Abstract
The concept of multidrug therapy with clopidogrel, acetylsalicylic acid and atorvastatin provides major advances for patients with acute coronary syndromes (ACS) treated invasively and conservatively to reduce the risk of ischemic events and improve survival. Dual antiplatelet therapy (DAPT) with acetylsalicylic acid and clopidogrel is currently the treatment of choice in acute coronary syndromes and prevention of thrombosis after coronary stent implementation. Present paper presents a simple, rapid and precise reversed phase high performance liquid chromatographic method for simultaneous estimation of clopidogrel hydrogen sulfate, acetylsalicylic acid and atorvastatin calcium from tablet dosage form. The cromatographic analysis was carried out using a ThermoFinnigan Chromatograph with UV detection and separation on an HDS Hypersil C18 column. The elution was isocratic with mobile phase consisting in mixture of 0.01M KH₂PO₄ buffer: acetonitrile: methanol. Chemicals were represented by clopidogrel bisulfat, atorvastatin calcium and acetylsalicylic acid standards with high purity. The quality control samples used in the accuracy and precision evaluation were spiked at low, medium and high concentration levels. Preliminary tests were performed to select optimum conditions for simultaneous separation of all three analytes such as mobile phase composition, proportion and pH. A satisfactory separation of all three drugs was achieved with a mobile phase of 0.01 M KH₂PO₄ buffer (pH adjusted to 2.6 with phosphoric acid):acetonitrile:methanol 20:40:40 v/v/v at flow rate of 0.8 ml/min. The proposed method was validated for linearity, precision and accuracy. The validated method can be successfully applied for simultaneous quantification of clopidogrel, acetylsalycilic acid and atorvastatin either in combination or in single dosage form in quality control analysis. The analytical conditions can also be performed for human plasma analysis.
Keywords: Acute Coronary Syndromes (ACS), Dual Antiplatelet Therapy (DAPT), Liquid Chromatography, Simultaneous Quantification
Introduction
Acute coronary syndromes (ACS) significantly contribute to cardiovascular mortality and morbidity worldwide and include clinical conditions like ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI) and unstable angina[1]. Dual antiplatelet therapy (DAPT) that combines acetylsalicylic acid and clopidogrel along with hypolipemic drugs may prove to be a therapeutic option for patients with ACS[2]. Multidrug therapy with clopidogrel, acetylsalicylic acid and atorvastatin (Fig 1) have been suggested as a therapeutic strategy to reduce cardiovascular risk[3]. The pleiotropic effect of atorvastatin (LDL reduction, plaque stabilization, decreased inflammation, improved endothelial function) offsets the potential drug-drug interaction and reduction in clinical platelet inhibition with clopidogrel.
Figure 1.
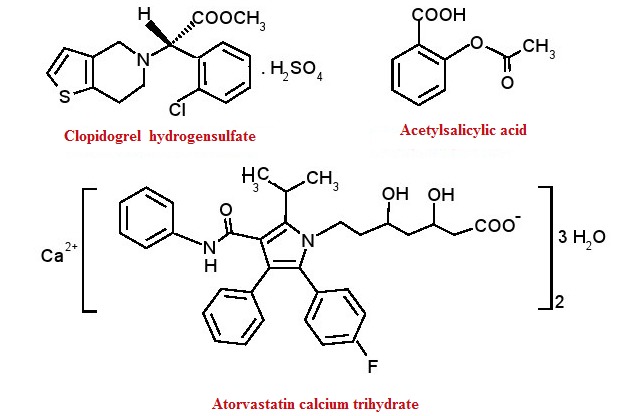
Chemical structure of clopidogrel, acetylsalicylic acid and atorvastatin
Clopidogrel hydrogen sulfate, methyl (2S)-(2-chlorophenyl)[6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]acetate sulfate (Fig. 1.) is an oral thienopyridine-class antiplatelet agent indicated for acute coronary syndrome , recent MI , recent stroke or established peripheral arterial disease [4]. Clopidogrel is a prodrug that requires biotransformation in the liver. Most of absorbed clopidogrel (85%-90%) is hydrolyzed by carboxylase to an inactive carboxylic acid metabolite, whereas the rest is metabolized by hepatic cytochrome P450 (CYP 450) isoenzymes in a two-step process. The metabolic pathway is under polymorphic genetic regulation with an increased incidence of major adverse cardiac events[5]. The thiophene ring of clopidogrel is oxidized to 2-oxo-clopidogrel, which then is hydrolyzed to a liable active thiolic metabolite [6,7] that demonstrates irreversible inhibition of the ADP receptor by disulfide bonding [8]. The elimination half-life of clopidogrel carboxylic acid is 8 hours [9].
