Abstract
Mesenchymal stem cells (MSCs) are broadly used in cell‐based regenerative medicine because of their self‐renewal and multilineage potencies in vitro and in vivo. To ensure sufficient amounts of MSCs for therapeutic purposes, cells are generally cultured in vitro for long‐term expansion or specific terminal differentiation until cell transplantation. Although physiologically up‐regulated reactive oxygen species (ROS) production is essential for maintenance of stem cell activities, abnormally high levels of ROS can harm MSCs both in vitro and in vivo. Overall, additional elucidation of the mechanisms by which physiological and pathological ROS are generated is necessary to better direct MSC fates and improve their therapeutic effects by controlling external ROS levels. In this review, we focus on the currently revealed ROS generation mechanisms and the regulatory routes for controlling their rates of proliferation, survival, senescence, apoptosis, and differentiation. A promising strategy in future regenerative medicine involves regulating ROS generation via various means to augment the therapeutic efficacy of MSCs, thus improving the prognosis of patients with terminal diseases.
Keywords: mesenchymal stem cell, multilineage, reactive oxygen species, regenerative medicine, self‐renewal
1. INTRODUCTION
Mesenchymal stem cells (MSCs) are broadly used in cell‐based regenerative medicine because of their self‐renewal and multilineage potencies in vitro and in vivo. These cells can be easily isolated from various tissues and then cultured over multiple passages for further application.1 After transplantation in vivo, they migrate and repair injured tissues or organs, thus making a contribution to tissue engineering; the fracture site is able to induce senescence or apoptosis based on the surrounding harsh conditions and oxidative stress. However, MSCs gradually lose their proliferation and differentiation potential after long‐term ex vivo culture. To ensure sufficient amounts of active MSCs for cell therapy, the surrounding microenvironment is considerably important for MSCs in vitro and in vivo.
Under physiological conditions, adenosine triphosphate (ATP) production can be generated through glycolysis and oxidative phosphorylation (OXPHOS), while mitochondrial respiration generate a certain amount of reactive oxygen species (ROS) as byproducts. NADH‐coenzyme Q oxidoreductase (Complex I) and ubiquinol‐cytochrome c oxidoreductase (Complex III) release electrons for generation of superoxide anion (O2‐.).2 Superoxide dismutase (SOD) will be activated for generation of hydrogen peroxide (H2O2) and then H2O2 will be decomposed to O2 and H2O after activation of catalase (CAT) or glutathione peroxidase (GPx).2 In addition, other metabolic intermediates including 2‐oxoglutarate dehydrogenase, pyruvate dehydrogenase, glycerol 3‐phosphate dehydrogenase also contribute to the upregulated generation of ROS in MSCs.3 ROS has been commonly thought of as harmful to cell functioning and can lead to apoptosis and senescence of MSCs in vitro. For example, Geissler et al4 demonstrated that MSCs upregulated the level of ROS after long term culture for several passages, accompanied with the impaired mitochondrial function and differentiation ability. In addition, the upregulated ROS can cause lipid peroxidation, oxidative modification of proteins, DNA damage and cellular senescence.5, 6 However, physiologically up‐regulated ROS production is essential for stem cell activities. In an undifferentiated state, MSCs have low levels of intracellular ROS and high levels of antioxidative enzymes7; while differentiated MSCs show the opposite properties.8 On the other hand, physiologically up‐regulated ROS are required for MSC proliferation, while inhibition of ROS blocks MSC self‐renewal9 (Figure 1).
Figure 1.
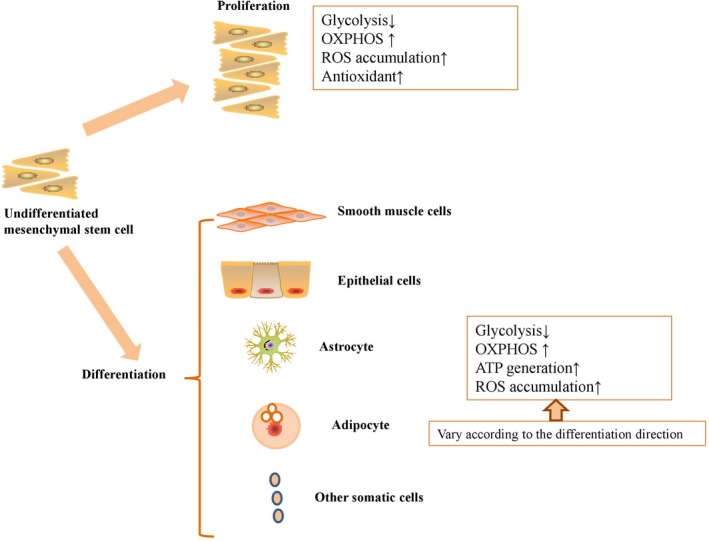
Physiologically up‐regulated ROS production is essential for MSC proliferation and differentiation
Commonly, ROS can be generated by mitochondria,10 endoplasmic reticulum (ER),11 cytosol,12 peroxisomes,13 plasma membrane,14 and extracellular space.15 Aerobic microenvironment brings out reduction of molecular oxygen, and then Ero1p will consequently yield stoichiometric H2O2.11 All‐trans arachidonic acid is able to improve the generation of ROS via xanthine dehydrogenase/xanthine oxidase interconversion in the in vitro liver cytosol.12 After peroxisomes are supplemented with uric acid, H2O2 will be generated at a rate which corresponds to 42%‐61% of the rate of uric acid oxidation.13 Fibroblast contains an ectoplasmic enzyme, distinct from NADPH oxidase, which can generate a major source of ROS after tissue damage.14 NADPH oxidase maintains endothelial cell xanthine oxidase activity for upregulating ROS generation in response to oscillatory shear stress.15 Although ROS are generated by various organelles, the mitochondrion is the main organelle of ATP and ROS generation.16 Respiratory chain electrons escape the mitochondrion, leading to superoxide ion and H2O2 generation due to superoxide dismutation via SOD catalysis in MSCs.17, 18, 19, 20 Overproduction of ROS enhances autophagy and apoptosis in MSCs through activation of c‐Jun NH(2)‐terminal kinase (JNK) signalling.21 In this process, colocalization of ataxia telangiectasia mutated (ATM), histone H2A.X, and p53‐binding protein 1 (53BP1) results in Bcl‐2‐associated X protein (BAX)‐Bcl‐2 homologous antagonist/killer (BAK) dimerization; subsequent release of cytochrome c to the cytoplasm and caspase release triggers apoptosis.22, 23 For cellular homeostasis, endogenous scavengers help to remove excessive ROS, including the following: enzymatic proteins such as SOD, peroxiredoxins, glutathione peroxidase and lysosomal catalases; and non‐enzymatic antioxidants such as vitamins, carotenoids and flavonoids.24, 25, 26, 27 Under physiological conditions, mitochondrially derived ROS include superoxide anion, hydroxyl radical, singlet oxygen, hydrogen peroxide, nitric oxide, and peroxynitrite.28 However, the incomplete oxidation of oxygen to water by mitochondrial complexes leads to excessive ROS production. Various conditions including ageing, long‐term culture and H2O2, adverse oxygen content, high glucose, proinflammatory cytokines and other toxic factors, abnormally up‐regulated ROS production can harm MSC activities.22, 29, 30, 31, 32, 33 On the other hand, although MSCs play critical roles on immunomodulatory properties for therapy in various diseases, the constant isolation from donors also bring out unrecoverable injury.34
In the current review, we mainly discuss the known ROS generation mechanisms and the regulatory routes for controlling MSC fates. Some key signalling pathways for maintaining energy metabolism and ROS homeostasis play particularly important roles in regulating MSC fates ex vivo and in vivo (Table 1). Based on these regulatory pathways, it may be possible to control MSC fates in vitro and in vivo by regulating ROS levels surrounding MSCs for future regenerative medicine.
Table 1.
