Malaria remains an important parasitic disease with a large morbidity and mortality burden. Plasmodium transmission-blocking (TB) compounds are essential for achieving malaria elimination efforts.
KEYWORDS: HTS, Pathogen Box, antimalarial agents, gametes, malaria, transmission
ABSTRACT
Malaria remains an important parasitic disease with a large morbidity and mortality burden. Plasmodium transmission-blocking (TB) compounds are essential for achieving malaria elimination efforts. Recent efforts to develop high-throughput screening (HTS) methods to identify compounds that inhibit or kill gametocytes, the Plasmodium sexual stage infectious to mosquitoes, have yielded insight into new TB compounds. However, the activities of these compounds against gametes, formed in the first minutes of mosquito infection, are typically not assessed, unless screened in a standard membrane feeding assay, a labor-intensive assay. We demonstrate here the generation of a Plasmodium model for drug screens against gametes and fertilization. The new P. berghei line, named Ookluc, was genetically and pharmacologically validated and scalable for HTS. Screening the Pathogen Box from the Medicines for Malaria Venture using the new model identified promising TB compounds. The use of Ookluc in different libraries of compounds may aid in the identification of transmission-blocking drugs not assessed in screens against asexual stages or gametocytes.
INTRODUCTION
In 2016, nearly half of the world population was at risk of malaria, and 216 million cases of the disease killed 445 thousand people (1). Malaria is transmitted to humans through the bite of infected Anopheles mosquitoes, and the symptomatic disease is caused by the multiplication of asexual stages of species of Plasmodium within erythrocytes. The transmission cycle is completed when mosquitoes ingest the sexual gametocyte stage upon biting an infected individual. When ingested during a mosquito blood meal, they activate and transform into male and female gametes in the mosquito midgut. Within the first hour, the gametes fertilize to form a zygote, which after 18 to 24 h becomes an ookinete (a motile zygote) (2). The ookinete invades the midgut epithelium and then settles at the basal lamina to form an oocyst (2). In the oocyst, thousands of sporozoites develop and bud after 12 to 16 days to migrate to the mosquito salivary glands (3), where they wait to be injected to the vertebrate host with another blood meal.
Malaria control is largely based on vector control with the use of insecticidal nets and long-lasting indoor residual insecticide spraying to protect humans from mosquito bites. Recent reports of mosquito resistance to insecticides are a matter of concern (4). The use of artemisinin combination therapies can efficiently clear the circulating asexual-stage parasites, with some antimalarials also possessing activity against the transmissible gametocyte. However, the emergence and spread of drug-resistant parasites in Asia reduce the effectiveness of the combination therapy and reduce their use for mass drug administration to reduce transmission (5).
The problem of mosquito and parasite resistance has led the World Health Organization (WHO) to launch, in 2015, The WHO Global Technical Strategy for Malaria 2016-2030 (http://www.who.int/malaria/en/), which aims at reducing the malaria incidence by 90% by 2030 and to eliminate malaria (interrupt local transmission) in at least 35 countries. One of the specific aims of the global strategy is to implement transmission-blocking (TB) chemotherapy.
The only drug approved for TB chemotherapy is primaquine. However, its use is limited by toxicity concerns, especially in patients carrying deficient alleles of the glucose-6-phosphate dehydrogenase (G6PD) enzyme. Thus, developing new TB antimalarials is important for malaria control and elimination efforts.
Efforts to identify and develop new TB compounds utilize high-throughput screening (HTS) methodologies that assess gametocytocidal activity (6). Continuous cultures of some lines of Plasmodium falciparum are compatible with gametocyte formation. Systems accessing gametocytogenesis or gametocyte clearance (7–14) and gametocyte viability (15, 16) in high throughput (HT) or semi-HT are available. However, to date only two models, developed using Plasmodium berghei, are able to assess gametocyte function, i.e., gametogenesis, zygote formation, and ookinete morphogenesis, in semi-HT (17) or HT (18).
Here, we present the generation of a P. berghei model to assess gametocyte function in HT and the application of the new model to identify TB compounds in the Pathogen Box from the Medicines for Malaria Venture (MMV).
RESULTS
Generation of Ookluc.