Acetylsalicilic acid, 2-(acetyloxy)benzoic acid, (Fig. 1.) remains a first-line therapy for patients with unstable angina/ NTEMI [10]. Acetylsalicylic acid is hydrolyzed by esterases in plasma or liver to salicylic acid [11]. The main half-life of aspirin is approximately 15-20 minutes. The antithrombotic effect of aspirin at low dose is explained by irreversibly acetylating a serine residue in the COX-1 enzyme expressed in platelets. Cox-2 was not found to be emlpoyed in platelets activity[12].
Atorvastatin, calcium (3R,5R)- 7-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate trihydrate, (Fig. 1.) provides greater protection against major cardiovascular events than does a standard regimen [13] and acts by competitively inhibiting hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA). Atorvastatin undergoes extensively metabolism by CYP 3A4 to ortho- and parahydroxylated derivatives. The low systemic bioavailability of 12% is due to presystemic clearance and first-pass metabolism in the liver, but half-life is 15-30 hours due to longer-lived active metabolites [14].
Current guideline recommedations and dosage regimen
Current guideline recommendations in optimizing clopidogrel therapy include a maintaining dose of 75 mg and a loading dose of 150-600 mg [15]. There are minimal increase in plasma concentration at doses higher than 600 mg clopidogrel. Dual antiplatelet therapy of clopidogrel and aspirin has survived despite the findings of MATCH [16] and CHARISMA [17] trials. For patients with non-ST-elevation myocardial infarction, guideline recommended ticagrelor as first line therapy, clopidogrel can be prescribed for patients intolerant to ticagrelor [18]. The clinicians need to carefully balance tailored therapy according to efficacity and safety profiles, compliance and economic data[19].Dosage regiment is summarized in Table 1.
Table 1.
Recommendations for Initial Antiplatelet Therapy in Patients with NSTE-ACS
| Recommendations | Dosing and considerations |
| Acetylsalicylic acid (Aspirin) | |
| Non–enteric-coated aspirin after presentation | 162 mg–325 mg |
| Aspirin maintenance dose indefinitely | 81 mg/d*–325 mg/d |
| P2Y12 Inhibitors | |
| P2Y12 inhibitor, in addition to aspirin, for up to 12 months for patients with initial ischemia-guided strategy:−Clopidogrel−Ticagrelor∗ | 300-mg or 600-mg loading dose, then 75 mg/d |
| 180-mg loading dose, then 90 mg BID | |
| Ticagrelor in preference to clopidogrel for patient treated with ischemia-guided strategy | N/A |
N/A, not available; NSTE-ACS, non–ST-elevation acute coronary syndromes;
The maintenance dose of aspirin with ticagrelor is 81 mg daily, BID twice daily
Literature survey reveals a new chemometric approach for simultaneous determination of clopidogrel and its carboxylic acid metabolite from dosage formulations[20] and various methods for simultaneous analysis of clopidogrel, acetylsalicylic acid and atorvastatin [21,22,23,24], clopidogrel and acetylsalicylic acid [25,26,27,28,29], atorvastatin and nicotinic acid [30], atorvastatin and acetylsalicylic acid [31] in tablets. Estimation of clopidogrel from tablets was performed by HPLC [31,32,34,35].
Matherials and Methods
Materials and reagents
Clopidogrel bisulfate, acetylsalicylic acid and atorvastatin calcium trihydrate standards were obtained from Sigma Aldrich. HPLC grade acetonitrile, methanol, orthophosphoric acid and water were obtained from Merck KGaA(Germany). Tablet dosage form ( clopidogrel 75mg, atorvastatin 20mg and acetylsalicylic acid 100mg per capsule) was purchased from local pharmacy.
