Abstract
Placental oxygenation varies throughout pregnancy. The detection of early changes in placental oxygenation as pregnancy progresses is important for early identification of preeclampsia or other complications. This invited commentary discusses a recent preclinical study on the application of 3-dimensional photoacoustic imaging (PAI) for assessment of regional variations in placental oxygenation and longitudinal analysis of differences in placental oxygenation throughout normal pregnancy and pregnancy associated with hypertension or placental insufficiency in mice. Three-dimensional PAI more accurately reflects oxygen saturation, hemoglobin concentrations, and changes in oxygen saturation in whole placenta compared to 2-dimensional imaging. These studies suggest that PAI is a sensitive tool to detect different levels of oxygen saturation in the placental and fetal vasculature in pathologic and normal pregnancy in mice.
Keywords: photoacoustic, placenta, hypoxia, oxygenation, preeclampsia, growth restriction
The placenta, a highly specialized organ, is an interface between the mother and the fetus that maintains appropriate fetal growth and development. It controls the exchange of oxygen and nutrients, and the removal of carbon dioxide and waste products between the maternal and fetal circulations. The placenta also protects the fetus against environmental factors and produces many hormones and cytokines necessary for pregnancy progression and fetal well-being. Placental development depends on oxygen levels and the mechanisms that control intrauterine oxygen concentrations.1 Oxygen levels are relatively low early in pregnancy to allow normal embryonic and placental development.2 During this time, extravillous cytotrophoblast cells differentiate and transform into an invasive phenotype to remodel the maternal uterine arterial tree. As a result, placental perfusion increases to supply sufficient oxygen to the growing placenta and the fetus. Later in pregnancy, oxygenation of the feto–maternal interface increases. However, abnormal vascular remodeling can lead to a series of changes in placental oxygenation, including persistent hypoxia and in some cases ischemia, with release of vasoactive and antiangiogenic factors into maternal circulation. These changes in uteroplacental circulation are thought to be associated with a subset of preeclamptic pregnancies. Therefore, detection of early changes in placental oxygenation throughout pregnancy may lead to the identification of pregnancies at risk for negative maternal and fetal outcomes, including preeclampsia and other hypertensive disorders of pregnancy.
Currently, Doppler ultrasound is widely used to assess uterine and umbilical blood flows in high-risk pregnancies. However, such measurements only indirectly relate to the development of the placental vascular network and oxygenation and the Doppler clinical value as a screening tool in intrauterine growth restriction or preeclampsia is controversial.3 Direct measurements of oxygen levels in the placenta (partial pressure of oxygen, pO2) are also possible using oxygen-sensing electrodes, but these invasive methods are not suitable for clinical use. Currently, no method can directly measure placental oxygenation in real time in the clinic. A few studies have demonstrated the utility of blood-oxygenation-level-dependent (BOLD) magnetic resonance imaging (MRI) for analysis of placental and fetal oxygenation. These early preclinical and clinical studies have shown the application of BOLD MRI for the detection of regional differences in placental oxygen saturation in response to changes in maternal oxygenation during singleton and monozygous twin human pregnancies,4-11
Recently, photoacoustic imaging (PAI) emerged as a promising noninvasive diagnostic modality to quantify placental and fetal oxygenation in real time. Photoacoustic imaging is based on the photoacoustic effect: a laser sends pulsed signals to the tissue; when absorbed light is converted into heat, thermal expansion of tissue occurs. The resultant sound waves are detected by ultrasound. Photoacoustic imaging can detect endogenous contrast such as hemoglobin (Hb). Differences in absorption spectra between oxygenated and deoxygenated Hb in the blood allow calculation of microvessel oxygen saturation in an area of imaged tissue. Use of PA tomography12 and ultrasound-guided PAI13 has been reported recently for analysis of fetal oxygenation in mice. The sensitivity of PAI was also demonstrated by changing levels of maternal fraction of inhaled oxygen (FiO2) from hyper-oxygenation (100%) to hypo-oxygenation (5%) at day 14 pregnant rats.14 However, whether differences exist in placental vascular oxygenation during pathologic pregnancy was not established.