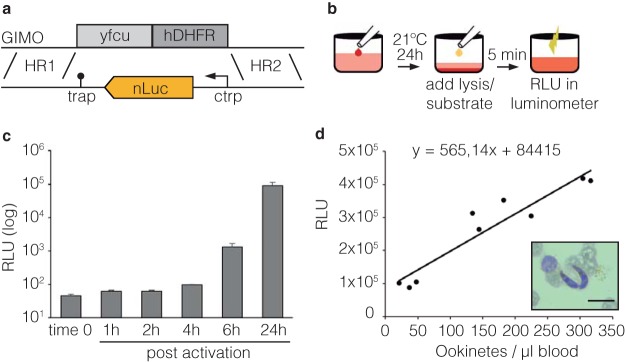
To generate a new Plasmodium experimental model for HT assessment of gametocyte function, we used the P. berghei line Gene In/Marker Out (GIMO) (19) to insert an expression cassette for nanoluciferase (nLuc; Promega) expression under the control of the ookinete-specific promoter, P. berghei CTRP (20) (Fig. 1a). Transfected parasites were negative selected with 5-fluorocytosine (5-FC), and the resulting mutants, named Ookluc, retain no selectable markers in the genome.
FIG 1.
Generation of the Ookluc line. (a) Strategy for generating the Ookluc reporter line. The coding sequence of nLuc (orange box) was cloned downstream the ctrp promoter (arrow) and upstream the 3′ UTR of the thrombospondin-related adhesive protein (TRAP; lollipop) into the plasmid pL0043 (19), containing the homology regions (HRs) for integration in the GIMO locus. Gray boxes indicate the resistant markers yeast fcu (yfcu) and human dihydrofolate reductase (hDHFR). (b) Ookinete conversion assay. Blood from an infected mouse is dispensed in a well of 96-well plate containing ookinete medium at 21°C and incubated for 24 h. Then, nLuc substrate is added, and the relative light units (RLU) were assessed by using a plate luminometer after a 5-min incubation. (c) Kinetics of nLuc activity, expressed as RLU (means + the SD), in Ookluc blood stages (blood) or different time points postactivation in conversion assay. The results are representative of 5 independent experiments. (d) Correlation between the number of ookinetes formed per μl of blood after 24 h conversion and the measured RLU. The RLU was measured without addition of lysis buffer, and the ookinetes were then counted by blood smears (for the image displayed: scale bar, 10 μm). Linear trendline and equation are shown. The results are representative of three independent experiments.
Circulating gametocytes in P. berghei-infected mouse blood can activate, form gametes, fertilize to form a zygote, and transform into ookinetes in vitro in conversion assays using ookinete medium (21). The assay to verify Ookluc conversion and nLuc activity combines infected mouse blood dispensed in ookinete medium at 21°C. After 0, 6, or 24 h, the nLuc substrate (Nano-Glo) was added with lysis buffer at 1:1 (vol/vol) to measure the emitted luminescence (relative light units [RLU]) in a plate luminometer (Fig. 1b). Ookluc had negligible nLuc activity in circulating blood stages (time zero, no incubation), and in in vitro fertilization assays (conversion) the signal has a relative increase of ∼40-fold after 6 h and ∼2,000-fold after 24 h (Fig. 1c), demonstrating that the ctrp promoter is sufficiently silenced to prevent detectable nLuc expression in blood stages and is activated only upon conversion into mosquito sexual stages. The nLuc activity after 24 h conversion directly correlated with the gametocytemia of the mouse used as blood donor for the assay (see Fig. S1 in the supplemental material) and with the number of formed ookinetes counted by blood smears (Fig. 1d), and the best assays regarding signal/background ratio, which was around 2,000, were achieved utilizing blood with gametocytemia above 0.4%.
Validation of Ookluc.
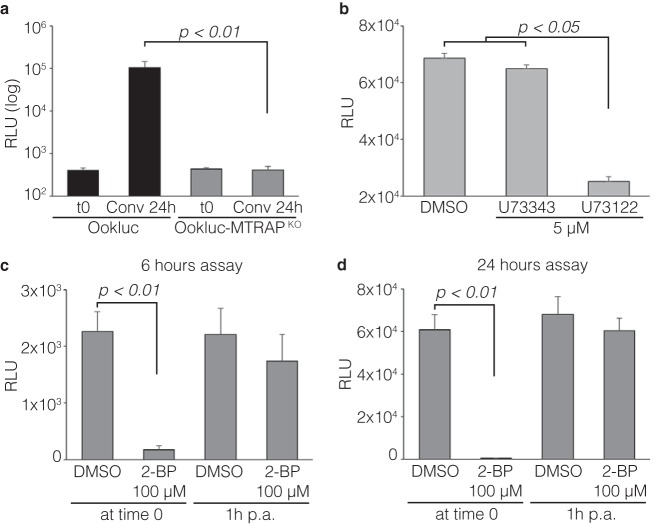
To verify whether the increase in nLuc activity in conversion assays with Ookluc was dependent on fertilization, we deleted the mtrap gene from the Ookluc genome. MTRAP-knockout (MTRAPKO) gametocytes can activate, but the gametes remain trapped inside the host cell (22). There was no increase in nLuc activity in Ookluc-MTRAPKO parasites after 24 h of conversion assay (Fig. 2a)—the absence of ookinetes was confirmed by blood smears (not shown)—showing that gametocyte activation per se is not sufficient for activation of the ctrp promoter; hence, nLuc expression and activity in Ookluc parasites depend on gamete egress and fertilization. Likewise, blocking gametogenesis with the phospholipase C inhibitor U73122 (23) reduced nLuc activity in Ookluc after 24 h of conversion assay; in contrast, the inactive analog U73343 or dimethyl sulfoxide (DMSO) had no effect (Fig. 2b). A reduction of ookinete formation with U73122 was confirmed by blood smears (data not shown). These results validate Ookluc as a tool for searching compounds with TB activity through inhibition of gametocyte activation, gametogenesis, or fertilization.
