Abstract
Background:
Total knee arthroplasty (TKA) provides good clinical outcomes for the treatment of end-stage osteoarthritis; however, discharge destination after TKA has major implications on postoperative adverse outcomes and readmissions. With the initiation of Bundled Payments for Care Improvement (BPCI), it is unclear how racial disparities in discharge destination after TKA will be affected by the new bundled payment for TKA.
Methods:
Bundled Payments for Care Improvement was implemented in July 01, 2014, at the University of Pennsylvania. We compared differences during early implementation (July 1, 2014, to, March 30, 2016) and during late policy implementation (April 1, 2016, to February 28, 2017) in patient characteristics (including race: African American [AA], white, and other race), discharge destination (skilled nursing facility [SNF], inpatient rehabilitation facility, home, home with home health, or other), and outcomes.
Results:
We identified 2276 patients who underwent TKA (43.8% AA, 48.2% white, and 8.0% other race). African American patients were more likely to be discharged to SNF as opposed to home than white patients both during the early BPCI (AA: 53.0%, n = 320; white: 32.4%, n = 210, P < .05) and late BPCI implementation (AA: 44.4%, n = 169, white: 26.9%, n = 120, P < .05), though all races showed trends to decreasing SNF use during the late BPCI implementation.
Discussion:
There were no significant differences in readmissions, length of stay, mortality, or intensive care unit days during early and late implementation of BPCI or when AA patients were compared to white patients.
Conclusion:
We found no significant changes in racial variations in discharge destination and outcomes after elective TKA. Bundled Payments for Care Improvement has encouraged better preoperative preparation of patients and discharge planning; however, payment reforms alone might not be sufficient to address variation in post-op management following elective surgery.
Keywords: payment reform, primary knee arthroplasty, racial disparity, discharge destination, readmission
Introduction
Total knee arthroplasty (TKA) is one of the most common elective inpatient surgeries for Medicare beneficiaries and requires lengthy recovery and rehabilitation periods.1 Almost 50% of patients who undergo total joint replacement in the United States are institutionalized for postoperative care and rehabilitation. Furthermore, postoperative care and rehabilitation after surgery accounted for a significant portion of the overall cost of care per episode.2-4 Nearly 36% to 55% of total joint arthroplasty (TJA) costs come from postacute care, with the highest costs in patients requiring acute inpatient rehab and skilled nursing facilities.5,6 In addition, postoperative discharge to a skilled nursing facility (SNF) after TJA is associated with worse outcomes including increased readmissions and higher rates of postdischarge complications.7
This is one of the reason why the Centers for Medicare and Medicaid Services (CMS), the largest payer of TJA,8 has for decades explored strategies to contain the cost of joint replacement. These strategies include the creation of the inpatient prospective payment system, the Acute Care Episode, Medicare Bundled Payment for Care Improvement (BPCI) initiative.3 The BPCI holds hospitals accountable for Medicare costs related to lower extremity joint replacement (total hip arthroplasty [THA] and TKA) for 90 days after patients’ hospital discharge.
Although the BPCI model was intended to improve collaboration with hospitals and postacute care facilities and improve quality metrics for TKA and THA, it unfolds in the setting of a well-documented disparity in health care. Although African American (AA) patients have similar clinical indications for joint replacement (arthritis-related activity, work limitations, and severe pain) and similar postoperative TJA complication rates as compared to white patients,9 they utilize elective joint replacement much less than white patients (41.5 per 10 000 for black patients vs 68.8 per 10 000 for white patients; P < .001).4 Although the reasons for this disparity are complex and involve patient-, clinician-, and system-level factors, what is becoming clear is that AA patients are more likely than white patients to be institutionalized (SNF/inpatient rehabilitation facility [IRF]) for post-op care and rehab following total joint replacement surgery.10 Furthermore, discharge to SNF or IRF is associated with significantly higher rate of postdischarge severe adverse events (nonhome: 3.0%, home: 1.7%), minor adverse events (nonhome: 1.9%, home: 1.1%), unplanned readmission (nonhome: 5.0%, home: 2.8%), and infectious complications (nonhome: 1.3%, home: 0.9%).7
There is relatively little research examining how new CMS policies such as BPCI model impact racial variations in discharge destination following joint replacement surgery. Using a sample of patients who received TKA at one large academic urban hospital in Philadelphia, Pennsylvania, we examined early and late policy trends in discharge destination for postacute care and rehabilitation comparing AA and white patients.