Instrumentation
Chromatographic analysis was carried out using a ThermoFinnigan chromatograph consisting of ternary solvent manager and PDA detector.
Chromatographic conditions
The separation was achieved on an HDS Hypersil C18 analytical column (250x4.6mm, 5µm particle size), the mobile phase consits in a mixture of 0.01 M KH₂PO₄ buffer (pH adjusted to 2.6 with phosphoric acid):acetonitrile:methanol 20:40:40 v/v/v at flow rate of 0.8 ml/min. The injection volume was 20µl and the UV detection was performed at 220nm for clopidogrel, 230nm for acetylsalicylic acid and 244nm for atorvastatin.
Preparation of stock and standard solutions
The stock solutions of clopidogrel bisulfate, acetylsalicylic acid and atorvastatin were prepared at a concentration of 100µg/ml in methanol. Standard solutions for calibration curves have been prepared at the concentrations of 0.0312, 0.0625, 0.125, 0.25, 0.5, 1, 5µg/ml for clopidogrel, 0.0312, 0.0625, 0.21, 0.37, 0.88, 1.93 µg/ml for acetylsalicylic acid and 0.04, 0.08, 0.156, 0.325, 0.625, 1.25µg/ml for atorvastatin. The quality control samples (QCs) used in the accuracy and precision evaluation were spiked at the levels :0.05, 0.3, 1 µg/ml for clopidogrel, 0.05, 0.5, 1 µg/ml for acetylsalicylic acid and 0.08, 0.1, 0.8 µg/ml for atorvastatin
Preparation of sample solutions
Powder of 20 tablets, each containing 75mg clopidogrel, 100mg acetylsalicylic acid and 20 mg atorvastatin, were weighed and analysed. A quantity of powder equivalent to 5 mg of clopidogrel bisulfate was weighed and transferred to a 50 ml volumetric flask and diluted with methanol to obtain stock solutions of clopidogrel, aspirin and atorvastatin of 100µg/ml. An aliquot of the dample stock solution (1ml) was transfered to a 100 ml volumetric flask and diluted to the mark with mobile phase to obtain working sample solution of clopidogrel, aspirin and atorvastatin of 1µg/ml.
Method validation
The method proposed was validated as described in ICH guidelines [36] in terms of linearity, limit of detection and quantification, accuracy, precision and robustness
1.Linearity -The linearity of the method was determined at six concentration levels ranging from 0.03-5µg/ml for clopidogrel, 0.03-2µg/ml for acetylsalicylic acid and 0.04-1.25µg/ml for atorvastatin. The calibration curves were constructed by plotting peak areas versus concentration of clopidogrel, acetylsalicylic acid and atorvastatin. The slope, Y-intercept and correlation coefficient were calculated.
2.Precision - Precision was investigated using the sample preparation procedure for six samples of commercial capsules (Plavix 75mg, Sortis 20mg and Aspirin Cardio 100mg). The precision of the method was evaluated by carrying out six independent assays of clopidogrel (0.05, 0.3, 1 µg/ml), acetylsalicylic acid (0.05, 0.5, 1µg/ml) and atorvastatin (0.08, 0.1, 0.8µg/ml) test samples against reference standard.
3.Limit of detection and quantification - The limit of detection (LOD) and quantification (LOQ) were estimated using signal-to-noise ratio of 3:1 and 10:1 as per ICH quidelines
4.Robustness - The robustness of the method was evaluated by assaying test solutions after deliberate changes in the analytical conditions.
Results
Preliminary tests were performed to select adequate parameters such as mobile phase composition and proportion, pH of buffer, detection wavelength. A satisfactory separation was obtained with a mobile phase of 0.01 M KH₂PO₄ buffer (pH adjusted to 2.6 with phosphoric acid):acetonitrile:methanol 20:40:40 v/v/v at flow rate of 0.8 ml/min. Quantification was obtained with PDA detection al 220nm for clopidogrel, 230nm for acetylsalicylic acid and 244nm for atorvastatin. The chromatogram of simultaneous detection of clopidogrel, acetylsalicylic acid and atorvastatin in tablets is presented in Fig. 2. The calibration curves of clopidogrel, acetylsalicylic acid and atorvastatin are shown in Fig. 3.
