Abstract
Allergies to various environmental factors, in particular mite species, represent a considerable healthcare burden with lost productivity resulting from symptoms including asthma, conjunctivitis, rhinitis, and atopic dermatitis. The complexity of mite species and the allergens that they produce complicates diagnosis and treatment; however, the advent of recombinant DNA technologies now allows clinicians to better pinpoint the specific sensitizing agents and creates new opportunities for avoidance or immunotherapy. Here we discuss the advantages and disadvantages of traditional and novel diagnostic and therapeutic platforms, with particular consideration given to multiplex tests able to generate patient-specific allergen profiles. Immunotherapies tailored to such profiles may be safer and more effective than generalized treatments, but many hurdles, including the costs associated with identifying the protein or protein combinations able to exert the safest and most beneficial effects, must be overcome before such therapies can be globally applied.
Keywords: allergy, diagnosis, domestic mite, immunotherapy
Introduction
Domestic mites are diverse, omnipresent arthropods that cause allergy in more than 10% of the general population, and 90% of individuals suffering from allergic asthma are sensitive to domestic mites. Current mainstay treatments for mite allergies—allergen avoidance and pharmacotherapy—are costly and, while effective, do not alter the course of disease and thus require continued therapy.1Allergen-specific immunotherapy (ASIT) may be better than mainstay treatments for changing the disease course. However, ASIT with mite extracts, which contain a mixture of allergens, non-allergens, and other proteins, are difficult to standardize and can cause severe side effects like anaphylaxis. A promising new ASIT methodology based on precision diagnosis and treatment of patients allergic to domestic mites may mitigate these deficiencies. Here, we review the causes of allergy to mites as affected by species diversity and allergen complexity, current methods for diagnosing these allergies, and the promise of precision diagnosis. We also discuss how traditional treatments may be improved with these new diagnostic tools.
Diversity in mite allergens
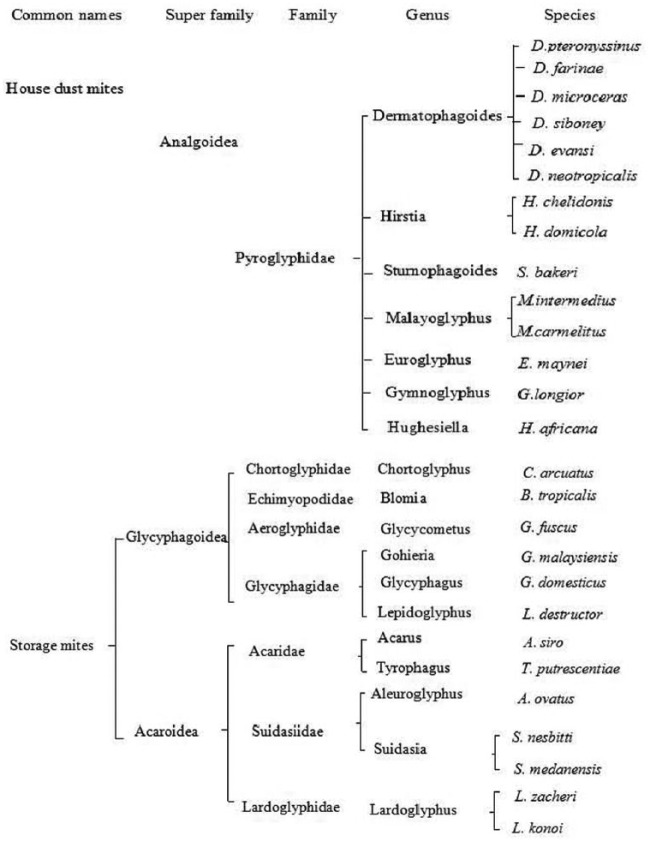
The general label “domestic mites” refers to the many mites frequently causing the development of immunoglobulin E (IgE) antibody responses and encompasses two broad categories: house dust mites (HDMs) and storage mites. Domestic mites include all indoor mites that belong to the subphylum Chelicerata, class Arachnida, subclass Acari, superorder Acariformes, and order Astigmata. HDM, belonging to the superfamily Analgoidea, family Pyroglyphidae (Figure 1), are present in human dwellings worldwide (Figure 1).2 The HDMs, especially Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df), and Euroglyphus maynei (Em) are the most common causes of human allergy. Storage mites, also known as flour mites, grain mites, and forage mites, belong to the families Acaridae and Glycyphagidae (Figure 1) and are commonly found in house dust, where they thrive in damp and humid conditions.2 The distribution of dust mite species in a geographical region is affected by the local climate, and different types of dust mites therefore dominate in different parts of the world (Table 1).