Methods
Study Sample
Our sample consisted of all patients who underwent primary TKA at the University of *** between 2014 and 2017. The Vizient database (formerly University HealthSystem Consortium [UHC], Oak Park, Illinois) was used to identify patients who underwent primary TKA from 2014 to 2017 at the University of Pennsylvania Penn Presbyterian Hospital Philadelphia, Pennsylvania. Total knee arthroplasty was identified using the common procedural terminology code corresponding to primary elective TKA (27447). Vizient hosts the Clinical Data Base/Resource Manager and provides line-item transactional details in the context of patient outcomes including mortality, length of stay, readmission rates, and hospital acquired conditions; and quality, safety, and resource utilization.11 Vizient also provided patient demographics such as age, sex, primary insurance carrier, and patient medical comorbidities including diabetes, heart failure, and renal failure. Multiple studies have utilized Vizient (formerly UHC) for quantitative research studies.12-15
We excluded patients with emergent surgery, those with acute fracture or active infection, and those who underwent bilateral TKA.
Study Measures
The primary outcome of interest for this analysis was discharge destination following TKA. Discharge options were categorized into IRF, SNF, home, or home with home health. Secondary outcomes of interest were length of stay, mortality, and readmissions.
The primary predictor of interest was patient race/ethnicity. Race/ethnicity was self-reported by the patient. The options were black, white, or other. Other race/ethnicity included Asian, Hispanic, and unknown, which were not reported on the tables to improve readability (n = 195). The secondary predictor of interest was early policy BPCI implementation versus late policy BPCI implementation on disposition and patient race/ethnicity.
The BPCI for primary TKA started in July 1, 2014. We defined “early policy implementation” as July 1, 2014, to March 30, 2016, and “late policy implementation” as April 1, 2016, to February 28, 2017. Length of stay, number of intensive care unit (ICU) days, direct costs, and readmissions were also looked at among races and before and after bundle. This study was approved by the University of Pennsylvania institutional review board.
Statistical Analysis
Paired t tests were used to compare differences in disposition among different races with referent group being white. P < .05 was defined as being statistically significant. Early and late BPCI implementation trends were compared with paired t tests. Linear regression analysis was utilized to plot trends in discharge destination for figures. We used Microsoft Excel (Redmond, Washington) for all calculations.
Results
We identified 2276 patients who underwent TKA at the University of Pennsylvania Penn Presbyterian Hospital between 2014 and 2017 (Table 1). A total of 43.8% were AA, 48.2% white, and 8.0% other. For simplicity, the 195 patients of other race were omitted from the table. Of the AA and white patients, over 50% of patients were female. One thousand two hundred fifty-three AA and white patients had surgery during the early BPCI period and 828 AA and white patients had surgery during the late BPCI period.
Table 1.
Sample Demographic and Clinical Characteristics Divided Into Early BPCI and Late BPCI Broken Down by Race.
