Dihydronaphthalene analogues as potent inhibitors of tubulin polymerization, cytotoxic agents, and vascular disrupting agents (VDAs).
Dihydronaphthalene analogues as potent inhibitors of tubulin polymerization, cytotoxic agents, and vascular disrupting agents (VDAs).
Abstract
The natural products colchicine and combretastatin A-4 (CA4) have provided inspiration for the discovery and development of a wide array of derivatives and analogues that inhibit tubulin polymerization through a binding interaction at the colchicine site on β-tubulin. A water-soluble phosphate prodrug salt of CA4 (referred to as CA4P) has demonstrated the ability to selectively damage tumor-associated vasculature and ushered in a new class of developmental anticancer agents known as vascular disrupting agents (VDAs). Through a long-term program of structure activity relationship (SAR) driven inquiry, we discovered that the dihydronaphthalene molecular scaffold provided access to small-molecule inhibitors of tubulin polymerization. In particular, a dihydronaphthalene analogue bearing a pendant trimethoxy aryl ring (referred to as KGP03) and a similar aroyl ring (referred to as KGP413) were potent inhibitors of tubulin polymerization (IC50 = 1.0 and 1.2 μM, respectively) and displayed low nM cytotoxicity against human cancer cell lines. In order to enhance water-solubility for in vivo evaluation, the corresponding phosphate prodrug salts (KGP04 and KGP152, respectively) were synthesized. In a preliminary in vivo study in a SCID-BALB/c mouse model bearing the human breast tumor MDA-MB-231-luc, a 99% reduction in signal was observed with bioluminescence imaging (BLI) 4 h after IP administration of KGP152 (200 mg kg–1) indicating reduced tumor blood flow. In a separate study, disruption of tumor-associated blood flow in a Fischer rat bearing an A549-luc human lung tumor was observed by color Doppler ultrasound following administration of KGP04 (15 mg kg–1).
1. Introduction
The natural products colchicine1 and combretastatin A-4 (CA4)2,3 provide a rich canopy for SAR-guided molecular interrogation directed towards the discovery of highly potent inhibitors of tubulin polymerization (Fig. 1). This rich, natural products-based structural landscape has enabled the exploration of a wide range of structural diversity, resulting in literally thousands of synthetic analogues and derivatives with structural and functional group modifications of both aryl rings and the ethylene bridge of CA4.4–9 Key structural features of the combretastatins include the trimethoxy phenyl ring, the p-methoxyphenyl moiety, cis-configuration of the aromatic rings and a distance of approximately 4–5 Å between the aryl rings.5,9,10 Our long-standing research agenda in this area has contributed a variety of functionalized molecular scaffolds including benzo[b]thiophene,11–13 indole,12,14–18 benzofuran,12,15,19 stilbenoid,5,9,20–22 and benzosuberene analogues23–26 that bear structural analogy to colchicine and CA4. Our original molecular design motif recognized salient aspects of structural similarity between non-steroidal,11 anti-estrogen agents (such as nafoxidine and LY117018)27,28 and CA4, which led us to the discovery of a collection of highly potent inhibitors of tubulin polymerization that function through a direct binding interaction at the colchicine site on the tubulin heterodimer.12,13,21,23–26,29,30 In addition to functioning as antiproliferative (cytotoxic agents), colchicine site inhibitors of tubulin polymerization can also function as vascular disrupting agents (VDAs). Selective disruption of tumor-associated microvessels results in loss of nutrients, oxygen deprivation, and ultimately necrosis.31–38 Therefore, this therapeutic approach offers a unique and promising strategy for the treatment of cancer, which is mechanistically distinct from that of the angiogenesis inhibiting agents (AIAs),39 such as bevacizumab (Avastin™)40 that target the angiogenesis process.31–34 The sub-set of colchicine site inhibitors of tubulin polymerization that function as VDAs induce rapid morphological changes (flat to round) in the endothelial cells lining microvessels, leading to microvessel occlusion, compromised vessel wall integrity, and necrosis.33,36,41–43 VDAs are selective for tumor-associated neovasculature due to the primitive nature of these vessels, which are typically incomplete or missing basement membranes, deficient in smooth muscle and pericytes, and therefore more reliant on endothelial cells to maintain the shape of the vessel wall.16,44–48
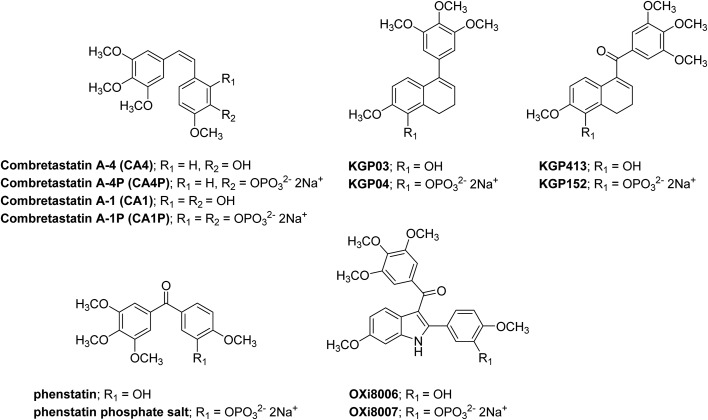
Fig. 1. Representative small-molecule inhibitors of tubulin assembly that bind to the colchicine site; including the combretastatin analogues (CA4, CA1),2,3 dihydronaphthalene analogues (KGP03 and KGP413), isocombretastatin analogue phenstatin,55,56 and indole analogue (OXi8006), along with the corresponding phosphate salt prodrugs.
Herein, we report the design, synthesis, and preliminary biological evaluation of a 5-hydroxy-6-methoxy-1-aryldihydronaphthalene analogue (referred to as KGP03, Fig. 1) along with its corresponding 1-aroyldihydronaphthalene analogue (referred to as KGP413, Fig. 1). Functionalized dihydronaphthalene analogues of this nature bear structural similarity to CA4 and have been previously reported by us5,21,25,49–53 and others.54 Our previous studies with indole analogues (such as OXi8006) along with Pettit's discovery of phenstatin (synthetic benzophenone analogue of CA4, Fig. 1)55,56 confirmed the tolerability (and potential benefit) of a carbonyl group juxtaposed between functionalized aryl rings in regard to inhibition of tubulin polymerization, cytotoxicity, and in vivo efficacy. These molecules along with CA4 have very limited water-solubility, and in their initial development of the combretastatins, Pettit and co-workers astutely developed phosphate prodrug salts of CA4 and CA1 (referred to as CA4P and CA1P, respectively) that dramatically increased water-solubility (Fig. 1).57–59 This strategy has also been applied to these dihydronaphthalene analogues to generate water-soluble phosphate prodrug salts (KGP04 and KGP152, respectively, Fig. 1). Synthetic strategies towards these molecules are reported along with inhibition of tubulin polymerization (cell-free assay) and cytotoxicity against NCI-H460 (non-small cell lung carcinoma), DU-145 (prostate carcinoma), and SK-OV-3 (ovarian adenocarcinoma) human cancer cell lines. The two water-soluble prodrugs (KGP04 and KGP152) were subjected to preliminary in vivo (mouse and rat) evaluation to assess their ability to disrupt tumor-associated vasculature and thus function as VDAs.
2. Results and discussion
2.1. Synthesis
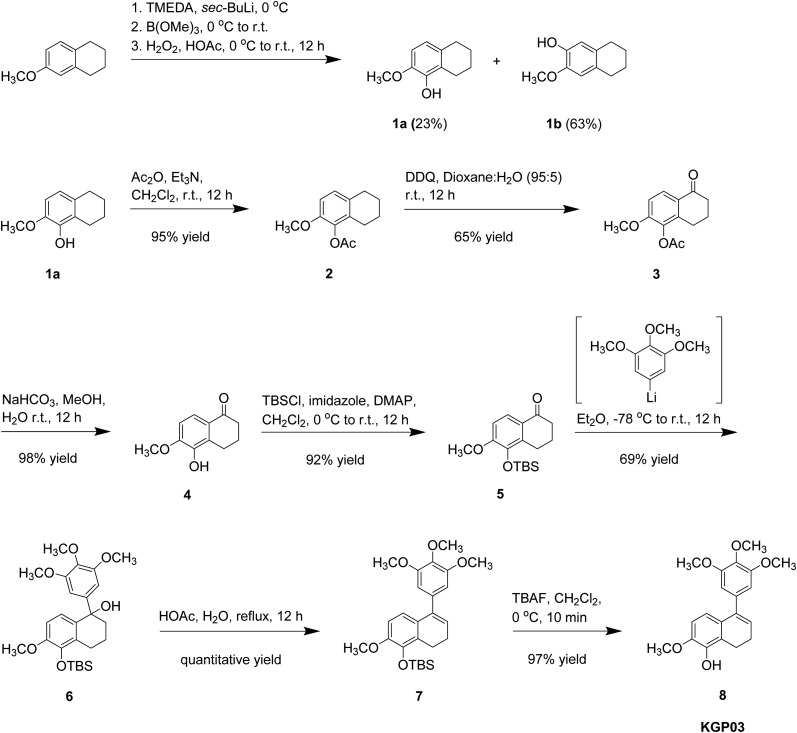
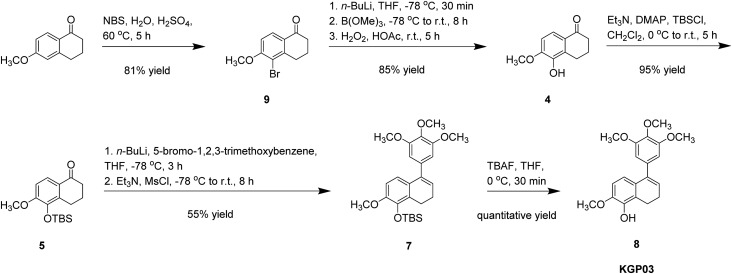
Our initial synthetic route towards KGP03 (Scheme 1) provided the target compound in a 9% overall yield from 6-methoxytetralin.21,25,49,54 Considering an alternative strategy to the synthesis of KGP03 in order to improve efficiency and overall yield, 6-methoxy-1-tetralone was selected as a starting material (Scheme 2). This precursor is more advanced, commercially available, and approximately half the cost of the tetralin used in the original synthesis. Regioselective bromination of the tetralone followed by lithium halogen exchange provided a means to direct the hydroxylation to the regiochemically desired position in a significantly higher yield (69% for 2 steps) compared to the ortho-directed C–H deprotonation of tetralin used in the original route (23% yield for 1 step).25,60,61 The phenolic tetralone 4 was subsequently converted to its corresponding silyl ether 5 upon treatment with TBSCl. Installation of the trimethoxy aryl ring and elimination of the resulting tertiary alcohol to generate KGP03 was accomplished in a one-pot reaction. The appropriate aryllithium intermediate (prepared in situ from the corresponding aryl bromide) was allowed to react with tetralone 5, and the resulting tertiary alcohol (in situ) was treated with triethylamine and mesyl chloride to furnish the KGP03-silyl ether derivative 7.62 Desilylation with TBAF afforded KGP03 in a 5-step synthesis with a 36% overall yield.
