Abstract
Micelles have been studied as drug delivery carriers for decades. Their use can potentially result in high drug accumulation at the target site through the enhanced permeability and retention effect. Nevertheless, the lack of stability of micelles in the physiological environment limits their efficacy as a drug carrier. In particular, micelles tend to disassociate and prematurely release the encapsulated drugs, lowering delivery efficacy and creating toxicity concerns. Many efforts to enhance the stability of micelles have focused mainly on decreasing the critical micelle forming concentration and improving blood circulation. Herein, we review different strategies including crosslinking and non-crosslinking approaches designed to stabilize micelles and offer perspectives on future research directions.
Keywords: Micelle, stability, crosslinking, critical micelle concentration (CMC), drug delivery
Summary
Different strategies to improve micelle stability were reviewed in this work. Specific examples with improved drug delivery efficacy owing to enhanced micelle stability were illustrated.
1. Introduction
Many different drug carriers have been developed for controlled drug delivery in recent decades, including micelles, liposomes, polymer or protein-drug conjugates, polymeric nanoparticles, and pathogens [1–13]. Among these drug carriers, micelles have a number of attractive features. Micelles are self-assembled microstructures formed by surfactants in an aqueous system and are usually < 50 nm in diameter [14]. Polymeric micelles are defined as organized auto-assemblies formed in a liquid, which are composed of amphiphilic macromolecules, in general amphiphilic di- or tri-block copolymers made of solvophilic and solvophobic blocks [15]. Polymeric micelles can exceed 100 nm in size and can still be regarded as micelles [16]. These self-assembled micelle structures can protect insoluble hydrophobic drugs, which mimic aspects of biological transport systems in terms of structure and function [17]. Micelles have been widely used as drug delivery carriers for a series of different molecules, including low molecular mass hydrophobic drugs, proteins, and genes [18–22].
Compared with other drug delivery strategies, micelles have two unmatched advantages. The first is their relatively small size. The hydrodynamic sizes of a micelle is usually less than 50 nm [23]. Considering the dimensions of physiological pores in the body’s vasculatures (e.g., pores of kidney glomeruli, inter-endothelial junctions for healthy tissues, tumors, cancerous tissues, etc.), nanomedicine utilizing smaller particle sizes (e.g., < 100 nm) is favored for blood circulation, tissue penetration, and cellular uptake [24–30]. Recent evidence has indicated that a sub-50 nm drug formulation is desirable to achieve the collective outcome of deeper tumor tissue penetration and more efficient cancer cell internalization, as well as the cellular response [31, 32]. Therefore, small micelles can markedly improve the in vivo performance of encapsulated drugs, leading to high accumulation at the target site (e.g., tumor tissue) due to the enhanced permeability and retention (EPR) effect [33–35].
The second advantage of micelles is their feasibility of large-scale manufacture. A micelle is the simplest assembled entity, with well-defined molecular structures and assembly behaviors. This enables simple drug formulation and ease of manufacturing. These attributes are vital; other advanced particle systems are complicated to prepare and have inherent problems that hinder their large-scale production in a stable and consistent manner. For example, periodic shortages due to difficulty in obtaining manufacture approval have resulted in limited availability of DOXIL® (Jassen Products, LP. Titusville, NJ), a polyethylene glycol (PEG)ylated liposome with encapsulated doxorubicin [36]. In contrast, a micelle formulation is simple to manufacture at a large scale, such as Taxotere® or Taxol®, which contain only the drug (docetaxel), the micelle (polysorbate 80), and the solvent (alcohol) [37]. The two advantages of micelles have spurred their acceptance as a first-line drug formulation technology[18] .
Despite the advantages, a fundamental limitation of micelles as drug delivery carriers is their low stability when encountering environmental changes. Once the concentration is below the critical micelle forming concentration (CMC), micelles can disassociate. This is typical when micelle formulations are injected into the blood. Disintegration of micelles due to their dilution in blood and other factors that include protein binding may result in a burst release of previously encapsulated drugs into the bloodstream. Such premature drug release can negate the potential benefits of a carefully optimized drug carrier, which include high drug loading, prolonged blood circulation due to the EPR, and targeting capability. The result can be an unfavorable drug biodistribution and a therapeutic outcome that is similar to that of an unprotected (non-encapsulated) drug [38]. For example, the micellar drug Taxotere® is quickly removed from the blood circulation once intravenously (i.v.) injected [39]. This can reduce the therapeutic performance of the encapsulated drug and raise concerns about toxicity [18, 40]. Therefore, how to stabilize micelles, especially in the physiological condition, is a significant challenge for their drug delivery applications.
In this review, we explore several factors that can potentially influence the stability of micelles and discuss different strategies to stabilize micelles. The current and potential therapeutic uses of micelles are considered with specific examples provided of the benefits of enhanced micelle stability on drug biodistribution and therapeutic outcome.
2. Micelle stability and evaluation methods
When a micelle solution is diluted to a very low concentration (typically < 10−3 mM), the surfactant content is not sufficient to drive the self-assembly of micelles. Instead, they tend to distribute at the air-water or aqueous-organic solvent interface which leads to the disintegration of the micelles. Thus, a minimal concentration of surfactant, the CMC, is required to maintain the structure of micelles [18]. CMC is determined by the micelle inherent properties and is critical to evaluate the stability of micelles at diluted concentration.
In addition to dilution, the structural stability of micelles is influenced by the complicated physiological environment, such as salt concentration, solvents, temperature, and pH. For example, after injection/infusion into the bloodstream, micelles may undergo several environmental changes, including significant dilution, exposure to pH changes, and contact with numerous proteins, lipids, and cells. The hydrophilic and hydrophobic domains of certain micelles are linked via ester, amide and other functional groups. The micelle structure may potentially be disrupted due to the hydrolysis of linkers when facing significant pH changes. Other disruptors include protein absorption through non-specific binding or electrostatic interaction. The resulting premature release of the encapsulated therapeutic content may lead to the accumulation of the drug in healthy tissues or organs, which could cause severe side effects and a further decrease of the pharmaceutical activity of the drug [41]. The fundamental strategy to improve the stability of micelles is to enhance intra-micellar interactions, which are often reflected by a decreased CMC. Therefore, measuring the CMC value can directly evaluate the efficacy of these strategies.
The classical method to measure CMC is based on the observation of the concentration-mediated change in physical properties of micelles by measuring electrical conductivity [42], surface tension [43], chemical shifts detected by nuclear magnetic resonance (NMR) [43], absorption [42], determination of self-diffusion coefficients [43], and fluorescence intensity [42]). Transmission electron microscopy (TEM) and dynamic light scattering (DLS) are commonly used to show the overall morphology and size of micelles. The determination of aggregation number, which is the number of small molecules or polymer chains assembled to form a micelle, is another way to evaluate the assembly behavior [44]. The micelle aggregation number can be determined by isothermal titration calorimetry [45, 46] or calculated from an apparent molecular mass of self-assembled micelle in a solvent obtained by static light scattering. In addition, fluorescence resonance energy transfer (FRET), which is sensitive to small changes in the distance between molecular groups, can be used to measure the integrity of micelles in solvents [47–49]. Typically, fluorescent energy donors and acceptors are encapsulated in the micelle hydrophobic domain, and the emission wavelength of the acceptors can be detected. Once a micelle dissociates upon dilution or other environmental changes, the resulting increased distance between donor and acceptor pair will lead to a decrease of the previous acceptor emission and an intensified emission of the donor.