In our article published in The FASEB Journal,15 we have extended these observations and demonstrated for the first time that 3-D PAI is a sensitive method for assessing regional variations in placental oxygenation in mice. Using a VevoLAZR device (FUJIFILM VisualSonics Inc, Toronto, Canada), we could directly analyze differences in placental oxygenation between normal pregnancy and pregnancy associated with hypertension or placental insufficiency in mice.
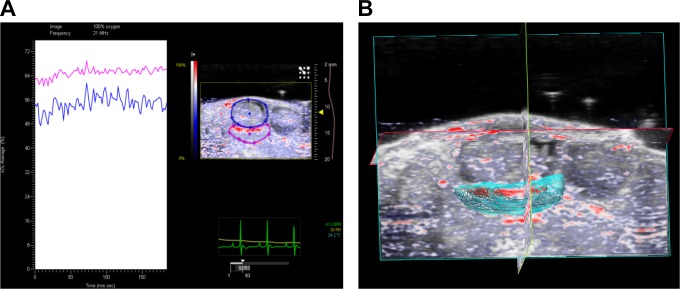
Photoacoustic imaging enables high-resolution 3-D imaging of tissue. Three-dimensional PAI more accurately reflects sO2 and Hb concentrations in whole tissue and therefore more completely represents changes in sO2 in the tissue vasculature compared to 2-dimensional (2-D) imaging. Three-dimensional images are created by acquiring a series of 2-D images and assembling them into a 3-D data set (Figure 1). The VevoLAZR software can also calculate 3-D volume, thus allowing the capture of differences in placental size between groups. Since rodents have multiple uteroplacental units, the 3-D model allows standardization of data from different placentas within 1 animal or between different animals within each study group. Thus, analysis of placental oxygenation accounts for the whole tissue volume; correlations between placental volume and oxygenation during normal and pathologic pregnancy can then be inferred. Three-dimensional imaging can be also used to record differences within regions of the rodent placenta, such as the labyrinth, decidual, and mesometrial triangle areas. Guided by the anatomical landmarks and histology, we have identified higher oxygenation of the placental labyrinth compared to the decidual and mesometrial triangle areas, as well as differences in lateral and central portions of the labyrinth during normal pregnancy.16 The ability to record regional changes in placental oxygenation is important for our understanding of the placental oxygenation status throughout pregnancy.
Figure 1.
Three-dimensional photoacoustic imaging of the placenta and the fetus (A) and the 3D rendering of the placenta (B) at day 14 of gestation in C57Bl/6 mouse. A, Representative photoacoustic spectra and the image of the uteroplacental unit with contours around the placenta (pink) and fetus (blue). B, Three-dimensional rendering of the placenta.
Changes in placental oxygenation can be identified in response to decreasing levels of maternal FiO2 ranging from hyperoxia (1) to normoxia (0.21) or hypoxia (0.05). When maternal oxygen was decreased from hyperoxia to normoxia, reciprocal changes in the fetal versus maternal portions of the placenta were recorded during normal mouse pregnancy using PAI.16 Although not tested in our study, placental insufficiency is potentially associated with a different capacity for oxygen transport and varied responses to changes in maternal oxygen. Clinical studies using BOLD MRI have demonstrated regional differences in human placental oxygenation when maternal oxygenation was changed from hyper-oxygenation to room air at normal gestation.6 These studies suggest that the placenta is sensitive to changes in maternal oxygen during gestation14 and that PAI can be used to analyze various states of placental oxygenation.6 Since vascular oxygenation depends on blood flow and vascularization, any abnormalities in these parameters may be signaled by regional differences in oxygenation. Thus, complementing PAI with contrast-enhanced ultrasound with microbubbles could provide more information about placental oxygenation in relation to its vascularization and perfusion.