Scheme 1. Synthesis of KGP03 from 6-methoxytetralin featuring early installation of phenolic moiety.
Scheme 2. Efficient alternative synthesis of KGP03 from 6-methoxytetralone.
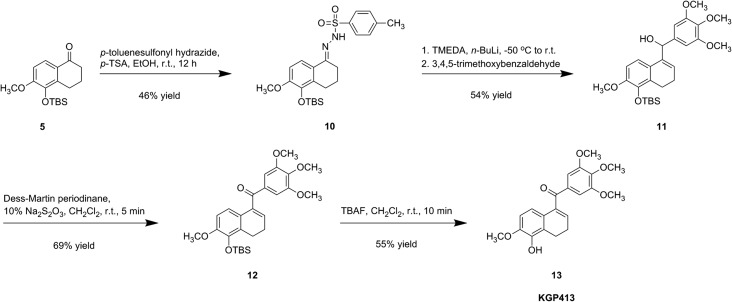
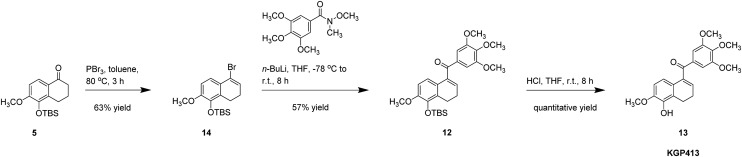
Our initial route to the carbonyl hinged analogue, KGP413, centered on a modified Shapiro coupling reaction (Scheme 3). Ketone 5 was converted to hydrazine 10, which provided the in situ generated vinyl lithium adduct that was subsequently treated with 3,4,5-trimethoxybenzaldehyde to afford secondary alcohol 11. Dess–Martin oxidation provided silyl-ether derivative 12, which was desilylated to afford KGP413.25,49 Our improved synthesis of KGP413 (Scheme 4), proceeded through a silyl ether tetralone intermediate that was highlighted in the KGP03 synthesis (Scheme 2). The ketone was converted to vinyl bromide 14 using phosphorus tribromide.63 The corresponding vinyllithium intermediate was prepared in situ followed by the addition of the trimethoxy aryl Weinreb amide to afford silyl-ether derivative 12. Desilylation using concentrated HCl afforded KGP413 in a 6-step synthesis with a 23% overall yield. This was a significant improvement from the original 9-step synthesis that resulted in a 1% overall yield.
Scheme 3. Synthesis of KGP413 from 6-methoxytetralin featuring a modified Shapiro coupling reaction.
Scheme 4. Efficient alternatives synthesis of KGP413 from 6-methoxytetralone featuring a Weireb amide coupling reaction.
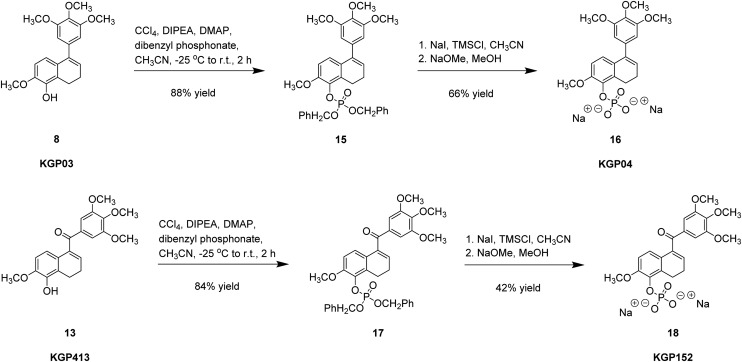
The reaction of either phenol (8 or 13) with in situ-generated dibenzyl chlorophosphite resulted in the expected dihydronaphthalene dibenzyl phosphate ester derivative (15 or 17, Scheme 5). Debenzylation was accomplished using in situ-generated iodomethylsilane, from chloromethylsilane and sodium iodide. The in situ-generated TMS–phosphate ester intermediate was quenched using sodium methoxide in methanol to afford the disodium phosphate salt prodrug (16 or 18).22,57
Scheme 5. Synthesis of KGP04 and KGP152 phosphate prodrug salt.
2.2. Biological evaluation
The two phenolic dihydronaphthalene analogues (KGP03 and KGP413 along with their corresponding water-soluble phosphate prodrug salts KGP04 and KGP152) were evaluated for their ability to inhibit the polymerization of purified tubulin (Table 1). Both KGP03 and KGP413 were potent inhibitors (IC50 = 0.46 and 0.85 μM, respectively) akin to CA4 (IC50 = 1.2 μM) while their corresponding phosphate prodrug salts were inactive (IC50 > 20 μM), as anticipated, since this cell free assay is devoid of phosphatase enzymes required for cleavage and regeneration of the parent phenolic analogues. Phosphate prodrug salts of this type are typically substrates for alkaline phosphatase enzymes, readily available in vivo. We confirmed that KGP04 underwent conversion (essentially quantitative) to its corresponding phenol (KGP03) in the presence of alkaline phosphatase (see ESI§ file for details). All four dihydronaphthalene compounds demonstrated low nM cytotoxicity (range of 2–52 nM) against the three cancer cell lines utilized in this study, and thus were comparable to the activity of CA4 and CA4P (Table 1). In order to assess the ability of these compounds to interfere with tumor-associated vasculature and thus function as VDAs, the two water-soluble prodrugs were evaluated against in vivo models (mouse and rat) of human cancer (breast and lung, respectively).
Table 1. Inhibition of tubulin polymerization, percent inhibition of colchicine binding, and cytotoxicity of the target dihydronaphthalene analogues.
| Compound | Inhibition of tubulin polymerization IC50 (μM) ± SD | % inhibition of colchicine binding ± SD | GI50 (μM) SRB assay |
||
| NCI-H460 | DU-145 | SK-OV-3 | |||
| KGP03 | 0.46 ± 0.007 | 90% ± 2 (1 μM), 98% ± 0.3 (5 μM) | 0.00451 ± 0.00104 | 0.00349 ± 0.000587 | 0.00219 ± 0.000774 |
| KGP04 | >20 | nd a | 0.00373 ± 0.00115 | 0.00327 ± 0.00139 | 0.00190 ± 0.000427 |
| KGP413 | 1.2 ± 0.007 | 81% ± 2 (1 μM), 98% ± 2 (5 μM) | 0.0216 ± 0.0227 | 0.00730 ± 0.00869 | 0.00622 ± 0.00296 |
| KGP152 | >20 | nd a | 0.0524 ± 0.0137 | 0.0126 ± 0.0125 | 0.0129 ± 0.0139 |
| CA4 b | 1.2 ± 0.05 | 84% ± 1.1 (1 μM), 98% ± 0.1 (5 μM) | 0.00500 ± 0.000359 | 0.00602 ± 0.000661 | 0.00506 ± 0.00145 |
| CA4P c | >20 | nd a | 0.00282 ± 0.000497 | 0.00336 ± 0.00105 | 0.00188 ± 0.000976 |
Preliminary in vivo evaluation of water-soluble prodrugs KGP04 and KGP413 as VDAs
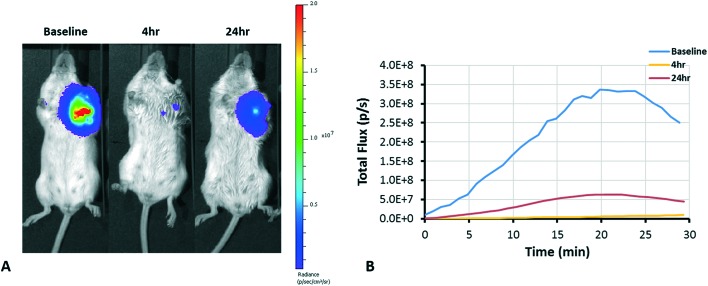
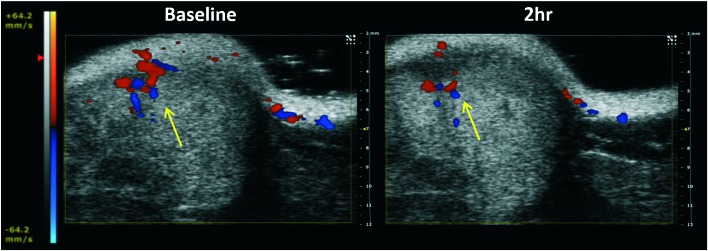
The dynamic and longitudinal effect of VDAs can be assessed with various non-invasive imaging modalities.38,64 Bioluminescence imaging (BLI) is a widely used optical imaging technique for preclinical research. The light emission of BLI is based on the expression of the luciferase enzyme and the presence of the substrate luciferin. The effect of VDAs can be observed through reduced delivery of substrate luciferin and consequent diminished light emission, as has been demonstrated with several VDAs.17,38,41,42,65 We performed a preliminary dose escalation study on three BALB/c SCID mice with orthotopic MDA-MB-231-luc tumors with respective doses of 100, 150, and 200 mg kg–1KGP152 administered IP. Vascular shutdown at 4 h following KGP152 was quite uniform with all three tumors showing at least 85% decrease in light emission. Fig. 2 shows the respective decrease of BLI signal 4 and 24 h after administration of KGP152 (200 mg kg–1). While dynamic BLI is very effective at revealing vascular disruption, it does require transfected tumor cells to express luciferase. As an alternative, ultrasound can reveal blood flow14,38,66,67 and color-Doppler based on the Doppler effect has been previously applied to demonstrate vascular shutdown following the administration of VDAs.14Fig. 3 shows an example of the use of color-Doppler ultrasound to evaluate the reduction of blood flow 2 h after administration of KGP04 to a nude rat with a subcutaneous A549 human lung tumor growing in the leg.