The key signalling pathways for maintaining energy metabolism and ROS homeostasis of MSCs ex vivo and in vivo
| Factor | Signalling pathway/mechanism | Effect on MSCs | Ref |
|---|---|---|---|
| Age | DNA methylation status↑ | Oxidative stress↑; mitochondrial function↓ | 29 |
| Long‐term culture | ROS‐induced suppression of c‐Maf↑ | Proliferative ability↓; differentiation capacity↓ | 30 |
| Long‐term culture | p38↑; MAPK↑; p53/p21↑ | Senescence↑ | 70 |
| H2O2 | p53↑; p21 ↑; p38↑; pRb↓ | Cell cycle↓; DNA damage↑ | 22 |
| Adipogenic differentiation | mTOR↑; NOX‐4↑; FOXO↑ | PPARγ↑ | 55, 56, 57, 58, 59 |
| Chondrogenic differentiation | AKT↑; ERK↑ | NOX‐2↑; NOX‐4↑ | 8 |
| Osteogenic differentiation | Wnt/β‐catenin↑ | ROS↑; apoptosis and necrosis rates↑ | 60, 61 |
| β‐ME | Notch1↓ | ROS↓; neural stem cell‐specific proteins↑ | 62 |
| Particulate matter | AKT↓ | MSC proliferation↓; ROS↑ | 36 |
| Old rat serum | Wnt/β‐catenin↑ | MSC proliferation↓; ROS↑ | 37 |
| FGF‐23 | p53↑; p21↑ | Senescence↑ | 33 |
| TNF‐α | NF‐κB↑ | Survival rate↑; migratory capacity↑ | 39 |
| Atmospheric oxygen | p53↑ | Viability↓; cell growth↓ | 31 |
| Hypoxia | AKT↑; mTOR↑ | Proliferative capacity↑; differentiation↑ | 43, 44 |
| Interleukin‐17 | MEK‐ERK↑ | MSC proliferation↑; migration↑; motility↑ | 83 |
| PEDF | p53↓; p16 ↓ | Cell survival rates in long‐term culture↑ | 85 |
| NAC | Wnt/β‐catenin↓ | Self‐renewal↑, multipotency↑; apoptosis rate↓; cell adhesion capacity↑ | 60, 89, 90, 91 |
| Fucoidan and carvedilol | p38‐MAPK↓; JNK↓; caspase 3↓; AKT↓ | H2O2‐induced injury↓ | 101, 102 |
| AGEs | ROS‐p38 mediated pathway↑ | Proliferative capacity↓; migratory capacity↓ | 49 |
| Apocynin | NOX↓ | Ageing process↓; osteogenesis↑ of ageing MSCs | 108 |
| Lycopene/Cirsium setidens | p38‐MAPK↓; JNK↓; ATM ↓; p53↓ | H2O2‐induced ROS generation↓; survival rate↑ | 109, 110 |
| CoQ10 | AKT/mTOR↑ | D‐galactose‐induced MSC ageing↓; ROS↓ | 137 |
| Menadione and 2,3‐dimethoxy‐1,4‐naphthoquinone | ERK1/2↑; JNK1/2↑ | Migration capacity↑ | 116 |
| Cholesterol | ROS/p53/p21Cip1/Waf1 signalling pathway ↑ | MSC senescence↑ | 117 |
| High‐density lipoprotein | PI3K/Akt pathway↑ | Cell viability↑; apoptosis↓ | 118 |
| miR‐210 inducers | ERK1/2↑; AKT ↑; c‐Met↓ | ROS‐induced apoptosis rate↓ | 82, 132 |
| Knockout of exon 4 of pNO40/PS1D in MSCs | p16↑; Rb ↑ | Ageing↑; osteogenic differentiation defect↑ | 124 |
2. SURROUNDING MICROENVIRONMENTS FOR REGULATING ROS PRODUCTION IN MSCS
Stem cells have been assumed to rely on anaerobic energy metabolism, as they are always in a hypoxic microenvironment before they are isolated from the source tissues. Because MSCs cultured in vitro are sensitive to the surrounding microenvironment, optimization of culture conditions is important for long‐term culture without loss of stem cell properties.
Contact culture at 100% confluence significantly increases ROS levels and promotes MSC senescence without influencing expression levels of telomerase reverse transcriptase (TERT) and p53.35 Moreover, when added to the culture medium, multiple factors have a negative impact on MSC properties. For example, particulate matter or old rat serum inhibits MSC proliferation and increases ROS formation by attenuating signalling via AKT phosphorylation and activating Wnt/β‐catenin signalling, respectively.36, 37 On the other hand, fibroblast growth factor 23 (FGF‐23) promotes MSC senescence by up‐regulating p53 and p21 expression levels,33 and TGF‐β1 significantly down‐regulates SOD2 and Id1 expression levels in MSCs, thus increasing levels of senescence‐related genes in a dose‐dependent manner.38 In contrast, preconditioning MSCs with tumor necrosis factor alpha (TNF‐α) strongly enhances their proliferative and migratory capacities; indeed, this cytokine effectively increases the survival rate and migratory capacity of MSCs even under oxidative stress in vivo.39 Furthermore, high glucose increases the expression levels of pluripotent markers in MSCs but does not alter the adipogenic and osteogenic differentiation capacities of the cells; it also decreases proliferation and migratory capacities of MSCs by enhancing ROS levels.32 Hypoxic conditions can decrease the expression levels of antioxidants, including glutathione, glutathione peroxidase and SOD1, and can increase the apoptosis rates of MSCs.40 Under culture conditions of extreme hypoxia and exposure to metal carcinogens, MSCs exhibit increased levels of oncogenic proteins and decreased levels of tumour suppressor proteins yet aberrant proliferation rates and redox mechanisms via intracellular ROS accumulation in vitro.41 In contrast, hypoxic conditions may also protect MSCs from injury, promoting proliferation, differentiation, and survival in a ROS‐mediated manner. A paradoxical discovery was that compared with normoxia, MSCs cultured under hypoxia showed a smaller size and greater cellular complexity, lower proliferation and mitochondrial activity, and reduced autophagy.42 It also moderately increases ROS production and promotes the proliferative capacity and differentiation potency of MSCs via phosphorylation of the AKT and mTOR pathways.43, 44 Intriguingly, long‐term hypoxic conditions upregulated the expression of HIF‐1α, and the hypoxia MSCs showed greater cell viability, decreased ROS levels and increased resistance to oxidative stress for alleviating both early radiation‐induced pneumonia and late pulmonary fibrosis when compared to normoxia MSCs.45 In addition, a low dose of hypoxic MSCs but not a high dose of hypoxic MSCs can effectively attenuate ischemia/reperfusion (I/R) induced permeability pulmonary oedema by decreasing ROS related inflammatory responses and anti‐apoptosis effect.46 On the other hand, Karlsen et al47 showed that hyperoxia of room air was not adverse for the proliferation, differentiation, or phenotype of bone marrow derived MSCs in vitro. Saini et al48 demonstrated that preconditioned with hyperoxia decreased the apoptosis of MSCs, while increased the the level of survival markers including Akt1, NF‐κB, and Bcl‐2. However, whether hyperoxia will alter the MSC activities via ROS related pathway need to to be further investigated. Advanced glycation products (AGEs) can dose‐dependently decrease the proliferative and migratory capacities of MSCs via activation of the ROS‐p38 mediated pathway in a dose‐dependent manner.49 In fact, addition of AGEs significantly enhances the levels of osteogenic markers while inhibiting the maturation of osteogenic MSCs; moreover, AGEs inhibit MSC adipogenic and chondrogenic differentiation.50
3. ROS GENERATION AND THE IN VITRO DIFFERENTIATION FATE OF MSCS
In general, undifferentiated MSCs have fewer mitochondria than differentiated MSCs, though the mitochondrial copy number, OXPHOS supercomplex, SOD expression, mitochondrial biogenesis and ROS levels are all significantly increased after specific differentiation.51, 52 ROS imbalance will lead to a significantly reduced MSC differentiation capacity,53 thus limiting MSC applications in vitro and in vivo. When adult MSCs are incubated under stress‐inducing conditions, they are considered to be in a state of cyclic stretch and produce more ROS and have weaker differentiation capacities.54 However, stimulation by different factors will induce MSCs to differentiate into various terminal somatic cells; according to current studies, different differentiation directions lead to various degrees of ROS production. The mammalian target of rapamycin (mTOR),55 mitochondrial biogenesis,56 NOX‐4,57 and forkhead box O (FOXO)58 pathways are activated after adipogenic differentiation of MSCs, and ROS expression levels are strongly enhanced. This excessive ROS causes a positive feedback on activating peroxisome proliferator‐activated receptor gamma (PPARγ) to accelerate adipogenic differentiation.59 Similarly, chondrogenic differentiation of MSCs has been demonstrated to activate AKT and extracellular signal‐regulated kinase (ERK) signalling and to up‐regulate expression of ROS‐related proteins, including NOX‐2 and NOX‐4. Excessive levels of ROS also lead to positive feedback on the chondrogenic progress.8 Osteogenic differentiation in MSCs significantly activates the Wnt/β‐catenin pathway and increases intracellular ROS levels, and the chromatin fragmentation caspase 3 expression induced by osteogenic differentiation consequently results in increased apoptosis and necrosis rates in MSCs.60, 61 As a type of antioxidant, β‐mercaptoethanol (β‐ME) is able to gradually increase the expression levels of neural stem cell‐specific proteins via down‐regulation of Notch1 expression and ROS production.62 However, Esmaeli et al63 demonstrated that although umbilical cord derived MSCs decreased the levels of polyunsaturated fatty acids and gradually enhanced the levels of saturated fatty acids after hepatogenic differentiation, these effects were independent of ROS production and lipid peroxidation at the end stage of hepatic differentiation. Therefore, the relationship between ROS generation and MSC differentiation fate should be elucidated before any conclusions regarding how to direct ROS towards controlling the direction of differentiation can be drawn.