3. Covalent crosslinking strategies to stabilize micelles
As previously discussed, enhanced stability of a micelle could be reflected by the decrease of its CMC. In general, polymeric micelles have a significantly lower CMC (10−6 - 10−7 M) than conventional small molecule micelles (10−3 - 10−4 M) [23, 50–52]. The low CMC is attributed to the long hydrophobic block of the amphiphilic copolymers, the presence of which, however, significantly increases the size of polymer micelles or spheres (typically from 100 nm to the μm scale). An increase in size generally lowers the in vivo performance of the encapsulated medicine [30–32]. In addition, the assembly of these long hydrophobic chains can barely achieve a thermodynamic equilibrium and is highly dependent on the specific method used to formulate the assembly [50]. As a consequence, the size and quality of the assembled polymeric micelles or spheres are sensitive to almost every formulating parameter, such as the polymer and drug concentration, stirring style and speed, and solvents [53–55].
It is highly desirable to develop micelles with further lowered CMC and increased stability with the goal of obtaining more efficient drug delivery carriers. Attempts have been made to co-inject empty micelles to increase the micelle concentration in the body to avoid micelle burst and drug loss [40, 56]. The effectiveness of this strategy remains questionable and is not yet well accepted as a stabilization method. Currently, the most popular and effective method, among many others, is crosslinking to decrease the CMC and stabilize micelles. In this section, different covalent crosslinking strategies to lower the CMC are introduced in 3.1 and specific crosslinking methods are discussed in 3.2.
3.1. Covalent crosslinking strategies
The dominant driving force for micelle formation is the reduction of free energy through the removal of hydrophobic fragments from the incompatible aqueous environment. Micelle formation is reversible and highly dependent on weak intermolecular interactions within the micelles. The most popular covalent crosslinking strategy involves the formation of covalent bonds/crosslinking within specific domains of the micelle, such as the shell and core domains. This reinforces the weak intermolecular interactions and thus stabilizes the micelles.
3.1.1. Shell crosslinked micelles
Shell crosslinking is a recognized way to stabilize polymeric micelles assembled from the copolymers. Early studies [57] focused on micelles derived from AB-type diblock copolymers, which possess a hydrophobic core (A) and hydrophilic shell (B). In these systems, crosslinking has to be controlled within the hydrophilic domains (shell) rather than between individual micelles to avoid the formation of large covalently bound aggregates. For this purpose, dilute concentration (0.1-0.5% solids) can effectively prevent extensive inter-micellar crosslinking [58]. Nevertheless, the diluted concentration can drastically limit the crosslinking efficiency. To circumvent this issue, ABC triblock copolymers have been studied. They have a significant advantage over AB diblock copolymers when preparing shell crosslinked micelles [58]. In addition to the A and B constituents, ABC triblock copolymers have an outer domain (C) termed the corona, which can bestow different functions on micelle systems. For micelles derived from ABC triblock copolymers, crosslinking is possible at a much higher concentration (e.g., 10% solids), since the additional outer C domain can effectively eliminate the chances for inter-micellar crosslinking and also provide potential sites for conjugating ligands for various functions. (Figure 1) Nevertheless, micelles based on ABC triblock copolymer with a crosslinked shell are relatively complicated and require sophisticated designs.
Figure 1.

Illustration of micelles assembled from AB diblock copolymers and ABC triblock copolymers
3.1.2. Core crosslinked micelles
Shell crosslinking is effective in stabilizing micelles but may modify the surface chemistry and the hydrophilicity of the shell region. This could potentially affect the blood circulation performance of the micelles, which is very dependent on the stealth property of polymer materials in the hydrophilic shell. To prevent the undesirable changes to the micelle surface characteristics, core crosslinking has been adopted as an alternative way to stabilize micelles. Core crosslinking is typically done after micelle formation. The reactive groups for core crosslinking are either already present in the core region or are added later. The groups do not interfere with the micelle formation process. Typical crosslinking methods include polymerization of radicals, addition of bifunctional crosslinkers, or click chemistry. Potential crosslinkers added following micelle formation must permeate the micelle’s hydrophobic domain before reacting with the substrate within the inner core of the micelle. This can be difficult to achieve, raising potential transport concerns. Although crosslinking of the shell or core region can efficiently improve the stability of the polymeric micelles, it does not influence the release of the encapsulated drugs, which are still released in a non-controllable manner[41]. Covalently entrapping drugs has been combined with core crosslinking to achieve proper drug retention within the micelles, such as during blood circulation. The encapsulated drugs can be released in a predefined manner via the cleavage of a specific linker in response to environmental stimuli, such as a pH-sensitive linker[59].
3.2. Covalent crosslinking methods
3.2.1. Photo/ultraviolet-induced dimerization
Compared with other crosslinking methods, photoinduced dimerization is advantageous because no additional crosslinking agents are required and no byproducts are formed during the reaction [60]. Furthermore, the size and shape of micelles are maintained [61]. The cross-linking density can be easily controlled by tuning the light wavelength or intensity. Radiation-induced dimerization was reported as early as 1998 by Liu [62], who obtained a crosslinked poly(2-cinnamoylethyl methacrylate) shell by photolysis of sample with ultraviolet (UV) light. The UV-induced dimerization of a cinnamic acid and its derivatives have been well explored [63, 64]. Typically, UV-irradiation at 365 nm induces dimerization of coumarin or cinnamoyl groups in aqueous media [61, 65]. This reaction is attractive because the crosslinking process is reversible. The newly formed bonds can be cleaved upon UV-irradiation at 254 nm [61, 66]. This potentially enables a controllable drug release at a special UV condition. However, the strategy is limited by its low tissue penetration ability and potential toxicity [67]. In addition to UV, visible light has also been used to induce micelle crosslinking involving di-selenide bonds, where the crosslinked micelles displayed excellent physiological stability and could encapsulate antitumor drugs [68, 69]. In addition, di-selenide bonds are sensitive to the redox environment, which makes controllable degradation and drug release at the targeted location feasible. In a tumor-bearing mouse model, diselenide-crosslinked micelles delivered significantly more drugs to tumors (1.69-fold higher) compared with non-crosslinked micelles [68].
