We evaluated MEDI8852, a human IgG1 monoclonal antibody that binds a highly conserved influenza A hemagglutinin stalk epitope, in outpatients with uncomplicated influenza A infection. A total of 126 subjects aged 18 to 65 years were enrolled during the 2015 to 2016 Northern and 2016 Southern Hemisphere seasons.
KEYWORDS: influenza, monoclonal antibody, safety, treatment
ABSTRACT
We evaluated MEDI8852, a human IgG1 monoclonal antibody that binds a highly conserved influenza A hemagglutinin stalk epitope, in outpatients with uncomplicated influenza A infection. A total of 126 subjects aged 18 to 65 years were enrolled during the 2015 to 2016 Northern and 2016 Southern Hemisphere seasons. Subjects with symptom onset ≤5 days before dosing were randomized to four cohorts: 750 mg (cohort 1) or 3,000 mg (cohort 2) MEDI8852 (single intravenous infusion) plus 75 mg oseltamivir, placebo plus 75 mg oseltamivir (cohort 3), and 3,000 mg MEDI8852 alone (cohort 4). Subjects were monitored through day 10 for solicited influenza symptoms, day 28 for adverse events (AEs), and day 101 for serious AEs and AEs of special interest. Nasopharyngeal samples were collected through day 7 for confirmation of influenza A infection, viral shedding, and oseltamivir and MEDI8852 susceptibility. Slightly more AEs were reported in subjects receiving MEDI8852 (cohorts 1, 2, and 4 combined: 39/93, 41.9%) than oseltamivir only (cohort 3: 10/32, 31.3%). Most AEs were mild or moderate. The most common AE was bronchitis (11/93, 11.8%; 1/32, 3.1%). The median (range) decrease in viral shedding (log10 virus genome copies/ml) was similar between the two groups (−3.58 [−6.2. 0.5]; −3.43 [−5.9, 0.9]). Genotypic analyses found a limited number of hemagglutinin and neuraminidase amino acid changes between viruses isolated before and after therapy; however, none appeared within a known oseltamivir-resistant site or MEDI8852-binding region. The safety profile of MEDI8852 supports its continued development for treatment of patients hospitalized with influenza A infection. (This study has been registered at ClinicalTrials.gov under identifier NCT02603952.)
INTRODUCTION
The World Health Organization estimates that influenza infection accounts for approximately 3 to 5 million cases of severe illness and up to 500,000 deaths globally each year (1). Vaccination is the most effective means to prevent morbidity and mortality caused by influenza infection; however, even when matched to the circulating influenza strain, vaccines have demonstrated suboptimal efficacy (2). Antiviral medications such as the small-molecule neuraminidase (NA) inhibitor oseltamivir are considered standard of care and are recommended in the United States to be administered as early as possible for individuals with confirmed or suspected influenza infection who have severe, complicated, or progressive illness; require hospitalization; or are at greater risk for influenza-related complications (3). However, antiviral medications are indicated for reducing the duration of symptoms and complications due to influenza infection only when administered early during the infection (i.e., within 48 h after symptom onset) and are less effective when given later (4, 5) and to individuals with severe influenza infection (6). Despite advances in vaccines and small-molecule antiviral therapeutics, there remains an unmet medical need for more effective treatment of influenza infection in populations at high risk for morbidity and mortality from this infection.
MEDI8852 is a human immunoglobulin G1 kappa monoclonal antibody that is derived from human memory B cells and binds to the conserved stalk region of the influenza hemagglutinin (HA) protein. MEDI8852 directly inhibits fusion between HA and cellular membranes, HA protein maturation, and host cell protease cleavage (thus blocking the cell-to-cell spread of virus) (7). MEDI8852 also clears infected cells through the Fc effector function via antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity (7). MEDI8852 has been demonstrated to bind to the HA of all influenza antigenic subtypes and neutralizes seasonal H1N1 and H3N2 viruses, as well as subtypes such as H2, H5, H6, H7, and H9, which have the potential to cause pandemics (7, 8). A first-in-human study demonstrated that a single intravenous (i.v.) dose of up to 3,000 mg of MEDI8852 had a safety profile comparable to that of placebo in healthy adult volunteers (9).
MEDI8852 is being developed as a treatment for influenza A infection; however, the safety profile of therapeutic monoclonal antibodies in individuals with either acute uncomplicated or severe influenza is largely unknown. Of interest is the potential risk that a therapeutic monoclonal antibody could mediate antibody-dependent enhancement of influenza infection, leading to disease exacerbation (10, 11). This risk of enhanced disease with influenza antibodies has been described in previous studies in which pigs vaccinated with an inactivated swine H1N2 influenza virus developed enhanced disease (pneumonia and lung damage) when challenged with a different (mismatched) pandemic H1N1 (pH1N1) virus (12, 13). Recent evidence suggests that the enhanced disease was caused by vaccine-induced, nonneutralizing anti-stalk antibodies that enabled the mismatched pH1N1 viruses to fuse more effectively with lung epithelial cells (14). Preclinical studies show that MEDI8852 is a broadly neutralizing antibody that effectively blocks the fusion process, providing survival benefit to both influenza-infected mice and ferrets with reduced lung virus titers and decreases in lung lesions assessed by histopathology (7). Antibody-dependent enhancement of influenza illness nevertheless remains of considerable interest to the field (15, 16), as no clinical data have been published to date on the association between therapeutic monoclonal antibodies and antibody-dependent enhancement of influenza infection.
In this study, we report the results of a safety study of a single i.v. dose of MEDI8852, administered alone or in conjunction with oseltamivir, in outpatient adults with acute, uncomplicated influenza A infection. Based on the results of pharmacokinetic modeling and simulation in our phase 1 study in healthy adult volunteers (NCT02350751), we evaluated two dose levels of MEDI8852 (750 and 3,000 mg) to identify a dose range for use in future safety and efficacy studies in individuals with influenza A infection (9). Although safety of the treatment regimens was the primary objective of this study, the exploratory efficacy of MEDI8852 was also evaluated.
RESULTS
Subject Disposition and Baseline Characteristics.