FIG 2.
nLuc activity in conversion assay with Ookluc depends on fertilization. (a) nLuc activity, expressed as RLU, in Ookluc and Ookluc-MTRAPKO blood stages (t0) or after 24 h of conversion assay. (b) nLuc activity, expressed as RLU, in Ookluc parasites after 24 h of conversion assay in the presence of 5 μM the phospholipase C inhibitor U73122 or its inactive analog U73343. Control, DMSO. (c and d) nLuc activity, expressed as RLU, in Ookluc parasites after 6 h (c) or 24 h (d) of conversion assay in the presence of 100 μM 2-BP, an inhibitor of palmitoylation, or DMSO control, added at time zero or 1 h postactivation (p.a.). All bars show the means + the SD and are representative of four independent experiments.
The increase in nLuc activity after 6 h of conversion assay with Ookluc showed that the ctrp promoter likely becomes active soon after zygote formation. Inhibition of palmitoylation with 2-bromopalmitic acid (2-BP) 1 h after Ookluc activation, blocking zygote to ookinete morphogenesis (24), did not reduce the nLuc activity after 6 h (Fig. 2c) or 24 h (Fig. 2d). This indicates that once the ctrp promoter is activated in zygotes it accumulates nLuc regardless of ookinete formation and precludes Ookluc as a tool for searching compounds with TB activity through inhibition of ookinete morphogenesis. The activity of 2-BP was validated by its effect on Ookluc conversion when added on time zero of the assay, completely blocking the nLuc signal after 6 h (Fig. 2c) or 24 h (Fig. 2d)—the absence of ookinetes was confirmed by blood smears (data not shown)—and revealing an essential role of palmitoylation in the first steps of Plasmodium sexual development in mosquitoes, i.e., gametocyte activation, gametogenesis, or fertilization.
The Ookluc conversion assay is scalable for HTS.
The level of variation of nLuc activity in conversion assays with Ookluc in a 96-well plate format represented a normal distribution, with 93.7% of the reads within the means ± two standard deviations (SD; Fig. S2a), and with a Z-factor of 0.6 (25), validating Ookluc as a tool for HTS. Different volumes of infected blood and medium can be used for the conversion assays, yielding robust RLU signals after 24 h (Fig. S2b). Importantly, assays with a reduced total volume (5 to 11 μl) are compatible with volume limitations of both 384- and 1536-well format assays, the prominent format used in most HTS compound libraries, while also reducing animal use and reagent expense.
Screening the Pathogen Box with Ookluc identifies TB compounds.
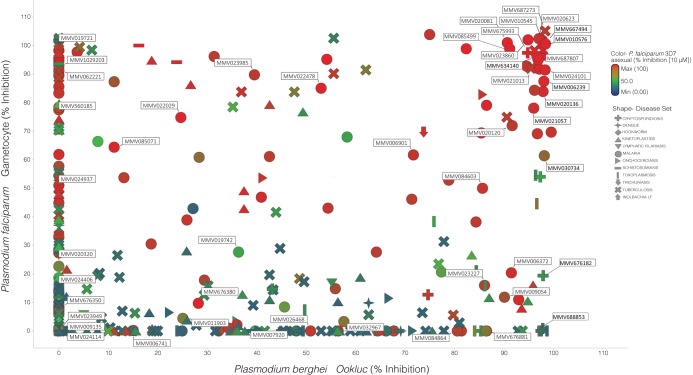
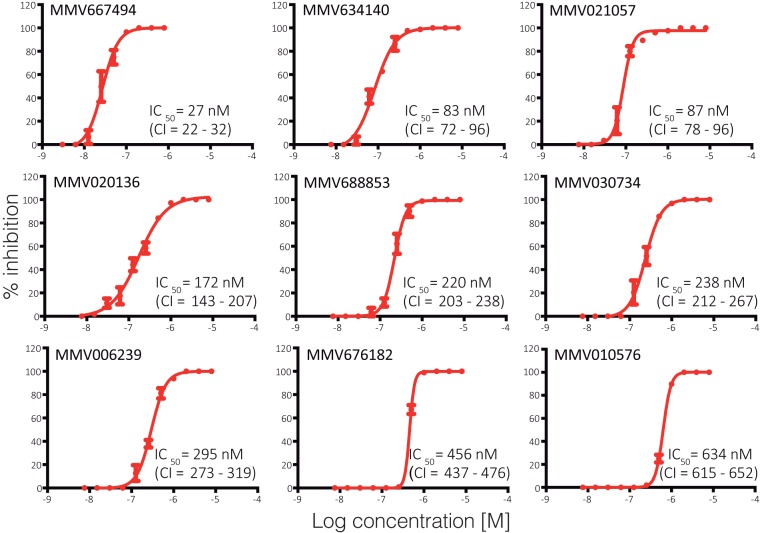
We then used the Ookluc model to screen for compounds with TB activity in the Pathogen Box (Medicines for Malaria Venture [MMV]). Compounds were initially tested at a concentration of 10 μM. From the ∼400 compounds in the Pathogen Box, 31 showed more than 95% inhibition of conversion, 45 showed more than 90% inhibition, and 75 showed more than 75% inhibition (Dataset S1). This screen identified compounds with TB potential and importantly demonstrated some overlap and divergence from a previous screen (26) against late-stage P. falciparum gametocytes (LSG; data kindly provided by V. Avery) (Fig. 3) and previous screens for compounds that existed in the MMV Malaria Box (https://chembl.gitbook.io/chembl-ntd/) (Fig. S3). This suggests the conservation of some drug targets between gametocytes and gametes, as well as the emergence/loss of additional targets between these closely related stages (Fig. 3). Moreover, the integration of the Ookluc and P. falciparum