4. ANTIOXIDATIVE CAPACITIES OF SENESCENT AND APOPTOTIC MSCS
Age, long‐term culture and H2O2 are important factors for inducing MSC senescence and can easily alter mitochondrial morphology, decrease antioxidant capacities, elevate ROS levels and increase the apoptosis rate. Reduced proliferation and differentiation capacities consequently decrease the therapeutic potential of MSC‐based treatments.4, 64, 65 Aged MSCs undergo senescence through increased DNA methylation, oxidative stress and mitochondrial function impairment.29 However, controversy remains regarding the pluripotency as well as the migration and antioxidative capacities of aged MSCs, as Lund et al66 demonstrated that all of the above features are equivalent in bone marrow derived MSCs from young and old individuals. Another studies conflict with the assertion that donor age negatively impacts MSC suppression of T cell proliferation67, 68; one of these studies analysed 53 human donors ranging within 13‐80 years demonstrated no significant correlation between age and T cell suppression capability.67 Moreover, although Li et al69 demonstrated that MSCs derived from old donors showed a more rapidly decreased survival rate than MSCs derived from young donors in the infarct region in acute myocardial infarction (MI) model, N‐acetyl‐L‐cysteine (NAC) which is a ROS scavenger can protected MSCs derived from old donors from apoptosis in vivo and significantly enhanced the therapeutic effects of MSCs. Long‐term culture always results in proliferative decline, cell cycle arrest and decreased differentiation capacity in adipose‐derived MSCs by ROS‐induced suppression of c‐Maf.30 However, long‐term culture of umbilical cord blood‐derived MSCs reinforces senescence by activating p38, MAPK, and p53/p21 pathways and enhancing ROS generation.70 According to the basic classification, long‐term cultured MSCs can be divided into early passaged MSCs and late‐passaged MSCs. With increasing passage number, ROS production and the apoptosis rate gradually increase, whereas antioxidant enzyme expression and differentiation capacities gradually decrease.71, 72 Furthermore, the morphology and release of inflammatory factors are positively related to passage number, as late‐passaged MSCs are flatter and more enlarged in morphology and release higher levels of inflammatory factors, including interferon gamma (IFNγ) and TNF‐α.73, 74 The apoptosis rate of MSCs may also be increased via the release of nuclear factor erythroid2‐related factor 2 (Nrf2), nuclear factor (NF)‐κB, Toll‐like receptor 4 (TLR4) and other inflammatory factors.75 Jeong et al76 argued that expression of apoptosis‐related genes in bone marrow derived MSCs is notably increased at the early stage of passaging but not altered in subsequent passaging stages. Regarding this debate, more studies should be performed to investigate the underlying mechanisms for ROS regulation in MSCs during in vitro culture. H2O2, an important factor that leads to oxidative stress‐induced premature senescence (OSIPS), has been demonstrated to inhibit MSC proliferation in a concentration‐dependent manner.6, 77 H2O2 enhances the release of NF‐κB and β‐catenin as well as the motility of MSCs by enhancing degradation of collagen 5 and fibronectin78 and further facilitates osteogenic differentiation of MSCs via up‐regulation of NFE2L2 expression.79 After treatment with 200 μm H2O2 for 3 to 4 passages, wharton's Jelly derived MSCs become morphologically heterogeneous and irregularly flattened, with significantly up‐regulated expression of senescence markers, including SA‐β‐galactosidase, p53, p21, p16 and lysosomal β‐galactosidase.80 Moreover, treatment with 0.1 mmol/L H2O2 reduced the proliferative capacity and pluripotent markers of adipose derived MSCs at 2 to 5 passages as a consequence.79 At the same time, H2O2 induces cell cycle arrest and the DNA damage response by activating p53, p21 and p38 MAPK signalling and inactivating pRb signalling.22 Overall, MSC senescence is closely linked to ROS‐related pathways and senescence‐related gene expression, which lead to apoptosis and necrosis in MSCs.
5. STRATEGIES TO CONTROL ROS LEVELS FOR MSC FATES IN VITRO AND IN VIVO
5.1. Growth factors or extracellular matrix components for ROS regulation in MSCs
To eliminate the increased ROS levels and their corresponding side effects, strategies have been developed to enhance cellular activities in vitro and in vivo. Exposure to basic fibroblast growth factor (bFGF) significantly enhances the proliferative activity and reduces the cellular senescence and apoptosis in MSCs by ameliorating ROS‐induced oxidative stress.81 Platelet‐derived growth factor (PDGF)‐BB contributes to ROS generation to mild degrees and increases the proliferation and migration capacities of adipose‐derived MSCs.82 In addition, interleukin‐17 (IL‐17) activates Rac1 GTPase, NOX1, and the MEK‐ERK pathway, resulting in ROS generation, and consequently stimulates MSC proliferation, migration and motility.83 Ex‐4 preconditioning protects MSCs against H2O2‐induced apoptosis in a dose‐dependent manner,84 and pigment epithelium‐derived factor (PEDF) increases MSC survival rates in long‐term culture by attenuating p53 and p16 signalling.85 In addition, various types of extracellular matrix components including decellularized cell‐deposited extracellular matrix and cardiogel derived from cardiac fibroblast strongly promote the self‐renewal and differentiation of MSCs by up‐regulating the expression of intracellular antioxidative enzymes and maintaining low levels of ROS even under excessive H2O2 conditions in vitro.86, 87 In addition, Lai et al88 reconstructed a native extracellular matrix which is consisted of collagen, fibronectin, small leucine‐rich proteoglycans and major components of basement membrane to maintain the cell activities of MSCs via downregulation of ROS level in vitro.
