Abstract
Background:
Multimorbidity is the co-occurrence of two or more diseases in the same individual. One method to identify this condition at an early stage is the use of specific markers for various combinations of morbidities. Nonetheless, evidence related to physiological markers in multimorbidity is limited.
Objective:
The aim was to perform a systematic review to identify physiological markers associated with multimorbidity.
Design:
Articles available on PubMed, Register of Controlled Trials, Academic Search Premier, CINAHL, Scopus, SocINDEX, Web of Science, LILACS, and SciELO, from their inception to May 2018, were systematically searched and reviewed. The project was registered in PROSPERO under the number CRD42017055522.
Results:
The systematic search identified 922 papers. After evaluation, 18 articles were included in the full review reporting at least one physiological marker in coexisting diseases or which are strongly associated with the presence of multimorbidity in the future. Only five of these studies examined multimorbidity in general, identifying five physiological markers associated with multimorbidity, namely, dehydroepiandrosterone sulfate (DHEAS), interleukin 6 (IL-6), C-reactive protein (CRP), lipoprotein (Lp), and cystatin C (Cyst-C).
Conclusions:
There is a paucity of studies related to physiological markers in multimorbidity. DHEAS, IL-6, CRP, Lp, and Cyst-C could be the initial focus for further investigation of physiological markers related to multimorbidity.
Keywords: Multimorbidity, physiological markers, comorbidity, molecular markers
Introduction
Multimorbidity is the co-occurrence of two or more diseases in the same individual, which are often associated with each other.1,2 It is more common in elderly,3 and it affects one in five adults and two-thirds of the elderly.4,5 Multimorbidity is more prevalent in groups from lower socioeconomic status.4 Although studies about multimorbidity have recently begun, evidence has already shown that it leads to a decrease in quality of life,6 functional decline,7 and an increased risk of mortality,8 in addition to difficulty to manage it adequately. Early diagnosis and management of multimorbidity may be important factors to minimize serious consequences. One method to identify this condition at an early stage is the use of specific physiological and molecular markers for various combinations of morbidities.9
Some attempts have been made to identify physiological markers associated with multimorbidity. Reviews have evaluated markers that are associated with pairs of diseases. For example, high levels of B-type natriuretic peptide are commonly associated with dyspnea and have prognostic value in the acute coronary syndromes, such as myocardial infarction, diastolic dysfunction, and atrial fibrillation in congestive heart failure patients.10 Cancer patients frequently have obesity and diabetes as comorbidity conditions. Current studies show that microRNAs such as miR-9 may serve as novel biomarkers and molecular targets for cancer therapy in patients with comorbidity conditions.11
Recently, other alternatives have been attempted to improve early detection of multimorbidity. Alemi et al. developed the multimorbidity index to account for the prognosis of patients with multiple diagnoses. The authors have reported that physiological markers may help not only in the prevention of certain conditions and comorbidities but also in the possibility of early diagnosis and treatment, increasing the chances of survival of patients and decreasing health expenditures.12,13
Using simple blood tests in hospital and/or outpatient laboratories may be an easy-to-access alternative for the population to detect specific physiological markers for a set of diseases. As there are few studies evaluating markers related to multiple concomitant diseases, the aim of this article is to perform a systematic review to identify physiological markers associated with multimorbidity.
Materials and methods
The search was conducted in the following electronic databases: MEDLINE (via PubMed), Register of Controlled Trials (Cochrane CENTRAL), Academic Search Premier, CINAHL, Scopus, SocINDEX, Web of Science, LILACS, and SciELO. The search was conducted in the first semester of 2018 and included all articles from the beginning of the bases until May 2018. Manuscripts written in the following languages were included, English, Portuguese, or Spanish. The Medical Subject Headings (MeSH) terms and their synonyms were used to search the terms in all fields. The search strategy used in PubMed is shown in Table 1.
Table 1.
Literature search strategy used for the PubMed database.
| #1 | “multimorbidity”[MeSH] OR “comorbidity” OR “co-morbidity” OR “multicomorbidity” OR “multi-morbidity” OR “-morbidity” OR “-morbid conditions” OR “multiple diseases” OR “multiple morbidities” OR “multimorbid” OR “multiple pathology” OR “disease clustering” OR “chronic diseases” OR “Severity of Illness Index” |
| #2 | “physiological markers” OR “Markers, Biological” OR “molecular markers” OR “Biology, Molecular” OR “Genetics, Molecular” OR “Molecular Genetic” OR “Biochemical markers” |
| #3 | #1 AND #2 |
The systematic review was reported according to the PRISMA statement and the AMSTAR 2.14,15 According to PICOS principles,14 we consider the following criteria: P means any patient who presented multimorbidity; I means patients with multimorbidity (who have two or more coexisting diseases); C stands for patients who have none or one disease; O means any physiological or molecular marker; and S stands for the study design, mainly observational/epidemiological studies.