3.2.2. Di-functional crosslinkers
The first reported crosslinker used to prepare shell-crosslinked micelles was p-(chloromethyl) styrene [57]. This di-functional crosslinker was used to form quaternary amines on the pyridine groups of the hydrophilic domain of a block copolymer of polystyrene and poly(4-vinyl pyridine), followed by a radical polymerization of the styrenyl side chains of the crosslinker at the micelle shell region [57]. Further efforts have included a series of di-functional crosslinkers taking advantage of the condensation reaction, conjugation, quaternization, and others to obtain shell crosslinking. For example, a diamine crosslinker can react with two carboxylic acid groups of poly(acrylic) acid in aqueous medium through a carbodiimide catalyzed condensation reaction to stabilize the shell layer of micelles [70, 71]. In a similar way, cystamine, a diamine crosslinker that contains a cleavable disulfide bond, can be used to stabilize micelles, which can be subsequently destabilized in a reducing environment [72]. Glutaraldehyde is a commonly used crosslinking agent, which can also react with primary amines, such as the poly(lysine) block of a micelle [73]. Through quaternization, the 1,2-bis(2-iodoethoxy)ethane (BIEE) crosslinker has been reacted with N,N-dimethyl aminoethyl methacrylate in aqueous solution to obtain shell crosslinking [74]. The Michael Addition reaction has been exploited in the use of a divinyl sulfone to crosslink hydroxylated blocks of a micelle in a basic condition [75]. Similar to the strategy of crosslinking hydrophilic shells, a di-functional crosslinker is also effective to crosslink the hydrophobic core of a micelle. Examples are the use of diamines [76–78], di-activated esters [79], and di-benzophenone [80].
3.2.3. Click crosslinking method
A click chemistry based crosslinking method was reported, in which acetylene groups in the shell of poly(acrylic acid)-poly(styrene) diblock copolymer micelles were crosslinked with azide-functionalized dendrimers [81]. The unreacted azide groups are also able to conjugate with a fluorescent label, which is essential for in vivo tracking and imaging, and other biologically active ligands. Such an azide-alkyne cycloaddition reaction was studied as a shell crosslinking strategy [23, 82, 83] and has also been applied to core crosslinking micelle systems [84, 85]. These crosslinked micelles have enhanced stability, improved drug loading capacity, and in vitro release profile [83], or low toxicity when incubating with cells [84]. Whether drug delivery is improved remains unclear.
3.2.4. Silicon chemistry method
A condensation reaction that produces the formation of siloxanes was reported as a method to crosslink micelle cores [86]. Siloxane bonds were formed upon the addition of triethylamine into precursor (trimethoxysilyl) propyl methacrylate (TMSP-MA), which induced the hydrolysis of the methoxy silane groups and the formation of crosslinked Si–O–Si linkage [87]. In a similar way, the poly ((triethoxysilyl) propyl methacrylate) block in a micelle system could form a crosslinked core through water-triggered hydrolysis and siloxane formation [88]. Another type of silicon chemistry-based method involves ring-opening polymerization of silacyclobutanes to crosslink micelle cores [89]. This reaction was reportedly catalyzed by hexachloroplatinic acid (H2PtCl6), and other series of similar crosslinker derivatives based on silacyclobutane have been reported [90].
3.2.5. Reversible boronate ester bond
Boronate ester bonds are formed between a boronic acid and an alcohol, diol, or molecule with multi-hydroxyl groups, such as glucose. The boronate ester bonds are reversible and sensitive to pH or competing substrates. The reversible boronate ester has been used to crosslink micelles to enhance stability and improve the release kinetics with external stimuli. For example, boronic acid containing polymers and catechol containing polymers were reported to form crosslinked micelles as drug delivery carriers [91, 92]. Using FRET technology to evaluate micelle integrity, such crosslinked micelles showed prolonged blood circulation. When triggered by the low pH in a typical tumor environment, the resulting micelles could quickly release their drug payload. The preferential accumulation of the drug in tumors was observed by imaging of harvested organs [91]. Nevertheless, the sensitivity of the boronate system to oxidative conditions, such as hydroxyl peroxide [93], is a concern with respect to toxicity because hydroxyl peroxide is necessary in the redox signaling pathway of normally-functioning cells [94].
4. Non-covalent crosslinking strategies
In addition to the majority of crosslinking methods that involve covalent bond formation, there are other crosslinking methods based on non-covalent molecular interactions. These include static electric interaction and hydrogen bonding, and have been applied to enhance the intra-micelle interaction and micelle stability [95].
4.1. Complexation of micelle cores
Diblock copolymers containing two hydrophilic blocks in which one of the blocks is ionic can self-assemble upon interaction with an oppositely charged species to form the so-called polyion core micelles [96] (Figure 2a). A typical example is the polyion micelle that can be assembled from the block copolymer poly(ethylene) glycol-b-poly(lysine) and poly(ethylene) glycol-b-poly(aspartic acid) [97]. The obtained polyion complexes do not significantly increase the hydrophobicity within the core, but do enhance micelle stability through electrostatic ionic interactions [97–99]. Compared with other crosslinking methods, polyion micelles are markedly less toxic because no small molecule crosslinker is used in the crosslinking process. In addition, polyion micelles typically display a high loading capacity for hydrophilic drugs [100] and can be used for DNA or RNA condensation [101, 102]. Furthermore, the stability of the formed complexes can be further enhanced through external crosslinking. For example, poly (ethylene oxide)-b-polymethacrylate anions (PEO-b-PMA) and divalent metal cations have been used to prepare core-complexed micelles [103]. In this process, PEO-b-PMA copolymers were self-assembled into block complexes in the presence of divalent ions, such as Ca2+ and Ba2+, followed by a second covalent crosslinking of available carboxylate groups in the micelle core region using diamine crosslinkers (Figure 2b). The second crosslinking maintained the core-shell morphology of the micelle and further increased core stability.
Figure 2.

Formation of polyion micelles (a) Polyion complex micelle formation from diblock copolymers. (b) Scheme of polymeric micelle with crosslinked ionic cores. Copyright © 2005 American Chemical Society[103].
In addition to static electric interaction, other complexation methods can be used to enhance the stability of micelle without compromising drug loading capacity. For example, π-π stacking of aromatic groups, as introduced by N-(2-benzoyloxypropyl methacrylamide (HPMAm-Bz) or the corresponding naphthoyl analog (HPMAm-Nt), was reported to strengthen the hydrophobic core and enhance the overall micelle stability [104]. The occurrence of π-π stacking in micelles was confirmed by solid-state NMR [104]. Using a particular polymeric micelle based on methoxy poly(ethylene glycol)-b-(N-(2-benzoyloxypropyl) methacrylamide) (mPEG-b-p(HPMAm-Bz)) block copolymers, paclitaxel was loaded with excellent capacity and strong drug retention without the need for chemical crosslinking and covalent drug attachment [105]. Substantially increased paclitaxel accumulation was observed compared with the unstable micellar formulations used as the control. This led to the reported complete tumor regression in xenograft models by the π-π stacking stabilized micelles.