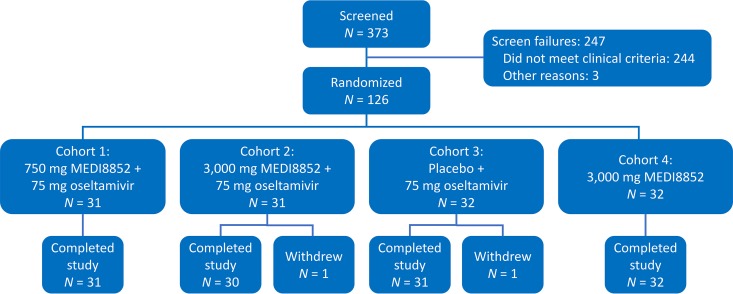
A total of 373 subjects were screened and 126 (of the planned 160) subjects were randomized into the study (Fig. 1). The study initially randomized 95 subjects at study sites in the United States during the 2015–2016 Northern Hemisphere influenza season. The study was extended into the 2016 Southern Hemisphere influenza season, and 31 additional subjects were randomized at study sites in South Africa. Because the influenza season in the Southern Hemisphere was waning, enrollment in the study was halted on 31 August 2016. A total of 124 subjects completed the study through day 101; 1 subject who was randomized to cohort 2 withdrew consent on day 2 (the subject did not have influenza A infection confirmed with a positive rapid antigen test at baseline and therefore did not receive study drugs), and 1 subject who was randomized to cohort 3 withdrew consent on day 78 (withdrawal of consent was not due to an adverse event [AE]).
FIG 1.
Study flow diagram outlining screening, randomization, dosing, and follow-up of subjects. One subject who was randomized to cohort 2 withdrew consent on day 2 (the subject did not have influenza A infection confirmed with a positive rapid antigen test at screening and therefore did not receive study drugs), and one subject who was randomized to cohort 3 withdrew consent on day 78 (withdrawal of consent was not due to an AE).
Treatment groups were generally balanced with respect to most demographic and baseline characteristics (see Table S1 in the supplemental material). Some variation was observed between the subjects who received MEDI8852 (cohorts 1, 2, and 4 combined) and those who received oseltamivir alone (cohort 3); approximately half of the MEDI8852 recipients were female (49%), in contrast to oseltamivir-only recipients (63%), and more than half of the MEDI8852 recipients had influenza symptoms for ≤48 h before randomization (54.3%), in contrast to oseltamivir-only recipients (43.8%).
Safety and tolerability.
AEs occurred at a slightly higher rate in MEDI8852 recipients than in oseltamivir-only recipients (41.9 and 31.3%, respectively; Table 1). This difference was mainly driven by the rate of AEs in the System Organ Class (SOC) of Infections and Infestations (using the Medical Dictionary for Regulatory Activities, version 18.1), where the corresponding values for MEDI8852 and oseltamivir-only recipients were 20.4 and 9.4%, respectively. Within the Infections and Infestations SOC, the preferred term of bronchitis occurred at a higher rate in MEDI8852 recipients than in oseltamivir-only recipients (11.8 and 3.1%, respectively). Other preferred terms (i.e., pharyngitis) within this SOC occurred at more similar rates (3.2 and 3.1%, respectively).
TABLE 1.
AEs in MEDI8852 and oseltamivir-only recipients
| Type of AE | No. (%) of AEsa |
||||
|---|---|---|---|---|---|
| Cohort 1: 750 mg MEDI8852 + OS (n = 31) | Cohort 2: 3,000 mg MEDI8852 + OS (n = 31) | Cohort 3: placebo + OS (n = 32) | Cohort 4: 3,000 mg MEDI8852 (n = 31) | Cohorts 1, 2, and 4 combined: total MEDI8852 (n = 93) | |
| Any | 11 (35.5) | 16 (51.6) | 10 (31.3) | 12 (38.7) | 39 (41.9) |
| Investigational product related | 4 (12.9) | 6 (19.4) | 5 (15.6) | 4 (12.9) | 14 (15.1) |
| Grade 3 | 0 | 3 (9.7) | 2 (6.3) | 0 | 3 (3.2) |
| SAE | 0 | 1 (3.2) | 1 (3.1) | 0 | 1 (1.1) |
| AESI (i.e., infusion-related reaction) | 0 | 1 (3.2) | 0 | 0 | 1 (1.1) |
| Occurring in ≥4% of subjects in any cohort | |||||
| Bronchitis | 4 (12.9) | 5 (16.1) | 1 (3.1) | 2 (6.5) | 11 (11.8) |
| Nausea | 2 (6.5) | 1 (3.2) | 2 (6.3) | 1 (3.2) | 4 (4.3) |
| Diarrhea | 0 | 2 (6.5) | 0 | 2 (6.5) | 4 (4.3) |
| Upper respiratory tract infection | 1 (3.2) | 3 (9.7) | 0 | 0 | 4 (4.3) |
| Pharyngitis | 2 (6.5) | 0 | 1 (3.1) | 1 (3.2) | 3 (3.2) |
| Dysgeusia | 1 (3.2) | 2 (6.5) | 1 (3.1) | 0 | 3 (3.2) |
| Bronchial hyperreactivity | 2 (6.5) | 0 | 0 | 0 | 2 (2.2) |
| Paresthesia | 0 | 0 | 2 (6.3) | 0 | 0 |
OS, oseltamivir.
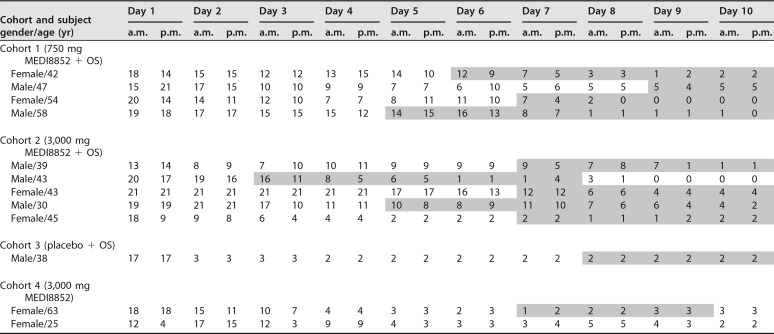
AEs of bronchitis occurred in 11 MEDI8852 recipients (4 subjects who received 750 mg of MEDI8852 and oseltamivir, 5 subjects who received 3,000 mg of MEDI8852 and oseltamivir, and 2 subjects who received 3,000 mg of MEDI8852) and 1 oseltamivir-only recipient (Table 2). Most bronchitis events occurred in subjects from a single site (75.0%; 9/12), which enrolled approximately one-third (34.9%; 44/126) of all subjects in the study. Nearly all events were either mild (grade 1, using Common Terminology Criteria for Adverse Events, version 4.0) or moderate (grade 2) in severity, except for one event that was severe (grade 3). Most bronchitis events started around day 7 and resolved by day 15. Three subjects with bronchitis had additional AEs, including one subject who had mild (grade 1) reactive airway disease consistent with possible lower respiratory tract involvement. It should be noted that all subjects with bronchitis were afebrile (temperature < 38°C) at the time of onset or at the time point prior to the diagnosis of bronchitis for which a temperature measurement was available, and no subjects had elevated white blood cell counts on day 7 of the study, when serum was drawn for all subjects. Influenza virus titers were either below the lower limit of quantitation (LLOQ) or were not detected by day 7 in 9 of 11 subjects for whom virus titer data were available. One subject had virus titers that initially decreased on day 5 from baseline (3.16 and 7.42 log10 genome copies per ml, respectively) but then increased on days 7 and 9 (4.40 and 6.61 log10 genome copies per ml, respectively); an analysis of the subject's HA and NA gene sequences did not reveal any changes when days 1, 3, 5, 7, and 9 sequences were compared with baseline sequences, and the subject's influenza symptom scores were low (≤2) from day 8 onward. All subjects with bronchitis (who also had influenza A infection at baseline confirmed with reverse transcription-PCR [RT-PCR]) were found to be infected with A/H1N1 strains. Furthermore, all subjects with bronchitis had solicited influenza symptom scores that were either stable or decreasing at the time of onset of bronchitis (Table 3).