Observational studies, which evaluated molecular, physiological, or genetic markers related to different multimorbidity, were selected. The inclusion criteria were studies reporting specific markers to a condition of at least two concomitant diseases. The exclusion criteria were papers that did not specify the marker or were related to one specific disease; studies evaluating seasonal or allergic diseases, general markers of oxidative stress, or markers of resting metabolic rate; and reviews or papers in a language other than English, Spanish, or Portuguese. Full texts of the selected articles after screening the titles and abstracts were assessed, and data were extracted using predetermined formats. The extracted data included author(s) and year of the publication, type of the study, sample, morbidities, and the evaluated biomarkers.
Two independent researchers (GDF and JAS) performed the literature search, screening titles and abstracts, full-paper reviews, study quality assessment, and data extraction. Disagreements were referred to a third author.
The project was registered in PROSPERO under number CRD42017055522.
Results
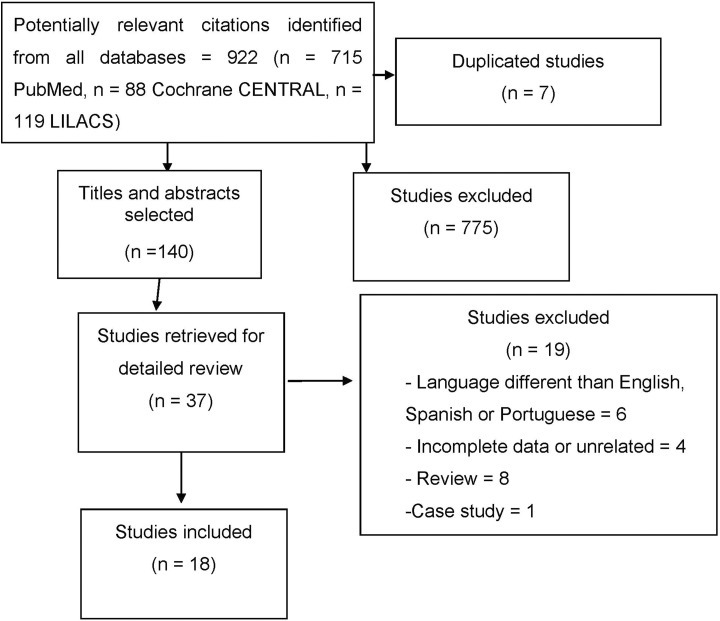
The search identified 922 papers. We found seven duplicate studies and another 775 were excluded as they did not include the outcome of interest. Thereafter, 140 papers were screened to title and abstract selection, and 37 papers were selected for full-text evaluation. Nineteen papers were excluded for the following reasons: languages other than English, Spanish, or Portuguese; literature review, case study, or incomplete data. In the end, 18 papers reporting at least one physiological marker with coexisting diseases were included in the systematic review (Figure 1).
Figure 1.
The flow diagram of studies included in the review.
Tables 2 and 3 show the characteristics of the included studies.
Table 2.
Characteristics of the included studies that analyzed multimorbidity.
| First author, year, country | Design | Sample | n | Measured biomarkers | Conclusions |
|---|---|---|---|---|---|
| Fabbri, 2015, Italy16 | Cohort | Aged 60 years or older | 1018 participants (InCHIANTI Study) | Inflammatory IL-6 and anabolic hormonal (DHEAS) biomarkers associated with multimorbidity that was evaluated at baseline and follow-up visits as number of diagnosed diseases from a predefined list of 15 candidate chronic condition. | Higher IL-6 and lower DHEAS was associated with higher number of
diseases, independent of age, sex, body mass index, and
education. 0 = no disease (n = 213) 1 = one disease (n = 347) 2, 3 = two or three diseases (n = 364) 4+ = four or more diseases (n = 94).