| White: Early BPCI (n = 649) | AA: Early BPCI (n = 604) | White: Late BPCI (n = 447) | AA: Late BPCI (n = 381) | |
|---|---|---|---|---|
| Demographic | ||||
| Female | 500 | 356 | 343 | 229 |
| Insurance | ||||
| Medicare | 276 | 213 | 190 | 134 |
| Medicaid | 28 | 124 | 19 | 78 |
| Private | 188 | 196 | 130 | 123 |
| HMO | 136 | 60 | 93 | 38 |
| Other | 21 | 10 | 14 | 6 |
| Mean age (SD) | 64.5 (9.7) | 61.5 (9.9)b | 64.3 (9.1) | 62.5 (10.1)b |
| Clinical characteristics | ||||
| DM | 38 (5.9%) | 204 (33.8%)b | 85 (19.0%)a | 116 (19.0%)a |
| CHF | 22 (3.4%) | 66 (10.9%)b | 20 (4.5%) | 22 (5.8%)a |
| Renal Failure | 58 (8.9%) | 77 (12.7%)b | 34 (7.6%) | 48 (12.6%)b |
Abbreviations: AA, African American; BPCI, Bundled Payment for Care Improvement; CHF, congestive heart failure; DM, diabetes mellitus; SD, standard deviation; HMO, Health Maintenance Organization.
a P < .05 compared to same race before bundle.
b P < .05 compared to white.
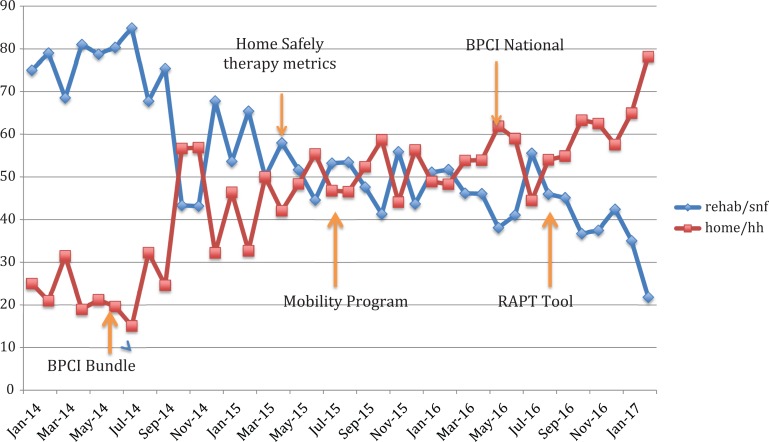
As shown in Figure 1, there was a statistically significant decrease in discharge to SNF—75% in January 2014 to 20% in January 2017 (P < .05). There was a corresponding increase in discharge to home with or without home health—25% in January 2014 to 80% in January 2017.
Figure 1.
Trends in discharge destination comparing home (home with/without home care) and institutionalization (acute rehab/skilled nursing facility [SNF]).
Differences Between AA and White Patients During Both Early and Late BPCI Periods
African American patients who underwent TKA were significantly younger than other races (average ages of AA, white, and other were 61.9, 64.4, and 64.0, respectively, P < .05). African American and other race TKA recipients were more likely to have Medicaid (22.4%), compared to white (4.3%, P < .05). African American TKA patients tended to have more diabetes mellitus (DM), congestive heart failure (CHF), and renal failure than white patients.
African American patients had significantly higher discharge to SNF than whites and others (49.5%, 30.1%, 34.6%, respectively, P < .05). Compared to AA patients, white patients and those of other race/ethnicity were more likely to be discharged to home with/without home health services (59.7% and 58.7% vs 42.2%, P < .05). However, we found no significant differences by race in the rates of discharge to IRF.
We found that 90-day readmissions rates were 5.5%, 3.4%, and 3.3% for AA, white, and other race/ethnicity patients, respectively. The cause of readmission was medical in 72.7% of AA patients and 83.3% of other race/ethnicity patients. In contrast, 43.2% of the readmitted white patients were secondary to medical causes. Surgical causes of readmission were significantly higher in white patients (45.9%) compared to AA patients (21.8%) or other (16.7%), P < .05.
There was no difference in mortality among white, AA, and other race. The average length of stay for white patients was significantly lower than for AA and other race/ethnicity recipients (2.5, 3.0, and 2.9, respectively; P < .05). There was no difference in the number of ICU days among white, AA, and other race.