TABLE 2.
Subjects with AEs of bronchitis, by cohorta
| Cohort and site no. | Subject gender/age (yr) | Bronchitis start/stop (days) | Grade | Additional TEAEs (start/stop) | Relevant concomitant medications | Potentially relevant medical history | Viral clearance/titersb log10 (genome copies/ml) | Influenza subtype |
|---|---|---|---|---|---|---|---|---|
| Cohort 1 (750 mg MEDI8852 + OS) | ||||||||
| 2001836 | Female/42 | 6/13 | 2 | None | Azithromycin, brompheniramine + pseudoephedrine | Renal cystectomy, cholecystectomy | Day 5: <3.097 (LOQ) | A/H1N1 |
| 2001836 | Male/47 | 9/18 | 2 | Pharyngitis (day 20/26) | Methylprednisolone sodium succinate, brompheniramine + dextromethorphan + pseudoephedrine, salbutamol, sulfamethoxazole + trimethoprim | None | Day 7: ND | A/H1N1 |
| 2001836 | Female/54 | 7/15 | 2 | Upper respiratory tract infection (day 7/12); bronchial hyperreactivity (day 9/12) | Azithromycin, salbutamol, methylprednisolone sodium succinate, prednisone | Tachycardia, depression | Day 7: <3.097 (LOQ); day 9: ND | A/H1N1 |
| 2001836 | Male/58 | 5/13 | 2 | Sinusitis (day 9/17) | Azithromycin, amoxicillin + clavulanic acid | Diabetes mellitus, hypertension | Day 5: 3.16; day 7: 4.40; day 9: 6.61 | A/H1N1 |
| Cohort 2 (3,000 mg MEDI8852 + OS) | ||||||||
| 2001152 | Male/39 | 7/11 | 2 | None | Salbutamol, beclomethasone dipropionate, amoxicillin + clavulanic acid | Influenza, obesity, bronchospasm | Day 5: <3.097 (LOQ); day 7: ND | A/H1N1 |
| 2001152 | Male/43 | 3/7 | 3 | None | Theophylline, salbutamol, fluticasone + salmeterol, amoxicillin + clavulanic acid | Influenza, asthma, hypertension | Day 5: <3.097 (LOQ); day 7: ND | A/H1N1 |
| 2001836 | Female/43 | 7/14 | 2 | None | Azithromycin, methylprednisolone Sodium succinate | None | Day 5: ND | A/H1N1 |
| 2001836 | Male/30 | 5/11 | 2 | None | Azithromycin, methylprednisolone Sodium succinate, salbutamol | Seasonal allergies | Day 9: <3.097 (LOQ) | A/H1N1 |
| 2001836 | Female/45 | 7/14 | 1 | None | Azithromycin | Asthma | Day 7: ND | A/H1N1 |
| Cohort 3 (placebo + OS) | ||||||||
| 2001836 | Male/38 | 8/15 | 2 | None | Azithromycin, brompheniramine + pseudoephedrine | Hypertriglyceridemia, diabetes mellitus | Day 5: ND | A/H1N1 |
| Cohort 4 (3,000 mg MEDI8852) | ||||||||
| 2001163 | Female/63 | 7/9 | 1 | None | Prednisone, alcophyllex liquid | Hypertension | Day 5: ND | A/H1N1 |
| 2001836 | Female/25 | 12/20 | 2 | None | Azithromycin, prednisone | Meningitis, asthma | No influenza A detected at baseline | ND |
LOQ, limit of quantitation; ND, not detected; OS, oseltamivir; TEAE, treatment-emergent adverse event.
Viral clearance/titers refer to the day by which virus titers were below the limit of quantitation (<3.097 log10) or were not detected during the AE of bronchitis or, if virus was not cleared, provides titer values.
TABLE 3.
Influenza symptom scores for subjects with AEs of bronchitis, by cohorta
Days during which subjects had AEs of bronchitis are shaded gray. For the last subject (Female/25), the onset of bronchitis was on day 12, which was after the period (days 1 to 10) during which influenza symptom scores were collected. OS, oseltamivir.
AEs that were related to investigational product occurred at similar rates in MEDI8852 and oseltamivir-only recipients (15.1 and 15.6%, respectively). In general, AEs were either mild (grade 1) or moderate (grade 2) in severity. Two serious AEs (SAEs) were reported during the study. One subject who received 3,000 mg of MEDI8852 and oseltamivir and had a history of hypertension and seasonal allergies had a severe (grade 3) investigational-product-related event of infusion-related reaction (which was also considered an AE of special interest [AESI]) on day 1. The infusion was stopped (the subject received a partial dose of MEDI8852), and the event resolved within 15 min after treatment with intramuscular dexamethasone, inhaled albuterol, and oxygen. Another subject who received placebo and oseltamivir and had an undisclosed history of recent syncope had a severe (grade 3) event of syncope on day 1. The infusion was completed and the event resolved without treatment. Both subjects completed their oseltamivir regimens and all study assessments.
Solicited influenza symptoms occurred at similar rates in MEDI8852 and oseltamivir-only recipients. The proportion of subjects who had any solicited influenza symptoms (a score of ≥1) through day 10 was the same in both MEDI8852 and oseltamivir-only recipients (100% in each group). Likewise, the median (range) number of days that subjects had any solicited symptoms was similar in MEDI8852 and oseltamivir-only recipients (10.0 [2 to 13] days in each group). There were no deaths or discontinuations from the study due to an AE. There were also no differences in routine chemistry and hematology results in MEDI8852 or oseltamivir-only recipients.
Virology.
Of the 125 subjects who had samples that were positive for influenza A by the rapid antigen test, 104 (83.2%; 78 from the United States and 26 from South Africa) had samples that were positive for influenza A infection by RT-PCR. The median viral loads were similar between cohorts at baseline (Table 4). A rapid decline in viral loads was observed in all cohorts; by day 5, the median viral load was below the LLOQ for the quantitative RT-PCR (qRT-PCR) assay (log10 3.1 genome copies per ml). The time to reduction of viral loads was similar between cohorts; the proportion of subjects who continued to shed virus after day 7 was similar in MEDI8852 and oseltamivir-only recipients (21.6 and 23.3%, respectively).