Early signs of placental hypoperfusion and onset of tissue hypoxia can be detected by monitoring placental oxygen saturation throughout pregnancy. We demonstrated stable placental oxygenation during normal mid-late mouse pregnancy but lower oxygenation in the placenta during hypertensive pregnancy. We also demonstrated lower total sO2 levels in the placenta of angiotensin converting enzyme 2 knockout (ACE2 KO) mouse, a model of uteroplacental insufficiency, compared with wild-type C57Bl/6 mice. Lower total placental sO2 levels in both models were corroborated by higher placental expression of markers of hypoxia.16 Furthermore, lack of difference in sO2 between labyrinth and decidual plus mesometrial triangle region in ACE2 KO compared to wild-type mice suggests different patterns of oxygenation between normal mouse pregnancy and placental insufficiency.16 Our study also showed that total fetal sO2 was similar between the normal and hypertensive pregnancies. However, hypertensive pregnancy may be associated with regional differences in fetal tissue oxygenation.17,18 Thus, in addition to placental oxygenation, PAI provides valuable information about fetal regional oxygenation thus permitting noninvasive, real-time analysis of fetal well-being. These studies suggest that ultrasound-guided PAI is a sensitive noninvasive tool to detect real-time differences in placental and fetal oxygenation during pathological and normal pregnancy.
Yamaleyeva et al15 and others16 have validated the use of PAI (VevoLAZR) for assessments of blood oxygenation using an in vitro approach. The accuracy and sensitivity of the PAI in measuring oxygen saturation was studied intensively with phantom experiments, where direct measurement from blood gas analysis, served as the ground true values, and PAI measurements were compared. Most importantly, data obtained in the phantom studies showed a significant correlation in oxygen saturation between signals recorded by PAI and direct measurements from blood gas analysis, suggesting that PAI is an accurate and linear method to measure oxygen saturation in vitro.15 In our previous work, we have also shown that both CO-oximetry and PAI measured a similar stepwise decline in femoral artery sO2 as the FiO2 was lowered, suggesting that PAI can accurately measure real-time sO2 in the macrocirculation.19 Since the direct placental sO2 measurements are nearly impossible in the mouse, we focused our study on relative changes in placental oxygenation in relation to control group, measurements over time or in response to different maternal oxygen levels that gave us relevant information on physiological responses of placental microvascular sO2. However, additional validation of PAI sO2 measurements in the placenta in vivo is desirable. Perhaps comparing PAI to other techniques such as BOLD-MRI could further validate PAI measurements of placental sO2. This would improve the overall understanding of the accuracy of PAI in the placenta.
To begin assessing relative variability of placental sO2 measured by PAI, the coefficients of variation (COVs) were calculated for each of the experimental protocols (table 1, supplemental data, Faseb J 15). Our data showed that the majority of experiments had COVs less than 10%, which is similar to other methods measuring sO2 such as BOLD MRI or arterial spin labeling measurements. Future studies should address the repeatability and reproducibility over time, as well as inter-subject and inter-experimental variability of PAI of the placenta.
In the discussed study, PAI was performed using a commercial VEVO LAZR system with LZ250 probe, which has a central frequency of 21 MHz and spatial resolution around 75 μm. As such, the resolution of measuring oxygenation within regions of the rodent placenta is limited by 75 μm. However, the spatial resolution can be further improved by imaging probes with higher central frequency, for example, LZ550 which has a spatial resolution around 44 micrometers. Higher resolution of the LZ550 probe is associated with lower depth of signal penetration and smaller total area of imaging. Therefore, the selection of the probe will depend on the depth of the location of the placenta within the animal.