Effects of Early Versus Late BPCI
Table 2 summarizes the postdischarge disposition for patients with TKA during early and late implementation of BPCI broken down by race. Unadjusted analysis found that all races (white, AA, and other) had significant decreases in discharge to SNF and acute rehab with concomitant increased use of home health services during late implementation of BPCI. African American patients continued to utilize SNF more so than other races both before and after the BPCI. Thus, there was no change in discharge destination patterns with early and late BPCI implementation.
Table 2.
Comparison of Outcomes During Early BPCI and Late BPCI Broken Down by Race.
| White: Early BPCI (n = 649) | AA: Early BPCI (n = 604) | White: Late BPCI (n = 447) | AA: Late BPCI (n = 381) | |
|---|---|---|---|---|
| Disposition IRF | 79 (12.2%) | 54 (8.9%) | 32 (7.2%)a | 24 (6.3%) |
| Disposition SNF | 210 (32.4%) | 320 (53.0%)b | 120 (26.9%)a | 169 (44.4%)a,b |
| Disposition HH | 322 (49.6%) | 219 (36.3%)b | 268 (60.0%)a | 181 (47.5%)a,b |
| Disposition home | 38 (5.9%) | 10 (1.7%)b | 27 (6.0%)a | 7 (1.8%)b |
| Readmissions, n (%) | 28 (2.6%) | 38 (3.8%) | 7 (1.7%) | 17 (4.5%)b |
| LOS (days) | 2.9 | 2.9 | 2.6 | 3.0 |
| Mortality | 0 | 1 (0.10%) | 0 | 0 |
| ICU days | 1.6 | 3.7 | 1.2 | 1.4 |
Abbreviations: AA, African American; BPCI, Bundled Payment for Care Improvement; HH, home health; ICU days, total number of days in intensive care unit per patient; IRF, inpatient rehabilitation facility; LOS, length of stay; SNF, skilled nursing facility.
a P < .05 compared to same race before bundle.
b P < .05 compared to white.
With BPCI, there was no change in the percentage of black or other races undergoing TKA. However, there were fewer AA patients with DM or CHF during late implementation of BPCI than early BPCI. There was an increase in DM in white patients during the late implementation of BPCI compared to early BPCI (see Table 1).
Discussion
In this sample of patients who underwent TKA in a large inner city hospital, we found that AA patients had the highest percentage of discharge to postacute facilities during both early and late BPCI implementation. This has important implications because discharge to postacute facilities is associated with higher cost and increased complications. Decreasing utilization to both SNF and IRF will result in fewer readmissions, which is an essential part of the BPCI. In addition, BPCI encourages improvements in preoperative care including optimization of medical conditions and preparation for discharge arrangements prior to surgery to improve overall outcomes.
These findings are in accordance to previous studies. Cram et al found that with National Surgical Quality Improvement Program (NSQIP) database, AA patients utilized SNF more frequently than other races.9 Similarly, patients of nonblack race/ethnicity were more likely to be discharged home (white odds ratio [OR]: 0.84, 95% confidence interval [CI]: 0.72-0.98, P 1/4 = .027; other OR: 0.80, 95% CI: 0.67-0.95, P 1/4 = .009).16 This suggests that the decision to go to SNF rather than home is complex and may involve patient-centered factors such as assistance available at home on discharge, patient values concerning rehabilitation, and home environment (eg, number of steps in the home, presence of first floor bathroom, etc).