LSG inhibition data with the data available from MMV regarding inhibition of P. falciparum asexual growth and P. berghei liver stages provides a comprehensive view of compound potential against Plasmodium stages (Fig. S3), an important tool to guide drug development by prioritizing compound classes. The 31 compounds inhibiting more than 95% of conversion at 10 µM were then tested at a final concentration of 1 µM, and those showing more than 95% inhibition at this lower concentration were subsequently screened using an initial concentration of 1 µM and serial 2-fold dilutions to determine the 50% inhibitory concentration (IC50) (Fig. 4). The nine most potent compounds (Fig. 4) did not inhibit nLuc activity in P. falciparum blood stages expressing nLuc (27) at a final concentration of 10 μM (Fig. S4a), and inhibition of ookinete formation was confirmed by direct blood smears of P. berghei conversion assays (Fig. S4b), confirming that their inhibitory activity in Ookluc conversion assays is not due to direct inhibition of nLuc.
FIG 3.
Multidimensional scatterplot of P. berghei Ookluc activity compared to P. falciparum gametocyte (stage IV and V) inhibition. The comparative activity of the MMV Pathogen Box compounds, previously screened as part of the MMV Malaria Box, is shown. The data represent the percent inhibition of the compounds utilizing the Ookluc assay screened at a single 10 μM concentration compared to the percent inhibitory activity, again at a single 10 μM concentration, against P. falciparum late-stage gametocytes (stages IV and V) (26). Colors reflect the growth inhibition level against P. falciparum 3D7 asexual parasites screened at 10 µM compound concentration, from blue (0% growth inhibition) to red (100% growth inhibition) (grey indicates no value). The symbols indicate the different annotated disease sets within the Pathogen Box.
FIG 4.
Dose-response curves and determination of IC50. Indicated compounds were 2-fold serially diluted in conversion assays, in triplicates, starting from 1 μM (100% inhibition) until no inhibition was observed. The calculated IC50 and top and bottom 95% confidence intervals (CI) for each compound are indicated in the graphs. Inhibition at each dilution point is shown as means ± the SD. The results are representative of three independent experiments.
DISCUSSION
This study presents a new P. berghei model, Ookluc, to study Plasmodium transmission. At least one parasite line similar to Ookluc has been previously reported (18). It uses a fluorescence reporter under the control of ctrp promoter, and the selectable marker for insertion of the reporter remains in the parasite genome. Ookluc has important improvements; specifically, the use of nLuc, a more sensitive reporter, and the absence of an integrated drug resistance marker make Ookluc ideal for drug screenings.
While most methodologies for screening TB compounds focus on gametocyte cultures and thus identify gametocytogenesis inhibitors or gametocytocidal compounds, the Ookluc model can assess parasites viability through ability to progress along the life cycle and complete sexual recombination. Blocking palmitoylation at 1 h after conversion culture (after gametogenesis and fertilization have occurred) did not impact the nLuc signal, showing that inhibitory compounds identified using the Ookluc model are likely acting prior to zygote formation. Therefore, Ookluc can identify TB compounds with activity against gametogenesis or sexual recombination, as well as those potentially killing mosquito stages, like reactive oxygen species inducers.