Fig. 2. BLI monitoring of tumor response to KGP152. KGP152 (200 mg kg–1) was administered IP to a BALB/C SCID mouse with a large MDA-MB-231-luc orthotopic breast tumor (2 cm3). (A) Images show signal intensity heat maps overlaid on gray scale photographs of mouse at successive time points. Following KGP152 administration, the BLI signal was dramatically lower at 4 h (99% signal reduction) and recovered slightly at 24 h (81% signal reduction from baseline). (B) Corresponding dynamic time courses of total flux obtained following luciferin administration at the three time points.
Fig. 3. Color Doppler ultrasound images show reduction of tumor blood perfusion. Images show baseline and 2 h after the administration of KGP04 (15 mg kg–1, IP) to a nude rat with a subcutaneous A549 human tumor xenograft (0.5 cm3). Red and blue indicate magnitude of flow into and out of plane. The layer of skin is apparent at the top of the images, together with structural heterogeneity in the tumor. Vascular shutdown is indicated by yellow arrow.
3. Conclusion
In summary, we utilized the dihydronaphthalene molecular scaffold to design and synthesize two promising inhibitors of tubulin polymerization (KGP03 and KGP413) and their corresponding water-soluble phosphate prodrug salts. Initial synthetic routes were further optimized towards these lead compounds. The analogues were evaluated for inhibition of tubulin polymerization and for their ability to compete for colchicine binding, which provided robust data [similar to CA4 (positive control compound)] for both phenolic dihydronaphthalene compounds (KGP03 and KGP413). Potent cytotoxicity (low nM GI50 values) was observed for the four compounds (KGP03/KGP04 and KGP413/KGP152) against the three human cancer cell lines (NCI-H460, DU-145, and SK-OV-3) used in this study. Preliminary in vivo BLI evaluation of dihydronaphthalene prodrug KGP152 (200 mg kg–1) against an MDA-MB-231-luc orthotopic breast tumor (SCID mouse model) showed a dramatic decrease in signal after 4 h. The signal recovered only slightly after 24 h. Preliminary in vivo assessment (nude rat bearing the A549 tumor) of KGP04 (15 mg kg–1) demonstrated evidence of vascular shutdown (imaged by color Doppler ultrasound). Thus, these dihydronaphthalene analogues appear to be promising candidates for further development as antiproliferative agents and VDAs.
4. Experimental section
4.1. Chemistry
4.1.1. General materials and methods
Dichloromethane, methanol, diethylether, and tetrahydrofuran (THF) were used in their anhydrous forms, as obtained from the chemical suppliers. Reactions were performed under an inert nitrogen atmosphere. Thin-layer chromatography (TLC) plates (precoated glass plates with silica gel 60 F254, 0.25 mm thickness) were used to monitor reactions. Purification of intermediates and products was carried out with a Biotage Isolera flash purification system using silica gel (200–400 mesh, 60 Å) or manually in glass columns. Intermediates and products synthesized were characterized on the basis of their 1H NMR (600, 500 MHz), 13C NMR (151 or 126 MHz) spectroscopic data using a Bruker Avance III 600 MHz or a Varian VNMRs 500 MHz instrument. Spectra were recorded in CDCl3, CD3OD, or C3D6O. All chemical shifts are expressed in ppm (δ), coupling constants (J) are presented in Hz, and peak patterns are reported as singlet (s), doublet (d), triplet (t), quartet (q), septet (sept), double doublet (dd), and multiplet (m).
Purity of the final compounds was further analyzed at 25 °C using an Agilent 1200 HPLC system with a diode-array detector (λ = 190–400 nm), a Zorbax XDB-C18 HPLC column (4.6 mm × 150 mm, 5 μm), and a Zorbax reliance cartridge guard-column; method A: solvent A, acetonitrile, solvent B, 0.1% TFA in H2O; or method B: solvent A, acetonitrile, solvent B, H2O; gradient, 10% A/90% B to 100% A/0% B over 0 to 40 min; post-time 10 min; flow rate 1.0 mL min–1; injection volume 20 μL; monitored at wavelengths of 210, 254, 230, 280, and 360 nm. Mass spectrometry was carried out under positive ESI (electrospray ionization) using a Thermo Scientific LTQ Orbitrap Discovery instrument.
Experimental procedures for intermediates in Scheme 1
4.1.1.1. 2-Methoxy-5,6,7,8-tetrahydronaphthalen-1-ol (1)61,68
To a well-stirred solution of 6-methoxy-1,2,3,4-tetrahydronapthalene (14.15 g, 86.75 mmol) in sec-BuLi (100 mL, 110 mmol) at 0 °C, freshly distilled TMEDA (13.6 mL) was added dropwise. The reaction mixture was then stirred at room temperature for 1 h. The reaction mixture was cooled to 0 °C and B(OMe)3 (12.5 mL, 110 mmol) was added dropwise. Then the reaction mixture was stirred for 1 h at room temperature. The reaction mixture was cooled to 0 °C, and glacial HOAc (7 mL) was added dropwise, followed by the dropwise addition of 35 wt% H2O2 (15 mL). Finally, the reaction mixture was stirred at room temperature for 12 h. Saturated NH4Cl (100 mL) was added, and product was extracted with Et2O (3 × 400 mL). The combined organic phases were washed with brine and dried over Na2SO4, filtered, and concentrated in vacuo. Purification by flash column chromatography (silica gel, EtOAc/hexanes, gradient 2 : 98 to 5 : 95) yielded 2-methoxy-5,6,7,8-tetrahydronaphthalen-1-ol 1 (3.50 g, 19.6 mmol, 23% yield) as an off-white solid. 1H NMR (CDCl3, 300 MHz): δ 6.68 (1H, d, J = 8.3 Hz,), 6.23 (1H, d, J = 8.8 Hz,), 5.65 (1H, s), 3.84 (3H, s), 2.71 (4H, t, J = 6.0 Hz), 1.71 (4H, m).
4.1.1.2. 2-Methoxy-5,6,7,8-tetrahydronaphthalen-1-yl acetate (2)25,49,68
To a well-stirred solution of 2-methoxy-5,6,7,8-tetrahydronaphthalen-1-ol 1 (1.25 g, 7.01 mmol) dissolved in anhydrous CH2Cl2 (25 mL) was added Et3N (1.5 mL, 10.8 mmol), Ac2O (1.0 mL, 11 mmol), and DMAP (0.10 g, 0.9 mmol). The reaction mixture was stirred for 12 hours at room temperature. The solvents were removed in vacuo, and the crude product was subjected to flash chromatography (silica gel, 3 : 97 EtOAc/hexanes) to afford 2-methoxy-5,6,7,8-tetrahydronaphthalen-1-yl acetate 2 (1.50 g, 6.81 mmol, 95% yield) as a white solid. 1H NMR (CDCl3, 300 MHz): δ 6.91 (1H, d, J = 8.4 Hz), 6.75 (1H, d, J = 8.4 Hz), 3.78 (3H, s), 2.71 (2H, m), 2.54 (2H, m), 2.32 (3H, s), 1.74 (4H, m).
4.1.1.3. 2-Methoxy-5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl acetate (3)25,49,68
A solution of DDQ (10.30 g, 22.70 mmol) in dioxane (40 mL) was added dropwise to a well stirred solution of 2-methoxy-5,6,7,8-tetrahydronaphthalen-1-yl acetate 2 (5.01 g, 23 mmol) in H2O/dioxane (5/95) at rt. The reaction mixture was stirred for 12 h, and the solid that precipitated was filtered and washed with EtOAc. The filtrates were evaporated under reduced pressure. To this was added saturated NaHCO3 (100 mL), and the solution was extracted with Et2O (3 × 200 mL). The combined organic phases were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The crude product was subjected to flash column chromatography (silica gel, 30 : 70 EtOAc/hexanes) to afford 2-methoxy-5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl acetate 3 (3.45 g, 14.7 mmol, 65% yield). 1H NMR (CDCl3, 300 MHz): δ 7.99 (1H, d, J = 8.7 Hz), 6.92 (1H, d, J = 8.7 Hz), 3.88 (3H, s), 2.79 (2H, t, J = 6.0 Hz), 2.60 (2H, m), 2.36 (3H, s), 2.10 (2H, p, J = 6.2 Hz).
4.1.1.4. 5-Hydroxy-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (4)25,49,68
Anhydrous K2CO3 (4.72 g, 34.1 mmol) was added to a well-stirred solution of 2-methoxy-5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl acetate 3 (4.0 g, 17.1 mmol) in MeOH (100 mL) and H2O (5 mL). The reaction mixture was stirred for 12 h at room temperature. The solvents were removed under reduced pressure followed by the addition of saturated NaHCO3 (50 mL). The solution was extracted with CH2Cl2 (3 × 100 mL), and the combined organic phases were washed with brine, dried over Na2SO4 and filtered. The organic phases were evaporated in vacuo to afford 5-hydroxy-6-methoxy-3,4-dihydronaphthalen-1(2H)-one 4 (3.24 g, 16.9 mmol, 98% yield) as red crystals. 1H NMR (CDCl3, 300 MHz): δ 7.67 (1H, d, J = 8.6 Hz), 6.83 (1H, d, J = 8.6 Hz), 5.72 (1H, s), 3.96 (3H, s), 2.93 (2H, t, J = 6.0 Hz), 2.60 (2H, m), 2.10 (2H, p, J = 6.1 Hz).
4.1.1.5. 5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (5)25,49,68
Tetralone 4 (3.24 g, 16.9 mmol) was dissolved in anhydrous CH2Cl2 (75 mL) at room temperature. Et3N (3.53 mL, 25.3 mmol) and DMAP (0.21 g, 1.69 mmol) were added, and the reaction mixture was stirred for 10 min. TBSCl (3.05 g, 20.2 mmol) was added in portions, and the reaction mixture was stirred for 12 h. The reaction was ended by the addition of H2O (50 mL). The organic layers were separated, and the aqueous phases were extracted with CH2Cl2 (2 × 100 mL). The combined organic phases were washed with brine, dried over Na2SO4, filtered and evaporated to dryness under reduced pressure. Separation of the crude product by flash column chromatography (silica gel, 10 : 90 EtOAc/hexanes) afforded TBS-protected tetralone 5 (4.38 g, 14.3 mmol, 92% yield) as a colorless oil. 1H NMR (CDCl3, 300 MHz): δ 7.72 (1H, d, J = 8.6 Hz), 6.82 (1H, d, J = 8.6 Hz), 3.85 (3H, s), 2.90 (2H, t, J = 6.0 Hz), 2.57 (2H, m), 2.07 (2H, p, J = 6.1 Hz), 1.01 (9H, s), 0.18 (6H, s).