Keswani et al found that SNF and IRF patients were more likely to have postdischarge severe adverse events (SNF: OR: 1.46, P .001; IRF: OR: 1.59, P = .001) and unplanned readmission (SNF: OR: 1.42, P = .001; IRF: OR: 1.38, P = .001).7 Severe adverse events included death, myocardial infarction, cerebrovascular accident, renal failure, pulmonary embolism, venous thromboembolism, sepsis, septic shock, unplanned intubation, peripheral nerve injury, deep wound infection, organ/space infection, and return to operating room. Minor adverse events included superficial wound infection, urinary tract infection, and pneumonia. Infectious complications including deep wound infection, superficial wound infection, organ/space infection, sepsis, or septic shock were also compiled for separate analysis.7 After stratifying patients by strongest independent risk factors (OR: 1.15, P < .05) for adverse outcomes after discharge, they found that home discharge is the optimal strategy for minimizing rate of severe 30-day adverse events after discharge (P < .05 for 5 of 6 risk levels) and unplanned 30-day readmissions (P < .05 for 6 of 7 risk levels).15
In a case–control data, patients exposed to the rapid recovery protocol had 45% increased odds of being discharged home compared to patients not exposed to the protocol5 Iorio et al recently reported on their decreased length of stay and increased discharge to home resulting in an overall cost savings with BPCI at a large high-volume academic center of 721 patients undergoing unilateral primary TKA and THA by utilizing changes in care coordination, clinical care pathways, and evidence-based protocols.17 However, joint replacement surgeons and their representative advocacy groups have expressed reservations about their implementation, including perceived disincentives to care for high-risk patients and uncertainties around gain sharing 18
We suspect that our decrease in SNF disposition was a concerted effort to improve postoperative outcomes with comprehensive multidisciplinary efforts before, during, and after surgery. A number of interventions occurred during this time contributed to the decrease in discharge to SNF including BPCI bundle, postoperative mobility program, definition of Home Safely metrics, and preoperative screening of Risk Assessment and Prediction Tool (RAPT) scores.
Our decreases in readmissions may be the result of optimizing patients with comorbidities before surgery, close monitoring patients with comorbidities perioperatively with medical consultation, and ensuring rigorous follow-up postoperatively of high-risk patients with both medical and surgical follow-up. Also, we have a team-based approach to encourage patients to discharge home rather than to SNF or IRF when possible including social work, home health services, and innovative programs such as wearable technology and Registered Nurse case manager to touch base with high-risk patients on discharge.
There are several limitations to consider in interpreting our findings. First, our data come from a single hospital. Therefore, the findings may not be generalizable to other hospitals or other parts of the country. Second, we did not conduct any modeling to adjust for confounding in comparing findings on study outcomes between the groups. It is conceivable all our observations can be accounted for by confounding9 and both race and comorbidity are likely playing a role in readmissions and disposition. African American patients tended to have higher percentage of DM, CHF, and renal failure. African American patients were also more likely to be insured by Medicaid, which is tied to lower socioeconomic status. As supported by other studies,9,10,16 we found similar findings of AA patients more likely to be discharged to nonhome destinations. Third, our study is a retrospective study. A prospective study would be better able to statistically analyze the drivers of the delta between the different race populations in this hospital and outcomes. Fourth, surgeon bias may have played a role in discharge disposition; however, bias is uncontrollable and cannot be measured. Finally, prebundle implementation data were not available (prior to July 1, 2014). It is conceivable that there may have been changes in disposition among AA and white patients pre-BPCI compared to late implementation of BPCI; however, the effects of BPCI on patient selection and outcomes were not abrupt and required time to implement. Therefore, we had chosen a wide time frame of early BPCI data to account for this. As described, implementation of programs in response to BPCI such as Home Safely Initiative, Mobility Program, and RAPT tool did not start until March 2015.
Conclusions
These limitations notwithstanding, we found that AA patients had the highest percentage of discharge to postacute facilities both during early and late implementation of BPCI. However, all groups showed significant decrease in discharge to SNF/IRF for post-op care and rehab since the introduction of the BPCI. Bundled Payments for Care Improvement has resulted in aggressive preoperative optimization of comorbidities and a team-based approach to encourage patients to discharge home after surgery. The decrease in SNF/IRF utilization and perioperative care of the patient likely contributed to the lower 90-day readmission rates to acute care hospital for all groups including AA patients during the time period after BPCI implementation. More research will be needed to determine whether these trends are applicable across institutions and over longer period of time.