TABLE 4.
Summary of virus shedding (per-protocol population)a
| Cohort | Baseline (day 1) |
Day 3 |
Day 5 |
Day 7 |
Day 9 |
Day 11 |
Day 13 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Median (range) | No. (%) | Median (range) | No. (%) | Median (range) | No. (%) | Median (range) | No. (%) | Median (range) | No. (%) | Median (range) | No. (%) | Median (range) | |
| Cohort 1:750 mg MEDI8852 + OS (n = 27) | 27 (100) | 6.38 (4.0–9.0) | 27 (100) | 4.91 (2.8–7.3) | 27 (100) | 2.80 (2.8–5.8) | 27 (100) | 2.80 (2.8–6.5) | 6 (22.2) | 2.80 (2.8–6.6) | 2 (7.4) | 2.80 (2.8–2.8) | 0 (0) | NA |
| Cohort 2: 3,000 mg MEDI8852 + OS (n = 23) | 23 (100) | 7.26 (3.8–8.4) | 22 (95.7) | 5.08 (2.8–7.2) | 21 (91.3) | 2.80 (2.8–5.8) | 22 (95.7) | 2.80 (2.8–6.1) | 3 (13.0) | 2.80 (2.8–6.6) | 3 (13.0) | 2.80 (2.8–2.8) | 0 (0) | NA |
| Cohort 3: placebo + OS (n = 30) | 30 (100) | 6.49 (2.8–8.7) | 29 (96.7) | 4.19 (2.8–8.1) | 28 (93.3) | 2.80 (2.8–6.1) | 30 (100) | 2.80 (2.8–4.8) | 7 (23.3) | 2.80 (2.8–5.0) | 3 (10.0) | 2.80 (2.8–2.8) | 0 (0) | NA |
| Cohort 4 3,000 mg MEDI8852 (n = 24) | 24 (100) | 6.58 (3.2–8.1) | 23 (95.8) | 4.75 (2.8–7.9) | 24 (100) | 2.80 (2.8–7.0) | 23 (95.8) | 2.80 (2.8–5.0) | 7 (29.2) | 2.80 (2.8–2.8) | 4 (16.7) | 2.80 (2.8–4.1) | 1 (4.2) | 2.80 (2.8–2.8) |
| Cohorts 1, 2, and 4 combined: total MEDI8852 (n = 74) | 74 (100) | 6.88 (3.2–9.0) | 72 (97.3) | 4.86 (2.8–7.9) | 72 (97.3) | 2.80 (2.8–7.0) | 72 (97.3) | 2.80 (2.8–6.5) | 16 (21.6) | 2.80 (2.8–6.6) | 9 (12.2) | 2.80 (2.8–4.1) | 1 (1.4) | 2.80 (2.8–2.8) |
Virus titers were measured by qRT-PCR as log10 genome copies per ml. The LLOQ was log10 3.097 genome copies per ml. Samples determined to have virus titers greater than the LLOQ (log10 2.796 genome copies per ml) were reported as a value (genome copies per ml). Samples containing virus titers below the LLOQ were imputed to 0.5. NA, not applicable; OS, oseltamivir.
NA sequencing.
None of the baseline NA sequences had changes at positions commonly reported or more frequently observed with oseltamivir resistance (H1N1: E119, H275, R293, or N295 [N1 numbering]; and H3N2: E119, H274, R292, or N294 [N2 numbering]) compared to the reference sequences (A/Bolivia/559/2013 [H1N1] or A/Hong Kong/4801/2014 [H3N2]).
Of the 97 subjects who had both a baseline and a last sample sequenced, 12 had changes in the NA gene; however, none of the changes occurred within a known oseltamivir-resistant site (Table S2). Only 2 of the 14 observed changes occurred at the same amino acid position (M15). A mixed population was observed in a day 3 sample from a subject who received 3,000 mg of MEDI8852, which corresponded to a known amino acid change associated with oseltamivir resistance (N1-R293) with a minor Sanger nucleotide peak that translated to amino acid K293. Due to low virus titers, sequence data from samples collected after day 3 could not be evaluated. This subject had decreasing solicited influenza symptoms (with a moderate cough through day 7) and had no AEs during the study.
HA sequencing.
When baseline HA sequences were compared to the reference sequences (A/Bolivia/559/2013 [H1N1] and A/Hong Kong/4801/2014 [H3N2]) at positions associated with the MEDI8852 binding region, unique changes were observed at positions L382 and V47 (Table S3). L382Q (H1N1, HA2-L38Q in H3 numbering) was observed in the baseline samples from seven subjects, and L382L/Q was observed in the baseline sample from one subject. Sequence alignment of HA from 5,028 H1 isolates obtained from the Influenza Virus Resource Database (National Center for Biotechnology Information) suggest that this position is polymorphic L/Q (76.2/23.4%). H1N1 isolates with the polymorphic change at this position were neutralized by MEDI8852 (MedImmune, unpublished data).
Unique changes were also observed at position V47 within the MEDI8852 binding region of HA (relative to the reference sequences V47F and V47I) in the baseline samples from two additional subjects (Table S3). Sequence alignment of HA suggests that this position is highly conserved (valine = 99.2%). However, this position appears to have some heterogeneity among group 1 influenza viruses. In addition to the dominant valine at this position, the virus panel tested during preclinical development of MEDI8852 also contained strains that had isoleucine, glutamine, and lysine. These viruses were neutralized efficiently by MEDI8852, suggesting that the HA-MEDI8852 interaction can tolerate some amino acid diversity at this position (MedImmune, unpublished data).
Of the 97 subjects who had both a baseline and a last sample sequenced, 6 had changes in the HA gene; however, none of the changes corresponded to amino acids located in the putative MEDI8852 binding region (Table S4). A mixed population was observed in a baseline sample from a subject who received 3,000 mg of MEDI8852, which corresponded to the predefined MEDI8852 binding region that contained amino acid sequence Leu/Gln382. However, only amino acid Gln382 was identified in sequence data from the day 5 sample. This residue position (i.e., position 382) appears to be polymorphic (Leu/Gln) within the H1 subtype. This subject had decreasing solicited influenza symptoms (with moderate myalgia and fatigue through day 2) and had no AEs during the study.
Virus susceptibility testing for MEDI8852.