5.2. Pharmaceuticals for ROS regulation in MSCs
To augment regimens for regulating ROS production in MSC‐based regenerative medicine, an increasing number of pharmaceuticals have been tested for particular and strong effects. As a strong antioxidant, NAC preserves MSC activities, including high self‐renewal, multipotency, a low apoptosis rate and strong cell adhesion capacity, by preventing activation of Wnt/β‐catenin signalling and excessive cellular ROS production.60, 89, 90, 91 Buthionine sulfoxide significantly increases albumin and ROS levels in hepatogenic MSCs, whereas NAC inhibits these related metabolic functions.92 However, NAC has also been demonstrated to inhibit adipocyte differentiation59 and to maintain the osteogenic differentiation capacity of MSCs by decreasing ROS levels.54 Moreover, a combination of NAC and L‐ascorbic acid 2‐phosphate promoted the growth of MSCs and suppressed oxidative stress‐induced cell death by enhancing mitochondrial integrity and function in vitro under H2O2‐induced oxidative stress.93 Preconditioning with Vitamin E eliminates H2O2‐induced injury in MSCs in vitro, and transplantation of these pretreated MSCs increases the proteoglycan content of cartilage matrix for tissue repair of osteoarthritis in vivo.94 In addition, metformin can be applied to rescue H2O2‐induced senescence, long‐term culture‐induced senescence and hypoxia/serum deprivation (Hy/SD)‐induced apoptosis in MSCs.95, 96, 97
Intriguingly, fullerol eliminates cellular ROS, effectively retarding the adipogenic differentiation capacity of MSCs while enhancing their osteogenic differentiation capacity.98, 99 Another compound, tricyclodecan‐9‐yl‐xanthogenate markedly increases the number of MSCs differentiating into neurons by increasing ROS levels and expression of antioxidative enzymes, but not by enhancing different SOD activities.100 Two medicines widely used clinically, fucoidan and carvedilol, protect in vitro MSCs from H2O2‐induced injury via inhibition of p38‐MAPK, JNK, caspase 3 and AKT as well as ROS‐related pathways.101, 102 Nicorandil attenuates Hy/SD‐induced apoptosis by reducing ROS generation, maintaining the stability of matrix metalloproteinases and decreasing the release of apoptosis‐related molecules.103 5‐Azacytidine was also found to drive aged MSCs into a dynamic state by increasing their proliferative capacity and decreasing oxidative stress and DNA methylation.29 In addition, some drugs have been demonstrated to act as double‐edged swords in clinical therapies due to their ROS scavenger functions or ROS‐mediated cellular toxicity. For instance, low and high doses of trichostatin A or isothiocyanates have protective and harmful effects on oxidative stress in MSCs through opposite regulatory mechanisms of SOD2 and FOXO1.104, 105
5.3. Extracts from natural products for ROS regulation in MSCs
In addition to drugs used in the clinic, a large number of extracts from natural products, including herbs, nutritional factors, and hormones, have important functions in regulating ROS generation in MSCs. Capsaicin (8‐methyl‐N‐vanillyl‐trans‐6‐nonenamide) extracted from red pepper induces cell cycle arrest and inhibits early adipogenic differentiation by increasing ROS and reactive nitrogen species (RNS) production in a dose‐ and time‐dependent manner.106 Gastrodin, the main active component in Gastrodia elata, significantly enhances the proliferative and osteogenic differentiation capacities of MSCs while inhibiting their adipogenic differentiation capacity via suppression of ROS generation.107 Apocynin, a phenolic compound, partially reverses the ageing process and enhances the potential for osteogenesis in ageing MSCs by suppressing NOX.108 Both lycopene and Cirsium setidens pretreatments suppressed H2O2‐induced ROS generation and increased survival of MSCs by attenuating the phosphorylated p38‐MAPK, JNK, ATM and p53 signalling pathways.109, 110 Berberine, a natural isoquinoline quaternary alkaloid extracted from the Chinese herb Huanglian, significantly prevents H2O2‐induced apoptotic progression by reducing ROS production and levels of apoptosis‐related proteins, while increasing SOD activity and p‐AKT and Bcl‐2 levels.111 Astragalus polysaccharide, which is isolated from Astragalus membranaceus, is able to counter the reductions in proliferation and pluripotency and to prevent the increased apoptosis and senescence in MSCs induced by ferric ammonium citrate (FAC).112
In addition to the extracts isolated from plants described above, compounds derived from animals also have antioxidative capacities for ROS regulation. Melatonin maintains MSC survival and their osteogenic differentiation capacity and chondrogenesis in an IL‐1β‐induced inflammatory environment by down‐regulating MMP expression and up‐regulating that of SOD.113, 114 Preconditioning with melatonin significantly increased the expression of the antioxidant enzyme catalase and Cu/Zn SOD‐1 and the other factors including pro‐angiogenic and mitogenic factors in MSCs in vitro; moreover, melatonin pre‐treatment enhanced the activity of engrafted adipose derived MSCs and enhanced their therapeutic potency in a rat model of myocardial infarction (MI).96 Combination of melatonin and apoptotic adipose‐derived MSCs was superior to apoptotic adipose‐derived MSCs alone in protecting the kidneys from sepsis‐induced injury, as this group well‐maintained the kidney function via decreasing the protein expressions of inflammatory, apoptotic, fibrotic markers, ROS and oxidative stress.115 A panel of polypeptides, namely, menadione and 2,3‐dimethoxy‐1,4‐naphthoquinone, effectively causes ROS production and promotes the migration capacity of human MSCs via stimulation of ERK1/2 and JNK1/2 signalling.116 Furthermore, cholesterol promotes MSC senescence via the ROS/p53/p21Cip1/Waf1 signalling pathway,117 while pretreatment with high‐density lipoprotein significantly increases cell viability and reduces the rate of apoptosis by decreasing ROS production via the phosphatidylinositol‐4,5‐bisphosphate 3‐kinase (PI3K)/AKT pathway.118 Parathyroid hormone, which increases Ca2+ levels in mammals, also effectively enhances cell viability and endogenous insulin‐like growth factor 1 levels by inhibiting ROS‐related pathways.119 17‐β‐Estradiol significantly protects MSCs against serum deprivation‐induced ROS overexpression and reduces lipid peroxidation, accompanied by a reduction in the Bax/Bcl‐2 ratio and caspase 3 level.120
As stem cell activities and differentiation capacities are important for MSC‐based therapeutic effects on repairing injury in vivo, drug treatments can also protect implanted MSCs from damage and then improve their biological activities for cell‐based treatments. The combination of bone marrow‐derived MSCs with plumbagin reduces the spinal cord water content after spinal cord injury by synergistically activating the Nrf2 pathway and regulating oxidative stress, inflammation and apoptosis in vivo.121 Injection of adipose‐derived MSCs and icariin markedly prevented apoptosis in transplanted MSCs and repaired erectile tissue structures in erectile dysfunction models, accompanied by increased levels of endothelial markers and smooth muscle markers in vivo.122
5.4. Gene modification for regulating ROS generation in MSCs