As an additional example, hydrogen bond formation between urea-functional groups in a PEG and polycarbonate copolymer was reported to strengthen the micelle cores [106]. The formation of hydrogen bond decreased the CMC of the micelle and improved micelle stability in the presence of destabilizing agents. By introducing both acid and urea-functional groups to the PEG and polycarbonate copolymer, the ratio of acid/urea groups could be further adjusted and optimized to achieve the desired micelle stability through the hydrogen bond formation between urea-urea and acid-urea [107].
4.2. Other complexation methods
Additional non-covalent complexation methods include macrocyclic host-guest complexation, which is an interaction used to bind two chemical entities. The goal of the approach is to design the structure of polymeric micelles [108], such as the interaction between β-cyclodextrin (β-CD) and adamantyl (ADA) [109]. Furthermore, the altered topology of the hydrophobic core was reported to enhance the stability of micelle and decrease the CMC [110]. In particular, PEGylated polymeric micelles with both flattened and curved corannulene core regions were prepared; only micelles with the curved corannulene displayed decreased CMC (Figure 3). The observations highlight the significance of topology in manipulating the stability of micelles. In the curved corannulene, the curvature-induced electron delocalization can create dipole-dipole interactions, which facilitate intermolecular electrostatic interactions that stabilize micelles.
Figure 3.

Illustration of the topology effect on the stability of micelles.[110] Copyright © The Royal Society of Chemistry 2017
5. Strategies to stabilize micelles without crosslinking
Since CMC is an inherent property of micelles, manipulating the structure of micelles to modify their micelle forming behavior has been frequently attempted without additional crosslinking. These efforts include altering hydrophilic/hydrophobic block ratios of the micelles [111], increase of the crystallinity of hydrophobic segments [112], and other non-crosslinking strategies. Typically, CMC decreases with increasing chain length of the hydrophobic block, which is explained by the enhanced hydrophobic interaction within the cores [113]. Furthermore, decoration of micelle cores with various fatty acids reportedly decreased the CMC of the modified micelles due to the introduction of hydrophobic segments by the fatty carbon chains to the micelle cores [114, 115]. Similarly, benzyl groups have been introduced to PEO–PCL copolymers. The resulting micelles displayed lowered CMC due to the increased rigidity and hydrophobicity of the micelle core [116]. Nevertheless, this design may significantly increase the size of polymer spheres (typically from 100 nm to the µm scale), which was reported to severely decrease the potential targeted delivery efficacy and lower the in vivo performance of the nanomedicine [30, 32, 117]. By changing the crystallinity of the hydrophobic segment, micelles with semi-crystalline core structures could be obtained. These displayed much lower CMC values than micelles with amorphous core structures [112]. A possible explanation is that the crystallization process itself may be an additional driving force for the micelle assembly, resulting in a lower CMC value.
As a notable non-crosslinking strategy to achieve stable micelles, the unimolecular micelle is unique in that the micelles literally contain only one molecule, and so do not require additional crosslinking for stabilization. Unimolecular micelles are formed by individual multi-arm star amphiphilic block copolymers or telodendrimer (e.g., linear-dendritic block copolymers [118]) and have been utilized as drug delivery carriers. One study explored engineered unimolecular micelles for targeted breast cancer therapies [119]. Unimolecular micelles were formed by esterification between the dendrimer core of poly(amidoamine)-poly(lactide)-OH (PAMAM-PLA-OH) and the hydrophilic domain of OCH3-PEG-COOH. The micelles displayed excellent in vitro and in vivo stability with high drug loading capacity. Significant tumor inhibition and high intratumoral drug concentration were also reported in the study. Other dendrimers, such as the commercially available hyperbranched Boltron® H40 polymer can also be used as the cores of unimolecular micelles (Figure 4). Furthermore, using a dendrimer as the core of the micelle system also enables the combination with covalently crosslinking to facilitate the drug release at the targeted site. For example, the cross-linkable telodendrimer pair, PEG-catechol-cholic acid were prepared and crosslinked via formation of a boronate ester [91]. This ester might hydrolyze in response to the acidic pH of the tumor environment, leading to a burst release of the encapsulated drugs. Biodistribution results confirmed the high drug accumulation in the tumor as the result of a quick drug release at the target site.
Figure 4.
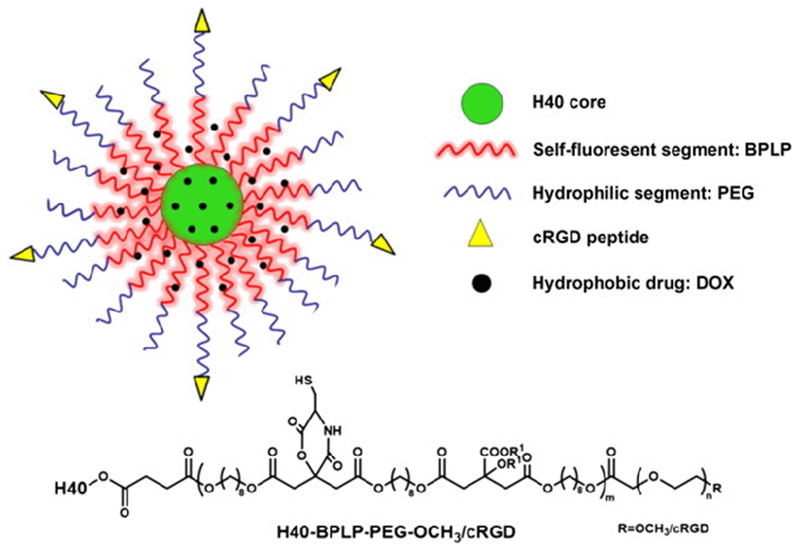
A schematic illustration of a unimolecular micelle nanoparticle made of the multi-arm, hyperbranched, star amphiphilic block copolymer, H40-BPLP-PEG-cRGD[120] Copyright©, 2015 Elsevier Ltd.
Recently, a zwitterionic polymer micelle with sharp polarity contrast between the hydrophilic zwitterionic domain and hydrophobic lipid domain was reported to show an undetectable ultra-low CMC below 10−6 mM, which was orders of magnitude lower than CMC (>10−3 mM) of common micelle systems [121] (Figure 5). Without chemical crosslinking or other decoration methods, which potentially complicate the micelle preparation and increase the micelle size, this particular zwitterionic micelle system did not disassociate and protected encapsulated drugs/nanoparticles at extremely dilute concentrations. The mechanism of ultra-stability of the zwitterionic micelles appeared to be due to the superhydrophilicity of the zwitterionic moieties promoting the hydrophobic-hydrophobic interaction within the micelle core. Even when the zwitterionic moiety or polymer alone was added to a micelle solution without chemical modification of the micelles, the existing micelles in the modified solution were stabilized at a concentration below their inherent CMC. A docetaxel formulation has been prepared using this zwitterionic polymer micelle system. The new formulation displayed significantly improved stability in serum and high drug retention upon dilution, and substantially enhanced drug delivery to the tumor region and an increased overall therapeutic outcome, compared with conventional micelles.