Unlike multiplicative Plasmodium asexual stages, late-stage gametocytes (LSG) are metabolically quiescent. Active metabolic pathways differ between these stages. Accordingly, >90% of antimalarial compounds active against asexual stages have no activity against LSG (28). Likewise, the transition from gametocytes to gametes and zygotes involve profound changes in metabolic activity (29), predicting that inactive compounds against gametocytes may be active against gametes and zygotes, or vice versa. Indeed, assays using Ookluc identified TB compounds previously not assessed in other screens against LSG. Therefore, integrating data obtained using the Ookluc model with data from screens against asexual stages, LSG and liver stages can provide valuable information for prioritizing compound classes active against all parasite stages. Moreover, Ookluc can be used as a tool for chemical genomics efforts in order to probe metabolic pathways that differ in parasite stages progressing through the life cycle.
Screening the Pathogen Box from MMV with Ookluc identified nine potent TB compounds with IC50 at the nM range. The compound MMV010576, with structure similarity to previously described kinase inhibitors (CHEMBL5903 and CHEMBL5311; Tanimoto value of 0.79), has also reported potent activity against asexual stages, LSG and liver stages, and thus represents a promising starting point for the development of compounds targeting at least four parasite developmental stages. The screen identified other known kinase inhibitors. MMV030734 is a specific inhibitor of Plasmodium calcium-dependent protein kinase 1 (CDPK1) (30), which is critical for parasite gametogenesis (31). MMV676182 and MMV688853 are bumped kinase inhibitors active against Toxoplasma and Cryptosporidium CDPK1 (32). The ortholog of TgCDPK1 in Plasmodium is CDPK4, a regulator of gametogenesis essential for parasite transmission (33). Analogs of MMV676182 were shown to specifically inhibit PfCDPK4 and block malaria transmission (34). The quinoline 4-carboxamides MMV667494 and MMV634140, inhibitors of Plasmodium translation elongation factor 2 (35), represent another class of compounds also identified as potent TB using Ookluc. They are related to the compound DDD107498, a potent multistage antimalarial with known TB activity (36). These potent TB compounds identified using Ookluc were previously identified using different methodologies. The ability of Ookluc screens to identify these compounds validate the model as a powerful tool for further screens using available libraries and identification of novel TB antimalarials.
Thus, we present here a powerful new tool to run HTS against Plasmodium sexual stages and find new TB compounds that may aid in the ambitious goal of eliminating malaria in the next 15 years.
MATERIALS AND METHODS
Animals and parasite strains.
C57BL/6 or BALB/c mice were bred and maintained in the animal facility of the Department of Parasitology at the Institute of Biomedical Sciences, University of Sao Paulo, under the protocol 132/2014-CEUA of research ethics approval for animal experimentation. The P. berghei ANKA recombinant Gene Insertion/Marker Out (GIMO) line (19) and the new Ookluc line were stored as frozen stocks in liquid nitrogen or at −80°C. Vial stocks were prepared by mixing 150 μl of parasitized mouse blood with 300 μl of Alsever's solution (Sigma-Aldrich, A3551) with 10% glycerol (Sigma-Aldrich, G5516). Mice were infected by intraperitoneal injection of 200-μl portions of thawed stocks. The parasitemia was followed daily through Giemsa staining (Laborclin, 620529) of thin blood smears in glass slides (Kasvi, K5-7105-1) counted by direct light microscopy with a 100× oil immersion objective (Nikon E200).
Construction of plasmids.
To construct the plasmid for generating the Ookluc strain, the 1,590 bp upstream the genomic sequence of the P. berghei “circumsporozoite protein and thrombospondin-related adhesive protein [TRAP]-related protein” (CTRP, PBANKA_0412900) were PCR amplified using forward (GGGCTGCAGCCACTTCCTCAAAATGAATAGG (the PstI restriction site is underlined) and reverse (GGATCCTTGTGTTTTGCTTTGTATTTAAA; the BamHI restriction site is underlined) primers. The coding sequence of nanoluciferase (nLuc) was PCR amplified from the plasmid PfNluc (27) using forward (GGATCCATGGTCTTCACACTCGAAG; the BamHI restriction site is underlined) and reverse (GATATCTTACGCCAGAATGCGTTCG; the EcoRV restriction site is underlined) primers. The 551-bp downstream 3′ untranslated region (3′ UTR) genomic sequences of the P. berghei thrombospondin-related adhesive protein (TRAP, PBANKA_1349800) were PCR amplified using forward (GATATCTTTTAATAAACATATATATCTAGAT; the EcoRV restriction site is underlined) and reverse (GCGGCCGCCATCGCTGCATTAATGATTT; the NotI restriction site is underlined) primers. The amplified sequences were cloned into plasmid pL0043 (19), which is designed for transfection of the P. berghei GIMO line, using the PstI, BamHI, EcoRV, and NotI restriction sites (FastDigest; Thermo Scientific) to generate plasmid p43-Ookluc. The plasmid for knocking out the merozoite thrombospondin-related adhesive protein (MTRAP; PBANKA_0512800) was already available (22).