This preclinical study supports the concept of applying PAI for the analysis of placental oxygenation in clinic. However, clinical translation of this technology in the field of feto–maternal medicine is still in its early stages. One of the major limitations that hinder the use of PAI technology in clinical practice is shallow penetration of light within the tissue. The maximum depth of imaging in our study was 18 to 20 mm. However, in order to image human placenta, PAI signal needs to be able to penetrate not just the placenta but also the surrounding tissues at the overall depth of approximately 10 to 15 cm at the end of gestation. The thickness and localization of the placenta, and thickness of uterine and abdominal walls should be considered when overall depth of imaging is estimated. A PAI depth up to 7 cm from the surface of the skin has been reported20 suggesting that at early stages of pregnancy it may be possible to image the placenta using transabdominal approach particularly when the placenta is attached to the anterior uterine wall. Furthermore, this technology is capable of providing assessment of variations in placental oxygenation both spatially and temporally throughout pregnancy. As such, it can be a very promising preclinical tool in the studies of pathologic pregnancy with a potential for further translational/clinical application in the field. The limitation of the current imaging depth of PAI technology can be solved with the further advancement of PAI technology, such as optical focusing to minimize optical scattering in diffusive tissue, nanotechnology for high performance imaging contrast with greatly enhanced PAI signals, PAI endoscopy to image inside of the body or specific organ of interest, and so on. For example, the recently developed time-reversed ultrasonically encoded optical focusing technique21 is capable of delivering light into any dynamically defined location inside a scattering medium by encoding diffused coherent light with focused ultrasonic wave. Such optical focusing method may significantly improve the PAI imaging depth and break through the current gap between preclinical studies and clinic applications of PAI technology in placental imaging. Furthermore, although current devices use laser energy within the safety limits established by the American National Standards Institute,22 the safety of PAI of placenta and fetus needs further investigation.
The significance of the PAI application during pregnancy is that accurate measurements of placental oxygenation may reveal the origin of hypoxic stimuli. It may also provide evidence about the timing and role of early placental vascular abnormalities in the pathogenesis of hypertensive pregnancy disorders such as preeclampsia. Using near-infrared fluorescent probes specifically targeted to hypoxic cells or other hypoxia-responsive imaging agents such as nanoparticles targeted to hypoxia markers can improve the detection of hypoxia by PAI in preeclampsia and other pregnancy complications associated with placental hypoxia. Furthermore, placental oxygenation at later stages of pregnancy may be rescued by delivering oxygen to the placenta using artificial oxygen carriers.23 Nanoscale-sized artificial oxygen carriers such as Hb vesicles reduced placental hypoxia, decreased levels of antiangiogenic proteins, and improved fetal growth restriction in a rat model of preeclampsia.23 Thus, accurate detection of placental hypoxia with PAI may enable identification of pregnancies at risk for development of preeclampsia or placental insufficiency, and the ability to therapeutically target hypoxic regions within the placenta to improve overall maternal and fetal outcomes.
Acknowledgment
We acknowledge the editorial assistance of Karen Klein, MA, through the Wake Forest Clinical and Translational Science Institute (UL1TR001420; PI: McClain).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH R21 HD086357, American Heart Association 13SDG17390009.