Figure 1.
The taxonomy of domestic mites and common species.
Table 1.
House dust mite allergens.
| Groups | Biochemical function | MWa (SDS-PAGE, kDa) | Speciesb | IgE bindingc (%) |
|---|---|---|---|---|
| 1 | Cysteine protease | 24, 25, 27, 39 | Bt, Df, Dp, Dm, Em | 33–87 |
| 2 | NPC2 family | 15 | Bt, Df, Dp, Em, Gy, Ld, Tp | 46–75 |
| 3 | Trypsin | 29 | Bt, Df, Dp, Em, Ah, Tp | 16–97 |
| 4 | Alpha-amylase | 57.9 | Bt, Df, Dp, Em, | 10–46 |
| 5 | Unknown | 14 | Bt, Dp, Ld | 20 |
| 6 | Chymotrypsin | 25 | Bt, Df, Dp | 8–41 |
| 7 | Unknown | 14, 23, 26, 30, 31 | Dp, Df, Ld | 37–53 |
| 8 | Glutathione S-transferase | 27, 32, | Bt, Df, Dp | 25–41 |
| 9 | Collagenolytic serine protease | 29 | Dp, Gd, Ld | 92 |
| 10 | Tropomyosin | 33, 36, 37, 42 | Bt, Ca, Df, Dp, Ld, Tp | 5.6–80.6 |
| 11 | Paramyosin | 98, 103, 110 | Bt, Df, Dp | 41.7–75 |
| 12 | Unknown | 14 | Bt | 50 |
| 13 | Fatty acid-binding protein | 15 | Bt, As, Df, Ld, Tp | 23 |
| 14 | Apolipophorin | 177 | Df, Dp, Em, Ld | 65.8–84.2 |
| 15 | Chitinase | 98/109 | Df | 41 |
| 16 | Gelsolin/villin | 53 | Df, Dp, Em, | 47 |
| 17 | Calcium binding protein | 53 | Df | 53 |
| 18 | Chitin-binding protein | 60 | Df | 54 |
| 19 | Anti-microbial peptide homologue | 7 | Bt | 10 |
| 20 | Arginine kinase | 40 | Df, Dp | 50 |
| 21 | 13/14 | Bt, Df, Dp | 50 | |
| 22 | 13 | Df, Dp | / | |
| 23 | Peritrophin-like protein domain (PF01607) | 14 | Dp | 74 |
| 24 | Ubiquinol–cytochrome c reductase binding protein homologue | 13 | Df, Dp | 29.4 |
| 25 | Triosephosphate isomerase | 34 | Df | 34.6 |
| 26 | Myosin alkali light chain | 18 | Df | 68.2 |
| 27 | Serpin | 48 | Df | 85.3 |
| 28 | Heat shock protein | 70 | Df, Tp | 70% |
| 29 | Peptidyl-prolyl cis-trans isomerase (cyclophilin) | 16 | Df | 85.36 |
| 30 | Ferritin | 16 | Df | 63.41 |
| 31 | Cofilin | 15 | Df | 25 |
| 32 | Secreted inorganic pyrophosphatase | 35 | Df | 68.4 |
| 33 | Alpha-tubulin | 52 | Df | 25 |
| 34 | Enamine/imine deaminase | 16 | Df, Tp | 68.42 |
| 35 | Chitinase-like protein | 14.4 | Df, Tp | 51.43 |
| 36 | Peritrophin-like protein domain | 23 | Df, Dp, Tp | 50 |
Data derived from http://www.allergen.org/.
Molecular Weight (MW) calculated from cDNA (sodium dodecylsulphate-polyacrylamide gel electrophoresis [SDS-PAGE] of natural allergen, if different).