Gene modification is widely used in regenerative medicine for reprogramming somatic cells into pluripotent cells. Recently, this technology has also been applied to alter intracellular ROS levels to maintain stem cell properties in vitro and in vivo. Although oxidative stress can easily induce diverse nuclear malformations, overexpression of TERT in MSCs protects them from nuclear damage and significantly improves the basal activities of antioxidant enzymes, SOD and catalase in MSCs.123 Knockout of exon 4 of pNO40/PS1D in MSCs results in elevated ROS levels and p16 and Rb expression, consequently accelerating ageing and osteogenic differentiation defects.124 Similarly, MSCs isolated from Tks4 knock‐out mouse have reduced osteogenic and adipogenic differentiation capacities compared with those isolated from wild‐type mice.125 Although knockdown of NOX4 suppressed ROS production and adipogenic differentiation in MSCs,126 Kim et al43 demonstrated that NOX4 silencing did not inhibit MSC adipocyte differentiation. Modification of genes in MSCs not only exerts important effects on their differentiation capacities but also influences cell senescence or apoptosis. Overexpression of TERT also protects long‐term cultured MSCs from senescence via similar mechanisms.127 Overexpression of PPARγ co‐activator 1α increases the survival rate of MSCs by reducing intracellular and mitochondrial ROS, even under conditions of high glucose, hypoxia and serum deprivation.128 Similarly, overexpression of proteasome subunit beta 5 (PSMB5) maintains the differentiation capacity of late‐passage MSCs, even under exposure to H2O2,129 and overexpression of heme oxygenase 1 in MSCs significantly attenuates H2O2‐induced injury of retinal ganglion cells by decreasing levels of cellular ROS and proapoptotic proteins. Thus, modified MSCs are highly effective at attenuating retinal I/R injury by maintaining structural thickness and decreasing apoptosis.130 In addition to general virus‐mediated overexpression of critical genes, microRNAs (miRNAs) and small molecules contribute to the regulation of MSC activities by modulating ROS levels. Overexpression of microRNA‐34a, a p53‐targeted miRNA, significantly enhances apoptosis, impairs cell vitality and aggravates senescence by promoting mitochondrial dysfunction and activating intrinsic apoptosis pathways; unfortunately, the harmful effects on MSCs can only be partially abolished by NAC.131 Specific miR‐210 inducers activate ERK1/2 and AKT pathways in MSCs but inhibit the c‐Met pathway, thus eliminating ROS‐induced apoptosis in MSCs.82, 132 Small inhibitory RNAs (siRNAs) targeting insulin‐like growth factor 1 and Nrf‐2 counteract the protective effects of parathyroid hormone in MSCs in vitro by enhancing ROS generation.119 In contrast, siRNA targeting caspase 3 strongly attenuates H2O2‐induced apoptosis of MSCs,133 and siRNA targeting p16 reverses LPS‐induced DNA damage and MSC senescence.134 As let‐7b has been identified as one of the mediators targeting caspase‐3 for eliminating ROS‐induced apoptosis and autophagy in MSCs, administration of let‐7b‐modified MSCs maintained ventricular function and accelerated myocardial repair in rat cardiac IR model.135 Intra‐myocardial injection of adenovirus‐ecSOD transfected MSCs exerted cardiac protection against MI partly via reduction of oxidative stress and enhancing the survival rate of MSCs.136
6. CONCLUSIONS
Although MSCs serve as promising resources for repairing tissue or organ functions, excessive ROS can be generated during MSC expansion and differentiation processes. In addition, MSC properties are partially dependent on oxidative stress; hence, novel approaches for optimizing ROS generation in MSCs can contribute to their regenerative applications (Figure 2). Multiple antioxidative enzymes and non‐enzymes participate in the physiological regulation of the redox balance of MSCs, and high levels of intracellular or extracellular ROS may trigger the DNA damage response, senescence and loss of potency of MSCs in vitro. These effects are strongly prohibitive for MSC proliferation and differentiation and thus not beneficial for MSC transplantation in vivo for experimental or clinical therapy. Moreover, transplanted MSCs face harsh environments that induce apoptosis and impair migration, markedly limiting the efficacy of MSC‐based therapeutics, thus the improvement of survival and antioxidant ability of MSCs in vivo should be confirmedMost importantly, clarification of the regulatory mechanisms maintaining the MSC redox balance in vitro and in vivo will help to retain stem cell activities even under adverse conditions. Moreover, improving the culture conditions for various resources derived from MSCs is critical for maintaining pluripotency and enhancing their differentiation capacity in vitro. Although the effects of some antioxidants vary somewhat among different stem cell types or different culture conditions, a correlation between the antioxidant defence level and stem cell fate decision does exist. Fortunately, some natural extracts that rarely exert side‐effects can be applied as strong antioxidants in oxidative stress‐induced microenvironments. In addition, gene modifications that were originally used to generate stem cells from somatic cells also serve as advanced technologies for ROS regulation in MSC‐based tissue engineering. All these strategies potentially help to improve the cell activities of MSCs but decrease the ROS level in vivo for enhancing the therapeutic effects. On the other hand, it is still necessary to further elucidate the mechanisms of physiological and pathological ROS generation, external ROS regulation via of multiple compounds and specific gene modifications. Only in this way will we effectively regulate ROS levels to take advantage of the mass multiplication and strong differentiation capacities of MSCs, which will allow for harnessing the immortality of MSCs for transplantation and therapy in regenerative medicine.
Figure 2.
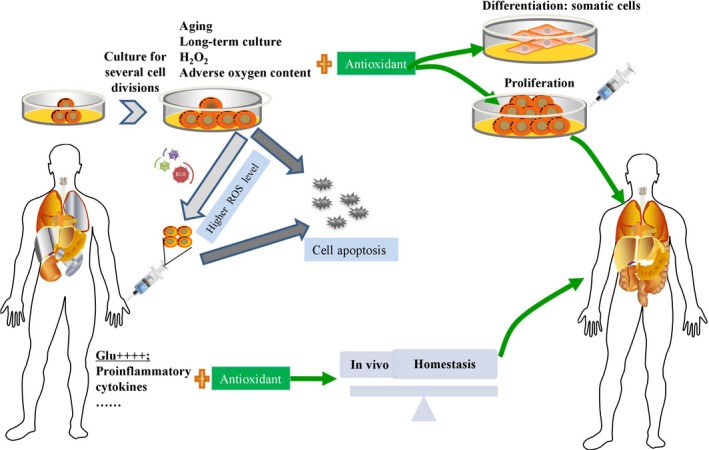
Antioxidants for MSCs contribute to the regenerative applications of MSCs
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No.: 81700553), the Stem Cell and Translational Research, the National Key Research and Development Program of China (No. 2016YFA0101001) and the China Postdoctoral Science Foundation (No. 2017M183789).
Hu C, Zhao L, Peng C, Li L. Regulation of the mitochondrial reactive oxygen species: Strategies to control mesenchymal stem cell fates ex vivo and in vivo. J Cell Mol Med. 2018;22:5196–5207. 10.1111/jcmm.13835
Chenxia Hu and Lingfei Zhao contributed equally to this work.
REFERENCES
- 1. Hu C, Li L. In vitro and in vivo hepatic differentiation of adult somatic stem cells and extraembryonic stem cells for treating end stage liver diseases. Stem Cells Int. 2015;2015:871972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quinlan CL, Perevoshchikova IV, Hey‐Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geissler S, Textor M, Kuhnisch J, et al. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One. 2012;7:e52700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adam‐Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639‐645. [DOI] [PubMed] [Google Scholar]
- 6. Kim JS, Kim EJ, Kim HJ, Yang JY, Hwang GS, Kim CW. Proteomic and metabolomic analysis of H2O2‐induced premature senescent human mesenchymal stem cells. Exp Gerontol. 2011;46:500‐510. [DOI] [PubMed] [Google Scholar]
- 7. Valle‐Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885‐1893. [DOI] [PubMed] [Google Scholar]
- 8. Kim KS, Choi HW, Yoon HE, Kim IY. Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem. 2010;285:40294‐40302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyublinskaya OG, Borisov YG, Pugovkina NA, et al. Reactive oxygen species are required for human mesenchymal stem cells to initiate proliferation after the quiescence exit. Oxid Med Cell Longev. 2015;2015:502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolisetty S, Jaimes EA. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci. 2013;14:6306‐6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gross E, Sevier CS, Heldman N, et al. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci USA. 2006;103:299‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakuma S, Kitamura T, Kuroda C, et al. All‐trans Arachidonic acid generates reactive oxygen species via xanthine dehydrogenase/xanthine oxidase interconversion in the rat liver cytosol in vitro. J Clin Biochem Nutr. 2012;51:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Donnell VB, Azzi A. High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid‐metabolizing enzyme. Biochem J. 1996;318(Pt 3):805‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290‐H2297. [DOI] [PubMed] [Google Scholar]
- 16. Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59:612‐619. [DOI] [PubMed] [Google Scholar]
- 17. Tormos KV, Anso E, Hamanaka RB, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960‐968. [DOI] [PubMed] [Google Scholar]
- 19. Drose S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre‐ and postconditioning. Biochim Biophys Acta. 2013;1827:578‐587. [DOI] [PubMed] [Google Scholar]