Figure 2.
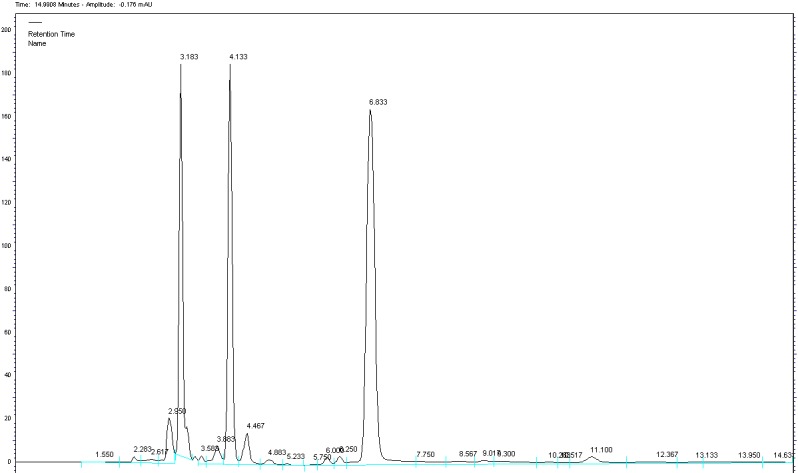
Chromatogram of clopidogrel (tR=6.833), acetylsalicylic acid (tR=3.183) and atorvastatin (tR=4.133) from tablets under optimized method KH₂PO₄:acetonitrile:methanol(20:40:40 v/v/v) at flow rate of 0.8 ml/min
Figure 3.
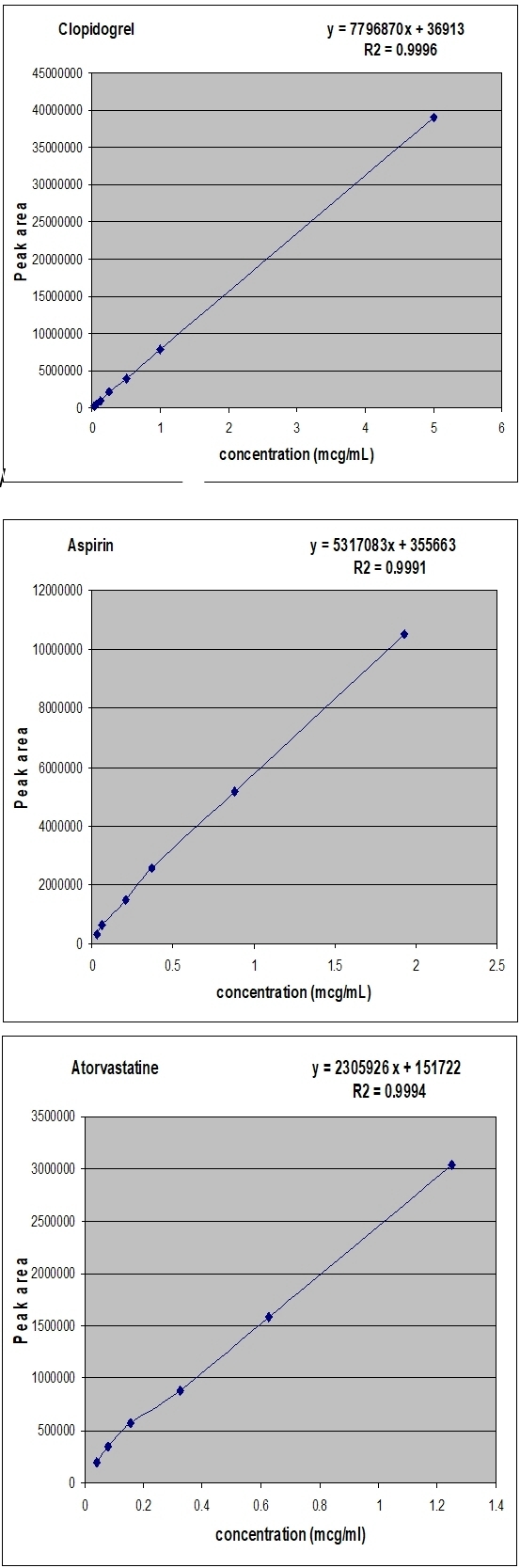
Calibation curves of clopidogrel, acetylsalicylic acid and atorvastatin
1.Linearity
calibration plot of peak area against concentration was linear in the range investigated. The low values of RSD and standard error show the method is precise. Statistical calculation was performed at the 5% level of significance. The results were represented in Table 2. The linear regression data for the calibration plot are indicative of a good linear relationship between peak area and concentration over a wide range. The correlation coefficient was indicative of high significance and shows that an excellent correlation exists between response factor and concentration of drug. The linear regression equation and the correlation coefficient were y = 2305926x + 151722 and 0,9994, respectively, for atorvastatin, y = 7796870x + 36913 and 0,9996, respectively, for clopidogrel and y = 5317083x + 355663 and 0,9991, respectively, for aspirin.