|
| Garrafa, 2017, Italy17 | Cohort | Older persons | 134 (60% women) (mean age was 77.7 ± 7.6 years). (ANZIANI IN-RETE study, a prospective population-based study) |
CRP, Lp, and Cyst-C | Reference values suggested by the literature: Lp <0.3 g/L,
Cyst-C value <1.11 mg/L and CRP < 5 mg/L). Higher levels of the markers were associated with higher number of diseases. Groups: Number of Chronic Disease 0/1, 2/3, 4, or more. (%) Percentage of person with normal value of markers. CRP: 0/1 = 92.9%, 2/3 = 82.2%, 4+ = 65.3%. Lp: 0/1 = 85.7%, 2/3 = 86.4%, 4+ = 60.3%. Cyst C: 0/1 = 91.7%, 2/3 = 61.5%, 4+ = 43.1%. |
| Friedman, 2015, USA18 | Cohort | Older persons | 1255 participants (MIDUS study) | IL-6, CRP, fibrinogen | Having two or more chronic conditions was positively associated with increased of the inflammation factor by 0.22 units (p < 0.001), mainly related to IL-6 and CRP. |
| Stepanova, 2015, USA19
|
Cross-sectional | Adults persons | 26,225 participants (NHANES study), of these 9992 had two or more chronic diseases (average of chronic diseases was 3.017 ± 0.021). | C-reactive protein | Having two or more chronic conditions is related to a higher
concentration (mg/dL) of CRP (p <
0.001). Two or more chronic diseases = 0.580 ± 0.012. One chronic disease = 0.392 ± 0.011. Healthy controls = 0.265 ± 0.011. |
| Schöttker, 2016, Germany20 | Cohort | Adults and older persons (aged 50–74 years) |
2547 participants (51.9% women) (median age was 70
years). Multimorbidity assessed by the Cumulative Illness Rating Scale–Geriatric version |
CRP | After adjusted for age and sex, the higher the concentration of
CRP (mg/L) is associated (OR (95% CI)) with
multimorbidity. CRP ≤ 3 = reference. CRP 3 to <10 = 1.62 (1.21; 2.19). CRP ≥10 = 2.84 (1.83; 4.40). |
IL-6: interleukin 6; DHEAS: dehydroepiandrosterone sulfate; IQR: interquartile range; CRP: C-reactive protein; Lp: lipoprotein; Cyst-C: cystatin C levels; OR: odds ratio; CI: confidence interval.
Table 3.
Characteristics of the included studies that analyzed at least two coexisting diseases (comorbidities).
| First author, year, country | Design | Sample | n | Measured biomarkers | Conclusions |
|---|---|---|---|---|---|
| Gabr, 2004, Saudi Arabia21 | Cross-sectional | IHD and diabetes in ESRD patients on chronic HD. | 73 patients (40 female) divided into 4 groups: 20 patients with IHD, 17 patients with diabetes, 19 patients with diabetes and IHD, and 17 patients without evidence of myocardial damage. | cTnT | 53% of diabetic and 37% of IHD patients had an increased cTnT;
59% of IHD and diabetic patients had an increased cTnT and 29%
of noncardiac disease patients had an increased cTnT. In dialyzed patients, cTnT (μg/L) without IHD is 0.04 and with IHD is 0.15. (p < 0.05). |
| Onyenekwe, 2008, Nigeria22 | Cross-sectional | Malaria and HIV coinfection | Malaria and HIV coinfection group (n = 45) and HIV infected group without concurrent malaria infection (n = 57) | Serum iron and albumin concentrations | Serum iron decreased and albumin increased in (1) Malaria–HIV
coinfection group compared with (2) HIV infection
group. Iron: (1) 107.5 (±53.5) × (2) 151.9 (±79.3) Albumin: (1) 42 (±9.6) × (2) 38.4 (±7.5) |
| Zhou, 2008, UK23 | Cross-sectional | ADHD and CD comorbidity | 141 ADHD + CD and 435 ADHD − CD | Genotypes of 20 DAT1 markers were analyzed. 51 candidate genes in which the association with DAT1 | Heterogeneity test found the two SNP (rs40184, rs2652511) with significant transmission pattern difference between ADHD + CD and ADHD − CD children (p = 0.016), indicating that DAT1 has a significantly greater genetic influence on ADHD with than without CD. |
| Luckhaus, 2009, Germany24 | Case–control | To detect comorbidity with Op at a subclinical level were studied concentrations of biochemical Op markers in subjects with MCI and mild AD compared to subjects with primary Op and age-matched cognitively normal controls | Control = 8, Op = 7, MCI = 19 AD = 20 | C-terminal collagen fragments (marking bone catabolism), Osteocalcin (marking bone remodeling and anabolism) | Equally increased C-terminal collagen in Op and AD (+ 68%) versus controls. Osteocalcin was concomitantly increased in Op (+63%) and in AD (+76%) versus controls. These results point to increased bone catabolism and concomitant remodeling in mild AD but not in cognitive impairment, associating the comorbidity of AD with Op in the early disease course. |