- 20. Perales‐Clemente E, Folmes CD, Terzic A. Metabolic regulation of redox status in stem cells. Antioxid Redox Signal. 2014;21:1648‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu GY, Jiang XX, Zhu X, et al. ROS activates JNK‐mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol Sin. 2015;36:1473‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borodkina A, Shatrova A, Abushik P, Nikolsky N, Burova E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging (Albany NY). 2014;6:481‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19:546‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebert R, Ulmer M, Zeck S, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24:1226‐1235. [DOI] [PubMed] [Google Scholar]
- 25. Song H, Cha MJ, Song BW, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555‐563. [DOI] [PubMed] [Google Scholar]
- 26. Koopman WJ, Nijtmans LG, Dieteren CE, et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431‐1470. [DOI] [PubMed] [Google Scholar]
- 27. Hanschmann EM, Lonn ME, Schutte LD, et al. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2‐Cys peroxiredoxin Prx3. J Biol Chem. 2010;285:40699‐40705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pryor WA, Houk KN, Foote CS, et al. Free radical biology and medicine: it's a gas, man!. Am J Physiol Regul Integr Comp Physiol. 2006;291:R491‐R511. [DOI] [PubMed] [Google Scholar]
- 29. Kornicka K, Marycz K, Marędziak M, Tomaszewski KA, Nicpoń J. The effects of the DNA methyltranfserases inhibitor 5‐Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J Cell Mol Med. 2017;21:387‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen PM, Lin CH, Li NT, et al. c‐Maf regulates pluripotency genes, proliferation/self‐renewal, and lineage commitment in ROS‐mediated senescence of human mesenchymal stem cells. Oncotarget. 2015;6:35404‐35418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boregowda SV, Krishnappa V, Chambers JW, et al. Atmospheric oxygen inhibits growth and differentiation of marrow‐derived mouse mesenchymal stem cells via a p53‐dependent mechanism: implications for long‐term culture expansion. Stem Cells. 2012;30:975‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng NC, Hsieh TY, Lai HS, Young TH. High glucose‐induced reactive oxygen species generation promotes stemness in human adipose‐derived stem cells. Cytotherapy. 2016;18:371‐383. [DOI] [PubMed] [Google Scholar]
- 33. Sato C, Iso Y, Mizukami T, et al. Fibroblast growth factor‐23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem Biophys Res Commun. 2016;470:657‐662. [DOI] [PubMed] [Google Scholar]
- 34. Wang LT, Jiang SS, Ting CH, et al. Differentiation of mesenchymal stem cells from human induced pluripotent stem cells results in downregulation of c‐Myc and DNA replication pathways with immunomodulation toward CD4 and CD8 cells. Stem Cells. 2018;36:903‐914. [DOI] [PubMed] [Google Scholar]
- 35. Ho JH, Chen YF, Ma WH, Tseng TC, Chen MH, Lee OK. Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: roles of Ras and oxidative stress. Cell Transplant. 2011;20:1209‐1220. [DOI] [PubMed] [Google Scholar]
- 36. Cui Y, Jia F, He J, et al. Ambient fine particulate matter suppresses in vivo proliferation of bone marrow stem cells through reactive oxygen species formation. PLoS One. 2015;10:e0127309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang DY, Wang HJ, Tan YZ. Wnt/beta‐catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One. 2011;6:e21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu J, Niu J, Li X, Wang X, Guo Z, Zhang F. TGF‐beta1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bai X, Xi J, Bi Y, et al. TNF‐alpha promotes survival and migration of MSCs under oxidative stress via NF‐kappaB pathway to attenuate intimal hyperplasia in vein grafts. J Cell Mol Med. 2017;21:2077‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Majumdar D, Bhonde R, Datta I. Influence of ischemic microenvironment on human Wharton's Jelly mesenchymal stromal cells. Placenta. 2013;34:642‐649. [DOI] [PubMed] [Google Scholar]
- 41. Crowder SW, Horton LW, Lee SH, et al. Passage‐dependent cancerous transformation of human mesenchymal stem cells under carcinogenic hypoxia. FASEB J. 2013;27:2788‐2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pezzi A, Amorin B, Laureano A, et al. Effects of hypoxia in long‐term in vitro expansion of human bone marrow derived mesenchymal stem cells. J Cell Biochem. 2017;118:3072‐3079. [DOI] [PubMed] [Google Scholar]
- 43. Kim JH, Kim SH, Song SY, et al. Hypoxia induces adipocyte differentiation of adipose‐derived stem cells by triggering reactive oxygen species generation. Cell Biol Int. 2014;38:32‐40. [DOI] [PubMed] [Google Scholar]
- 44. De Barros S, Dehez S, Arnaud E, et al. Aging‐related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol Ther. 2013;21:399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li B, Li C, Zhu M, et al. Hypoxia‐induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation‐induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem. 2017;44:1295‐1310. [DOI] [PubMed] [Google Scholar]
- 46. Liu YY, Chiang CH, Hung SC, et al. Hypoxia‐preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion‐induced lung injury. PLoS One. 2017;12:e0187637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karlsen TA, Mirtaheri P, Shahdadfar A, Fløisand Y, Brinchmann JE. Effect of three‐dimensional culture and incubator gas concentration on phenotype and differentiation capability of human mesenchymal stem cells. J Cell Biochem. 2011;112:684‐693. [DOI] [PubMed] [Google Scholar]
- 48. Saini U, Gumina RJ, Wolfe B, Kuppusamy ML, Kuppusamy P, Boudoulas KD. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem. 2013;114:2612‐2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang K, Wang XQ, He YS, et al. Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol. 2010;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kume S, Kato S, Yamagishi S, et al. Advanced glycation end‐products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647‐1658. [DOI] [PubMed] [Google Scholar]
- 51. Hofmann AD, Beyer M, Krause‐Buchholz U, Wobus M, Bornhäuser M, Rödel G. OXPHOS supercomplexes as a hallmark of the mitochondrial phenotype of adipogenic differentiated human MSCs. PLoS One. 2012;7:e35160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang Y, Luo B, Deng Z, et al. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ. 2016;4:e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun N, Yang L, Li Y, et al. Effect of advanced oxidation protein products on the proliferation and osteogenic differentiation of rat mesenchymal stem cells. Int J Mol Med. 2013;32:485‐491. [DOI] [PubMed] [Google Scholar]
- 54. Tan J, Xu X, Tong Z, et al. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res. 2015;3:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu W, Chen Z, Zhang J, et al. Critical role of phosphoinositide 3‐kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem. 2008;310:11‐18. [DOI] [PubMed] [Google Scholar]
- 56. Forni MF, Peloggia J, Trudeau K, Shirihai O, Kowaltowski AJ. Murine Mesenchymal Stem Cell Commitment to Differentiation Is Regulated by Mitochondrial Dynamics. Stem Cells. 2016;34:743‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schröder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239‐245. [DOI] [PubMed] [Google Scholar]
- 58. Higuchi M, Dusting GJ, Peshavariya H, et al. Differentiation of human adipose‐derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang W, Zhang Y, Lu W, Liu K. Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PLoS One. 2015;10:e0120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang DY, Pan Y, Zhang C, et al. Wnt/beta‐catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 2013;374:13‐20. [DOI] [PubMed] [Google Scholar]
- 61. Fujita H, Yamamoto M, Ogino T, et al. Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem Funct. 2014;32:77‐86. [DOI] [PubMed] [Google Scholar]
- 62. Shi Y, Hu Y, Lv C, Tu G. Effects of reactive oxygen species on differentiation of bone marrow mesenchymal stem cells. Ann Transplant. 2016;21:695‐700. [DOI] [PubMed] [Google Scholar]
