). Integrating the current PD results in the context of human PK exposures helps to elucidate the potential efficacy of this agent in clinical practice. Human PK studies with rezafungin demonstrated that 400 mg/kg administered i.v. once every 7 days resulted in an AUC from 0 to 168 h of 1,840 mg · h/liter (1). Incorporating human protein binding (97.4%) and converting the exposure to an average daily (24-h) AUC yields a result of approximately 7 mg · h/liter. Using these free-drug estimates and the stasis target identified in this study yields an MIC ceiling estimate of 2 to 4 mg/liter. If the 1-log-kill target exposures from our study are used, then the MIC ceiling is 1 to 2 mg/liter. In a recent surveillance study examining the rezafungin MIC distribution for 100 C. auris clinical isolates, only 4 isolates (4%) had MIC values of ≥1 mg/liter (10). Thus, rezafungin therapy in humans would be expected to exceed the PK/PD targets for the vast majority of patients with C. auris infections.
FIG 1.
(A) In vivo rezafungin dose-response curves for 4 C. auris strains. Each symbol represents the mean and standard deviation (error bars) of the change in burden in the kidneys of 3 mice. The horizontal dashed line represents the burden at the start of therapy. (B) Relationships between treatment effects for all strains and the PK/PD index AUC/MIC for rezafungin. Each symbol represents the mean change in burden in the kidneys of 3 mice. The horizontal dashed line represents the burden at the start of therapy. Both total (red) and free (blue) drug concentrations are shown; best-fit lines based on the Hill equation are shown for both. Also shown are the PD parameters maximum effect (Emax), 50% effective dose (ED50), slope of the curve (N), and coefficient of determination for the free-drug AUC/MIC.
TABLE 2.
Stasis and 1-log kill doses and associated AUC/MIC (total and free-drug) values in the neutropenic murine disseminated candidiasis modela
| Strain | MIC (mg/liter) | Stasis |
1-log kill |
||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | Ave 24-h tAUC/MIC | Ave 24-h fAUC/MIC | Dose (mg/kg) | Ave 24-h tAUC/MIC | Ave 24-h fAUC/MIC | ||
| B11220 | 0.06 | 0.87 | 144.78 | 1.16 | 6.99 | 987.68 | 7.90 |
| B11785 | 0.125 | 6.09 | 421.39 | 3.4 | 10.65 | 685.80 | 5.49 |
| B11799 | 0.25 | 8.76 | 288.72 | 2.3 | 16.17 | 488.81 | 3.91 |
| B11211 | 2 | 23.04 | 82.95 | 0.66 | NA | ||
| Mean | 9.69 | 234.46 | 1.88 | 11.27 | 720.76 | 5.77 | |
| Median | 7.43 | 216.75 | 1.73 | 10.65 | 685.80 | 5.49 | |
| SD | 9.48 | 151.53 | 1.21 | 4.62 | 251.26 | 2.01 | |
Ave, average; tAUC/MIC, total-drug AUC/MIC; fAUC/MIC, free-drug AUC/MIC; NA, not achieved.
In summary, rezafungin exhibited potent in vivo activity against a group of clinical C. auris strains. As in other echinocandin studies, the PK/PD index AUC/MIC strongly predicted efficacy. PK/PD free-drug 24-h AUC/MIC target exposures of 1.88 and 5.77 led to net stasis and 1-log kill, respectively. Integration of these targets with human PK studies suggests that the clinically studied dose of 400 mg administered i.v. once a week would meet or exceed the PD target for >90% of C. auris isolates. These findings suggest that rezafungin may be a useful option for patients with C. auris infections, and further clinical study is warranted.
ACKNOWLEDGMENT
This study was supported by funding from Cidara Therapeutics, Inc.
REFERENCES
- 1.Sandison T, Ong V, Lee J, Thye D. 2017. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 61:e01627-16. doi: 10.1128/AAC.02687-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. 2017. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 antifungal surveillance program. Int J Antimicrob Agents 50:352–358. doi: 10.1016/j.ijantimicag.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2016. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendrup MC, Meletiadis J, Zaragoza O, Jorgensen KM, Marcos-Zambrano LJ, Kanioura L, Cuenca-Estrella M, Mouton JW, Guinea J. 2018. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect doi: 10.1016/j.cmi.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. 2018. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother 62:e02154-17. doi: 10.1128/AAC.02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamoth F, Kontoyiannis DP. 2018. The Candida auris alert: facts and perspectives. J Infect Dis 217:516–520. doi: 10.1093/infdis/jix597. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi: 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 9.Lepak AJ, Zhao M, Berkow EL, Lockhart SR, Andes DR. 2017. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob Agents Chemother 61:e00791-17. doi: 10.1128/AAC.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkow EL, Lockhart SR. 2018. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn Microbiol Infect Dis 90:196–197. doi: 10.1016/j.diagmicrobio.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard— 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 13.Lepak AJ, Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hager CL, Larkin EL, Long LA, Ghannoum MA. 2018. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]