4.1.1.6. 5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-1-(3,4,5-trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-1-ol (6)49,68
To a well stirred solution of 3,4,5-trimethoxybromobenzene (7.57 g, 30.6 mmol) in anhydrous Et2O (300 mL) at –78 °C, was added n-BuLi (20.8 mL, 24.5 mmol) dropwise. The reaction was stirred until the temperature reached –30 °C. The TBS-protected tetralone 5 (6.26 g, 20.4 mmol) dissolved in Et2O (50 mL) was added dropwise to the reaction mixture, and the reaction mixture was stirred until it reached room temperature. H2O (100 mL) was added, and the organic layer was separated. The aqueous phase was extracted with Et2O (2 × 250 mL), and the combined organic phases were washed with brine. Removal of solvent under reduced pressure afforded crude product, which was subjected to flash column chromatography (silica gel, 20 : 80 EtOAc/hexanes) to afford tertiary alcohol 6 (6.86 g, 14.5 mmol, 69% yield) as a colorless oil. 1H NMR (CDCl3, 300 MHz): δ 6.67 (2H, m), 6.55 (2H, s), 3.89 (3H, s), 3.78 (9H, s), 2.89 (2H, m), 2.06 (2H, m), 1.60 (2H, m), 1.02 (9H, s), 0.23 (3H, s), 0.20 (3H, s).
4.1.1.7. tert-Butyl((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)oxy)dimethylsilane (7)49,68
A mixture of tertiary alcohol 6 (6.86 g, 14.1 mmol), glacial acetic acid (120 mL) and H2O (400 mL) was refluxed for 12 h. The reaction was cooled and extracted with CH2Cl2 (2 × 300 mL). The combined organic phases were washed with brine, dried over Na2SO4 and filtered. Evaporation of the solvents under reduced pressure followed by drying under high vacuum afforded tert-butyl((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)oxy)dimethylsilane 7 as a colorless oil (6.43 g, 14.1 mmol, quantitative yield). The crude product was carried on to the next step without further characterization.
4.1.1.8. 2-Methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-ol (8)49,68
The crude product 7 (6.43 g, 14.1 mmol) was dissolved in CH2Cl2 (150 mL) and cooled to 0 °C. To this was added TBAF (22 mL, 22 mmol) dropwise. The reaction was stirred for 10 min and ended by the addition of H2O (100 mL). The organic layers were separated, and the aqueous phases were extracted with CH2Cl2 (2 × 300 mL). The combined organic phases were washed with brine and dried over Na2SO4. Filtration followed by evaporation of solvents afforded the crude product, which was separated by flash column chromatography (silica gel, 40 : 60 EtOAc/hexanes) to afford 8 (4.50 g, 13.1 mmol, 97% yield) as an off-white solid. 1H NMR (CDCl3, 300 MHz): δ 6.62 (1H, d, J = 8.4 Hz), 6.58 (1H, d, J = 8.4 Hz), 6.56 (2H, s), 5.97 (1H, t, J = 4.6 Hz), 5.74 (1H, s), 3.89 (3H, s), 3.88 (3H, s), 3.84 (6H, s), 2.88 (2H, t, J = 8.2 Hz), 2.37 (2H, td, J = 8.2, 4.6 Hz). 13C NMR (CDCl3, 75 MHz): δ 152.9, 145.8, 141.9, 139.0, 136.9, 128.9, 125.4, 122.3, 117.4, 107.2, 105.9, 60.9, 55.9, 22.8, 20.2. HPLC retention time – 14.00 min.
Experimental procedures for intermediates in Scheme 2
4.1.1.9. 5-Bromo-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (9)60
6-Methoxy-1-tetralone (1.06 g, 6.02 mmol) was stirred in 60 mL of H2O. N-Bromosuccinimide (1.07 g, 6.01 mmol) was added, and the reaction mixture was heated to 60 °C. H2SO4 (0.67 mL) was then added to the reaction mixture, which was heated for 5 h. The reaction mixture was extracted with EtOAc, and the organic layers were dried over Na2SO4 and filtered. The solvent was then removed in vacuo, and the resulting solid was dissolved in methanol and recrystallized. The crystals were isolated by filtration and washed with cold methanol to afford the product 9 (1.22 g, 4.78 mmol, 81% yield) as a white solid. 1H NMR (CDCl3, 600 MHz): δ 8.09 (1H, d, J = 8.7 Hz), 6.91 (1H, d, J = 8.7 Hz), 4.00 (3H, s), 3.06 (2H, t, J = 6.2 Hz), 2.66 (2H, m), 2.15 (2H, p, J = 6.3 Hz). 13C NMR (CDCl3, 151 MHz): δ 196.8, 159.8, 145.5, 128.4, 127.6, 113.0, 109.6, 56.5, 38.0, 30.5, 22.5. HRMS: obsd 255.0016 [M + H+], calcd for C11H12BrO2+: 255.0015.
4.1.1.10. 5-Hydroxy-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (4)61
To a well-stirred solution of 5-bromo-6-methoxy-3,4-dihydronaphthalen-1(2H)-one 9 (0.50 g, 1.96 mmol) in THF at –78 °C, n-BuLi (4.90 mL, 7.84 mmol) was added dropwise. The reaction mixture was then stirred at –78 °C for 1 h and then allowed to warm to room temperature. B(OMe)3 (0.45 mL, 3.92 mmol) was added dropwise, and the reaction mixture was stirred for 1 h at room temperature. Glacial acetic acid (0.22 mL) was added dropwise, followed by the addition of 35 wt% H2O2 (0.48 mL) added dropwise. The reaction mixture was then stirred at room temperature for 12 h. Saturated NH4Cl (20 mL) was added to the solution, and the mixture was extracted with EtOAc (3 × 50 mL). The combined organic phases were washed with brine and dried over Na2SO4, filtered, and concentrated in vacuo. Purification by flash column chromatography (silica gel, EtOAc/hexanes) afforded the phenol 4 (0.32 g, 1.66 mmol, 85% yield) as a tan solid. 1H NMR (CDCl3, 600 MHz): δ 7.70 (1H, d, J = 8.6 Hz), 6.86 (1H, d, J = 8.6 Hz), 5.76 (1H, s), 3.98 (3H, s), 2.95 (2H, t, J = 6.2 Hz), 2.65 (2H, m), 2.13 (2H, p, J = 6.4 Hz). 13C NMR (CDCl3, 151 MHz): δ 197.9, 149.9, 141.9, 130.4, 126.8, 120.0, 108.3, 56.1, 38.8, 22.9, 22.7. HRMS: obsd 215.0679 [M + Na+], calcd for C11H12NaO3+: 215.0679.
4.1.1.11. 5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (5)49
5-Hydroxy-6-methoxy-3,4-dihydronaphthalen-1(2H)-one 4 (1.05 g, 5.46 mmol) was stirred in 10 mL of CH2Cl2 at 0 °C. Triethylamine (0.84 mL, 6.03 mmol) and DMAP (0.27 g, 2.18 mmol) were then added. The reaction mixture was stirred for an additional 10 min before adding TBSCl (0.90 g, 5.97 mmol). The reaction was quenched with 100 mL of H2O after 1 h. The reaction mixture was extracted with CH2Cl2 (3 × 100 mL), and the organic layer was dried over Na2SO4. The organic layer was then filtered, and the solvent was removed in vacuo. The crude mixture was purified by flash chromatography (silica gel, EtOAc/hexanes) to afford silyl ether 5 (1.58 g, 5.16 mmol, 95% yield) as an orange oil. 1H NMR (CDCl3, 600 MHz): δ 7.54 (1H, d, J = 8.6 Hz), 6.64 (1H, d, J = 8.7 Hz), 3.67 (3H, s), 2.72 (2H, t, J = 6.1 Hz), 2.42 (2H, m), 1.89 (2H, p, J = 6.4 Hz), 0.83 (9H, s), 0.00 (6H, s). 13C NMR (CDCl3, 151 MHz): δ 198.0, 154.0, 141.3, 136.1, 126.8, 121.5, 109.2, 54.9, 38.7, 26.1, 24.4, 22.9, 18.9, –3.8. HRMS: obsd 307.1727 [M + H+], calcd for C17H27O3Si+: 307.1724.
4.1.1.12. tert-Butyl((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)oxy)dimethylsilane (7)25,62
To a solution of 3,4,5-trimethoxybromobenzene (1.62 g, 6.55 mmol) in anhydrous THF (20 mL), n-BuLi (2.62 mL, 6.55 mmol) was added dropwise at –78 °C. The reaction mixture was stirred for 30 min at –78 °C. A solution of 5-((tert-butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one 5 (1.00 g, 3.27 mmol) in THF (10 mL) was added dropwise, and the reaction mixture was then allowed warm to 0 °C over 3 h. The reaction was then cooled to –78 °C, and triethylamine (3.68 mL, 26.16 mmol) and methanesulfonyl chloride (MsCl) (1.01 mL, 13.08 mmol) were added dropwise to the solution. The reaction mixture was allowed to warm to room temperature over a period of 8 h. The reaction mixture was quenched with H2O (100 mL), and the reaction mixture was then extracted with EtOAc (3 × 100 mL). The combined organic phases were washed with brine, dried over Na2SO4, filtered, and the solvent was removed in vacuo. The resulting crude product was then subjected to flash column chromatography (silica gel, EtOAc/hexanes) to afford tert-butyl((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)oxy)dimethylsilane 7 (0.82 g, 1.80 mmol, 55% yield). 1HNMR (CDCl3, 600 MHz): δ 6.44 (1H, d, J = 8.4 Hz), 6.40 (1H, d, J = 8.5 Hz), 6.37 (2H, s), 5.75 (1H, t, J = 4.6 Hz), 3.69 (3H, s), 3.65 (6H, s), 3.58 (3H, s), 2.69–2.64 (2H, m), 2.13 (2H, td, J = 7.9, 4.7 Hz), 0.83 (9H, s), 0.00 (6H, s). 13C NMR (CDCl3, 151 MHz): δ 152.9, 149.7, 141.4, 139.8, 137.0, 137.0, 128.7, 128.1, 124.9, 118.9, 108.1, 106.0, 60.9, 56.1, 26.1, 23.1 21.7, 18.9, –3.9.