Parasite transfection.
For the transfections, the plasmids were electroporated into synchronized P. berghei asexual schizonts using an Amaxa (Lonza) Nucleofector electroporator set at program U33 and using a human T cell Nucleofector kit (Lonza, VPA-1002) as previously described (37).
For transfection of the P. berghei GIMO, plasmid p43-Ookluc was linearized with SacII (FastDigest; Thermo Scientific), and transformed parasites were negative selected by the intraperitoneal administration of one dose per day of 0.4 g/kg (body weight) of 5-fluorocytosine (Sigma-Aldrich, F7129) to mice infected with the transfected parasites, starting 24 h after transfection.
To knock out mtrap from Ookluc, the sequence targeting the mtrap locus for replacement of the coding sequence by cassettes for mCherry fluorescence and pyrimethamine resistance was removed from a plasmid previously available (22). After transfection, transformed parasites (Ookluc-MTRAPKO) were positive selected by the administration of pyrimethamine (Sigma-Aldrich, 46706) for 72 h in the drinking water (70 mg/liter) to mice infected with the transfected parasites, starting 24 h after transfection. After selection, the transformed parasites were cloned by limiting dilution and infection of mice.
Conversion assays.
For conversion assays, parasitized mouse blood was obtained by cardiac puncture in heparinized syringes (heparin sodium salt from Sigma-Aldrich, H3393) and added to ookinete medium (1/20). The ookinete medium (21) consisted of RPMI 1640 (Thermo Scientific, 61870) with 0.025 M HEPES (Thermo Scientific, 15630080), penicillin-streptomycin-neomycin (Sigma-Aldrich, P4083), hypoxanthine 50 mg/liter (Sigma-Aldrich, H9636), and xanthurenic acid 100 μM (Sigma-Aldrich, D120804) at a pH of 8.3.
The assays were kept at 21°C in an incubator for 0, 6, or 24 h. The luciferase activity was determined by measuring the RLU using a microplate reader (SpectraMax i3; Molecular Devices) after the addition of 1 volume of the substrate/lysis buffer (Nano-Glo luciferase assay system; Promega). For some experiments, lysis buffer was not added so the cells are not destroyed and can be counted by blood smears. The phospholipase C inhibitor U73122 (Sigma-Aldrich, U6756) and the inactive analog U73343 (Sigma-Aldrich, U6881) were used at final concentrations of 5 μM, which blocks gametocyte activation (23). The palmitoylation inhibitor 2-bromopalmitic acid (2-BP; Sigma-Aldrich, 21604) was added at a final concentration of 100 μM, a concentration known to block ookinete morphogenesis when the drug is mixed in the conversion assay at 1 h after gametocyte activation in ookinete medium (24). Alternatively, the same concentration of 2-BP was added at time zero of the conversion assay.
For the drug screenings, the 400 compounds from the Pathogen Box (MMV) were initially diluted in ookinete medium to a final concentration of 10 μM. The conversion assays were performed as described, and the luciferase activity was measured after 24 h. A second round of screening tested the best hits (>95% inhibition of conversion) at a final concentration of 1 μM. The IC50 was determined for the best compounds (>95% inhibition of conversion). For IC50 determination, the compounds were initially diluted to a final concentration of 1 μM and serially diluted by a 2-fold factor until no inhibition of conversion was observed. The curves of inhibition and IC50 determination were made using GraphPad Prism version 6.0.
Statistical and comparative analysis.
Student t test analysis was used to calculate statistical differences between sample groups. The Z-factor for the high-throughput assay was calculated as described previously (25), with positive controls being wells with conversion assays as described in Fig. 1b and negative controls being wells with conversion assays with nonparasitized blood or wells with nonactivated Ookluc (in both cases, the RLU signal is the same). Previously assessed compound activity against Plasmodium was downloaded (https://chembl.gitbook.io/chembl-ntd/; dataset 21; MMV Pathogen Box).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2013/13119-6), Instituto Serrapilheira (grant G-1709-16618), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant 405996/2016-0) and the Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health. J.C., I.D., and X.A.G. were supported by the FAPESP fellowships 2016/16649-4, 2014/23083-1, and 2016/16649-4, respectively. M.H.B. is a fellow of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). M.S.R. was supported by CNPq fellowship 870219/1997-9.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01053-18.