Allergen described for the species designated by initials: Blomia tropicalis, Acarus siro, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Chortoglyphus arcuatus, Dermatophagoides microceras, Euroglyphus maynei, Glycyphagus domesticus, Lepidoglyphus destructor, Tyrophagus putrescentiae.
Binding (% patients, variation due to patient selection).
Specific induction of IgE, as suggested by Caraballo,3 is not the only criteria for an allergen. It should also induce allergic reactivity and symptoms, and using IgE reactivity alone can confound diagnosis. Another cause of difficult diagnosis is allergen complexity. Currently, over 30 clinically relevant mite species have been identified. These diverse mites produce a wide variety of allergenic compounds. Studies worldwide have revealed that at least three of these species, Dp and Df of the Pyroglyphidae family and Blomia tropicalis (Bt) of the Echimyopodidae family, each generate over 20 IgE-binding components. These allergens are named using the first 3 letters of the genus, the first letter of the species, and the assigned number of the allergen group. Thus, the known allergens from the three predominant species are Der p 1 to Der p 36, Der f 1 to Der f 36, and Blo t 1 to Blo t 21 (http://www.allergen.org/).3 Numerous mite allergens have been purified, sequenced, and cloned.
Based on the frequency of patient sensitization and the amount of specific IgE, group 1 (Der p 1 and Der f 1) and group 2 (Der p 2 and Der f 2) proteins are considered as major allergens. Der p 1 and Der p 2 are reported to bind 50%–60% of total HDM-reactive IgEs in nearly all HDM-allergic subjects, with the summed Der p 1 and Der p 2 titers tightly correlating with binding to extracts.4 Der p 23 may represent an additional major allergen, with 74% of 347 European HDM-allergic patients exhibiting Der p 23-reactive IgE antibodies.5 Groups 4, 5, 7, and 21 allergens are considered as mid-tier allergens, with approximately 50% of patients expressing IgEs reactive to each protein. The mid-tier allergens bind individually and collectively in proportion to the major allergens and constitute over 30% of the total titer.4 Group 3, 6, 8, 9, 10, 11, 13, 15, 16, 17, 18, and 20 proteins are deemed minor allergens because of their low IgE binding.6 Despite the high prevalence of dust mite allergy, the majority of disease worldwide may be accounted for by a relatively small number of allergens.
Thomas and colleagues have reported genetic polymorphisms in the major HDM allergen Der p 1 in residues 19, 81, and 215 as well as sporadic changes in other residues. Der p 2 and Der f 2 show a similar frequency of variations with clusters of amino acid substitutions at species-specific locations without structural concordance. Half of the 48 analyzed sequences of Der p 1 differ, and there are frequent clusters of amino acid substitutions for Der p 2.7 In contrast, Der f 1 was found to have few amino acid sequence substitutions, but two-dimensional immunoblotting revealed the high heterogeneity of Der f 1, Der f 2, and Der f 3. Thus, sequence variation or changes in posttranslational processing within and among species complicates diagnosis.
Species diversity and allergen complexity are not the only factors that confound diagnosis. HDMs produce many proteins and macromolecules that might stimulate innate immunity. In addition, contaminating microbial compounds in dust mites may also play a critical role as adjuvant factors to trigger typical Th2-biased allergic responses. Group 1, 3, 6, and 9 proteases and group 2, 7, 13, and 14 lipid-binding proteins can amplify allergic response by direct cell activation or by facilitated transport of microbial lipid compound adjuvants, respectively. Even dust mite allergens with low IgE-binding activities might induce allergic pathogenesis by activating innate immune cells.8 In addition, mites, eggs, larval forms, and allergen-containing dust mite fecal pellets have all been detected in human lungs.
Together, species diversity, allergen complexity, and allergen polymorphism contribute to a vast number of agents that may cause HDM-induced allergy. Numerous methods have been devised to identify specific allergens.