4.1.1.13. 2-Methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-ol (8)49
tert-Butyl((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)oxy)dimethylsilane 7 (0.82 g, 1.80 mmol) was dissolved in THF and cooled to 0 °C. TBAF (0.71 g, 2.70 mmol) was added to the solution. The reaction mixture was stirred 30 min and then quenched with 50 mL of H2O. The aqueous layer was extracted with EtOAc (3 × 100 mL), and the organic layer was dried over Na2SO4. The organic layer was then filtered, and the solvent was removed in vacuo. Purification by flash chromatography (silica gel, EtOAc/hexanes) afforded 2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-ol 8 (0.62 g, 1.80 mmol, quantitative yield) as a white solid. 1H NMR (CDCl3, 600 MHz): δ 6.65 (1H, d, J = 8.4 Hz), 6.61 (1H, d, J = 8.4 Hz), 6.58 (2H, s), 5.99 (1H, t, J = 4.7 Hz), 5.74 (1H, s), 3.91 (6H, s), 3.86 (6H, s), 2.93 (2H, t, J = 8.4 Hz), 2.40 (2H, td, J = 8.0, 4.7 Hz). 13C NMR (CDCl3, 151 MHz): δ 152.9, 145.8, 142.0, 139.5, 136.9, 129.0, 125.4, 122.3, 117.4, 107.2, 105.8, 61.0, 56.1, 56.0, 22.8, 20.2. HRMS: obsd 343.1541 [M + H+], calcd for C20H23O5+: 343.1540. HPLC retention time 7.47 min.
Experimental procedures for intermediates in Scheme 3
4.1.1.14. N′-(5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1(2H)-ylidene)-4-methylbenzenesulfonohydrazide (10)49
To a well-stirred solution of tetralone 5 (6.20 g, 13.1 mmol) in anhydrous EtOH (120 mL) was added p-toluenesulfonyl hydrazide (3.77 g, 20.3 mmol). Solution was achieved within 5 min, at which point p-TSA monohydrate (0.17 g, 1.01 mmol) was added, and the reaction mixture was stirred at room temperature for 12 h. Hydrazone 10 precipitated as a white solid, which was then filtered, washed with ice-cold EtOH, and dried under reduced pressure to afford 10 (5.20 g, 11.0 mmol, 54% yield) as a white solid. 1H NMR (CDCl3, 500 MHz): δ 7.93 (2H, d, J = 8.3 Hz), 7.86 (1H, s), 7.61 (1H, d, J = 8.7 Hz), 7.31 (2H, d, J = 8.0 Hz), 6.73 (1H, d, J = 8.8 Hz), 3.79 (3H, s), 2.71–2.65 (2H, m), 2.42 (2H, t, J = 6.6 Hz), 2.40 (3H, s), 1.80 (2H, p, J = 6.6 Hz), 0.97 (9H, s), 0.13 (6H, s).
4.1.1.15. (5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1-yl)(3,4,5-trimethoxyphenyl)methanol (11)49
n-BuLi (13.5 mL, 33.8 mmol) was added to freshly distilled TMEDA (30 mL), and the mixture was cooled to –50 °C. At this point, hydrazine 10 (4.00 g, 8.44 mmol) was added, and the reaction mixture was stirred until the temperature reached 25 °C. To the reaction mixture 3,4,5-trimethoxybenzaldehyde (6.62 g, 33.8 mmol) was added, and the reaction was stirred for 1 h. H2O (25 mL) was added, and the product was extracted with EtOAc (2 × 100 mL). The combined organic phases were washed with 10% aqueous CuSO4 solution (100 mL) and brine, dried over Na2SO3, filtered, and evaporated under reduced pressure. Purification of the crude product by flash column chromatography (silica gel, 16 : 84 EtOAc/hexanes) yielded alcohol 11 (2.22 g, 4.56 mmol, 54% yield) as a pale-yellow oil. The resulting secondary alcohol was carried on to the next step without characterization.
4.1.1.16. (5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1-yl)(3,4,5-trimethoxyphenyl)methanone (12)49
To a well-stirred solution of Dess–Martin periodinane (1.30 g, 2.24 mmol) in dry CH2Cl2 (20 mL) at room temperature was added a solution of alcohol 11 (1.00 g, 2.04 mmol) in anhydrous CH2Cl2 (20 mL) followed by 10% aqueous Na2S2O4 (0.05 mL). After stirring for 5 min, a solution of 10% aqueous Na2S2O3 and saturated NaHCO3 (30 mL, 1 : 1 ratio) was added, and the reaction mixture was stirred for 5 min. The product was extracted into CH2Cl2 (3 × 50 mL). The combined organic phases were filtered, and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (silica gel, 10 : 90 EtOAc/hexanes) to afford ketone 12 (0.68 g, 1.41 mmol, 69% yield) as a pale-yellow oil. 1H NMR (CDCl3, 500 MHz): δ 7.14 (2H, s), 6.80 (1H, d, J = 8.4 Hz), 6.63 (1H, d, J = 8.5 Hz), 6.32 (1H, t, J = 4.7 Hz), 3.91 (3H, s), 3.84 (6H, s), 3.77 (3H, s), 2.88 (2H, t, J = 8.0 Hz), 2.43 (2H, td, J = 7.9, 4.8 Hz), 1.01 (9H, s), 0.17 (6H, s).
4.1.1.17. (5-Hydroxy-6-methoxy-3,4-dihydronaphthalen-1-yl)(3,4,5-trimethoxyphenyl)methanone (13)49
Ketone 12 (0.68 g, 1.41 mmol) was dissolved in CH2Cl2 (25 mL), and the reaction mixture was cooled to 0 °C. To this was added TBAF (2.82 mL, 2.82 mmol) dropwise. The reaction mixture was stirred for 10 min and terminated by the addition of H2O (25 mL). The organic layers were separated, and the aqueous phases were extracted with CH2Cl2 (2 × 25 mL). The combined organic phases were washed with brine and dried over Na2SO4. Filtration followed by evaporation of solvents afforded the crude product, which was separated by flash column chromatography (silica gel, 40 : 60 EtOAc/hexanes) to afford 13 (0.29 g, 0.78 mmol, 55% yield) as an off-white solid. 1H NMR (CDCl3, 500 MHz): δ 7.16 (2H, s), 6.77 (1H, d, J = 8.4 Hz), 6.65 (1H, d, J = 8.4 Hz), 6.33 (1H, t, J = 4.7 Hz), 5.74 (1H, s), 3.92 (3H, s), 3.88 (3H, s), 3.85 (6H, s), 2.91 (2H, t, J = 8.0 Hz), 2.47 (2H, td, J = 8.0, 4.7 Hz). 13C NMR (CDCl3, 151 MHz): δ 196.3, 152.8, 146.4, 142.4, 142.2, 138.3, 133.0, 133.0, 125.9, 121.4, 117.5, 107.8, 107.5, 60.9, 56.2, 55.9, 22.5, 19.8. HRMS: obsd 371.1489 [M + H+], calcd for C21H23O6+ 371.1489.
Experimental procedures for intermediates in Scheme 4
4.1.1.18. ((5-Bromo-2-methoxy-7,8-dihydronaphthalen-1-yl)oxy)(tert-butyl)dimethylsilane (14)63
To a solution of 5-((tert-butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one 5 (0.66 g, 2.17 mmol) in toluene (50 mL) phosphorus tribromide (3.25 mL, 1 M in dichloromethane) was added. The reaction mixture was heated to 80 °C for 3 h. Water was added, and the mixture was extracted with EtOAc (3 × 50 mL). The organic layer was dried over Na2SO4, filtered, concentrated under reduced pressure, and purified by flash chromatography (silica gel, EtOAc/hexanes) to afford vinyl bromide 14 (0.51 g, 1.4 mmol, 63% yield) as an orange oil. 1H NMR (CDCl3, 500 MHz): δ 7.16 (1H, d, J = 8.5 Hz), 6.69 (1H, d, J = 8.5 Hz), 6.29 (1H, t, J = 4.8 Hz), 3.80 (3H, s), 2.86–2.82 (2H, m), 2.29 (2H, td, J = 8.0, 4.9 Hz), 1.00 (9H, s), 0.16 (6H, s). 13C NMR (CDCl3, 151 MHz): δ 150.6, 141.1, 128.7, 127.7, 126.7, 121.1, 120.1, 108.2, 54.9, 26.1, 26.0, 25.0, 21.5, –4.0.
4.1.1.19. (5-((tert-Butyldimethylsilyl)oxy)-6-methoxy-3,4-dihydronaphthalen-1-yl)(3,4,5-trimethoxyphenyl)methanone (12)
To a solution of vinyl bromide 14 (0.51 g, 1.4 mmol) in THF (50 mL) at –78 °C, n-BuLi (0.56 mL, 2.5 M in hexanes) was added. The reaction mixture was stirred for 15 min, then N,3,4,5-tetramethoxy-N-methylbenzamide dissolved in THF was added. The reaction was allowed to warm to room temperature over a period of 8 h. Upon completion, water was added, and the mixture was extracted with EtOAc (3 × 50 mL). The organic layer was dried over Na2SO4, filtered, concentrated under reduced pressure, and purified by flash chromatography (silica gel, EtOAc/hexanes) to afford dihydronaphthalene analog 12 (0.32 g, 0.66 mmol, 47% yield) as a clear oil. 1H NMR (CDCl3, 500 MHz): δ 7.14 (2H, s), 6.80 (1H, d, J = 8.4 Hz), 6.63 (1H, d, J = 8.5 Hz), 6.32 (1H, t, J = 4.7 Hz), 3.91 (3H, s), 3.84 (6H, s), 3.77 (3H, s), 2.88 (2H, t, J = 8.0 Hz), 2.43 (2H, td, J = 7.9, 4.8 Hz), 1.01 (9H, s), 0.17 (6H, s). 13C NMR (CDCl3, 126 MHz): δ 196.4, 152.8, 150.2, 142.3, 141.5, 138.6, 133.1, 133.0, 127.3, 125.8, 119.2, 108.7, 107.4, 60.9, 56.2, 54.8, 26.0, 22.8, 21.3, 18.8, –4.0.