Table 2.
Liniarity data
| Substance | Linearity range (mg/mL) | Equation for regression line | Correlation coefficient |
| Clopidogrel | 0.03 – 5 | y = 7796870x + 36913 | R2=0,9996 |
| Acetylsalicylic acid | 0.03 – 2 | y = 5317083x + 355663 | R2=0,9991 |
| Atorvastatin | 0.04 – 1,25 | y = 2305926x + 151722 | R2=0,9994 |
2.Precision
Precision was determined as both repeatability and intermediate precision, in accordance with ICH guidelines. Repeatability of sample was determined as intra-day variation and intermediate precision was determined by measurement of inter-day variation. Acceptance criteria for repeatability states that RSD should be less than 1%. Intermediate precision (within laboratory variation) will be demonstrated on different days. Acceptance criteria for Intermediate Precision states that the statistical RSD≤2%. Precision results are summaried in table 3.
Table 3.
Results Analysis of Precision of the method
| Concentration (µg/mL) | Repeatability (intra-day precision)* | Intermediate precision (inter-day)* | ||
| (mean±SD) | %RSD | (mean±SD) | %RSD | |
| Atorvastatin | ||||
| 0, 08 | 332739,2±2323,29 | 0,70 | 331387,64±1432,46 | 0,43 |
| 0, 1 | 375688,20±3630,55 | 0,97 | 375729,24±2458,76 | 0,65 |
| 0, 8 | 1989075,20±2424,92 | 0,12 | 1982701,04±3982,23 | 0,20 |
| Clopidogrel | ||||
| 0,05 | 382313,80±2595,05 | 0,68 | 382698,16±2395,40 | 0,63 |
| 0, 3 | 2259476,80±38976,01 | 1,73 | 2240926,96±28044,73 | 1,25 |
| 1 | 7543808,80±37450,56 | 0,50 | 7544630,96±20355,44 | 0,27 |
| Acetylsalicylic acid | ||||
| 0,05 | 505056,20±4928,94 | 0,98 | 504833,84±2561,27 | 0,51 |
| 0, 5 | 5152663,80±28132,37 | 0,55 | 5158230,56±37172,29 | 0,72 |
| 1 | 10747879,20±26705,52 | 0,50 | 10748269,84±24570,32 | 0,23 |
*Indicates mean of five determination, RSD=Relative Standard Deviation, SD=Standard Deviation
3.Limit of detection and quantification
The LODs for clopidogrel, acetylsalicylic acid and atorvastatin were found to be 0.03, 0.1 and 0.05µg/ ml for clopidogrel, acettylsalicyic acid and atorvastatin , while LOQ were 0.1, 0.3 and 0.17 µg/ml respectively
4.Robustness
The method was found to be robust and small changes in mobile phase compposition and flow rate did not produce notable chanage in the retention times of the drugs.
Conclusion
A simple, rapid, precise and robust chromatographhic method has been proposed for the simultaneous detection of clopidogrel, acetylsalicylic acid and atorvastatin in tablet dosage form. The validated method can be successfully applied for simultaneous quantification of the drugs in quality control tablet analysis or human plasma samples.
Acknowledgments
"This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 20007-2013, project no. POSDRU/159/1.5/S/ 133377".