Diagnosing allergies to HDMs: current technologies
Clinical history drives the diagnosis of allergy, but there are a large number of testing options for confirming the diagnosis and identifying causal allergens from mite extracts. These methods include in vivo assays such as skin prick test (SPT), patch, and basophil activation tests (BAT) and in vitro techniques such as radioallergosorbent tests (RAST), enzyme-linked immunosorbent assays (ELISA), microarrays, fluoroenzyme immunoassays, and the UniCAP assay system. In vivo assays have the disadvantages of potentially causing allergic reactions in patients. Disadvantages for in vitro assays include false positives in patients with a high total IgE level and false negatives in patients with high levels of IgG antibodies. In addition, studies of other species indicate that the quantity of allergen-specific IgE may not directly reflect the biologically relevant mast cell-fixed antibody and the severity of clinical reactions, although this remains unknown for mite species.9
SPT is a prevalent classical method for diagnosing allergy, but like all in vivo tests, it may elicit undesirable allergic responses in patients. SPT is relatively easy to perform and generally yields results in 20 min. It is a preferred first-line testing method for the evaluation of allergy because of high sensitivity, rapidity, and inexpensiveness; however, it can generate false-positive results from cross reactivity. SPT is not indicated in patients using anti-histamines or having dermatitis or severe eczema.
The atopy patch test (APT) detects T-cell-mediated reactions underlying allergic diseases, although there are mixed opinions regarding its use for aeroallergens.10 In patients with allergic rhinitis (AR), APT was frequently positive, especially when patients had a positive history for atopic dermatitis. Considering that APT often is the only positive test in patients with respiratory allergy, the test should be included in the diagnostic work-up of AR.
BAT, referred to as the “allergic reaction in a test tube,” is a blood-based test for allergens. The core of BAT testing is the quantitative detection of activation markers on the basophil surface, preferably in whole blood. In contrast to skin testing, BAT can be performed with patients undergoing treatment with anti-histamines. Another advantage is that BAT demonstrates functional responses because positive results simply occur after successful cross-linking of the high-affinity IgE receptor (FcεRІ) by allergens resulting in the activation of basophils. However, inconsistent results produced by different laboratories due to diverse in-house protocols and commercial kits are potential pitfall of BAT. Sturm et al.11 reported that basophil reactivity started to decline after 4 h and the percentage of activated basophils could be influenced by the cytometry system that was used.
RAST is an in vitro test of specific IgE levels. It is a sensitive radioimmunoassay that uses radiolabeled anti-IgE antibodies to detect serum IgEs bound to specific antigens immobilized on a solid substrate (e.g. paper disks). Although sensitive and specific, its use has been limited since the rise of even more sensitive fluoroenzyme immunoassays, such as ELISAs. ELISA can be used to measure either total or specific IgE levels. To measure total IgE, anti-human IgE is first fixed to a solid surface (e.g. a microtiter plate or plastic bead), which is then incubated with human serum. The bound serum IgE is then detected by incubation with an enzymatically modified anti-human IgE designed to produce either a colorimetric of fluorescent product. The assay for specific IgE binding is similar except that the solid surface is first coated with the antigen of interest instead of anti-human IgE. Modified versions have been developed to increase sensitivity and specificity. Also, considering that some IgEs can be cross-reactive, competitive ELISAs in which various soluble antigens are used to compete away IgE binding to the solid phase have been developed to ensure specificity. However, ELISA is not well suited for high-throughput analysis of multiple allergens.
SPT and sIgE assays are the most commonly used diagnostic tools for confirming sensitization. Generally, good agreement between SPT and sIgE blood assay has been identified for most aeroallergens. Nevertheless, some studies have revealed discordant results between SPT and sIgE assays. The discrepancies may be attributable to patient characteristics, the skin tester’s skill, quality and stability of the allergen extracts, the biological reagents used in the laboratory assay, and assay methods. Thus, it may be necessary to use both SPT and sIgE assays to identify allergic sensitization.
Diagnosing allergies to HDMs: precision medicine
Whole mite extracts are used in diagnostic and therapeutic products, but commercial standardized extracts against all mite species (see Figure 1) are not currently available. Phadia, one of the major producers of products to diagnose and treat allergies, offers extracts from the most prevalent species (4 of the 14 species of dust mites, and 5 of the 13 species of storage mites), but sensitization to rare and more geographically restricted species is still difficult to assess. Also, using whole extracts for diagnostic purposes has several disadvantages. First, crude mite extract can reveal that a patient is generally sensitized to mite allergens but not the specific allergenic components. Second, these extracts are difficult to standardize and may show considerable batch-to-batch variation, although both the European Union and the United States have issued regulatory requirements for allergen testing and recommend reference standards for commercial products. Third, the concentration of allergens found in mite extracts does not reflect concentrations found in the environment. Finally, in vivo use of crude extracts may also induce sensitization against additional allergenic proteins in the extract, although few studies have directly addressed this issue. New methods that allow for better identification of allergy causing components are a prerequisite for defining the specific causes of an individual’s allergic response and improving the treatment of HDM-induced allergy.