ORCID iD: Liliya M. Yamaleyeva  http://orcid.org/0000-0001-7801-1269
http://orcid.org/0000-0001-7801-1269
References
- 1. Soares MJ, Iqbal K, Kozai K. Hypoxia and placental development. Birth Defects Res 2017;109(17):1309–1329. doi:10.1002/bdr2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 2000;21(suppl A):S25–S30. [DOI] [PubMed] [Google Scholar]
- 3. Bamfo JE, Odibo AO. Diagnosis and management of fetal growth restriction. J Pregnancy 2011;2011:640715 doi:10.1155/2011/640715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorensen A Peters D Frund E Lingman G, Christiansen O Uldbjerg N. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol 2013;42(3):310–314. doi:10.1002/uog.12395. [DOI] [PubMed] [Google Scholar]
- 5. Sorensen A, Peters D, Simonsen C, et al. Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Prenat Diagn 2013;33(2):141–145. doi:10.1002/pd.4025. [DOI] [PubMed] [Google Scholar]
- 6. Sorensen A, Sinding M, Peters DA, et al. Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol Rep 2015;3(10):e12582 doi:10.14814/phy2.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo J, Abaci Turk E, Bibbo C, et al. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep 2017;7(1):3713 doi:10.1038/s41598-017-03450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ginosar Y, Gielchinsky Y, Nachmansson N, et al. BOLD-MRI demonstrates acute placental and fetal organ hypoperfusion with fetal brain sparing during hypercapnia. Placenta. 2018;63:53–60. doi:10.1016/j.placenta.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 9. Huen I, Morris DM, Wright C, et al. R1 and R2* changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med. 2013;70(5):1427–1433. Article. doi:10.1002/mrm.24581. [DOI] [PubMed] [Google Scholar]
- 10. Schabel MC, Roberts VHJ, Lo JO, et al. Functional imaging of the non-human primate placenta with endogenous BOLD contrast. Magn Reson Med 2016;76(5):1551–1562. doi: 10.1002/mrm.26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siauve N, Chalouhi GE, Deloison B, et al. Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol. 2015;213(suppl 4):S103–S114. doi:10.1016/j.ajog.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 12. Laufer J, Norris F, Cleary J, et al. In vivo photoacoustic imaging of mouse embryos. J Biomed Opt. 2012;17(6):061220 doi:10.1117/1.JBO.17.6.061220. [DOI] [PubMed] [Google Scholar]
- 13. Bayer CL, Wlodarczyk BJ, Finnell RH, Emelianov SY. Ultrasound-guided spectral photoacoustic imaging of hemoglobin oxygenation during development. Biomed Opt Express. 2017;8(2):757–763. doi:10.1364/BOE.8.000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arthuis CJ, Novell A, Raes F, et al. Real-time monitoring of placental oxygenation during maternal hypoxia and hyperoxygenation using photoacoustic imaging. Plos One. 2017;12(1):e0169850 doi:10.1371/journal.pone.0169850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamaleyeva LM Sun Y Bledsoe T Hoke A,Gurley SB,Brosnihan KB. Photoacoustic imaging for in vivo quantification of placental oxygenation in mice. FASEB J. 2017;31(12):5520–5529. doi:10.1096/fj.201700047RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rich LJ, Seshadri M. Photoacoustic imaging of vascular hemodynamics: validation with blood oxygenation level-dependent MR imaging. Radiology. 2015;275(1):110–118. doi:10.1148/radiol.14140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamaleyeva LM, Brosnihan KB, Bledsoe T. Fetal status evaluation using photoacoustics and 3D power Doppler ultrasound imaging In: AHA High Blood Pressure Research Scientific Sessions. San Francisco, CA, 2017;70(suppl 1):AP300 Hypertension. [Google Scholar]
- 18. Andescavage N, duPlessis A, Metzler M, et al. In vivo assessment of placental and brain volumes in growth-restricted fetuses with and without fetal Doppler changes using quantitative 3D MRI. J Perinatol 2017;37(12):1278–1284. doi:10.1038/jp.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith LM, Varagic J, Yamaleyeva LM. Photoacoustic imaging for the detection of hypoxia in the rat femoral artery and skeletal muscle microcirculation. Shock (Augusta, Ga) 2016;46(5):527–530. doi:10.1097/SHK.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 2012;335(6075):1458–1462. doi:10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu X, Liu H, Wang LV. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat Photonics 2011;5(3):154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouchard R, Sahin O, Emelianov S. Ultrasound-guided photoacoustic imaging: current state and future development. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61(3):450–466. doi:10.1109/TUFFC.2014.2930. [DOI] [PubMed] [Google Scholar]
- 23. Li H, Ohta H, Tahara Y, et al. Artificial oxygen carriers rescue placental hypoxia and improve fetal development in the rat pre-eclampsia model. Sci Rep. 2015;5:15271 doi:10.1038/srep15271. [DOI] [PMC free article] [PubMed] [Google Scholar]