| Conroy, 2011, USA25 | Cross-sectional | Mid-adolescents | 106 school children (65 males), 11–15 years |
RBP4 correlated with metabolic (lipids, glucose), and inflammatory (TNF-α, IL-6, CRP, adiponectin) markers for adiposity and alanine ALT. | Circulating concentrations of RBP4 are correlated with multiple risk factors for adiposity-related comorbidities. Serum RBP4 was significantly correlated with ALT and triglycerides and inversely correlated with adiponectin. RBP4 is a marker that may offer preventive measures in those who are at increasing risk for comorbidities. |
| Jabbar, 2011, UK26 | Case–control | Periodontal disease in postmenopausal women with and without Op | 185 postmenopausal Op women and 185 age- and sex-matched control | Plasma cytokines, vitamin D, bone mineral density, OPG and
RANKL. |
Higher proportion of the women with Op had periodontal disease compared with control (87.6% vs. 37.8%, p < 0.001). Plasma vitD was lower, and RANKL (0.66 ± 0.67 vs. 0.37 ± 0.38) and OPG (18.70 ± 9.70 vs. 10.44 ± 5.85) were significantly higher in the women with Op than in control. CRP (mg/L) was higher in patients (6.42 ± 7.72) than in control (4.03 ± 4.24). |
| Taurines, 2011, Germany27 | Case–control | Children and adolescents with ADHD and ASD: highly comorbid with ADHD | ADHD (n = 51) and ASD (n = 26), diagnosed according to ICD-10 criteria as well as healthy controls (n = 39) | mRNA expression of monoaminergic candidate genes (DRD4, DRD5) | The concentrations of DRD4 in the blood were significantly lower in ADHD and ASD children (19 of 26 comorbid with ADHD) compared to healthy controls (p = 0.005). Just ASD patients revealed a significantly decreased DRD5 mRNA expression in comparison to the two other groups (p = 0.001). mRNA expression may be useful tools in (differential) diagnostic procedures of ADHD with or without ASD. |
| Adamczyk, 2012, Poland28 | Cross-sectional | Degenerative AS in relation to the AVCS and concomitant CAD | 88 patients: 68 patients with degenerative AS (group A), including 44 patients with severe AS (A1; 25 patients with CAD) and 24 patients with moderate AS (A2; 13 patients with CAD), and 20 matched subjects as controls (18 patients with CAD) | Markers of calcification: OPG | In moderate AS, serum OPG levels were higher in subjects with concomitant CAD versus without CAD (5.84 ±1.4 vs. 4.03 ±1.3 pmol/L, p = 0.036). |
| AlMutairi, 2013, Kuwait29 | Case–control | Psoriasis and comorbidities (diabetes, obesity, heart disease, hypertension, and metabolic syndrome). | 100 patients (71 male) with stable plaque psoriasis (psoriasis area and severity index ≥10), compared with equal number of matched healthy volunteers | Cathelicidin (LL-37), and vitamin D in psoriasis patients with comorbidities | Reference values for comorbidities: hypertension (BP ≥ 140/90 mmHg), diabetes (fasting blood sugar > 125mg%), dyslipidemia (triglyceride ≥150 mg%, and/or LDL≥160 mg%), obesity (BMI > 30kg/m2). Metabolic syndrome was diagnosed in the presence of three or more criteria of the National Cholesterol Education Program’s Adult treatment Panel III. The vitamin D levels were lower in patients with psoriasis and comorbidities (29.53 nmol/L ± 9.38 nmol/L vs. 53.5 nmol/L ± 19.6 nmol/L). Furthermore, the levels of serum LL-37 were significantly high (18.16 ng/mL vs. 7.92 ng/mL). |
| Patange, 2013, USA30 | Case–control | Pediatric patients with CKD and comorbidity cardiovascular disease | 34 children with CKD (no history of cardiac disease congenital or structural). The control group consisted of 33 age- and sex-matched children free of any underlying cardiac or renal disease | Parameters of vitamin D and cardiovascular structure (LVMI) | LVMI was correlated with vitamin D (r = −0.54; p = 0.05). Cases (CKD) versus reference range: vitamin D (ng/dL): 18.79 ± 9.95 versus 30–100 ng/dL. Comparing dialysis (n = 20) versus nondialysis (n = 14) = LVMI 46.94 ± 12.12 versus 35.68 ± 10.57. CKD more severe high the risk of cardiovascular disease. |