4.1.1.20. (5-Hydroxy-6-methoxy-3,4-dihydronaphthalen-1-yl)(3,4,5-trimethoxyphenyl)methanone (13)
To a solution of silyl ether 12 (0.32 g, 0.66 mmol) in THF concentrated HCl (5 mL) was added. The reaction was stirred at room temperature for 3 h. Upon completion, saturated aqueous NaHCO3 was added, and the mixture was extracted with EtOAc (3 × 50 mL). The organic layer was dried over Na2SO4, filtered, concentrated under reduced pressure, and purified by flash chromatography (silica gel, EtOAc/hexanes) to afford 13 (0.24 g, 0.66 mmol, quantitative yield) as a white solid. To prevent the dihydronaphthalene ring from aromatizing the reaction mixture was not heated above room temperature during the deprotection reaction or during the purification process. 1H NMR (CDCl3, 500 MHz): δ 7.16 (2H, s), 6.77 (1H, d, J = 8.4 Hz), 6.65 (1H, d, J = 8.4 Hz), 6.33 (1H, t, J = 4.7 Hz), 5.74 (1H, s), 3.92 (3H, s), 3.88 (3H, s), 3.85 (6H, s), 2.91 (2H, t, J = 8.0 Hz), 2.47 (2H, td, J = 8.0, 4.7 Hz). 13C NMR (CDCl3, 151 MHz): δ 196.3, 152.8, 146.4, 142.4, 142.2, 138.3, 133.0, 133.0, 125.9, 121.4, 117.5, 107.8, 107.5, 60.9, 56.2, 55.9, 22.5, 19.8. HRMS: obsd 371.1489 [M + H+], calcd for C21H23O6+ 371.1489. HPLC retention time – 5.18 min.
Experimental procedures for intermediates in Scheme 5
4.1.1.21. Dibenzyl (2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl) phosphate (15)47,49
To a well-stirred solution of 13 (1.40 g, 4.09 mmol) in CH3CN (25 mL) at –25 °C, CCl4 (25 mL) was added, and the reaction mixture was stirred for approximately 15 min. DIPEA (1.51 mL, 8.61 mmol) and DMAP (0.05 g, 0.41 mmol) were added, followed by the addition of dibenzyl phosphonate (1.36 mL, 6.14 mmol), and the reaction mixture was stirred for approximately 2 h. The reaction was quenched with a 0.5 M KH2PO4 solution (30 mL). The solution was extracted with EtOAc (3 × 100 mL). The combined organic phases were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography (silica gel, 40 : 60 EtOAc/hexanes) afforded dibenzyl-KGP03-phosphate 15 (2.16 g, 3.58 mmol, 88% yield) as a yellow oil. The resulting dibenzyl ester was carried on to the next step without characterization.
4.1.1.22. Disodium 2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl phosphate (16)47,49
To a solution of dibenzyl-KGP03-phosphate 15 (2.16 g, 3.58 mmol) in acetonitrile (60 mL), sodium iodide (1.07 g, 7.16 mmol) was added. Before dropwise addition of chlorotrimethylsilane (0.91 mL, 7.16 mmol), the mixture was stirred for 2 min, and approximately 30 min later the reaction was terminated with 1% aqueous sodium thiosulfate (1 mL). Removal of the acetonitrile in vacuo afforded a crude mixture, which was dissolved in water–dichloromethane and washed with water (4 × 60 mL). Concentration (facilitated by toluene azeotrope) of the aqueous layer resulted in isolation of the crude phosphoric acid intermediate, which was subjected to drying under vacuum for approximately 1 h, and then dissolved in dry methanol (30 mL). Next, sodium methoxide (0.39 g, 7.16 mmol) was added. The mixture was stirred 6 h and additional methanol was added to effect dissolution. After filtration of the solution, concentration of the methanol in vacuo led to an off-white solid, which was reprecipitated from water–ethanol to yield 16 (1.10 g, 2.36 mmol, 66% yield). 1H NMR (D2O, 300 MHz): δ 6.67 (1H, d, J = 8.4 Hz), 6.65 (2H, s), 6.64 (1H, d, J = 8.4 Hz), 5.98 (1H, t, J = 4.6 Hz), 3.75 (6H, s), 3.74 (6H, s), 2.86 (2H, t, J = 8.2 Hz), 2.23 (2H, td, J = 8.2, 4.6 Hz). 13C NMR (D2O, 75 MHz): δ 152.2, 151.6, 140.0, 140.0, 138.5, 137.8, 135.8, 131.5, 131.5, 128.2, 128.1, 126.7, 120.7, 109.1, 106.3, 61.0, 56.0, 55.5, 22.5, 21.8. HPLC retention time 3.07 min.
4.1.1.23. Dibenzyl (2-methoxy-5-(3,4,5-trimethoxybenzoyl)-7,8-dihydronaphthalen-1-yl) phosphate (17)47,49
To a well-stirred solution of 13 (0.74 g, 2.00 mmol) in CH3CN (15 mL) at –25 °C CCl4 (15 mL) was added, and the reaction mixture was stirred for approximately 15 min. DIPEA (0.51 mL, 2.91 mmol) and DMAP (0.02 g, 0.16 mmol) were added, followed by the addition of dibenzyl phosphonate (0.53 mL, 2.40 mmol), and the reaction mixture was stirred for approximately 2 h. The reaction was quenched with a 0.5 M KH2PO4 solution (30 mL). The solution was extracted with EtOAc (3 × 100 mL). The combined organic phases were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography (silica gel, 40 : 60 EtOAc/hexanes) afforded dibenzyl-KGP413-phosphate 17 (0.67 g, 1.06 mmol, 84% yield) as a yellow oil. The resulting dibenzyl ester was carried on to the next step without characterization.
4.1.1.24. Disodium 2-methoxy-5-(3,4,5-trimethoxybenzoyl)-7,8-dihydronaphthalen-1-yl phosphate (18)47,49
To a solution of dibenzyl-KGP413-phosphate 17 (0.67 g, 1.06 mmol) in acetonitrile (20 mL), sodium iodide (0.32 g, 2.12 mmol) was added. Before dropwise addition of chlorotrimethylsilane (0.27 mL, 2.12 mmol), the mixture was stirred for 2 min, and approximately 30 min later the reaction was terminated with 1% aqueous sodium thiosulfate (1 mL). Removal of the acetonitrile in vacuo afforded a crude mixture, which was dissolved in water–dichloromethane and washed with water (4 × 20 mL). Concentration (facilitated by toluene azeotrope) of the aqueous layer resulted in isolation of the crude phosphoric acid intermediate, which was subjected to drying under vacuum for approximately 1 h, and then dissolved in dry methanol (10 mL). Next, sodium methoxide (0.12 g, 2.12 mmol) was added. The mixture was stirred 6 h, and additional methanol was added to effect dissolution. After filtration of the solution, concentration of the methanol in vacuo led to an off-white solid, which was reprecipitated from water–ethanol to yield 18 (0.22 g, 0.45 mmol, 42% yield). 1H NMR (D2O, 300 MHz): δ 7.11 (2H, s), 6.77 (1H, d, J = 8.4 Hz), 6.69 (1H, d, J = 8.4 Hz), 6.44 (1H, t, J = 4.7), 3.75 (6H, s), 3.75 (3H, s), 3.70 (3H, s), 2.86 (2H, t, J = 8.0 Hz), 2.34 (2H, td, J = 8.0, 4.7 Hz). 13C NMR (D2O, 75 MHz): δ 200.1, 152.3, 152.2, 152.1, 141.5, 140.1, 139.0, 137.3, 133.5, 130.8, 130.8, 124.9, 120.9, 109.5, 108.5, 107.9, 61.0, 56.1, 55.4, 22.6, 21.0. 31P NMR (D2O, 122 MHz): δ 0.92, 0.79. HRMS: obsd 495.0791 [M + H+], calcd for C21H22Na2O9P+ 495.0791. HPLC retention time 8.05 min.
4.2. Biological evaluation
4.2.1. Cell lines and sulforhodamine B (SRB) assay
Inhibition of growth of human cancer cells was assessed using the sulforhodamine B assay (SRB), as previously described.69–71 Cancer cell lines (DU-145, SK-OV-3, and NCI-H460) (obtained from ATCC) were plated at 7000–8000 cells per well into 96-well plates using DMEM supplemented with 5% fetal bovine serum/1% gentamicin sulfate and incubated for 24 h at 37 °C in a humidified incubator in a 5% CO2 atmosphere. Compounds to be tested were dissolved in DMSO to generate a 10 mg mL–1 stock solution. Serial dilutions were made and added to the plates. Doxorubicin and paclitaxel were used as positive controls. After a 48 h treatment, the cells were fixed with trichloroacetic acid (10% final concentration), washed, dried, stained with SRB dye, washed to remove excess dye, and dried. SRB dye was solubilized, and absorbances were measured at wavelength 540 nm and normalized to values at wavelength 630 nm using an automated Biotek plate reader. A growth inhibition of 50% (GI50 or the drug concentration causing 50% reduction in the net protein increase) was calculated from the absorbance data.
4.2.2. Colchicine binding assay72
Inhibition of [3H]colchicine binding to tubulin was measured using 100 μL reaction mixtures containing 1.0 μM tubulin, 5.0 μM [3H]colchicine (from Perkin-Elmer), 5% (v/v) dimethyl sulfoxide, potential inhibitors at 1.0 or 5.0 μM, as indicated, and components that stabilize the colchicine binding activity of tubulin (1.0 M monosodium glutamate [adjusted to pH 6.6 with HCl in a 2.0 M stock solution], 0.5 mg mL–1 bovine serum albumin, 0.1 M glucose-1-phosphate, 1.0 mM MgCl2, and 1.0 mM GTP). Incubation was for 10 min at 37 °C, when the binding reaction in control reaction mixtures is 40–60% complete. Reactions were stopped with 2.0 mL of ice-cold water, and the reaction mixtures were placed on ice. Each sample was poured onto a stack of two DEAE-cellulose filters (from Whatman), followed by 6 mL of ice-cold water. The samples were aspirated under reduced vacuum. The filters were washed three times with 2 mL water and placed into vials containing 5 mL of Biosafe II scintillation cocktail. Samples were counted 18 h later in a Beckman scintillation counter. Samples with inhibitors were compared to samples with no inhibitor, and percent inhibition was determined. All samples were corrected for the amount of radiolabel bound to the filters in the absence of tubulin.