Component-resolved diagnosis (CRD) has been developed using allergen molecules derived from natural allergen sources or produced by recombinant expression of allergen-encoding cDNAs. For clinical purposes, the Phadia ImmunoCAP system is used in the majority of laboratories for quantitative measurements of IgE levels directed against individual purified antigens. This system is a solid phase immuno-sandwich assay in which excess antigen is bound to a cellulose matrix in the form of pellets. Sera from patients are applied to the pellets and antibodies directed against the given antigen are allowed to bind. Antibodies of specific subtypes (e.g. IgE and IgG) are subsequently detected by anti-immunoglobins coupled to an enzymatic tag. Automation of this system allows multiple antigen beads to be quickly tested, but its throughput does not compare to newer semi-quantitative technologies such as antigen microarrays.
For microarrays, multiple antigens are spotted onto a single slide and exposed to a small amount of patient serum. Bound IgEs are then detected by fluorescently tagged anti-IgEs. Commercially available microarrays like the ImmunoCAP immunosorbent allergen chip (ISAC) can now assess patient sensitivity to over 100 antigens to simultaneously interrogate and define a patient’s complete reactivity profile. Despite studies indicating that the ImmunoCAP ISAC may not be sufficiently sensitive particularly when IgE levels are low and the fact that the chips lacks some important allergens, this product was the first allergen microarray approved for diagnostic purposes in the European Union. Further development of the ISAC has led to the creation of the MeDALL chip, which can be used for the specific and sensitive monitoring of IgE and IgG reactivity profiles toward more than 170 allergen molecules. This chip has been tested using sera collected in European birth cohorts, but is not yet approved for diagnostic purposes.
For patients with confirmed allergies to HDMs, the use of CRD with HDM components has been tested for diagnosing mite-induced allergy. Using ImmunoCAP and ImmunoCAP ISAC with commercially available HDM proteins revealed Der p 1 and Der p 2 as relevant clinical markers for HDM-induced allergy.12 The results suggest that CRD-based tests using major mite allergens (e.g. Der p 1 and Der p 2) may help improve patient selection for immunotherapy.
The precision of such component-derived allergy diagnoses may eventually allow for targeted immunotherapy based on the sensitization profile, but significant challenges remain. Some concerns that have been reported include inconsistencies in diagnosis between different diagnostic methods, for example, there was a discrepancy between results from SPT and CAP for diagnosing allergic sensitization among inhalant allergens. In addition, the complex automated systems used to perform both the single-plex (ImmunoCAP) and multiplex (ImmunoCAP ISAC) assays are typically only available in hospitals or large medical centers, which means these tests, unlike SPT, cannot be easily performed at all primary points of care. Also, insurance companies do not routinely reimburse for allergen microarrays, the out of pocket cost is too high for many patients, and studies suggest that multiplex assays are not any more accurate (and in fact, may be less accurate) at diagnosing general allergies. Finally, it is poor practice to test patients for reactions to allergens not indicated based on their clinical symptoms. Therefore, allergen microarrays may best be used for research or as tertiary diagnostic tools for patients with known polysensitization. In this population, chips can help identify causal elements and help to rule out cross-reactive antigens. Despite these challenges, CRD may eventually give rise to new forms of specific immunotherapy based on recombinant allergen molecules.13
Traditional treatment of dust mite allergy
Traditional treatment options for dust mite allergy include medications focused on mitigating symptoms. However, these medications are not available to the cause of the symptoms and are therefore unable to cure the allergy. These drugs worse still, many side effects are emerged, such as drowsiness. Allergen immunotherapy with dust mite allergens is the only available treatment option shown to have a disease-modifying effect with sustained benefit. Allergen immunotherapy is used to gradually induce tolerance1 by modulating the immunologic mechanism.