| Felipe, 2015, Chile31 | Cross-sectional | Presumed healthy men | 66 | Serum ferritin: association between hyperferritinemia and metabolic syndrome parameters | There are correlations between serum ferritin and metabolic syndrome parameters (HDL cholesterol r = −0.285, triglycerides r = 0.388, and fasting glucose r = 0.481). There is an increase of the serum ferritin with the quantity of RF of metabolic syndrome. Zero RF = 152.8 and three or more RF = 286.2. Besides that oxidative stress markers, hepatic damage markers (GGT, SGOT), and HOMA index were correlated with serum ferritin. |
| Sinha, 2015, UK32 | Cohort | Children with non-dialysis stages 3–5 of CKD | 83 children (51 boys; mean age 12.1 ± 3.2 years) with a mean estimated GFR of 32.3 ± 14.6 mL/min | Serum intact FGF23 with indexed LVMI | CKD-associated mineral bone disease is a significant factor contributing to the increased cardiovascular risk in patients with CKD. Was observed no significant relationship of FGF23 with LVMI (r = 0.16, p = 0.15). Cannot clarify the roles of FGF23 with LV mass and their roles in the evolution of the development of adverse cardiovascular outcomes. |
| Robaczewska, 2016, Poland33 | Case–control | Patients with depression, hypertension, or comorbid depression + hypertension and healthy age- and sex-matched controls | Four groups of patients aged 50 or more. n = 15 depression, n = 20 hypertension; n = 16 hypertension + depression; and n = 19 healthy age- and sex-matched controls | HO-1 | HO-1 was decreased in patients with depression and was significantly associated with the severity of symptoms (decreased enzyme expression was correlated with increased severity of depressive). The relationship between HO-1 and the prevalence of depressive symptoms was confirmed by logistic regression, which allowed classification of hypertensive patients as depressed based on HO-1 concentrations (p < 0.01). Decreased expression of HO-1 may contribute to the risk of depression in hypertension. |
IHD: ischemic heart disease; ESRD: end-stage renal disease; HD: hemodialysis; cTnT: cardiac troponin T; ADHD: attention deficit hyperactivity disorder; CD: conduct disorder; MCI: mild cognitive impairment; AD: Alzheimer’s disease; Op: osteoporosis; RBP4: retinol-binding protein 4; TNF-α: tumor necrosis factor-α; IL-6: interleukin 6; CRP: C-reactive protein; ALT: aminotransferase; OPG: osteoprotegerin; ASD: autism spectrum disorders; AS: aortic stenosis; AVCS: aortic valve calcium score; CAD: coronary artery disease; CKD: chronic kidney disease; LVMI: left ventricular mass index; RF: risk factors; GFR: glomerular filtration rate; FGF23: fibroblast growth factor 23; HO-1: heme oxygenase; SNP: single nucleotide polymorphisms; HDL: high density lipoprotein; ICD: international classification of diseases; GGT: gamma glutamyl transferase; SGOT: serum glutamic oxalacetic transaminase; HOMA: homeostatic model assessment.
In relation to disease conditions, the studies are heterogeneous, and only five evaluated multimorbidity in a more widespread construct (Table 2). Although only five studies dealt exclusively with multimorbidity in general, our search selected some studies, which associated markers for specific comorbidities. We only considered papers evaluating at least two of the following diseases related to different markers: three studies related renal and cardiovascular disease, two studies with behavioral diseases, two with bone diseases associated with periodontal diseases and Alzheimer, one study related to depression and hypertension, one related to different cardiac conditions, one related to psoriasis and metabolic comorbidities, one study about infectious diseases, and two studies with healthy people that evaluated markers predicting multimorbidity (Table 3).
Discussion
This article aims to review the literature to identify physiological markers associated with patterns of multimorbidity. Based on the systematic search, we found 18 studies from 2004 to 2017, which evaluated physiological markers related to different concomitant diseases or which are strongly associated with the presence of multimorbidity in the future.