Figure 5.

Ultra-low-CMC micelles and their unusual ability to stabilize cargoes in extremely diluted conditions with micelle concentrations far below CMCs of common micelles [121] Conventional micelles dissociate at a concentration below CMC, and thus cannot stabilize a hydrophobic cargo at that concentration. Polar groups of conventional micelles were either non-ionic (e.g., Polysorbate 80, which is hydrophobic) or only contained one ionic group (e.g., sodium dodecyl sulfate). For zwitterionic micelles, as shown, the polar groups contained multiple zwitterionic moieties to form a polymer. Copyright © 2018, Springer Nature
6. Perspectives
Numerous polymeric micelle systems have been developed to increase micelle stability with the goal of improving drug delivery. Nevertheless, various issues remained that prevented these strategies from being utilized in a clinical setting. Firstly, many current efforts involve sophisticated structure designs and/or complicated preparation procedures. Micelles are a simple and easy means of drug formulating. Increasing the complexity would pose a potential hurdle for consistent and large-scale manufacturing, which is a prerequisite for regulatory approval for clinical trials. The complexity further increases the difficulty to characterize micellar drug formulations and to repeat the results of the formulations and potential advantageous therapeutic outcomes. Challenges remain in enhancing the stability of micelles and their formulations through simple strategies without comprising the feasibility for large-scale manufacturing. Secondly, despite many studies concerning modification of micelle structure and their stability or correlation with CMC, more fundamental data are needed on how these potential modifications impact the micelle stability. For example, it is recognized that micelles in water will not disassociate at a concentration above the particular CMC. However, when these micelles are exposed to serum proteins, the majority of the micelle molecules will bind the proteins at this concentration rather than remaining as a micelle assembly. Potential theories or knowledge supporting and explaining similar observations will be beneficial for future design of micelles with ultra-stability. Lastly, despite the potential of many new materials in drug formulations, including the zwitterionic polymer materials [122–124], the consensus is still to use PEG almost exclusively as the hydrophilic component of micelles or nanoparticle systems, since PEG is approved by FDA for similar injection use. More research is needed to facilitate the translation of new material platforms to expand the list of approved materials for formulation scientists to develop more efficient medicines.
References
- [1].Verma G; Hassan P Self assembled materials: Design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys 2013, 15, 17016–17028. [DOI] [PubMed] [Google Scholar]
- [2].Kamaly N; Xiao ZY; Valencia PM; Radovic-Moreno AF; Farokhzad OC Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev 2012, 41, 2971–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Service RF Nanoparticle trojan horses gallop from the lab into the clinic. Science 2010, 330, 314–315. [DOI] [PubMed] [Google Scholar]
- [4].Zhang L; Gu FX; Chan JM; Wang AZ; Langer RS; Farokhzad OC Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Therapeut 2008, 83, 761–769. [DOI] [PubMed] [Google Scholar]
- [5].Service RF Nanotechnology takes aim at cancer. Science 2005, 310, 1132–1134. [DOI] [PubMed] [Google Scholar]
- [6].Yoo JW; Irvine DJ; Discher DE; Mitragotri S Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov 2011, 10, 521–535. [DOI] [PubMed] [Google Scholar]
- [7].Scheinberg DA; Villa CH; Escorcia FE; McDevitt MR Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol 2010, 7, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petros RA; DeSimone JM Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov 2010, 9, 615–627. [DOI] [PubMed] [Google Scholar]
- [9].Kim S; Kim JH; Jeon O; Kwon IC; Park K Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm 2009, 71, 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo ST; Huang L Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv 2014, 32, 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo ST; Miao L; Wang YH; Huang L Unmodified drug used as a material to construct nanoparticles: Delivery of cisplatin for enhanced anti-cancer therapy. J. Control. Release 2014, 174, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tong R; Tang L; Ma L; Tu CL; Baumgartner R; Cheng JJ Smart chemistry in polymeric nanomedicine. Chem. Soc. Rev 2014, 43, 6982–7012. [DOI] [PubMed] [Google Scholar]
- [13].Hu CMJ; Zhang L; Aryal S; Cheung C; Fang RH; Zhang LF Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kulkarni VS; Shaw C Chapter 2 - Surfactants, lipids, and surface chemistry In Essential Chemistry for Formulators of Semisolid and Liquid Dosages Kulkarni VS; Shaw C, Eds.; Academic Press: Boston, 2016; pp 5–19. [Google Scholar]
- [15].Vert M; Doi Y; Hellwich K-H; Hess M; Hodge P; Kubisa P; Rinaudo M; Schué F Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem 2012, 84, 377–410. [Google Scholar]
- [16].Cabral H; Matsumoto Y; Mizuno K; Chen Q; Murakami M; Kimura M; Terada Y; Kano MR; Miyazono K; Uesaka M et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol 2011, 6, 815–823. [DOI] [PubMed] [Google Scholar]
- [17].Feng X; Wang CX; Lin BR; Xu F Methoxy poly(ethylene glycol)-conjugated linoleic acid polymeric micelles for paclitaxel delivery. Colloid J 2006, 68, 779–783. [Google Scholar]
- [18].Kim S; Shi YZ; Kim JY; Park K; Cheng J-X Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv 2010, 7, 49–62. [DOI] [PubMed] [Google Scholar]
- [19].Rösler A; Vandermeulen GWM; Klok HA Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev 2001, 53, 95–108. [DOI] [PubMed] [Google Scholar]
- [20].Alibolandi M; Ramezani M; Abnous K; Sadeghi F; Hadizadeh F Comparative evaluation of polymersome versus micelle structures as vehicles for the controlled release of drugs. J. Nanopart. Res 2015, 17, 76. [Google Scholar]
- [21].Muthu MS; Kulkarni SA; Liu YT; Feng S-S Development of docetaxel-loaded vitamin E TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nanomedicine 2012, 7, 353–364. [DOI] [PubMed] [Google Scholar]
- [22].Fukushima S; Miyata K; Nishiyama N; Kanayama N; Yamasaki Y; Kataoka K PEGylated polyplex micelles from triblock catiomers with spatially ordered layering of condensed pDNA and buffering units for enhanced intracellular gene delivery. J. Am. Chem. Soc 2005, 127, 2810–2811. [DOI] [PubMed] [Google Scholar]
- [23].O’Reilly RK; Hawker CJ; Wooley KL Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev 2006, 35, 1068–1083. [DOI] [PubMed] [Google Scholar]
- [24].Fox ME; Szoka FC; Fréchet JMJ Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res 2009, 42, 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Venkatachalam MA; Rennke HG The structural and molecular basis of glomerular filtration. Circul. Res 1978, 43, 337–347. [DOI] [PubMed] [Google Scholar]
- [26].Jain RK Transport of molecules across tumor vasculature. Cancer Metast. Rev 1987, 6, 559–593. [DOI] [PubMed] [Google Scholar]
- [27].Alexis F; Pridgen E; Molnar LK; Farokhzad OC Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Owens DE; Peppas NA Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm 2006, 307, 93–102. [DOI] [PubMed] [Google Scholar]
- [29].Vonarbourg A; Passirani C; Saulnier P; Benoit JP Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [DOI] [PubMed] [Google Scholar]
- [30].Moghimi SM; Hunter AC; Murray JC Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev 2001, 53, 283–318. [PubMed] [Google Scholar]
- [31].Tang L; Yang XJ; Yin Q; Cai KM; Wang H; Chaudhury I; Yao C; Zhou Q; Kwon M; Hartman JA et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiang W; Kim BYS; Rutka JT; Chan WCW Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol 2008, 3, 145–150. [DOI] [PubMed] [Google Scholar]
- [33].Duncan R; Sat YN Tumor targeting by enhanced permeability and retention (EPR) effect. Ann. Oncol 1998, 9, 39. [Google Scholar]
- [34].Maeda H; Wu J; Sawa T; Matsumura Y; Hori K Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [DOI] [PubMed] [Google Scholar]
- [35].Stirland DL; Nichols JW; Miura S; Bae YH Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release 2013, 172, 1045–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].http://www.doxil.com/doxil-supply-shortage.