REFERENCES
- 1.World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Bennink S, Kiesow MJ, Pradel G. 2016. The development of malaria parasites in the mosquito midgut. Cell Microbiol 18:905–918. doi: 10.1111/cmi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klug D, Frischknecht F. 2017. Motility precedes egress of malaria parasites from oocysts. Elife 6:e19157. doi: 10.7554/eLife.19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alout H, Labbe P, Chandre F, Cohuet A. 2017. Malaria vector control still matters despite insecticide resistance. Trends Parasitol 33:610–618. doi: 10.1016/j.pt.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Blasco B, Leroy D, Fidock DA. 2017. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat Med 23:917–928. doi: 10.1038/nm.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkholtz LM, Coetzer TL, Mancama D, Leroy D, Alano P. 2016. Discovering new transmission-blocking antimalarial compounds: challenges and opportunities. Trends Parasitol 32:669–681. doi: 10.1016/j.pt.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Lucantoni L, Fidock DA, Avery VM. 2016. Luciferase-based, high-throughput assay for screening and profiling transmission-blocking compounds against Plasmodium falciparum gametocytes. Antimicrob Agents Chemother 60:2097–2107. doi: 10.1128/AAC.01949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucantoni L, Silvestrini F, Signore M, Siciliano G, Eldering M, Dechering KJ, Avery VM, Alano P. 2015. A simple and predictive phenotypic high content imaging assay for Plasmodium falciparum mature gametocytes to identify malaria transmission blocking compounds. Sci Rep 5:16414. doi: 10.1038/srep16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. 2014. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One 9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka TQ, Dehdashti SJ, Nguyen DT, McKew JC, Zheng W, Williamson KC. 2013. A quantitative high-throughput assay for identifying gametocytocidal compounds. Mol Biochem Parasitol 188:20–25. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M. 2011. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis 203:1445–1453. doi: 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Liu M, Liang X, Siriwat S, Li X, Chen X, Parker DM, Miao J, Cui L. 2014. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS One 9:e93825. doi: 10.1371/journal.pone.0093825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alessandro S, Camarda G, Corbett Y, Siciliano G, Parapini S, Cevenini L, Michelini E, Roda A, Leroy D, Taramelli D, Alano P. 2016. A chemical susceptibility profile of the Plasmodium falciparum transmission stages by complementary cell-based gametocyte assays. J Antimicrob Chemother 71:1148–1158. doi: 10.1093/jac/dkv493. [DOI] [PubMed] [Google Scholar]
- 15.Miguel-Blanco C, Lelievre J, Delves MJ, Bardera AI, Presa JL, Lopez-Barragan MJ, Ruecker A, Marques S, Sinden RE, Herreros E. 2015. Imaging-based high-throughput screening assay to identify new molecules with transmission-blocking potential against Plasmodium falciparum female gamete formation. Antimicrob Agents Chemother 59:3298–3305. doi: 10.1128/AAC.04684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azevedo R, Markovic M, Machado M, Franke-Fayard B, Mendes AM, Prudencio M. 2017. Bioluminescence method for in vitro screening of plasmodium transmission-blocking compounds. Antimicrob Agents Chemother 61:e02699-16. doi: 10.1128/AAC.02699-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delves MJ, Ramakrishnan C, Blagborough AM, Leroy D, Wells TN, Sinden RE. 2012. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int J Parasitol 42:999–1006. doi: 10.1016/j.ijpara.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, Franke-Fayard BM, Janse CJ, Khan SM. 2011. A novel “gene insertion/marker out” (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One 6:e29289. doi: 10.1371/journal.pone.0029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuda M, Sawai T, Chinzei Y. 1999. Structure and expression of an adhesive protein-like molecule of mosquito invasive-stage malarial parasite. J Exp Med 189:1947–1952. doi: 10.1084/jem.189.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagborough AM, Delves MJ, Ramakrishnan C, Lal K, Butcher G, Sinden RE. 2013. Assessing transmission blockade in Plasmodium spp. Methods Mol Biol 923:577–600. doi: 10.1007/978-1-62703-026-7_40. [DOI] [PubMed] [Google Scholar]