No randomized clinical trials (RCTs) were found in the search strategy, most part of the studies carried out observational analyses, and none cited a methodology including blind review. Nevertheless, from the 18 included studies, 15 presented a control group for comparability: patients who had only one of the morbidities of the study16–24,27,28,30,33 or without morbidity (matched according to sex and age).19,26,29 Although all included papers assessed association between at least two different morbidities, only five assessed specifically markers for multimorbidity in general regardless of the disease. The three studies, which did not present control groups, were related to correlation between variables, which may increase the risk of comorbidities.25,31,32 In general, even considering that the included papers are not RCT or long follow-up periods, the presence of a control group in most studies can be used to assist with a more thorough data analysis. Nevertheless, suggestions are put forward as the results are not conclusive, because the aim is to observe cross-sectional data. The quality evaluation of the studies followed the parameters of the Newcastle–Ottawa scale.34
Physiological markers and multimorbidity
Only five studies evaluated multimorbidity in general. The results cross-sectional data show that the elevated interleukin 6 (IL-6) and lower dehydroepiandrosterone sulfate (DHEAS) are associated with a greater burden of multimorbidity and suggest that investing in these markers may be an alternative for earlier detection and target interventions aimed at reducing multimorbidity.16 Although high levels of serum cytokines such as IL-6 and tumor necrosis factor α (TNF-α) are considered risk factors for several chronic diseases, the second study emphasizes that these markers are not available in the majority of clinical laboratories. Therefore, other markers should be studied, such as C-reactive protein (CRP), lipoprotein (Lp), and cystatin C (Cyst-C) levels. People in the group of those with zero or one disease showed normal levels for the markers, as the number of chronic diseases increased the percentage of a person with a normal value of the markers decreased, showing that they are markers which can also be considered for detection of multimorbidity.17 Three other studies also suggested CRP as a possible marker for multimorbidity, associating the increase of its concentration with two or more chronic conditions.18–20 These data should be analyzed with caution, because cross-sectional and longitudinal studies have limitations to identify cause or consequence.
Physiological markers, patterns of multimorbidity, and specific combinations of diseases
In relation to the studies described in Table 3, combinations of pairs of diseases were normally controlled by individuals with only one of the associated diseases, thus observing the pattern of specific markers between groups. Three studies related renal and cardiovascular diseases. In ischemic heart disease (IHD) and diabetes in end-stage renal disease (ESRD) patients, it was observed that cardiac troponin T (cTnT) was increased in IHD and diabetic patients compared to control and isolated diseases, suggesting that increased cTnT appears to be the most specific biochemical marker to predict subclinical myocardial damage in ESRD patients.21 It is suggested that chronic kidney disease severely increases the risk of cardiovascular disease30; however, it does not seem to be associated with mineral bone disease.32
Two studies analyzed attention deficit hyperactivity disorder (ADHD) and conduct disorder (CD) comorbidity. The genetic heterogeneity showed that variations in the dopamine active transporter 1 (DAT1) gene confer significantly different risks to ADHD children to either have or not have CD.23 These data corroborate with Gizer who showed co-occurrence among psychiatric disorders and that the candidate gene studies of psychological disorders are involved in monoaminergic function (DAT1) giving the relevance of these neurotransmitter systems to brain–behavior relations.35 Taurines et al. also related monoaminergic genes to comorbidities with autism spectrum disorders.27
One study reported different cardiac conditions and concluded that the presence of coronary artery disease in moderate aortic stenosis was associated with increased serum osteoprotegerin (OPG) levels, suggesting the effect of atherosclerosis on early valve calcification.28 Moreover, one study evaluated depression and hypertension and showed that decreased expression of heme oxygenase may contribute to the risk of comorbidity of depression in hypertension cases.33
Two studies reported osteoporosis with comorbidities. It is clear that in Alzheimer’s disease there is an increase in bone catabolism, associating the comorbidity of Alzheimer’s disease with osteoporosis in the early disease course detected at the level of biochemical blood markers, such as C-terminal collagen fragments and osteocalcin.24 Other comorbidity is periodontal disease, which is more common in women with osteoporosis and is associated with lower vitamin D and higher concentrations of RANKL and OPG.26