- 63. Esmaeli S, Allameh A, Soleimani M, Rahbarizadeh F, Frouzandeh‐Moghadam M. The role of albumin and PPAR‐alpha in differentiation‐dependent change of fatty acid profile during differentiation of mesenchymal stem cells to hepatocyte‐like cells. Cell Biochem Funct. 2014;32:410‐419. [DOI] [PubMed] [Google Scholar]
- 64. Feng X, Xing J, Feng G, et al. p16(INK4A) mediates age‐related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev. 2014;141–142:46‐55. [DOI] [PubMed] [Google Scholar]
- 65. Kornicka K, Marycz K, Tomaszewski KA, Marędziak M, Śmieszek A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid Med Cell Longev. 2015;2015:309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lund TC, Kobs A, Blazar BR, Tolar J. Mesenchymal stromal cells from donors varying widely in age are of equal cellular fitness after in vitro expansion under hypoxic conditions. Cytotherapy. 2010;12:971‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Siegel G, Kluba T, Hermanutz‐Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow‐derived mesenchymal stromal cells. BMC Med. 2013;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Landgraf K, Brunauer R, Lepperdinger G, Grubeck‐Loebenstein B. The suppressive effect of mesenchymal stromal cells on T cell proliferation is conserved in old age. Transpl Immunol. 2011;25:167‐172. [DOI] [PubMed] [Google Scholar]
- 69. Li L, Guo Y, Zhai H, et al. Aging increases the susceptivity of MSCs to reactive oxygen species and impairs their therapeutic potency for myocardial infarction. PLoS One. 2014;9:e111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jin HJ, Lee HJ, Heo J, et al. Senescence‐associated MCP‐1 secretion is dependent on a decline in BMI1 in human mesenchymal stromal cells. Antioxid Redox Signal. 2016;24:471‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fu WL, Li J, Chen G, Li Q, Tang X, Zhang CH. Mesenchymal stem cells derived from peripheral blood retain their pluripotency, but undergo senescence during long‐term culture. Tissue Eng Part C Methods. 2015;21:1088‐1097. [DOI] [PubMed] [Google Scholar]
- 72. Jeong SG, Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun. 2015;460:971‐976. [DOI] [PubMed] [Google Scholar]
- 73. Yu KR, Lee JY, Kim HS, et al. A p38 MAPK‐mediated alteration of COX‐2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS One. 2014;9:e102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim HS, Shin TH, Lee BC, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392‐1403 e1‐e8. [DOI] [PubMed] [Google Scholar]
- 75. Chen X, Yan L, Guo Z, et al. Adipose‐derived mesenchymal stem cells promote the survival of fat grafts via crosstalk between the Nrf2 and TLR4 pathways. Cell Death Dis. 2016;7:e2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jeong SG, Cho GW. Accumulation of apoptosis‐insensitive human bone marrow‐mesenchymal stromal cells after long‐term expansion. Cell Biochem Funct. 2016;34:310‐316. [DOI] [PubMed] [Google Scholar]
- 77. Gu Y, Li T, Ding Y, et al. Changes in mesenchymal stem cells following long‐term culture in vitro. Mol Med Rep. 2016;13:5207‐5215. [DOI] [PubMed] [Google Scholar]
- 78. Yun SP, Lee SJ, Oh SY, et al. Reactive oxygen species induce MMP12‐dependent degradation of collagen 5 and fibronectin to promote the motility of human umbilical cord‐derived mesenchymal stem cells. Br J Pharmacol. 2014;171:3283‐3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tao J, Wang H, Zhai Y, et al. Downregulation of Nrf2 promotes autophagy‐dependent osteoblastic differentiation of adipose‐derived mesenchymal stem cells. Exp Cell Res. 2016;349:221‐229. [DOI] [PubMed] [Google Scholar]
- 80. Choo KB, Tai L, Hymavathee KS, et al. Oxidative stress‐induced premature senescence in Wharton's jelly‐derived mesenchymal stem cells. Int J Med Sci. 2014;11:1201‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nawrocka D, Kornicka K, Szydlarska J, Marycz K. Basic fibroblast growth factor inhibits apoptosis and promotes proliferation of adipose‐derived mesenchymal stromal cells isolated from patients with type 2 diabetes by reducing cellular oxidative stress. Oxid Med Cell Longev. 2017;2017:3027109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim JH, Park SG, Song SY, Kim JK, Sung JH. Reactive oxygen species‐responsive miR‐210 regulates proliferation and migration of adipose‐derived stem cells via PTPN2. Cell Death Dis. 2013;4:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang H, Kim HJ, Chang EJ, et al. IL‐17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332‐1343. [DOI] [PubMed] [Google Scholar]
- 84. Zhou H, Yang J, Xin T, et al. Exendin‐4 protects adipose‐derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt‐Sfrp2 pathways. Free Radic Biol Med. 2014;77:363‐375. [DOI] [PubMed] [Google Scholar]
- 85. Cao Y, Yang T, Gu C, Yi D. Pigment epithelium‐derived factor delays cellular senescence of human mesenchymal stem cells in vitro by reducing oxidative stress. Cell Biol Int. 2013;37:305‐313. [DOI] [PubMed] [Google Scholar]
- 86. Liu X, Zhou L, Chen X, et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord‐derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sreejit P, Verma RS. Cardiogel supports adhesion, proliferation and differentiation of stem cells with increased oxidative stress protection. Eur Cell Mater. 2011;21:107‐121. [DOI] [PubMed] [Google Scholar]
- 88. Lai Y, Sun Y, Skinner CM, et al. Reconstitution of marrow‐derived extracellular matrix ex vivo: a robust culture system for expanding large‐scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19:1095‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang Q, Zhu H, Zhou WG, et al. N‐acetylcysteine‐pretreated human embryonic mesenchymal stem cell administration protects against bleomycin‐induced lung injury. Am J Med Sci. 2013;346:113‐122. [DOI] [PubMed] [Google Scholar]
- 90. Xiao Y, Li X, Cui Y, et al. Hydrogen peroxide inhibits proliferation and endothelial differentiation of bone marrow stem cells partially via reactive oxygen species generation. Life Sci. 2014;112:33‐40. [DOI] [PubMed] [Google Scholar]
- 91. Rodrigues M, Turner O, Stolz D, Griffith LG, Wells A. Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to fas ligand. Cell Transplant. 2012;21:2171‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Allameh A, Ahmadi‐Ashtiani H, Emami Aleagha MS, Rastegar H. The metabolic function of hepatocytes differentiated from human mesenchymal stem cells is inversely related to cellular glutathione levels. Cell Biochem Funct. 2014;32:194‐200. [DOI] [PubMed] [Google Scholar]
- 93. Li CJ, Chen PK, Sun LY, Pang CY. Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxid Med Cell Longev. 2017;2017:8510805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bhatti FU, Mehmood A, Latief N, et al. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide‐induced oxidative stress in vitro and improves their therapeutic potential in surgically‐induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2017;25:321‐331. [DOI] [PubMed] [Google Scholar]
- 95. Marycz K, Tomaszewski KA, Kornicka K, et al. Metformin decreases reactive oxygen species, enhances osteogenic properties of adipose‐derived multipotent mesenchymal stem cells in vitro, and increases bone density in vivo. Oxid Med Cell Longev. 2016;2016:9785890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu P, Liu J, Shi J, et al. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J Cell Mol Med. 2015;19:2232‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang F, Zhou H, Du Z, et al. Cytoprotective effect of melatonin against hypoxia/serum deprivation‐induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol. 2015;748:157‐165. [DOI] [PubMed] [Google Scholar]
- 98. Liu Q, Jin L, Shen FH, Balian G, Li XJ. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells–a potential novel treatment for intervertebral disc degeneration. Spine J. 2013;13:1571‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu H, Yang X, Zhang Y, Dighe A, Li X, Cui Q. Fullerol antagonizes dexamethasone‐induced oxidative stress and adipogenesis while enhancing osteogenesis in a cloned bone marrow mesenchymal stem cell. J Orthop Res. 2012;30:1051‐1057. [DOI] [PubMed] [Google Scholar]