Selected nasopharyngeal samples that had quantifiable virus titer (qRT-PCR > LLOQ) were chosen to culture and expand the virus in order to perform susceptibility testing with MEDI8852. A total of 35 of the 53 clinical samples yielded detectable virus levels that were 50% of the tissue culture infective dose (TCID50). Unfortunately, no virus was cultivable from any of the selected postbaseline samples, even though these samples had a quantifiable amount of virus via qRT-PCR. MEDI8852 susceptibility testing was performed on all isolated viruses, of which 27 yielded valid results for 50% inhibitory concentrations (IC50s) and 8 failed from insufficient viral input. For the isolated H1N1 isolates (n = 23), the median IC50 was 44 nM with a range of 19 to 101 nM; for the H3N2 isolates (n = 4), the median IC50 was 48 nM with a range of 43 to 50 nM. The IC50s for the clinical samples were comparable to or below that of the two control viruses (A/California/7/2009 [H1N1] and A/Hong Kong/4801/2014 [H3N2]) (289 and 74 nM, respectively), suggesting that these isolates were susceptible to MEDI8852. In addition, five of the virus isolates contained the unique (relative to reference) L382Q (three samples), V47I (one sample), and V47F (one sample) sequence within the MEDI8852 binding region. These five viruses had a median IC50 (median, 40 nM; range, 36 to 62 nM) similar to that of the rest of the panel, confirming that the HA-MEDI8852 interaction can tolerate some amino acid diversity at these positions.
Serum levels of MEDI8852.
On day 1 (postinfusion), the mean serum levels of MEDI8852 were 131 μg/ml (750 mg of MEDI8852 and oseltamivir), 619 μg/ml (3,000 mg of MEDI8852 and oseltamivir), and 652 μg/ml (3,000 mg of MEDI8852). On day 7, the mean values were 55.4, 243, and 270 μg/ml, respectively.
Efficacy.
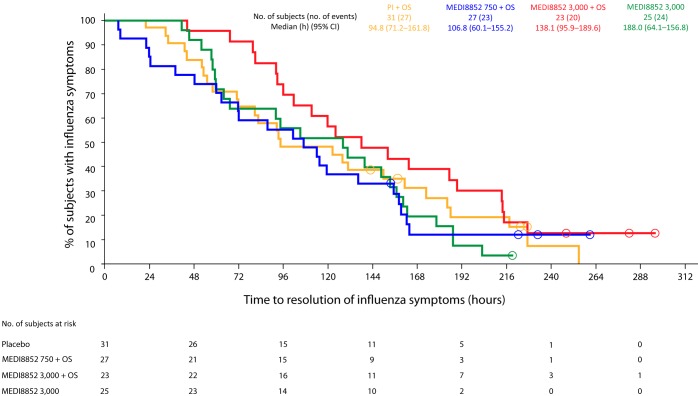
Overall, treatment with MEDI8852 alone at a dose of 3,000 mg appeared to have similar effects on alleviation of influenza symptoms as oseltamivir alone, and the combination of MEDI8852 at doses of 750 or 3,000 mg with oseltamivir did not appear to provide additional benefit beyond that provided by each individual treatment alone (Table 5). The median (95% confidence interval) time to resolution of influenza symptoms (hours) for subjects whose initial positive rapid antigen test for influenza virus was confirmed by RT-PCR was similar in 3,000 mg of MEDI8852 (128.00 [64.05 to 156.82]), 750 mg of MEDI8852 plus oseltamivir (106.75 [60.12 to 155.17), 3,000 mg of MEDI8852 plus oseltamivir (138.10 [95.87 to 189.55]), and oseltamivir-only (94.83 [71.17 to 161.75]) recipients, all values having broad overlapping confidence intervals (Fig. 2). There was also no clear differentiation between the treatment groups in the prespecified, stratified analysis of subjects who had influenza symptoms for either ≤48 h or >48 h before treatment (MedImmune, unpublished data). Similarly, the treatment groups did not appear to differ in time to resolution of specific influenza symptoms (nasal congestion, sore throat, cough, aches and pains, fatigue, headache, and chills/sweats); cough took the longest time to resolve in all groups (Table S5). The median duration and severity of influenza symptoms (score-hours) and the median time to return of the ability to perform usual activities (hours) were also similar between the treatment groups, and no clear impact was seen when evaluated by the duration of influenza symptoms before treatment (Table 5; MedImmune, unpublished data).
TABLE 5.
Summary of efficacy, influenza A virus-infected subjects confirmed by RT-PCR (intent-to-treat population)a
| Cohort | Median (range) |
||
|---|---|---|---|
| Median time to resolution of any symptoms, h (95% CI)a | Median duration and severity of symptoms, total score (score-h)b | Median time to return of ability to perform usual activities (h)c | |
| Cohort 1: 750 mg MEDI8852 + OSd (n = 27) | 106.75 (60.12–155.17) | 903.30 (157.50–2493.70) | NAe (45–288) |
| Cohort 2: 3,000 mg MEDI8852 + OS (n = 23) | 138.10 (95.87–189.55) | 1,204.90 (674.20–3288.00) | 238.4 (108–286) |
| Cohort 3: placebo + OS (n = 31) | 94.83 (71.17–161.75) | 1,007.10 (379.40–2,790.10) | 254.3 (47–288) |
| Cohort 4: 3,000 mg MEDI8852 (n = 25) | 128.00 (64.05–156.82) | 876.50 (406.40–2992.10) | 237.3 (115–241) |
| Cohorts 1, 2, and 4 combined: total MEDI8852 (n = 75) | 119.25 (94.40–148.50) | 1189.80 (157.50–3288.00) | 238.4 (45–288) |
Confidence intervals (CI) were calculated based on the medians obtained from Kaplan-Meier analysis.
Score-hours were assessed by area-under-the-curve analysis derived on a by-subject basis, using the linear trapezoidal rule with all available data from baseline to the last time point with influenza symptoms measurement up to day 13.
The median time to return of ability to perform usual activities is summarized by Kaplan-Meier curves. Subjects with missing values are censored.
OS, oseltamivir.
NA, not applicable. The median time could not be calculated because there were not enough subjects who returned to ability to perform usual activities.
FIG 2.
Time to resolution of influenza symptoms for subjects infected with influenza A virus (confirmed by RT-PCR at baseline). Open circles represent censored subject data. M8852, MEDI8852; OS, oseltamivir.
DISCUSSION
To date, no clinical data have been published on the safety and efficacy of a monoclonal antibody for the treatment of influenza A infection when the virus is acquired through natural infection during an influenza season. Two studies have evaluated the safety and pharmacokinetics of broadly neutralizing monoclonal antibodies that target the conserved stalk region of the influenza HA protein in healthy adult volunteers challenged with influenza A virus (17, 18). However, it may be difficult to extrapolate the findings from these studies to subjects with naturally acquired infection, since both studies enrolled subjects who were seronegative to the challenge strains and the mode of infection and induction of symptoms in challenge studies may differ from that seen in outpatients or inpatients with influenza illness (19). We conducted this study to fully characterize the safety and tolerability profile of MEDI8852 and to evaluate its potential efficacy in a population of outpatient adults with acute, uncomplicated influenza A infection before initiating studies in a target population of more severely ill patients hospitalized with influenza A infection.