4.2.3. Inhibition of tubulin polymerization43
Tubulin polymerization experiments were performed in 0.25 mL reaction mixtures (final volume) that contained 1 mg mL–1 (10 μM) purified bovine brain tubulin, 0.8 M monosodium glutamate (pH 6.6), 4% (v/v) dimethyl sulfoxide, 0.4 mM GTP, and different compound concentrations. All reaction components except GTP were preincubated for 15 min at 30 °C in 0.24 mL. The mixtures were cooled to 0 °C, and 10 μL of 10 mM GTP was added. Reaction mixtures were transferred to cuvettes held at 0 °C in Beckman DU-7400 and DU-7500 spectrophotometers equipped with electronic temperature controllers. The temperature was jumped to 30 °C, taking about 30 s, and polymerization was followed at 350 nm for 20 min. The IC50 was defined as the compound concentration that inhibited extent of polymerization by 50% after 20 min.
4.2.4. In vivo color Doppler ultrasound imaging with KGP04
A549 human lung cancer cells (3 × 106, in 200 μL serum free medium with 50% Matrigel®; cell line provided by Professor John Minna (UT Southwestern)) were implanted subcutaneously in the right hind thigh of anesthetized nude rats (8–10 week old female; T-cell-deficient, athymic homozygous nude rat; Charles River, NCI at Frederick, Frederick, MD).73 The tumor was allowed to grow until it reached about 9 mm diameter. At this time the rat was anesthetized with isoflurane and color-Doppler ultrasound was performed using a Vevo 2100 [Fuji (VisualSonics), Toronto, Canada] before and 2 h after administration of KGP04 IP (15 mg kg–1 dissolved in 500 μl saline) in situ.14 All animal procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee under APN101222 and 102 169.
4.2.5. In vivo bioluminescence imaging (BLI) with KGP15217
MDA-MB-231-luc cells (1 × 106 in 100 μL of PBS with 50% Matrigel®; original cell line from ATCC, with transfected cell line recently provided by Dr. Edward Graves, Stanford University) were injected directly into the left upper mammary fat pad of three anesthetized BALB/c SCID mice. Tumors were allowed to grow over about 3 months and then BLI was performed using an IVIS® Spectrum system (Perkin-Elmer (Xenogen), Alameda, CA). d-Luciferin (128 mg kg–1 in PBS in a total volume of 80 μL; Gold Biotechnology Inc., St. Louis, MO) was administered subcutaneously (SC) in the foreback neck region. Immediately after luciferin injection, a series of BLI images was acquired over a period of 30 min using auto exposure time.17 Following baseline BLI, mice were injected intraperitoneally (IP) respectively with 100, 150, and 200 mg kg–1KGP152 in saline. Dynamic BLI was repeated 4 and 24 h post-treatment with administration of fresh luciferin on each occasion. All animal procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee under APN101222 and 102169.
Conflicts of interest
A significant portion of the work presented in this manuscript was funded through Mateon Therapeutics, Inc. (formerly OXiGENE Inc.), and this relationship is properly indicated in the acknowledgement section. Dr. David Chaplin is a former employee and current member of the Board of Directors of Mateon Therapeutics, Inc. He is included as a co-author on this manuscript for his valuable contributions to the overall project. In addition, one of the authors (KGP) is a former paid consultant with Mateon Therapeutics, Inc. and current shareholder. We are pleased with this productive and useful long-term scientific collaboration and funding relationship with Mateon Therapeutics, Inc., and it is important to note that there is absolutely no actual conflict of interest associated with the science presented in this manuscript.
Supplementary Material
Acknowledgments
The research was supported in part by the National Cancer Institute of the National Institutes of Health (Grant No. 5R01CA140674 to K. G. P., M. L. T., and R. P. M.), the Cancer Prevention and Research Institute of Texas (CPRIT, Grant No. RP140399 to K. G. P., M. L. T., and R. P. M., Grant No. RP170696 to K. G. P., M. L. T., and MIRA RP120670-P3 to RPM), and Mateon Therapeutics, Inc. (grant to K. G. P. and M. L. T.) for their financial support of this project. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. The authors also thank Dr. Craig Moehnke and Dr. Michelle Nemec (Director) for the use of the shared Molecular Biosciences Center at Baylor University, Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University), Dr. Christine A. Herdman (help with spectral characterization), and Dr. Kevin Klausmeyer (X-ray analysis). Imaging was facilitated with the assistance of the Southwestern Small Animal Imaging Resource (SW-SAIRP), which is supported in part by NCI U24 CA126608, the Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant, 1P30CA142543, and the Department of Radiology. The IVIS Spectrum was acquired with the assistance of NIH Shared Instrumentation Grant S10RR024757. We are grateful to Professor J. Hill (Cardiology, UTSW) for providing access to the Vevo 2100, which was acquired under the NIH Shared Instrumentation Grant S10 RR031859.
Footnotes
†Dedicated to the memory of Dr. Anjan Ghatak, an inspiring and creative synthetic organic, medicinal chemist who made seminal contributions to the science described in this manuscript.
‡All animal procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee under APN101222 and 102169.
§Electronic supplementary information (ESI) available: Supplementary data including (1H NMR, 13C NMR, 31P NMR, HRMS, HPLC) for target compounds and intermediates (1H NMR, 13C NMR, only), phosphatase enzyme cleavage data, and X-ray crystallography (CCDC 1018601 and 1842517) for KGP03 and KGP413 associated with this article can be found in the online supplementary data file. CCDC 1018601 and 1842517. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8md00322j
References
- Ludford R. J. JNCI, J. Natl. Cancer Inst. 1945;6:89–101. [Google Scholar]
- Pettit G. R., Singh S. B., Hamel E., Lin C. M., Alberts D. S., Garcia-Kendal D. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Singh S. B., Niven M. L., Hamel E., Schmidt J. M. J. Nat. Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Singh S. B., Boyd M. R., Hamel E., Pettit R. K., Schmidt J. M., Hogan F. J. Med. Chem. 1995;38:1666–1672. doi: 10.1021/jm00010a011. [DOI] [PubMed] [Google Scholar]
- Pinney K., Pettit G., Trawick M., Jelinek C. and Chaplin D., in Anticancer Agents from Natural Products, Second Edition, CRC Press, 2011, pp. 27–64. [Google Scholar]
- Griggs J., Metcalfe J. C., Hesketh R. Lancet Oncol. 2001;2:82–87. doi: 10.1016/S1470-2045(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Bukhari S. N. A., Kumar G. B., Revankar H. M., Qin H.-L. Bioorg. Chem. 2017;72:130–147. doi: 10.1016/j.bioorg.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Hsieh H. P., Liou J. P., Mahindroo N. Curr. Pharm. Des. 2005;11:1655–1677. doi: 10.2174/1381612053764751. [DOI] [PubMed] [Google Scholar]
- Rajak H., Dewangan P. K., Patel V., Jain D. K., Singh A., Veerasamy R., Sharma P. C., Dixit A. Curr. Pharm. Des. 2013;19:1923–1955. doi: 10.2174/1381612811319100013. [DOI] [PubMed] [Google Scholar]
- Tron G. C., Pirali T., Sorba G., Pagliai F., Busacca S., Genazzani A. A. J. Med. Chem. 2006;49:3033–3044. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- Pinney K. G., Carlson K. E., Katzenellenbogen J. A. Steroids. 1992;57:222–232. doi: 10.1016/0039-128x(92)90106-j. [DOI] [PubMed] [Google Scholar]
- Pinney K. G., Wang F., Hadimani M. and Mejia M., US20070082872A1, 2007.