- 100. Wang N, Xie K, Huo S, Zhao J, Zhang S, Miao J. Suppressing phosphatidylcholine‐specific phospholipase C and elevating ROS level, NADPH oxidase activity and Rb level induced neuronal differentiation in mesenchymal stem cells. J Cell Biochem. 2007;100:1548‐1557. [DOI] [PubMed] [Google Scholar]
- 101. Han YS, Lee JH, Jung JS, et al. Fucoidan protects mesenchymal stem cells against oxidative stress and enhances vascular regeneration in a murine hindlimb ischemia model. Int J Cardiol. 2015;198:187‐195. [DOI] [PubMed] [Google Scholar]
- 102. Chen M, Chen S, Lin D. Carvedilol protects bone marrow stem cells against hydrogen peroxide‐induced cell death via PI3K‐AKT pathway. Biomed Pharmacother. 2016;78:257‐263. [DOI] [PubMed] [Google Scholar]
- 103. Zhang F, Cui J, Lv B, Yu B. Nicorandil protects mesenchymal stem cells against hypoxia and serum deprivation‐induced apoptosis. Int J Mol Med. 2015;36:415‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jeong SG, Cho GW. Trichostatin A modulates intracellular reactive oxygen species through SOD2 and FOXO1 in human bone marrow‐mesenchymal stem cells. Cell Biochem Funct. 2015;33:37‐43. [DOI] [PubMed] [Google Scholar]
- 105. Zanichelli F, Capasso S, Di Bernardo G, et al. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis. 2012;17:964‐974. [DOI] [PubMed] [Google Scholar]
- 106. Ibrahim M, Jang M, Park M, et al. Capsaicin inhibits the adipogenic differentiation of bone marrow mesenchymal stem cells by regulating cell proliferation, apoptosis, oxidative and nitrosative stress. Food Funct. 2015;6:2165‐2178. [DOI] [PubMed] [Google Scholar]
- 107. Huang Q, Shi J, Gao B, et al. Gastrodin: an ancient Chinese herbal medicine as a source for anti‐osteoporosis agents via reducing reactive oxygen species. Bone. 2015;73:132‐144. [DOI] [PubMed] [Google Scholar]
- 108. Sun J, Ming L, Shang F, Shen L, Chen J, Jin Y. Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci Rep. 2015;5:18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim JY, Lee JS, Han YS, et al. Pretreatment with lycopene attenuates oxidative stress‐induced apoptosis in human mesenchymal stem cells. Biomol Ther (Seoul). 2015;23:517‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lee JH, Jung HK, Han YS, et al. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016;14:3777‐3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li W, Liu Y, Wang B, et al. Protective effect of berberine against oxidative stress‐induced apoptosis in rat bone marrow‐derived mesenchymal stem cells. Exp Ther Med. 2016;12:4041‐4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang F, Yan G, Li Y, et al. Astragalus polysaccharide attenuated iron overload‐induced dysfunction of mesenchymal stem cells via suppressing mitochondrial ROS. Cell Physiol Biochem. 2016;39:1369‐1379. [DOI] [PubMed] [Google Scholar]
- 113. Liu X, Xu Y, Chen S, et al. Rescue of proinflammatory cytokine‐inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med. 2014;68:234‐246. [DOI] [PubMed] [Google Scholar]
- 114. Liu X, Gong Y, Xiong K, et al. Melatonin mediates protective effects on inflammatory response induced by interleukin‐1 beta in human mesenchymal stem cells. J Pineal Res. 2013;55:14‐25. [DOI] [PubMed] [Google Scholar]
- 115. Chen HH, Lin KC, Wallace CG, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose‐derived mesenchymal stem cell against sepsis‐induced kidney injury. J Pineal Res. 2014;57:16‐32. [DOI] [PubMed] [Google Scholar]
- 116. Novo E, Busletta C, Bonzo LV, et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow‐derived hepatic pro‐fibrogenic cells. J Hepatol. 2011;54:964‐974. [DOI] [PubMed] [Google Scholar]
- 117. Zhang M, Du Y, Lu R, et al. Cholesterol retards senescence in bone marrow mesenchymal stem cells by modulating autophagy and ROS/p53/p21Cip1/Waf1 pathway. Oxid Med Cell Longev. 2016;2016:7524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xu J, Qian J, Xie X, et al. High density lipoprotein protects mesenchymal stem cells from oxidative stress‐induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species. Int J Mol Sci. 2012;13:17104‐17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oh YI, Kim JH, Kang CW. Protective effect of short‐term treatment with parathyroid hormone 1‐34 on oxidative stress is involved in insulin‐like growth factor‐I and nuclear factor erythroid 2‐related factor 2 in rat bone marrow derived mesenchymal stem cells. Regul Pept. 2014;189:1‐10. [DOI] [PubMed] [Google Scholar]
- 120. Mirzamohammadi S, Mehrabani M, Tekiyehmaroof N, Sharifi AM. Protective effect of 17beta‐estradiol on serum deprivation‐induced apoptosis and oxidative stress in bone marrow‐derived mesenchymal stem cells. Hum Exp Toxicol. 2016;35:312‐322. [DOI] [PubMed] [Google Scholar]
- 121. Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37:1075‐1082. [DOI] [PubMed] [Google Scholar]
- 122. Wang X, Liu C, Xu Y, et al. Combination of mesenchymal stem cell injection with icariin for the treatment of diabetes‐associated erectile dysfunction. PLoS One. 2017;12:e0174145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Trachana V, Petrakis S, Fotiadis Z, et al. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress‐induced genomic damage. Cytotherapy. 2017;19:808‐820. [DOI] [PubMed] [Google Scholar]
- 124. Lin YM, Wu CC, Chang YC, et al. Target disruption of ribosomal protein pNO40 accelerates aging and impairs osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2016;469:903‐910. [DOI] [PubMed] [Google Scholar]
- 125. Dulk M, Kudlik G, Fekete A, et al. The scaffold protein Tks4 is required for the differentiation of mesenchymal stromal cells (MSCs) into adipogenic and osteogenic lineages. Sci Rep. 2016;6:34280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kanda Y, Hinata T, Kang SW, Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89:250‐258. [DOI] [PubMed] [Google Scholar]
- 127. Estrada JC, Torres Y, Benguria A, et al. Human mesenchymal stem cell‐replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jiang XY, Lu DB, Jiang YZ, Zhou LN, Cheng LQ, Chen B. PGC‐1alpha prevents apoptosis in adipose‐derived stem cells by reducing reactive oxygen species production in a diabetic microenvironment. Diabetes Res Clin Pract. 2013;100:368‐375. [DOI] [PubMed] [Google Scholar]
- 129. Lu L, Song HF, Wei JL, et al. Ameliorating replicative senescence of human bone marrow stromal cells by PSMB5 overexpression. Biochem Biophys Res Commun. 2014;443:1182‐1188. [DOI] [PubMed] [Google Scholar]
- 130. Li L, Du G, Wang D, Zhou J, Jiang G, Jiang H. Overexpression of heme oxygenase‐1 in mesenchymal stem cells augments their protection on retinal cells in vitro and attenuates retinal ischemia/reperfusion injury in vivo against oxidative stress. Stem Cells Int. 2017;2017:4985323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang F, Cui J, Liu X, et al. Roles of microRNA‐34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res Ther. 2015;6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu J, Huang Z, Lin L, et al. miR‐210 over‐expression enhances mesenchymal stem cell survival in an oxidative stress environment through antioxidation and c‐Met pathway activation. Sci China Life Sci. 2014;57:989‐997. [DOI] [PubMed] [Google Scholar]
- 133. Hua P, Liu LB, Liu JL, et al. Inhibition of apoptosis by knockdown of caspase‐3 with siRNA in rat bone marrow mesenchymal stem cells. Exp Biol Med (Maywood). 2013;238:991‐998. [DOI] [PubMed] [Google Scholar]
- 134. Feng X, Feng G, Xing J, et al. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res. 2014;356:369‐380. [DOI] [PubMed] [Google Scholar]
- 135. Cheng J, Zhang P, Jiang H. Let‐7b‐mediated pro‐survival of transplanted mesenchymal stem cells for cardiac regeneration. Stem Cell Res Ther. 2015;6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pan Q, Qin X, Ma S, et al. Myocardial protective effect of extracellular superoxide dismutase gene modified bone marrow mesenchymal stromal cells on infarcted mice hearts. Theranostics. 2014;4:475‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang D, Yan B, Yu S, et al. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D‐galactose through Akt/mTOR signaling. Oxid Med Cell Longev. 2015;2015:867293. [DOI] [PMC free article] [PubMed] [Google Scholar]