In this study, AEs occurred at a higher rate in MEDI8852 recipients than in oseltamivir-only recipients, a difference that was mainly driven by a slightly higher rate of bronchitis in MEDI8852 recipients. As this was the first study of MEDI8852 in patients with influenza A infection, and due to the nonclinical finding of antibody-dependent enhancement of influenza illness in swine, all bronchitis events were reviewed in detail for possible association with MEDI8852 administration. Nearly all cases of bronchitis were either mild (grade 1) or moderate (grade 2) in severity and were associated with few additional AEs. In addition, nearly all bronchitis events occurred in afebrile individuals whose solicited influenza symptom scores were either stable or decreasing during the time of diagnosis and whose viral loads were less than the LLOQ at the time of onset. Finally, it should be noted that most (9/12) of the bronchitis events occurred at a single study site. All individuals at this site who had residual influenza symptoms on days 5 to 7 were treated with antibiotics, and a subset (5/9) were also treated with steroids, possibly to more rapidly resolve these symptoms, which may have reflected clinical practice at the site. Given the low-grade severity of these bronchitis events and the associated influenza symptoms, as well as the general lack of increase in influenza virus shedding during the events, these findings are not considered to be consistent with antibody-dependent enhancement of influenza illness. In general, AEs related to investigational product occurred at similar rates in MEDI8852 and oseltamivir-only recipients, and the most commonly reported AEs (bronchitis, nausea, diarrhea, and upper respiratory tract infection) in all cohorts were those that were expected in a population with acute, uncomplicated influenza A illness.
Virus samples collected from subjects in the study were genotyped to determine whether MEDI8852 binding site- or oseltamivir resistance-associated mutations were present at baseline or before treatment or arose over the course of treatment. Sanger sequence analysis of the NA and HA genes demonstrated that there were a limited number of amino acid changes between the baseline sample and the last sample sequenced; however, none of the changes appeared within the MEDI8852 binding region or within a known oseltamivir-resistant site. The amino acid positions in which changes were observed varied across all subjects, regardless of treatment, and were outside the MEDI8852 binding region. In addition, we saw no susceptibility differences between virus controls and the baseline samples in which virus could be isolated and expanded. Overall, these data suggest that there was no drug-specific selective pressure favoring the development of escape mutations in this study.
Reduction in virus titers did not differ significantly between the treatment groups, which suggests that MEDI8852, when administered either alone or in addition to standard of care (i.e., oseltamivir), had effects that were similar to those of standard-of-care alone. These findings may be due to the fact that subjects in this study were relatively healthy and had mild illness; most subjects had infections with A/H1N1 strains. As a result, subjects may have started to rapidly clear virus before and after enrollment, which would have minimized the ability to differentiate between MEDI8852 and oseltamivir treatment. In fact, most subjects in the study had rapid declines in virus titers by day 3, and almost all subjects had virus titers that were less than the LLOQ by day 5. A similar phenomenon was observed in a randomized, placebo-controlled, double-blind study of oseltamivir (75 and 150 mg administered orally twice a day for 5 days) that had recruited a large (n = 629) cohort of outpatient adults with acute, uncomplicated influenza infection (20). All subjects in that study had virus titers that had rapidly declined by day 3, and the differences in virus titers between the placebo and oseltamivir groups were not statistically significant. In a post hoc analysis focusing on subjects with more severe symptoms (defined as those whose baseline symptom scores were greater than the median score), the time to resolution of all influenza symptoms, as well as the time to resolution of individual symptoms, were consistently lower in the three treatment groups that received MEDI8852 than in the group that received oseltamivir alone; however, this finding should be interpreted with caution due to its post hoc nature, the limited number of subjects involved, and the broad confidence intervals associated with the times to resolution (Table S6).
A limitation of our study is the absence of a placebo-only arm, which may have complicated the assessment of the effect of MEDI8852 on reductions in influenza viral load after treatment (i.e., if oseltamivir treatment did not result in reductions in viral load). However, a placebo-only arm was not included in the study because its primary purpose was to gather safety data on the coadministration of MEDI8852 and oseltamivir prior to the initiation of a larger phase 2b study, in which both treatments would be coadministered to more seriously ill patients hospitalized with influenza A infection.
Another limitation of the present study is that we did not evaluate the pharmacokinetics of MEDI8852 in the upper respiratory tract. Although we have previously evaluated the serum pharmacokinetics of MEDI8852 in healthy volunteers (9) and have data on both day 1 and day 7 mean serum levels of MEDI8852 from the present study, MEDI8852 concentration at the site of infection (i.e., the upper respiratory tract) remains unknown. It is possible that the highest dose of MEDI8852 evaluated in this study (3,000 mg) may have been too low to achieve a nasal concentration needed to neutralize influenza A strains. In fact, a recent study with a similar monoclonal antibody administered to healthy volunteers challenged with influenza A virus found that the nasal concentrations of the antibody were not dose proportional (17). The study determined that a single i.v. dose of 3,600 mg (or higher) would be required to achieve a nasal concentration needed to neutralize multiple influenza A strains. Furthermore, a pharmacokinetic model developed from the study data predicted that an even higher dose might be needed to decrease virus shedding in the upper respiratory tract. Given that severely ill patients hospitalized with influenza A infection are likely to have higher viral loads in the upper and lower respiratory tracts, higher doses of MEDI8852 may be needed to achieve efficacious outcomes and should be evaluated in future studies.
In summary, this study demonstrated that MEDI8852, administered alone and in conjunction with oseltamivir, had an acceptable AE profile in outpatient adults with acute, uncomplicated influenza A infection. The relatively small number of subjects in the study, and the fact that the study was conducted in adults who were generally healthy, may have made it difficult to observe a clinically meaningful reduction in overall disease burden with MEDI8852 treatment. The dose range and efficacy of MEDI8852 remains to be fully evaluated in an adequately powered clinical study, especially in more severely ill patients hospitalized with influenza A illness.
MATERIALS AND METHODS
Study design.
This phase 2a, randomized, partial double-blind, active-controlled, dose-ranging study (ClinicalTrials.gov, NCT02603952) was conducted at 24 centers in the United States and South Africa during the 2015 to 2016 Northern Hemisphere and 2016 Southern Hemisphere influenza seasons. The study was carried out in accordance with the Declaration of Helsinki and the International Council for Harmonisation's Harmonised Tripartite Guideline E6(R1): Good Clinical Practice (21). The study protocol was approved by the institutional review boards or independent ethics committees at each study site and by national regulatory authorities.
Subjects.