- Pinney K. G., Bounds A. D., Dingeman K. M., Mocharla V. P., Pettit G. R., Bai R., Hamel E. Bioorg. Med. Chem. Lett. 1999;9:1081–1086. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]
- Hadimani M. B., MacDonough M. T., Ghatak A., Strecker T. E., Lopez R., Sriram M., Nguyen B. L., Hall J. J., Kessler R. J., Shirali A. R., Liu L., Garner C. M., Pettit G. R., Hamel E., Chaplin D. J., Mason R. P., Trawick M. L., Pinney K. G. J. Nat. Prod. 2013;76:1668–1678. doi: 10.1021/np400374w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonough M. T., Shi Z., Pinney K. G. Tetrahedron Lett. 2015;56:3624–3629. doi: 10.1016/j.tetlet.2015.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T. E., Odutola S. O., Lopez R., Cooper M. S., Tidmore J. K., Charlton-Sevcik A. K., Li L., MacDonough M. T., Hadimani M. B., Ghatak A., Liu L., Chaplin D. J., Mason R. P., Pinney K. G., Trawick M. L. Cancer Lett. 2015;369:229–241. doi: 10.1016/j.canlet.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Hallac R. R., Lopez R., Denney R., MacDonough M. T., Li L., Liu L., Graves E. E., Trawick M. L., Pinney K. G., Mason R. P. Am. J. Nucl. Med. Mol. Imaging. 2015;5:143–153. [PMC free article] [PubMed] [Google Scholar]
- Hadimani M. B., Kessler R. J., Kautz J. A., Ghatak A., Shirali A. R., O'Dell H., Garner C. M., Pinney K. G. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2002;58:o330–o332. doi: 10.1107/s0108270102003669. [DOI] [PubMed] [Google Scholar]
- Kessler R. J., Synthesis and Evaluation of New Inhibitors of Tubulin Polymerization and Their Corresponding Prodrugs as Potential Vascular Targeting Agents, Master's Thesis, Baylor University, 2002. [Google Scholar]
- Shirali A., Sriram M., Hall J. J., Nguyen B. L., Guddneppanavar R., Hadimani M. B., Ackley J. F., Siles R., Jelinek C. J., Arthasery P., Brown R. C., Murrell V. L., McMordie A., Sharma S., Chaplin D. J., Pinney K. G. J. Nat. Prod. 2009;72:414–421. doi: 10.1021/np800661r. [DOI] [PubMed] [Google Scholar]
- Devkota L., Lin C.-M., Strecker T. E., Wang Y., Tidmore J. K., Chen Z., Guddneppanavar R., Jelinek C. J., Lopez R., Liu L., Hamel E., Mason R. P., Chaplin D. J., Trawick M. L., Pinney K. G. Bioorg. Med. Chem. 2016;24:938–956. doi: 10.1016/j.bmc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanpure R. P., Nguyen B. L., Strecker T. E., Aguirre S., Sharma S., Chaplin D. J., Siim B. G., Hamel E., Lippert J. W., Pettit G. R., Trawick M. L., Pinney K. G. J. Nat. Prod. 2011;74:1568–1574. doi: 10.1021/np200104t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanpure R. P., George C. S., Strecker T. E., Devkota L., Tidmore J. K., Lin C.-M., Herdman C. A., MacDonough M. T., Sriram M., Chaplin D. J., Trawick M. L., Pinney K. G. Bioorg. Med. Chem. 2013;21:8019–8032. doi: 10.1016/j.bmc.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman C. A., Devkota L., Lin C.-M., Niu H., Strecker T. E., Lopez R., Liu L., George C. S., Tanpure R. P., Hamel E., Chaplin D. J., Mason R. P., Trawick M. L., Pinney K. G. Bioorg. Med. Chem. 2015;23:7497–7520. doi: 10.1016/j.bmc.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram M., Hall J. J., Grohmann N. C., Strecker T. E., Wootton T., Franken A., Trawick M. L., Pinney K. G. Bioorg. Med. Chem. 2008;16:8161–8171. doi: 10.1016/j.bmc.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Tanpure R. P., George C. S., Sriram M., Strecker T. E., Tidmore J. K., Hamel E., Charlton-Sevcik A. K., Chaplin D. J., Trawick M. L., Pinney K. G. MedChemComm. 2012;3:720–724. doi: 10.1039/C2MD00318J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Sano Y., Shiina I. Molecules. 2010;15:6773–6794. [Google Scholar]
- Scholl S. M., Huff K. K., Lippman M. E. Endocrinology. 1983;113:611–617. doi: 10.1210/endo-113-2-611. [DOI] [PubMed] [Google Scholar]
- Herdman C. A., Strecker T. E., Tanpure R. P., Chen Z., Winters A., Gerberich J., Liu L., Hamel E., Mason R. P., Chaplin D. J., Lynn Trawick M., Pinney K. G. MedChemComm. 2016;7:2418–2427. doi: 10.1039/C6MD00459H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanpure R. P., Harkrider A. R., Strecker T. E., Hamel E., Trawick M. L., Pinney K. G. Bioorg. Med. Chem. 2009;17:6993–7001. doi: 10.1016/j.bmc.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann D. W., Chaplin D. J., Horsman M. R. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hill S. A., Hobson B. Eur. J. Cancer Clin. Oncol. 1983;19:271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Kanthou C., Baguley B. C. Nat. Rev. Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- Mattern J., Volm M. Cytotechnology. 1998;27:249–256. doi: 10.1023/A:1008033326059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman M. R., Bohn A. B., Busk M. Exp. Oncol. 2010;32:143–148. [PubMed] [Google Scholar]
- Kanthou C., Tozer G. M. Int. J. Exp. Pathol. 2009;90:284–294. doi: 10.1111/j.1365-2613.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton R. A., Gernert K. M., Nettles J. H., Aneja R. Med. Res. Rev. 2011;31:443–481. doi: 10.1002/med.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Mason R. P., Zhao D., Liu L., Trawick M. L., Pinney K. G. Integr. Biol. 2011;3:375–387. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Siemann D. W., Chaplin D. J., Horsman M. R. Cancer Invest. 2017;35:519–534. doi: 10.1080/07357907.2017.1364745. [DOI] [PubMed] [Google Scholar]; (c) Siemann D. W., Bibby M. C., Dark G. G., Dicker A. P., Eskens F. A. L. M., Horsman M. R., Marmé D., Lorusso P. M. Clin. Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]; (d) Tozer G. M., Prise V. E., Wilson J., Cemazar M., Shan S., Dewhirst M. W., Barber P. R., Vojnovic B., Chaplin D. J. Cancer Res. 2001;61:6413–6422. [PubMed] [Google Scholar]; (e) Tozer G. M., Kanthou C., Parkins C. S., Hill S. A. Int. J. Exp. Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe P. E. Clin. Cancer Res. 2004;10:415–427. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Hillan K. J., Novotny W. Biochem. Biophys. Res. Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- Zhao D., Richer E., Antich P. P., Mason R. P. FASEB J. 2008;22:2445–2451. doi: 10.1096/fj.07-103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folaron M., Seshadri M. Mol. Imaging Biol. 2016;18:860–869. doi: 10.1007/s11307-016-0963-8. [DOI] [PubMed] [Google Scholar]
- Hamel E. Cell Biochem. Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- Siemann D. W. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Horsman M. R. Int. J. Hyperthermia. 2008;24:57–65. doi: 10.1080/02656730701829710. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Thornhill A., Melody N., Knight J. C. J. Nat. Prod. 2009;72:380–388. doi: 10.1021/np800608c. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Chaplin D. J., Walicke P. A. Expert Opin. Invest. Drugs. 2009;18:189–197. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney K. G., Mocharla V. P., Chen Z., Garner C. M., Ghatak A., Hadimani M., Kessler J., Dorsey J. M., Edvardsen K., Chaplin D. J., Prezioso J. and Ghatak U. R., US20040043969A1, 2004.
- Shirali A. R., Inhibitors of Tubulin Assembly: Designed Ligands Featuring Benzo[b]thiophene, Dihydronaphthalene and Aroylchromene Molecular Core Structure, Ph.D. Dissertation, Baylor University, 2002. [Google Scholar]
- Hadimani M. B., Studies Toward the Discovery of New Classes, Studies Toward the Discovery of New Classes of Privileged Molecules as Colchicine-Site Binding Ligands for Tubulin: Structure-Based Design, Synthesis, and Bioactivity of Small Ligands Targeted at Tumor Vasculature, of Privileged Molecules as Colchicine-Site Binding Ligands for Tubulin: Structure-Based Design, Synthesis, and Bioactivity of Small Ligands Targeted at Tumor Vasculature, Ph.D. Dissertation, Baylor University, 2004. [Google Scholar]
- Dogra A., Design and Synthesis of Dihydronaphthalene Vascular Disrupting Agents and Indolequinone-Based Bioreductives, Master's Thesis, Baylor University, 2006. [Google Scholar]
- Jelinek C. J., Discovery and Development of Dihydronaphthalene-Based Vascular Targeting Agents and Combretastatin-Related Analogs, Master's Thesis, Baylor University, 2004. [Google Scholar]
- Rasolofonjatovo E., Provot O., Hamze A., Rodrigo J., Bignon J., Wdzieczak-Bakala J., Desravines D., Dubois J., Brion J. D., Alami M. Eur. J. Med. Chem. 2012;52:22–32. doi: 10.1016/j.ejmech.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Toki B., Herald D. L., Verdier-Pinard P., Boyd M. R., Hamel E., Pettit R. K. J. Med. Chem. 1998;41:1688–1695. doi: 10.1021/jm970644q. [DOI] [PubMed] [Google Scholar]
- Pettit G. R., Grealish M. P., Herald D. L., Boyd M. R., Hamel E., Pettit R. K. J. Med. Chem. 2000;43:2731–2737. doi: 10.1021/jm000045a. [DOI] [PubMed] [Google Scholar]
- Pettit R. and Rhodes M. R., WO1999035150A1, 1999.
- Pettit G. R., Rhodes M. R. Anti-Cancer Drug Des. 1998;13:183–191. [PubMed] [Google Scholar]
- Pettit G. R., Lippert J. W. Anti-Cancer Drug Des. 2000;15:203–216. [PubMed] [Google Scholar]
- Pravst I., Zupan M., Stavber S. Tetrahedron Lett. 2006;47:4707–4710. [Google Scholar]
- Ghatak A., Dorsey J. M., Garner C. M., Pinney K. G. Tetrahedron Lett. 2003;44:4145–4148. [Google Scholar]
- Darwish A., Chong J. M. J. Org. Chem. 2007;72:1507–1509. doi: 10.1021/jo062145f. [DOI] [PubMed] [Google Scholar]
- Shagufta A. I., Raghunandan R., Maulik P. R., Panda G. Tetrahedron Lett. 2005;46:5337–5341. [Google Scholar]
- Liang W., Ni Y., Chen F. Oncotarget. 2016;7:15444–15459. doi: 10.18632/oncotarget.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Beck H., Wang X., Hsieh H.-P., Mason R. P., Liu X. PLoS One. 2012;7:e43314. doi: 10.1371/journal.pone.0043314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhasan M. K., Liu L., Lewis M. A., Magnusson J., Mason R. P. PLoS One. 2012;7:e46106. doi: 10.1371/journal.pone.0046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Chen Y., Liu J., Yang Y., Lv Q., Wang J., Zhang L., Xie M. Ultrasound Med. Biol. 2018;44:840–852. doi: 10.1016/j.ultrasmedbio.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Sriram M., Design, Synthesis, Biochemical and Biological Evaluation of Benzocyclic and Enediyne Analogs of Combretastatins as Potential Tubulin Binding Ligands in the Treatment of Cancer, Ph.D. Dissertation, Baylor University, 2007. [Google Scholar]
- Siles R., Ackley J. F., Hadimani M. B., Hall J. J., Mugabe B. E., Guddneppanavar R., Monk K. A., Chapuis J.-C., Pettit G. R., Chaplin D. J., Edvardsen K., Trawick M. L., Garner C. M., Pinney K. G. J. Nat. Prod. 2008;71:313–320. doi: 10.1021/np070377j. [DOI] [PubMed] [Google Scholar]
- Vichai V., Kirtikara K. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Biochim. Biophys. Acta, Gen. Subj. 1981;675:226–231. doi: 10.1016/0304-4165(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Zhou H., Zhang Z., Denney R., Williams J. S., Gerberich J., Stojadinovic S., Saha D., Shelton J. M., Mason R. P. Oncotarget. 2017;8:37464–37477. doi: 10.18632/oncotarget.16395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.