- [37].Lamb M; Laugenour K; Liang OW; Alexander M; Foster CEI; Lakey JRT In vitro maturation of viable islets from partially digested young pig pancreas. Cell Transplant 2014, 23, 263–272. [DOI] [PubMed] [Google Scholar]
- [38].Blanco E; Shen HF; Ferrari M Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 2015, 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gao Y; Chen LL; Gu WW; Xi Y; Lin LP; Li YP Targeted nanoassembly loaded with docetaxel improves intracellular drug delivery and efficacy in murine breast cancer model. Mol. Pharmaceutics 2008, 5, 1044–1054. [DOI] [PubMed] [Google Scholar]
- [40].Feng L; Mumper RJ A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett 2013, 334, 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Talelli M; Barz M; Rijcken CJF; Kiessling F; Hennink WE; Lammers T Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dominguez A; Fernandez A; Gonzalez N; Iglesias E; Montenegro L Determination of critical micelle concentration of some surfactants by three techniques. J. Chem. Educ 1997, 74, 1227. [Google Scholar]
- [43].Al-Soufi W; Piñeiro L; Novo M A model for monomer and micellar concentrations in surfactant solutions: Application to conductivity, NMR, diffusion, and surface tension data. J. Colloid Interface Sci 2012, 370, 102–110. [DOI] [PubMed] [Google Scholar]
- [44].Moroi Y Micelles: Theoretical and Applied Aspects; Springer Science & Business Media: New York, 1992. [Google Scholar]
- [45].Olesen NE; Westh P; Holm R Determination of thermodynamic potentials and the aggregation number for micelles with the mass-action model by isothermal titration calorimetry: A case study on bile salts. J. Colloid Interface Sci 2015, 453, 79–89. [DOI] [PubMed] [Google Scholar]
- [46].Bouchemal K; Agnely F; Koffi A; Djabourov M; Ponchel G What can isothermal titration microcalorimetry experiments tell us about the self‐organization of surfactants into micelles? J. Mol. Recogn 2010, 23, 335–342. [DOI] [PubMed] [Google Scholar]
- [47].Lu J; Owen SC; Shoichet MS Stability of self-assembled polymeric micelles in serum. Macromolecules 2011, 44, 6002–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen HT; Kim S; He W; Wang HF; Low PS; Park K; Cheng J-X Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo förster resonance energy transfer imaging. Langmuir 2008, 24, 5213–5217. [DOI] [PubMed] [Google Scholar]
- [49].Chen HT; Kim S; Li L; Wang SY; Park K; Cheng JX Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu W; Ling PX; Zhang TM Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv 2013, 2013, 340315 DOI: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Topel Ö; Çakir BA; Budama L; Hoda N Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq 2013, 177, 40–43. [Google Scholar]
- [52].Adams ML; Lavasanifar A; Kwon GS Amphiphilic block copolymers for drug delivery. J. Pharm. Sci 2003, 92, 1343–1355. [DOI] [PubMed] [Google Scholar]
- [53].Avgoustakis K Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Curr. Drug Deliv 2004, 1, 321–333. [DOI] [PubMed] [Google Scholar]
- [54].Cheng JJ; Teply BA; Sherifi I; Sung J; Luther G; Gu FX; Levy-Nissenbaum E; Radovic-Moreno AF; Langer R; Farokhzad OC Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Astete CE; Sabliov CM Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed 2006, 17, 247–289. [DOI] [PubMed] [Google Scholar]
- [56].Önyüksel H; Jeon E; Rubinstein I Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett 2009, 274, 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Thurmond KB; Kowalewski T; Wooley KL Water-soluble knedel-like structures: The preparation of shell-cross-linked small particles. J. Am. Chem. Soc 1996, 118, 7239–7240. [Google Scholar]
- [58].Bütün V; Billingham NC; Armes SP Synthesis of shell cross-linked micelles with tunable hydrophilic/hydrophobic cores. J. Am. Chem. Soc 1998, 120, 12135–12136. [Google Scholar]
- [59].Bütün V; Wang X-S; de Paz Báñez MV; Robinson KL; Billingham NC; Armes SP; Tuzar Z Synthesis of shell cross-linked micelles at high solids in aqueous media. Macromolecules 2000, 33, 1–3. [Google Scholar]
- [60].Talelli M; Iman M; Varkouhi AK; Rijcken CJF; Schiffelers RM; Etrych T; Ulbrich K; van Nostrum CF; Lammers T; Storm G et al. Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 2010, 31, 7797–7804. [DOI] [PubMed] [Google Scholar]
- [61].Ding JX; Zhuang XL; Xiao CS; Cheng YL; Zhao L; He CL; Tang ZH; Chen XS Preparation of photo-cross-linked pH-responsive polypeptide nanogels as potential carriers for controlled drug delivery. J. Mater. Chem 2011, 21, 11383–11391. [Google Scholar]
- [62].Jin Q; Liu XS; Liu GY; Ji J Fabrication of core or shell reversibly photo cross-linked micelles and nanogels from double responsive water-soluble block copolymers. Polymer 2010, 51, 1311–1319. [Google Scholar]
- [63].Ding JF; Liu GJ Polystyrene-block-poly(2-cinnamoylethyl methacrylate) nanospheres with cross-linked shells. Macromolecules 1998, 31, 6554–6558. [Google Scholar]
- [64].Cohen MD; Schmidt GMJ 383. Topochemistry. Part I. A survey. J. Chem. Soc 1964, 1996–2000. [Google Scholar]
- [65].Schmidt GMJ 385. Topochemistry. Part III. The crystal chemistry of some trans-cinnamic acids. J. Chem. Soc 1964, 2014–2021. [Google Scholar]
- [66].Yusa S-I; Sugahara M; Endo T; Morishima Y Preparation and characterization of a pH-responsive nanogel based on a photo-cross-linked micelle formed from block copolymers with controlled structure. Langmuir 2009, 25, 5258–5265. [DOI] [PubMed] [Google Scholar]
- [67].Lendlein A; Jiang HY; Jünger O; Langer R Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [DOI] [PubMed] [Google Scholar]