All subjects provided written informed consent before undergoing any study procedures. Eligible subjects were male or nonpregnant female adults, aged 18 to 65 years, with positive results for a rapid influenza antigen test at screening. Subjects also had symptomatic presumptive influenza A infection with an onset of symptoms ≤5 days before the administration of study drugs; symptoms were defined as the presence of a fever of ≥38.0°C, one or more moderate systemic symptom (headache, malaise, myalgia, sweats, and/or chills or fatigue), and one or more moderate respiratory symptom (cough, sore throat, or nasal symptoms). Major exclusion criteria included current hospitalization for influenza A infection, receipt of antiviral therapy within the past 2 weeks, receipt of immunoglobulin or blood products within the past 6 months, current clinical evidence of pneumonia, and active bacterial infection requiring oral or parenteral antibiotics.
Study treatments.
Subjects were randomized 1:1:1:1 into four cohorts. Cohort 1 received 750 mg of MEDI8852 and 75 mg of oseltamivir (Roche, Kaiseraugust, Switzerland), cohort 2 received 3,000 mg of MEDI8852 and 75 mg of oseltamivir, cohort 3 received placebo and 75 mg of oseltamivir (referred to as oseltamivir alone), and cohort 4 received 3,000 mg of MEDI8852. In each cohort, MEDI8852 or placebo was administered as single i.v. infusion on day 1 and oseltamivir was administered orally twice daily for 5 days beginning on day 1. Except for cohort 4, where placebo capsules matched to the appearance of oseltamivir could not be provided, subjects and study site personnel were blinded to treatment allocation during the study. Randomization was stratified by duration of illness (≤48 versus >48 h).
Clinical assessments.
Subjects recorded the severity of seven influenza symptoms (cough, nasal obstruction, sore throat, fatigue, headache, myalgia, and feverishness) twice daily on days 1 to 10, using a 4-point scale (0, absent; 1, mild; 2, moderate; 3, severe) that was previously used in pivotal studies for oseltamivir and zanamivir (20, 22). Subjects who did not show signs of clinical improvement by day 10 continued to record the severity of solicited influenza symptoms on days 11 and 13, if needed, until clinical improvement was observed. Subjects also recorded their ability to perform usual activities on an 11-point visual analog scale (0, unable to perform usual activity; 10, fully able to perform normal activity) on days 1 to 10 (and on days 11 and 13, if needed).
AEs were defined as any untoward medical event that occurred in a patient or clinical investigation subject administered a pharmaceutical product and that did not necessarily have a causal relationship with this treatment (21). SAEs were defined as any AE that resulted in death, was immediately life threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect in the offspring of a subject, or was an important medical event that may jeopardize the subject or may require medical intervention to prevent one of the outcomes listed here. AESIs were defined as AEs that were of scientific and medical interest specific to understanding of the investigational product and that may require close monitoring and rapid communication by the investigator to the study sponsor (MedImmune).
Assessments included AEs from screening to day 28, SAEs, and AESIs from screening to day 101; vital signs at screening and on day 1 (during and after investigational product infusion); temperature on days 1 to 7; and routine chemistry and hematology laboratories from screening to day 7.
Virology assessments.
Nasopharyngeal swab samples were collected for confirmation of influenza A virus positivity, using a multiplex real-time qualitative RT-PCR assay (Lyra influenza A+B assay; Quidel Corporation, San Diego, CA), and virus shedding was assessed using a qRT-PCR assay (based on Lyra influenza A+B assay) at screening; during treatment on days 1, 3, and 5; and during follow-up on day 7. Subjects who did not show signs of clinical improvement by day 7 had additional nasopharyngeal swab samples collected on days 9, 11, and 13, if needed, until clinical improvement was observed. Evaluation of infectious virus titers was an exploratory endpoint for the study. However, due to inconsistent results generated with the TCID50 assay, these data are not presented here.
Genotypic analyses of baseline and posttreatment samples containing quantifiable influenza A virus (i.e., greater than the LLOQ for the qRT-PCR assay, log10 3.1 genome copies per ml) were performed by Sanger sequencing of the NA gene. In cases where a mixed population was observed, only the major changes were reported unless the mixed population was observed within a known oseltamivir-resistant site. Sequence data were analyzed to identify all occurrences of amino acid changes in NA between an individual's baseline sample and their last sample sequenced. In addition, all baseline NA sequences were compared to reference sequences (A/Bolivia/559/2013 [H1N1] and A/Hong Kong/4801/2014 [H3N2]) to identify changes relative to the reference sequences at positions known to be associated with oseltamivir resistance (H1N1: E119, H275, R293, or N295; and H3N2: E119, H274, R292, or N294).
Sanger sequencing of the HA gene from baseline and posttreatment samples containing quantifiable influenza A virus was also performed. In cases where a mixed population was observed, only the major changes were reported unless the mixed population was observed within a MEDI8852 binding region. In addition, all baseline HA sequences were compared with reference sequences (A/Bolivia/559/2013 [H1N1] and A/Hong Kong/4801/2014 [H3N2]) to identify changes at positions known to be associated with the 30–amino acid MEDI8852 binding region (7; MedImmune, unpublished data).
Quantification of virus susceptibility.
Selected nasopharyngeal samples with quantifiable viral load were processed to isolate, expand, and quantitate the influenza virus, using Madin-Darby canine kidney (MDCK) cells, according to the supplier's standard procedures (Viroclinics, Rotterdam, Netherlands). Virus susceptibility to MEDI8852 was measured by using a MDCK cell-based microneutralization assay (ViroSpot; Viroclinics). The concentration that produced IC50s from each virus isolate and from two control virus strains (A/California/7/2009 [H1N1] and A/Hong Kong/4801/2014 [H3N2]) were calculated.
Serum levels of MEDI8852.
Serum was collected on day 1 (postinfusion) and on day 7 to estimate the serum levels of MEDI8852.
Study endpoints.
The primary endpoint of the study was the safety and tolerability of MEDI8852, as measured by AEs, SAEs, and AESIs, and the incidence of solicited influenza symptoms. The secondary endpoints were the quantification of influenza virus shedding by qRT-PCR, oseltamivir resistance-associated mutations, and virus susceptibility to MEDI8852. The exploratory endpoints were time to resolution of influenza symptoms, duration and severity of influenza symptoms, and time to return of the ability to perform usual activities.
Statistical analysis.
The as-treated population, used to assess safety, included all subjects who were randomized and received any portion of their protocol-specified treatment regimen. Subjects were analyzed according to the treatment they received. The per-protocol population, used to assess virus shedding and resistance analyses, included all subjects who received any portion of their protocol-specified treatment regimen and had valid assay results from nasopharyngeal samples obtained at any posttreatment time point (i.e., after the single i.v. infusion of MEDI8852 or placebo on day 1). Subjects without confirmed (by RT-PCR) influenza A infection at baseline were excluded from the per-protocol population. The intent-to-treat population, used to assess exploratory efficacy, included all randomized subjects.