References
- 1.Myocardial infarction redefined-a consensus document of the Joint European Society of Cardiology /American College of Cardiology Comitee for the redefinition of myocardial. Myocardial infarction redefined-a consensus document of the Joint European Society of Cardiology /American College of Cardiology Comitee for the redefinition of myocardial infarction [Google Scholar]
- 2.Patrick WL, Patel C, Guddeti R, et al. Is there a need for triple therapy? Role of anticoagulation with dual antiplatelet therapy in acute coronary syndromes (ATLAS Study and TRAP Study) CurrCardiol Repl. 2011 doi: 10.1007/s11886-013-0411-1. [DOI] [PubMed] [Google Scholar]
- 3.Bates E, Lau W, Angiolillo DJ, et al. Clopidogrel-Drug Interactions. Journal of American College of Cardiology. 2011;57(11) doi: 10.1016/j.jacc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 4. Plavix prescribing information,Bristol-Meyers Squibb/Sanofi .
- 5.Nishio R, Shinke T, Otake H, et al. Effect of Cytochrome P450 2C19 Polymorphism on Target Lesion Outcome After Drug-Eluting Stent Implantation in Japanese Patients Receiving Clopidogrel. Official Journal of the Japanese Circulation Society. 2012;76:2348–2355. doi: 10.1253/circj.cj-12-0476. [DOI] [PubMed] [Google Scholar]
- 6.Hagihara K, Kazui M, Kurihara A, et al. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Metab Dispos. 2009;37:2145–2145. doi: 10.1124/dmd.109.028498. [DOI] [PubMed] [Google Scholar]
- 7.Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 8.Ding Z. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys 17 and Cys 270. Blood. 2003;101(10):3908–3914. doi: 10.1182/blood-2002-10-3027. [DOI] [PubMed] [Google Scholar]
- 9.Ksycinska H, Rudzki P, Bukowska-Kiliszek M, et al. Determination of clopidogrel metabolite (SR26334) in human plasma by LC-MS. J Pharm Biomed Anal. 2006;41(2):533–539. doi: 10.1016/j.jpba.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 10.AHA/ACC Guideline for the Management of Patients With Non-ST- Elevation Acute Coronary Syndromes:Executive Summary:A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Benedek IH. Variability in the pharmacokinetics and pharmacodynamics of low dose aspirin in healthy male volunteers. J Clin Pharmacol. 1995;35(12):1181–1186. doi: 10.1002/j.1552-4604.1995.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 12.Patrono C, Garcia Rodriguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2005;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 14.Bhindi R, Ormerod O, Newton J, et al. Interaction between statins and clopidogrel:is there anything clinically relevant? Q J Med. 2008;101:915–925. doi: 10.1093/qjmed/hcn089. [DOI] [PubMed] [Google Scholar]
- 15.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alonso F, et al. Variability in Individual Responsiveness to Clopidogrel. Journal of the Americn College of Cardiology. 2007;49(14) doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high risk patients (MATCH): randomised, double-blind, placebo controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Topol EJ, et al. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004;148(2):263–268. doi: 10.1016/j.ahj.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Thomson RM, Anderson DC, et al. Aspirin and Clopidogrel for Prevention of Ischemic Stroke. Curr Neurol Neurosci Rep. 2013;13:327–327. doi: 10.1007/s11910-012-0327-y. [DOI] [PubMed] [Google Scholar]