Psoriasis and comorbidities (diabetes, obesity, heart disease, hypertension, and metabolic syndrome) can be evaluated with cathelicidin (LL-37) and vitamin D. Vitamin D levels were lower in patients with psoriasis and comorbidities, but serum LL-37 levels were significantly elevated.29 Furthermore, one study analyzed the coinfection of malaria and HIV compared to HIV-infected individuals without concurrent malaria infection and showed that serum iron and albumin concentrations were indicators related with malaria infection in HIV-infected subjects.22
Two studies with healthy people evaluating markers that predict multimorbidity were included. In adolescents, the establishment of retinol-binding protein 4 as a marker may offer health-care providers a tool to begin aggressive preventive measures in those who are at increasing risk for adiposity-related comorbidities but have not yet been afflicted by them.25 In healthy men, significant correlations were found between serum ferritin concentration and metabolic syndrome parameters.31
Related findings
A birth cohort study aimed at assessing the association of obesity with physiological markers at 22 years of age, showing direct association for IL-6 and CRP and an inverse association for adiponectin, suggesting early care for this population at risk.36 A meta-analysis of prospective studies suggests the association of IL-6 and CRP with type 2 diabetes risk (relative risk of 1.31 per 1 log pg/mL increment in IL-6 levels and of 1.26 per 1 log mg/L increment in CRP levels).37
Therefore, a systematic review has shown the elevation of the concentrations of pro-inflammatory cytokines (IL-6, TNF-α) in metabolic syndrome, suggesting that the measurement of these biomarkers may aid in the detection of these disorders,38 and agrees with findings from the past decade that have already indicated that laboratory-measured pro-inflammatory markers are needed to target specific treatment in the metabolic syndrome.39
Limitations
As limitations of this study, the included papers were cross-sectional analyses; however, it is not clear whether the marker is a cause or a consequence related to different pairs of diseases. In addition, only five studies are performed with nonspecific concomitant diseases, showing the existing gap in the literature. Therefore, more studies controlling confounding variables and monitoring different conditions are needed to find markers that allow the prevention or early treatment of different multimorbidity.
Conclusions
This study was important for grouping physiological markers associated with different types of comorbidities to facilitate early diagnosis and intervention in these patients. Nonetheless, there is a paucity of studies related to physiological markers and multimorbidity. In the initial efforts, despite cross-sectional analyses, DHEAS, IL-6, CRP, Lp, and Cyst-C seem to be the choices for further investigation regarding the effects of physiological markers related to multimorbidity.
Footnotes
Authors’ note: All authors have read and agreed the Statement for Authors.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Department of Medical Sciences, Faculty of Health Sciences, University of Beira Interior.
ORCID iD: José Augusto Simões  http://orcid.org/0000-0003-2264-7086
http://orcid.org/0000-0003-2264-7086
Chamara Senaratna  http://orcid.org/0000-0002-5879-6174
http://orcid.org/0000-0002-5879-6174
References
- 1. Prazeres F, Santiago LM, Simões JA. Defining multimorbidity: from English to Portuguese using a Delphi technique. Biomed Res Int 2015; 2015: Article ID 965025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reste JY, Nabbe P, Manceau B, et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. JAMDA 2013; 14(5): 319–325. [DOI] [PubMed] [Google Scholar]
- 3. Nunes BP, Thumé E, Facchini LA. Multimorbidity in older adults: magnitude and challenges for the Brazilian health system. BMC Public Health 2015; 15: 1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380(9836): 37–43. [DOI] [PubMed] [Google Scholar]
- 5. Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10(2): 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fortin M, Lapointe L, Hudon C, et al. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2004; 2: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryan A, Wallace E, O’Hara P, et al. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes 2015; 13: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nunes BP, Flores TR, Mielke GI, et al. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–138. [DOI] [PubMed] [Google Scholar]
- 9. Sturmberg JP, Bennett JM, Martin CM, et al. “Multimorbidity” as the manifestation of network disturbances. J Eval Clin Pract 2016; 23(1): 199–208. [DOI] [PubMed] [Google Scholar]
- 10. Burke MA, Cotts WG. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev 2007; 12: 23–36. [DOI] [PubMed] [Google Scholar]
- 11. Ali AS, Ali S, Ahmad A, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obes Rev 2011; 12: 1050–1062. [DOI] [PubMed] [Google Scholar]