- 22.Bargieri DY, Thiberge S, Tay CL, Carey AF, Rantz A, Hischen F, Lorthiois A, Straschil U, Singh P, Singh S, Triglia T, Tsuboi T, Cowman A, Chitnis C, Alano P, Baum J, Pradel G, Lavazec C, Menard R. 2016. Plasmodium merozoite TRAP family protein is essential for vacuole membrane disruption and gamete egress from erythrocytes. Cell Host Microbe 20:618–630. doi: 10.1016/j.chom.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raabe AC, Wengelnik K, Billker O, Vial HJ. 2011. Multiple roles for Plasmodium berghei phosphoinositide-specific phospholipase C in regulating gametocyte activation and differentiation. Cell Microbiol 13:955–966. doi: 10.1111/j.1462-5822.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos JM, Kehrer J, Franke-Fayard B, Frischknecht F, Janse CJ, Mair GR. 2015. The Plasmodium palmitoyl-S-acyl-transferase DHHC2 is essential for ookinete morphogenesis and malaria transmission. Sci Rep 5:16034. doi: 10.1038/srep16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 26.Duffy S, Sykes ML, Jones AJ, Shelper TB, Simpson M, Lang R, Poulsen SA, Sleebs BE, Avery VM. 2017. Screening the medicines for malaria venture pathogen box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob Agents Chemother 61:e00379-. doi: 10.1128/AAC.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo MF, Nie CQ, Elsworth B, Charnaud SC, Sanders PR, Crabb BS, Gilson PR. 2014. Plasmodium falciparum transfected with ultra bright NanoLuc luciferase offers high sensitivity detection for the screening of growth and cellular trafficking inhibitors. PLoS One 9:e112571. doi: 10.1371/journal.pone.0112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plouffe DM, Wree M, Du AY, Meister S, Li F, Patra K, Lubar A, Okitsu SL, Flannery EL, Kato N, Tanaseichuk O, Comer E, Zhou B, Kuhen K, Zhou Y, Leroy D, Schreiber SL, Scherer CA, Vinetz J, Winzeler EA. 2016. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe 19:114–126. doi: 10.1016/j.chom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngwa CJ, Scheuermayer M, Mair GR, Kern S, Brugl T, Wirth CC, Aminake MN, Wiesner J, Fischer R, Vilcinskas A, Pradel G. 2013. Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito. BMC Genomics 14:256. doi: 10.1186/1471-2164-14-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowther GJ, Hillesland HK, Keyloun KR, Reid MC, Lafuente-Monasterio MJ, Ghidelli-Disse S, Leonard SE, He P, Jones JC, Krahn MM, Mo JS, Dasari KS, Fox AM, Boesche M, El Bakkouri M, Rivas KL, Leroy D, Hui R, Drewes G, Maly DJ, Van Voorhis WC, Ojo KK. 2016. Biochemical screening of five protein kinases from Plasmodium falciparum against 14,000 cell-active compounds. PLoS One 11:e0149996. doi: 10.1371/journal.pone.0149996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal A, Molina-Cruz A, Brzostowski J, Liu P, Luo Y, Gunalan K, Li Y, Ribeiro JMC, Miller LH. 2018. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc Natl Acad Sci U S A 115:774–779. doi: 10.1073/pnas.1715443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BG, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CL, White AC Jr, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. 2004. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117:503–514. doi: 10.1016/S0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 34.Ojo KK, Eastman RT, Vidadala R, Zhang Z, Rivas KL, Choi R, Lutz JD, Reid MC, Fox AM, Hulverson MA, Kennedy M, Isoherranen N, Kim LM, Comess KM, Kempf DJ, Verlinde CL, Su XZ, Kappe SH, Maly DJ, Fan E, Van Voorhis WC. 2014. A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J Infect Dis 209:275–284. doi: 10.1093/infdis/jit522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baragana B, Norcross NR, Wilson C, Porzelle A, Hallyburton I, Grimaldi R, Osuna-Cabello M, Norval S, Riley J, Stojanovski L, Simeons FR, Wyatt PG, Delves MJ, Meister S, Duffy S, Avery VM, Winzeler EA, Sinden RE, Wittlin S, Frearson JA, Gray DW, Fairlamb AH, Waterson D, Campbell SF, Willis P, Read KD, Gilbert IH. 2016. Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. J Med Chem 59:9672–9685. doi: 10.1021/acs.jmedchem.6b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baragana B, Hallyburton I, Lee MC, Norcross NR, Grimaldi R, Otto TD, Proto WR, Blagborough AM, Meister S, Wirjanata G, Ruecker A, Upton LM, Abraham TS, Almeida MJ, Pradhan A, Porzelle A, Luksch T, Martinez MS, Luksch T, Bolscher JM, Woodland A, Norval S, Zuccotto F, Thomas J, Simeons F, Stojanovski L, Osuna-Cabello M, Brock PM, Churcher TS, Sala KA, Zakutansky SE, Jimenez-Diaz MB, Sanz LM, Riley J, Basak R, Campbell M, Avery VM, Sauerwein RW, Dechering KJ, Noviyanti R, Campo B, Frearson JA, Angulo-Barturen I, Ferrer-Bazaga S, Gamo FJ, Wyatt PG, Leroy D, Siegl P, Delves MJ, Kyle DE, et al. 2015. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522:315–320. doi: 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. 2006. High-efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.