- [68].Xu L; Zhang WY; Cai HB; Liu F; Wang Y; Gao Y; Zhang WA Photocontrollable release and enhancement of photodynamic therapy based on host-guest supramolecular amphiphiles. J. Mater. Chem. B 2015, 3, 7417–7426. [DOI] [PubMed] [Google Scholar]
- [69].Deepagan VG; Kwon S; You DG; Nguyen VQ; Um W; Ko H; Lee H; Jo D-G; Kang YM; Park JH In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. [DOI] [PubMed] [Google Scholar]
- [70].Zhai SD; Hu XL; Hu YJ; Wu BY; Xing D Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [DOI] [PubMed] [Google Scholar]
- [71].Zhang Q; Remsen EE; Wooley KL Shell cross-linked nanoparticles containing hydrolytically degradable, crystalline core domains. J. Am. Chem. Soc 2000, 122, 3642–3651. [Google Scholar]
- [72].Huang HY; Kowalewski T; Remsen EE; Gertzmann R; Wooley KL Hydrogel-coated glassy nanospheres: A novel method for the synthesis of shell cross-linked knedels. J. Am. Chem. Soc 1997, 119, 11653–11659. [Google Scholar]
- [73].Li YT; Lokitz BS; McCormick CL RAFT synthesis of a thermally responsive ABC triblock copolymer incorporating N-acryloxysuccinimide for facile in situ formation of shell cross-linked micelles in aqueous media. Macromolecules 2006, 39, 81–89. [Google Scholar]
- [74].Rodríguez-Hernández J; Babin J; Zappone B; Lecommandoux S Preparation of shell cross-linked nano-objects from hybrid-peptide block copolymers. Biomacromolecules 2005, 6, 2213–2220. [DOI] [PubMed] [Google Scholar]
- [75].Pilon LN; Armes SP; Findlay P; Rannard SP Synthesis and characterization of shell cross-linked micelles with hydroxy-functional coronas: A pragmatic alternative to dendrimers? Langmuir 2005, 21, 3808–3813. [DOI] [PubMed] [Google Scholar]
- [76].Liu SY; Weaver JVM; Save M; Armes SP Synthesis of pH-responsive shell cross-linked micelles and their use as nanoreactors for the preparation of gold nanoparticles. Langmuir 2002, 18, 8350–8357. [Google Scholar]
- [77].Zhang JY; Jiang XZ; Zhang YF; Li YT; Liu SY Facile fabrication of reversible core cross-linked micelles possessing thermosensitive swellability. Macromolecules 2007, 40, 9125–9132. [Google Scholar]
- [78].Duong HTT; Nguyen TLU; Stenzel MH Micelles with surface conjugated RGDpeptide and crosslinked polyurea core viaRAFT polymerization. Polym. Chem 2010, 1, 171–182. [Google Scholar]
- [79].Huang C-Q; Hong C-Y; Pan C-Y Formation of flower-like aggregates from self-assembling of micelles with PEO shells and cross-linked polyacrylamide cores. Chin. J. Polym. Sci 2008, 26, 341–352. [Google Scholar]
- [80].Shim MS; Kwon YJ Acid-transforming polypeptide micelles for targeted nonviral gene delivery. Biomaterials 2010, 31, 3404–3413. [DOI] [PubMed] [Google Scholar]
- [81].Kim JS; Youk JH Preparation of core cross-linked micelles using a photo-cross-linking agent. Polymer 2009, 50, 2204–2208. [Google Scholar]
- [82].Joralemon MJ; O’Reilly RK; Hawker CJ; Wooley KL Shell click-crosslinked (SCC) nanoparticles: A new methodology for synthesis and orthogonal functionalization. J. Am. Chem. Soc 2005, 127, 16892–16899. [DOI] [PubMed] [Google Scholar]
- [83].Zhao Y Surface-cross-linked micelles as multifunctionalized organic nanoparticles for controlled release, light harvesting, and catalysis. Langmuir 2016, 32, 5703–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dai Y; Wang HQ; Zhang XJ Reduction-responsive interlayer-crosslinked micelles prepared from star-shaped copolymer via click chemistry for drug controlled release. J. Nanopart. Res 2017, 19, 383. [DOI] [PubMed] [Google Scholar]
- [85].Withey AB; Chen GJ; Nguyen TLU; Stenzel MH Macromolecular cobalt carbonyl complexes encapsulated in a click-cross-linked micelle structure as a nanoparticle to deliver cobalt pharmaceuticals. Biomacromolecules 2009, 10, 3215–3226. [DOI] [PubMed] [Google Scholar]
- [86].Jiang XZ; Zhang JY; Zhou YM; Xu J; Liu SY Facile preparation of core‐crosslinked micelles from azide‐containing thermoresponsive double hydrophilic diblock copolymer via click chemistry. J. Polym. Sci. Part A: Polym. Chem 2008, 46, 860–871. [Google Scholar]
- [87].Du JZ; Chen YM; Zhang YH; Han CC; Fischer K; Schmidt M Organic/inorganic hybrid vesicles based on a reactive block copolymer. J. Am. Chem. Soc 2003, 125, 14710–14711. [DOI] [PubMed] [Google Scholar]
- [88].Du JZ; Armes SP pH-responsive vesicles based on a hydrolytically self-cross-linkable copolymer. J. Am. Chem. Soc 2005, 127, 12800–12801. [DOI] [PubMed] [Google Scholar]
- [89].Zhang YF; Gu WY; Xu HX; Liu SY Facile fabrication of hybrid nanoparticles surface grafted with multi‐responsive polymer brushes via block copolymer micellization and self‐catalyzed core gelation. J. Polym. Sci. Part A: Polym. Chem 2008, 46, 2379–2389. [Google Scholar]
- [90].Matsumoto K; Hasegawa H; Matsuoka H Synthesis of sodium-polystyrenesulfonate-grafted nanoparticles by core-cross-linking of block copolymer micelles. Tetrahedron 2004, 60, 7197–7204. [Google Scholar]
- [91].Delgado PA; Matloka P; Zuluaga F; Wagener KB Synthesis and thermal crosslinking of carbosiloxane and oligo(oxyethylene) polymers. J. Polym. Sci. Part A: Polym. Chem 2012, 50, 431–440. [Google Scholar]
- [92].Chen WX; Cheng YF; Wang BH Dual‐responsive boronate crosslinked micelles for targeted drug delivery. Angew. Chem., Int. Ed 2012, 51, 5293–5295. [DOI] [PubMed] [Google Scholar]
- [93].Li YP; Xiao WW; Xiao K; Berti L; Luo JT; Tseng HP; Fung G; Lam KS Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew. Chem., Int. Ed 2012, 51, 2864–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lin VS; Dickinson BC; Chang CJ Chapter two - Boronate-based fluorescent probes: Imaging hydrogen peroxide in living systems. Methods Enzymol 2013, 526, 19–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rhee SG H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [DOI] [PubMed] [Google Scholar]