- 12. Alemi F, Levy KR, Kheirbek RE. The multi-morbidity index: a tool for assessing the prognosis of patients from history of illness. eGEMs 2016; 4(1): 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zierer J, Pallister T, Tsai PC, et al. Exploring the molecular basis of age-related disease comorbidities using a multi-omics graphical model. Sci Rep 2016; 6: 37646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62(10): e1–e34. [DOI] [PubMed] [Google Scholar]
- 15. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci 2015; 70(1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrafa E, Casnici N, Squazzoni F, et al. C-reactive protein, lipoprotein (a) and cystatin C levels increase with multimorbidity in older persons. Eur J Intern Med 2017; 42: e25–e26. [DOI] [PubMed] [Google Scholar]
- 18. Friedman EM, Christ SL, Mroczek DK. Inflammation partially mediates the association of multimorbidity and functional limitations in a national sample of middle-aged and older adults: the MIDUS study. J Aging Health 2015; 27(5): 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stepanova M, Rodriguez E, Birerdinc A, et al. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget 2015; 6(3): 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schöttker B, Saum KU, Jansen EH, et al. Associations of metabolic, inflammatory and oxidative stress markers with total morbidity and multi-morbidity in a large cohort of older German adults. Age Ageing 2016; 45(1): 127–135. [DOI] [PubMed] [Google Scholar]
- 21. Gabr AM, Ibrahim IA, Aloulou SM, et al. Cardiac troponin T and end stage renal disease. Saudi Med J 2004; 25(8): 1015–1019. [PubMed] [Google Scholar]
- 22. Onyenekwe CC, Ukibe N, Meludu SC, et al. Possible biochemical impact of malaria infection in subjects with HIV co-infection in Anambra state, Nigeria. J Vector Borne Dis 2008; 45: 151–156. [PubMed] [Google Scholar]
- 23. Zhou K, Chen W, Buitelaar J, et al. Genetic heterogeneity in ADHD: DAT1 gene only affects probands without CD. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 24. Luckhaus C, Mahabadi B, Grass Kapanke B, et al. Blood biomarkers of osteoporosis in mild cognitive impairment and Alzheimer’s disease. J Neural Transm 2009; 116: 905–911. [DOI] [PubMed] [Google Scholar]
- 25. Conroy R, Espinal Y, Fennoy I, et al. Retinol binding protein 4 is associated with adiposity-related co-morbidity risk factors in children. J Pediatr Endocrinol Metab 2011; 24: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jabbar S, Drury J, Fordham J, et al. Plasma vitamin D and cytokines in periodontal disease and postmenopausal osteoporosis. J Periodont Res 2011; 46: 97–104. [DOI] [PubMed] [Google Scholar]
- 27. Taurines R, Glunblatt E, Schecklmann M, et al. Altered mRNA expression of monoaminergic candidate genes in the blood of children with attention deficit hyperactivity disorder and autism spectrum disorder. World J Biol Psychiatry 2011; 12(S1): 104–108. [DOI] [PubMed] [Google Scholar]
- 28. Adamczyk T, Mizia-Stec K, Mizia M, et al. Biomarkers of calcification and atherosclerosis in patients with degenerative aortic stenosis in relation to concomitant coronary artery disease. Pol Arch Med Wewn 2012; 122(1–2): 14–21. [DOI] [PubMed] [Google Scholar]
- 29. AlMutairi N, Eassa BE, Nair V. Measurement of vitamin D and cathelicidin (LL37) levels in patients of psoriasis with comorbidities. Indian J Dermatol Venereol Leprol 2013; 79: 492–496. [DOI] [PubMed] [Google Scholar]
- 30. Patange AR, Valentini RP, Gothe MP, et al. Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr Cardiol 2013; 34: 536–542. [DOI] [PubMed] [Google Scholar]
- 31. Felipe A, Guadalupe E, Druso P, et al. Serum ferritin is associated with metabolic syndrome and red meat consumption. Oxid Med Cell Longev 2015; 2015: 769739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinha MD, Turner C, Booth CJ, et al. Relationship of FGF23 to indexed left ventricular mass in children with non-dialysis stages of chronic kidney disease. Pediatr Nephrol 2015; 30(10): 1843–1852. [DOI] [PubMed] [Google Scholar]
- 33. Robaczewska J, Kędziora-Kornatowska K, Kucharski R, et al. Decreased expression of heme oxygenase is associated with depressive symptoms and may contribute to depressive and hypertensive comorbidity. Redox Rep 2016; 21(5): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25(9): 603–605. [DOI] [PubMed] [Google Scholar]
- 35. Gizer IR. Molecular genetic approaches to understanding the comorbidity of psychiatric disorders. Dev Psychopathol 2016; 28: 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menezes AMB, Oliveira PD, Wehrmeister FC, et al. Association between interleukin-6, C-reactive protein and adiponectin with adiposity: findings from the 1993 Pelotas (Brazil) birth cohort at 18 and 22 years. Cytokine 2018; 110: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes. A systematic review and meta analysis. Diabetes Care 2013; 36(1): 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srikanthan K, Feyh A, Visweshwar H, et al. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci 2016; 13(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Odrowaz-Sypniewska G. Markers of pro-inflammatory and pro-thrombotic state in the diagnosis of metabolic syndrome. Adv Med Sci 2007; 52: 246–50. [PubMed] [Google Scholar]