The first generation of dust mite immunotherapy was developed using the subcutaneous injection of crude dust mite extracts. Subcutaneous immunotherapy (SCIT) can improve the symptoms associated with dust mite-induced AR.13 However, SCIT requires frequent office visits over a long period, which can be inconvenient to many patients and therefore reduces their compliance. In addition, one out of nine patients are estimated to experience injection-related systemic reactions and in some cases life-threatening anaphylaxis. SCIT with modified hypoallergenic extracts appears safer than using whole extracts and is widely used in Europe; however, this treatment option has not gained acceptance in the United States.
Sublingual immunotherapy (SLIT) is considered a simpler and safer alternative to SCIT. SLIT involves placing extracts or possibly purified allergens in either liquid or tablet formulations under a patient’s tongue. The first dose is administered in a clinical setting in case of an adverse systemic reaction. Following doses can be administered at home. The efficacy and safety of SLIT has been investigated in adult as well as pediatric patients. SLIT tablets have been proven effective in improving symptoms of AR and asthma, reducing the risk of asthma exacerbation, and reducing the need for pharmacotherapy. SLIT treatment appears to be generally well tolerated, with mild to moderate side effects (e.g. oral pruritus) and Odactra, a tablet formulation containing Df and Dp extracts, has recently been approved for the treatment of HDM-associated AR in the US (https://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm544326.htm). A comparison of studies using standard and modified mite allergens (e.g. antigen fragments or genetically modified proteins that induce a protective IgG response without eliciting IgE binding) suggests that SLIT like SCIT with modified mite allergens might be safer compared to standard allergens or whole extracts. Unfortunately, despite these encouraging results, compliance with SLIT protocols appears to be even worse than compliance with SCIT.
While studies have shown immunotherapy’s potential for treating dust mite-induced allergy, bringing it to clinical settings across the world remains challenging. Unlike pharmacotherapy, immunotherapy using standard allergens is unlikely to work for all populations. First, even though only a small number of HDM proteins account for most HDM-induced allergy, each allergen component induces antibody binding to a different level.6 Second, while Der p 1, 2, and 23 affect the majority of HDM-allergic patients in many countries, other proteins such as Der p 4, 5, 7, and 21 are potent sensitizers in specific geographic regions and some patients in these studies exhibited reactivity to these proteins in the absence of binding to Der p 1, 2, and 23.14 Third, varied IgE binding and reactivity to HDM allergens have also been reported in different populations. Finally, the polymorphisms of the main allergens necessitate that targeted immunotherapy will need to incorporate variants to be effectve.7 This underscores the need to develop multiple patient-specific treatments that can encompass the variety of different antigens responsible for HDM-induced allergies.
Precision treatment of dust mite allergy
Diagnostic and therapeutic tools based on a patient’s specific allergen reactivity profile will likely prove more effective than generic treatments. First reported in the early 1900s, allergen-specific immunotherapy (AIT) is now garnering increased attention because, like immunotherapy derived from crude extracts, it has the potential to produce long-lasting benefits. Historically, the poor quality of purified allergens used for AIT meant the technique had a high risk of serious side effects. Today, improved techniques, in particular recombinant DNA technology, can be used to develop AIT with defined composition as well as modified allergens with reduced allergic activity but retained or enhanced immunogenicity.
Based on recombinant allergens, AIT is likely to be more efficient and safer than immunotherapy using allergen extracts. In recent years, a number of clinical studies on both wild-type and hypoallergenic derivatives of recombinant allergen vaccines have reported encouraging results. A strategy based around using one or two specific allergens responsible for the patient’s symptoms has some potential advantages, including reduced allergic side effects, straightforward production, and effective administration.4 To improve the safety profile of AIT, modified HDM allergens have recently been developed, including hypoallergenic derivatives of the group 1 and group 2 allergens. Efforts have also been undertaken to develop vaccines using combinations of hypoallergenic group 1 and 2 proteins.