- [96].Priftis D; Leon L; Song ZY; Perry SL; Margossian KO; Tropnikova A; Cheng JJ; Tirrell M Self‐Assembly of α‐helical polypeptides driven by complex coacervation. Angew. Chem., Int. Ed 2015, 54, 11128–11132. [DOI] [PubMed] [Google Scholar]
- [97].Oberoi HS; Laquer FC; Marky LA; Kabanov AV; Bronich TK Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum(II). J. Control. Release 2011, 153, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Harada A; Kataoka K Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar]
- [99].Bütün V; Lowe AB; Billingham NC; Armes SP Synthesis of zwitterionic shell cross-linked micelles. J. Ame. Chem. Soc 1999, 121, 4288–4289. [Google Scholar]
- [100].Zhang XH; Ai CJ; Ma JH; Xu J; Yang SG Synthesis of zwitterionic shell cross-linked micelles with pH-dependent hydrophilicity. J. Colloid Interface Sci 2011, 356, 24–30. [DOI] [PubMed] [Google Scholar]
- [101].Dai Y; Wang HQ; Zhang XJ Polyion complex micelles prepared by self-assembly of block-graft polycation and hyperbranched polyanion. J. Nanopart. Res 2017, 19, 298. [Google Scholar]
- [102].Ueda T; Oshida H; Kurita K; Ishihara K; Nakabayashi N Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J 1992, 24, 1259–1269. [Google Scholar]
- [103].Rungsardthong U; Deshpande M; Bailey L; Vamvakaki M; Armes SP; Garnett MC; Stolnik S Copolymers of amine methacrylate with poly(ethylene glycol) as vectors for gene therapy. J. Control. Release 2001, 73, 359–380. [DOI] [PubMed] [Google Scholar]
- [104].Bronich TK; Keifer PA; Shlyakhtenko LS; Kabanov AV Polymer micelle with cross-linked ionic core. J. Am. Chem. Soc 2005, 127, 8236–8237. [DOI] [PubMed] [Google Scholar]
- [105].Shi Y; van Steenbergen MJ; Teunissen EA; Novo L; Gradmann S; Baldus M; van Nostrum CF; Hennink WE Π–Π stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837. [DOI] [PubMed] [Google Scholar]
- [106].Shi Y; van der Meel R; Theek B; Oude Blenke E; Pieters EHE; Fens MHAM; Ehling J; Schiffelers RM; Storm G; van Nostrum CF et al. Complete regression of xenograft tumors upon targeted delivery of paclitaxel via Π–Π stacking stabilized polymeric micelles. ACS Nano 2015, 9, 3740–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kim SH; Tan JPK; Nederberg F; Fukushima K; Colson J; Yang C; Nelson A; Yang Y-Y; Hedrick JL Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials 2010, 31, 8063–8071. [DOI] [PubMed] [Google Scholar]
- [108].Yang C; Ebrahim Attia AB; Tan JPK; Ke XY; Gao SJ; Hedrick JL; Yang Y-Y The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 2012, 33, 2971–2979. [DOI] [PubMed] [Google Scholar]
- [109].Loh XJ Supramolecular host-guest polymeric materials for biomedical applications. Mater. Horiz 2014, 1, 185–195. [Google Scholar]
- [110].Wang J; Jiang M Polymeric self-assembly into micelles and hollow spheres with multiscale cavities driven by inclusion complexation. J. Am. Chem. Soc 2006, 128, 3703–3708. [DOI] [PubMed] [Google Scholar]
- [111].Dong XP; Guo XL; Liu GQ; Fan AP; Wang Z; Zhao YJ When self-assembly meets topology: An enhanced micelle stability. Chem. Commun 2017, 53, 3822–3825. [DOI] [PubMed] [Google Scholar]
- [112].Attwood D; Elworthy PH; Kayne SB Membrane osmometry of aqueous micellar solutions of pure nonionic and ionic surfactants. J. Phys. Chem 1970, 74, 3529–3534. [Google Scholar]
- [113].Glavas L; Olsén P; Odelius K; Albertsson A-C Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li F; Danquah M; Mahato RI Synthesis and characterization of amphiphilic lipopolymers for micellar drug delivery. Biomacromolecules 2010, 11, 2610–2620. [DOI] [PubMed] [Google Scholar]
- [115].Ahmad Z; Shah A; Siddiq M; Kraatz HB Polymeric micelles as drug delivery vehicles. RSC Adv 2014, 4, 17028–17038. [Google Scholar]
- [116].Lavasanifar A; Samuel J; Kwon GS The effect of alkyl core structure on micellar properties of poly(ethylene oxide)-block-poly(L-aspartamide) derivatives. Colloids Surf. B: Biointerfaces 2001, 22, 115–126. [DOI] [PubMed] [Google Scholar]
- [117].Falamarzian A; Lavasanifar A Chemical modification of hydrophobic block in poly(ethylene oxide) poly (caprolactone) based nanocarriers: Effect on the solubilization and hemolytic activity of amphotericin B. Macromol. Biosci 2010, 10, 648–656. [DOI] [PubMed] [Google Scholar]
- [118].Choi J; Moquin A; Bomal E; Na L; Maysinger D; Kakkar A Telodendrimers for physical encapsulation and covalent linking of individual or combined therapeutics. Mol. Pharmaceutics 2017, 14, 2607–2615. [DOI] [PubMed] [Google Scholar]
- [119].Brinkman AM; Chen GJ; Wang YD; Hedman CJ; Sherer NM; Havighurst TC; Gong SQ; Xu W Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials 2016, 101, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen GJ; Wang LW; Cordie T; Vokoun C; Eliceiri KW; Gong SQ Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials 2015, 47, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lu Y; Yue ZG; Xie JB; Wang W; Zhu H; Zhang ES; Cao ZQ Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng 2018, 1, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cao ZQ; Zhang L; Jiang SY Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 2012, 28, 11625–11632. [DOI] [PubMed] [Google Scholar]
- [123].Cao ZQ; Jiang SY Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar]
- [124].Yusa S-I; Fukuda K; Yamamoto T; Ishihara K; Morishima Y Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670. [DOI] [PubMed] [Google Scholar]