Acknowledgments
The authors thank Finnah Pio for assistance with REDCAP and the Vizient databases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Ibrahim is supported in part by a K24 Mid-Career Development Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24AR055259). The views expressed in this editorial are those of the author and do not represent the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
References
- 1. Press MJ, Rajkumar R, Conway PH. Medicare’s new bundled payments design, strategy, and evolution. JAMA. 2016;315(2):131–132. [DOI] [PubMed] [Google Scholar]
- 2. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: general information. 2018. http://innovation.cms.gov/initiatives/bundled-payments/. Accessed September 20, 2018.
- 4. Ibrahim SA, Kim H, McConnell KJ. The CMS comprehensive care model and racial disparity in joint replacement. JAMA. 2016;316(12):1258–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. London DA, Vilensky S, O’Rourke C, Schill M, Woicehovich L, Froimson MI. Discharge disposition after joint replacement and the potential for cost savings: effect of hospital policies and surgeons. J Arthroplasty. 2016;31(4):743–748. [DOI] [PubMed] [Google Scholar]
- 6. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705–2709. [DOI] [PubMed] [Google Scholar]
- 7. Keswani A, Tsai MC, Fields A, Lovy AJ, Moucha CS, Bozic KJ. Discharge destination after total joint arthroplasty: an analysis of postdischarge outcomes, placement risk factors, and recent trends. J Arthroplasty. 2016;31( 6 ):1155–1162. [DOI] [PubMed] [Google Scholar]
- 8. HCPUnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. http://hcupnet.ahrq.gov. [PubMed]
- 9. Cram P, Hawker G, Matelski J, et al. Disparities in knee and hip arthroplasty outcomes: an observational analysis of the ACS-NSQIP clinical registry. J Racial Ethn Health Disparities. 2018:5(1):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jorgenson ES, Richardson DM, Thomasson AM, Nelson C, Ibrahim SA. Race, rehabilitation, and 30-day readmission after elective total knee arthroplasty. Geriatr Orthop Surg Rehabil. 2015;6(4):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubenfire A. VHA-UHC alliance to be called Vizient Modern Healthcare. 2015. http://www.modernhealthcare.com/article/20151119/NEWS/151119860. Accessed September 20, 2018.
- 12. Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the University HealthSystem Consortium. Crit Care Med. 2015;43(9):1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marino M, Spencer H, Hohmann S, Bodenner D, Stack BC., Jr Costs of outpatient thyroid surgery from the University Health System Consortium (UHC) database. Otolaryngol Head Neck Surg. 2014;150(5):762–769. [DOI] [PubMed] [Google Scholar]
- 14. Rutledge JW, Spencer H, Moreno MA. Predictors for perioperative outcomes following total laryngectomy: a University HealthSystem Consortium Discharge Database Study. Otolaryngol Head Neck Surg. 2014;151(1):81–86. [DOI] [PubMed] [Google Scholar]
- 15. Fang M, Noiseux N, Linson E, Cram P. The effect of advancing age on total joint replacement outcomes. Geriatr Orthop Surg Rehabil. 2015;6(3):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inneh IA, Clair AJ, SLover JD, Iorio R. Disparities in discharge destination after lower extremity joint arthroplasty: analysis of 7924 patients in an urban setting. J Arthroplasty. 2016;31(12):2700–2704. [DOI] [PubMed] [Google Scholar]
- 17. Iorio R, Clair AJ, Inneh IA, Slover JD, Bosco JA, Zuckerman JD. Early results of Medicare’s Bundled Payment Initiative for a 90-day total joint arthroplasty episode of care. J Arthroplasty. 2016;31(2):343–350. [DOI] [PubMed] [Google Scholar]
- 18. Kamath AF, Courtney PM, Bozic KJ, Mehta S, Parsley BS, Froimson MI. Bundled payment in total joint care: survey of AAHKS membership attitudes and experience with alternative payment models. J Arthroplasty. 2015;30(12):2045–2056. [DOI] [PubMed] [Google Scholar]