- 19.Russolillo A, Di Minno MND, Tufano A, et al. Filling the gap between science and clinical practice: Prevention of stroke recurrence. Thrombosis Research. 2012;129(2012):3–8. doi: 10.1016/j.thromres.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Anuta V, Sarbu I, Mircioiu I, Velescu BS. Development of a new HPLC method for simultaneous determination of clopidogrel and its major metabolite using a chemometric approach. Curr Health Sci J. 2015;41(4) doi: 10.12865/CHSJ.41.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Londhe SV, Deshmukh RS, Mulgund SV, et al. Development and validation of a reversed-phase HPLC method for simultaneous determination of aspirin. atorvastatin calcium and clopidogrel bisulphate in capsules. Indian J Pharm Sci. 2011;73:23–29. doi: 10.4103/0250-474X.89753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanitha Sri M. Simultaneous determination of aspirin, clopidogrel bisulphate and atorvastatin calcium in capsule dosage form by RP-HPLC. J Bioequiv Availab. 2012 [Google Scholar]
- 23.Kaila HO, Ambasana MA, Shah AK, et al. A Simple and Rapid Ultra-Performance Liquid Chromatographic Assay Method for the Simultaneous determination of Aspirin, Clopidogrel Bisulphate and Atorvastatin Calcium in Capsule Dosage Form. IJCRGG. 2011;3(1):459–465. [Google Scholar]
- 24.Rasheed A, Pattanayak S, Rama Rao T, et al. Analytical Method Development and Validation for the Simultaneous Estimation of Aspirin, Clopidogrel Bisulphate and Atorvastatin Calcium in Tablet Dosage Form. Am J Pharm Tech Res. 2014;4(4) [Google Scholar]
- 25.Kahsay G, Schepdael A, Adams E. Development and validation of a liquid chromatographic method for purity control of copidogrel-acetylsalicylic acid in combined oral dosage forms. J Pharm Biomed Anal. 2012;61:271–276. doi: 10.1016/j.jpba.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Sultana N, Arayne M, Ali K, et al. Simultaneous Determination of Clopidogrel and Aspirin by RP-HPLC from Bulk Material and Dosage Formulations Using Multivariate Calibration Technique. Journal of Chromatographic Science. 2011;(49) doi: 10.1093/chrsci/49.2.165. [DOI] [PubMed] [Google Scholar]
- 27.Patel RB, Shankar MB, Patel MR, et al. Simultaneous estimation of acetylsalicylic acid and clopidogrel bisulfate in pure powder and tablet formulation by high-performance column liquid chromatography and high- performance thin-layer chromatography. J AOAC Int. 2008;91(4):750–755. [PubMed] [Google Scholar]
- 28.Anandkumar K, Ayyappan T, Raghu Raman V, et al. RP-HPLC Analysis of aspirin and clopidogrel bisulphate in combination. Indian J Pharm Sci. 2007;69(4):597–599. [Google Scholar]
- 29.Gandhimathi M, Ravi TK. High performance liquid chromatographic determination of aspirin and clopidogrel in tablets. Indian J Pharm Sci. 2007;69(1):123–125. [Google Scholar]
- 30.Shah DA, Bhatt KK, Metha RS, et al. RP-HPLC method for the determination of atorvastatin calcium and nicotinic acid in combined tablet dosage form. Indian J Pharm Sci. 2007;69(5):700–703. [Google Scholar]
- 31.Shah DA, Bhatt KK, Metha RS, et al. Development and validation of a RP- HPLC method for determination of atorvastatin calcium and aspirin in a capsule dosage form. Indian J Pharm Sci. 2007;69(4):546–549. [Google Scholar]
- 32.Mitakos A, Panderi I. A validated LC method for the determination of clopidogrel in pharmaceutical preparations. J Pharm Biomed Anal. 2002;28:431–438. doi: 10.1016/s0731-7085(01)00620-3. [DOI] [PubMed] [Google Scholar]
- 33.Gomez Y, Adams E, Hoogmartens J. Analysis of purity in 19 drug product tablets containing clopidogrel:18 copies versus the original brand. J Pharm Biomed Anal. 2002;34:341–348. doi: 10.1016/S0731-7085(03)00533-8. [DOI] [PubMed] [Google Scholar]
- 34.Sippel J, Sfair LL, Schapoval EE, et al. New high-performance liquid chromatographic method for determination of clopidogrel in coated tablets. J AOAC Int. 2008;91:67–72. [PubMed] [Google Scholar]
- 35.Dermis S, Aydogan E. Rapid and Accurate Determination of Clopidogrel In Tablets By Spectrophotoetric And Chromatographic Techniques. Commun Fac Sci Univ Ank. 2009;55(1):1–16. [Google Scholar]
- 36.Proceeding of the International Conference on Harmonization. Geneva, Switzerland: 2005. IHC. Validation of analytical procedures and methodology . [Google Scholar]