Treating the allergic symptoms for asthmatics creates an economic burden for families. A review of cost of care estimates country-specific expenditures of between US$495 and US$1993 per patient per year adjusted for inflation since 1998. The largest proportion of this cost is medication to treat general symptoms. Cost-benefit analysis indicates that immunotherapy may be more cost-effective than long-term treatment for asthma symptoms, particularly when considering the societal burden of lost productivity. Recombinant vaccines will likely offer the same long-term savings as traditional immunotherapies; however, there are hurdles to overcome before multiple recombinant HDM allergen products can reach the market. Approval requires large multi-center double-blind placebo-controlled trials for each individual component and where patients are polysensitized, possible combinations of components. For less common sensitizers (e.g. proteins other than major allergens Der p 1 and Der p 2 and possibly Der p 23), it may be difficult to recruit enough patients to perform such large-scale studies. In addition, suppliers may not recoup the cost of such studies when these therapies are targeted at limited populations.
Considering the many varying factors associated with mite allergy, customized treatment based on regional and even individual signatures may be an ideal way to improve treatment efficacy and possibly reduce overall cost. Moving toward precision medicine in mite allergy, however, will largely depend upon the success of precision diagnostics, the quality of vaccines, and regulatory approvals.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the National Natural Sciences Foundation of China (NSFC31272369, NSFC31572319), 333 Project of Jiangsu Province in 2017 (grant no. BRA2017216), Medical Innovation Team of Jiangsu Province (grant no. CXTDB 2017016), Major Program of Wuxi Health and Family Planning Commission (Z201606 and Z201701), and Primary Research & Development Plan of Jiangsu Province (Grant No.BE2018627).
Human and animal rights and informed consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
ORCID iD: Yubao Cui  https://orcid.org/0000-0001-7736-8012
https://orcid.org/0000-0001-7736-8012
References
- 1. Eifan AO, Calderon MA, Durham SR. (2013) Allergen immunotherapy for house dust mite: Clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opinion on Biological Therapy 13: 1543–1556. [DOI] [PubMed] [Google Scholar]
- 2. Cui Y. (2014) When mites attack: Domestic mites are not just allergens. Parasites & Vectors 7: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caraballo L. (2017) Mite allergens. Expert Review of Clinical Immunology 13: 297–299. [DOI] [PubMed] [Google Scholar]
- 4. Vrtala S, Huber H, Thomas WR. (2014) Recombinant house dust mite allergens. Methods 66: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weghofer M, Grote M, Resch Y, et al. (2013) Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. Journal of Immunology 190: 3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas WR. (2015) Hierarchy and molecular properties of house dust mite allergens. Allergology International 64: 304–311. [DOI] [PubMed] [Google Scholar]
- 7. Piboonpocanun S, Malainual N, Jirapongsananuruk O, et al. (2006) Genetic polymorphisms of major house dust mite allergens. Clinical and Experimental Allergy 36: 510–516. [DOI] [PubMed] [Google Scholar]
- 8. Jacquet A. (2011) The role of the house dust mite-induced innate immunity in development of allergic response. International Archives of Allergy and Immunology 155: 95–105. [DOI] [PubMed] [Google Scholar]
- 9. Cui Y. (2014) Immunoglobulin E-binding epitopes of mite allergens: From characterization to immunotherapy. Clinical Reviews in Allergy & Immunology 47(3): 344–353. [DOI] [PubMed] [Google Scholar]
- 10. Turjanmaa K, Darsow U, Niggemann B, et al. (2006) EAACI/GA2LEN position paper: Present status of the atopy patch test. Allergy 61(12): 1377–1384. [DOI] [PubMed] [Google Scholar]
- 11. Sturm GJ, Kranzelbinder B, Sturm EM, et al. (2009) The basophil activation test in the diagnosis of allergy: Technical issues and critical factors. Allergy 64: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Caldas E, Iraola V, Boquete M, et al. (2006) Mite immunotherapy. Current Allergy and Asthma Reports 6: 413–419. [DOI] [PubMed] [Google Scholar]
- 13. Kazemi-Shirazi L, Niederberger V, Linhart B, et al. (2002) Recombinant marker allergens: Diagnostic gatekeepers for the treatment of allergy. International Archives of Allergy and Immunology 127: 259–268. [DOI] [PubMed] [Google Scholar]
- 14. Thomas WR. (2018) IgE and T-cell responses to house dust mite allergen components. Molecular Immunology 100: 120–125. [DOI] [PubMed] [Google Scholar]