For the exploratory efficacy analyses, time to resolution of influenza symptoms and time to return of the ability to perform usual activities were summarized by the Kaplan-Meier method for each treatment group. Symptom resolution was considered to have occurred at the start of the first 24-h period in which all influenza symptoms were scored as ≤1 (mild or none) and remained so for 24 h. The time to the return of the ability to perform usual activities was the time at which a subsequently maintained normalization was initially identified. Subjects who did not return to their ability to perform usual activities were censored at the time of dropout or at the time the last activity level was assessed, whichever occurred earlier. Duration and severity of influenza symptoms were assessed using an area-under-the-curve analysis that was derived on a by-subject basis with the linear trapezoidal rule, in which all available data from baseline to the last time point with influenza symptoms were measured up to day 13.
Because all analyses were descriptive in nature and no hypothesis was being tested statistically, no formal sample size calculations were performed. With 94 subjects exposed to MEDI8852 (cohorts 1, 2, and 4 combined), there was a 90% probability of observing at least one AE if the true event rate was 1.9%; if no AEs were observed, the study provided 95% confidence that the true event rate was <2.5%.
A planned interim analysis was performed after all subjects had completed assessments through day 28, unless withdrawn or lost to follow-up.
Supplementary Material
ACKNOWLEDGMENTS
Editorial assistance was provided by Jeffery Brubaker and Deborah Shuman of MedImmune.
This study was sponsored and funded by MedImmune, the global biologics research and development arm of AstraZeneca. The study research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors were involved in one or more aspects of the following: conception or design of the study or the acquisition, analysis, or interpretation of the data. All authors were equally involved in the writing of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00694-18.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2017. Summary of the 2015-2016 influenza season. US Department of Health and Human Services, Washington, DC: https://www.cdc.gov/flu/about/season/flu-season-2015-2016.htm. [Google Scholar]
- 2.De Villiers PJ, Steele AD, Hiemstra LA, Rappaport R, Dunning AJ, Gruber WC, Forrest BD. 2009. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 28:228–234. doi: 10.1016/j.vaccine.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 3.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, Centers for Disease Control and Prevention . 2011. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 60:1–24. [PubMed] [Google Scholar]
- 4.Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, Griffiths M, Waalberg E, Ward P, IMPACT Study Group . 2003. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, Rode A, Kinnersley N, Ward P. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845–1850. doi: 10.1016/S0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 6.Louie JK, Yang S, Acosta M, Yen C, Samuel MC, Schechter R, Guevara H, Uyeki TM. 2012. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 55:1198–1204. doi: 10.1093/cid/cis636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallewaard NL, Corti D, Collins PJ, Neu U, McAuliffe JM, Benjamin E, Wachter-Rosati L, Palmer-Hill FJ, Yuan AQ, Walker PA, Vorlaender MK, Bianchi S, Guarino B, De Marco A, Vanzetta F, Agatic G, Foglierini M, Pinna D, Fernandez-Rodriguez B, Fruehwirth A, Silacci C, Ogrodowicz RW, Martin SR, Sallusto F, Suzich JA, Lanzavecchia A, Zhu Q, Gamblin SJ, Skehel JJ. 2016. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paules CI, Lakdawala S, McAuliffe JM, Paskel M, Vogel L, Kallewaard NL, Zhu Q, Subbarao K. 2017. The hemagglutinin A stem antibody MEDI8852 prevents and controls disease and limits transmission of pandemic influenza viruses. J Infect Dis 216:356–365. doi: 10.1093/infdis/jix292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallory RM, Ali SO, Takas T, Kankam M, Dubovsky F, Tseng L. 2017. A phase 1 study to evaluate the safety and pharmacokinetics of MEDI8852, an anti-influenza A monoclonal antibody, in healthy adult volunteers. Biologicals 50:81–86. doi: 10.1016/j.biologicals.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Chan-Hui PY, Swiderek KM. 2016. Immunological considerations for developing antibody therapeutics for Influenza A. Hum Vaccin Immunother 12:474–477. doi: 10.1080/21645515.2015.1079676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishnan B, Viswanathan K, Tharakaraman K, Dancik V, Raman R, Babcock GJ, Shriver Z, Sasisekharan R. 2016. A structural and mathematical modeling analysis of the likelihood of antibody-dependent enhancement in influenza. Trends Microbiol 24:933–943. doi: 10.1016/j.tim.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauger PC, Vincent AL, Loving CL, Lager KM, Janke BH, Kehrli ME Jr, Roth JA. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–2719. doi: 10.1016/j.vaccine.2011.01.082. [DOI] [PubMed] [Google Scholar]
- 13.Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. 2008. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet Microbiol 126:310–323. doi: 10.1016/j.vetmic.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. 2013. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 5:200ra114. doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 15.McKimm-Breschkin JL, Fry AM. 2016. Meeting report: 4th ISIRV antiviral group conference: novel antiviral therapies for influenza and other respiratory viruses. Antiviral Res 129:21–38. doi: 10.1016/j.antiviral.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparrow E, Friede M, Sheikh M, Torvaldsen S. 2017. Therapeutic antibodies for infectious diseases. Bull World Health Organ 95:235–237. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng R, Lee AP, Maia M, Lim JJ, Burgess T, Horn P, Derby MA, Newton E, Tavel JA, Hanley WD. 2018. Pharmacokinetics of MHAA4549A, an anti-influenza A monoclonal antibody, in healthy subjects challenged with influenza A virus in a phase IIa randomized trial. Clin Pharmacokinet 57:367–377. doi: 10.1007/s40262-017-0564-y. [DOI] [PubMed] [Google Scholar]
- 18.Sloan S, Babcock GJ, Szretter K, Bedard SS, Hay C, Williams J, Hershberger E, Trevejo J. 2016. Evaluation of efficacy and emergence of resistance to VIS410, a human monoclonal antibody, in a human challenge model of infection with a p2009 H1N1 virus, abstr International Society for Influenza and Other Respiratory Virus Diseases, Options IX for the Control of Influenza, Chicago, IL. [Google Scholar]
- 19.Balasingam S, Wilder-Smith A. 2016. Randomized controlled trials for influenza drugs and vaccines: a review of controlled human infection studies. Int J Infect Dis 49:18–29. doi: 10.1016/j.ijid.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 283:1016–1024. [DOI] [PubMed] [Google Scholar]
- 21.International Council on Harmonisation. 1996. Guideline for good clinical practice, E6(R1). ICH, Geneva, Switzerland: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. [Google Scholar]
- 22.Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, Bohnen AM, Hirst HM, Keene O, Wightman K, GG167 Influenza Study Group . 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med 337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.