Abstract
Sprinters have been found to possess longer muscle fascicles than non-sprinters, which is thought to be beneficial for high-acceleration movements based on muscle force-length-velocity properties. However, it is unknown if their morphology is a result of genetics or training during growth. To explore the influence of training during growth, thirty guinea fowl (Numida meleagris) were split into exercise and sedentary groups. Exercise birds were housed in a large pen and underwent high-acceleration during their growth period (age 4–14 weeks), while sedentary birds were housed in small pens to restrict movement. Morphological analyses (muscle mass, PCSA, optimal fascicle length, pennation angle) of a hip extensor muscle (ILPO) and plantarflexor muscle (LG), which differ in architecture and function during running, were performed post-mortem. Muscle mass for both ILPO and LG was not different between the two groups. Exercise birds were found to have ~12% and ~14% longer optimal fascicle lengths in ILPO and LG, respectively, than the sedentary group despite having ~3% shorter limbs. From this study we can conclude that optimal fascicle lengths can increase as a result of high-acceleration training during growth. This increase in optimal fascicle length appears to occur irrespective of muscle architecture and in the absence of a change in muscle mass. Our findings suggest high-acceleration training during growth results in muscles that prioritize adaptations for strain and shortening velocity over isometric strength. Thus, the adaptations observed suggest these muscles produce higher force during dynamic contractions, which is beneficial for movements requiring large power outputs.
Keywords: fascicle length, acceleration, training, guinea fowl, ontogeny
INTRODUCTION
Musculoskeletal morphology often mirrors an animal’s locomotor function. For example, cursorial species have evolved long legs, a tapered limb mass distribution, and long elastic tendons that improve running economy (Wilson et al., 2001). In contrast, burrowing and climbing vertebrate species are often characterized by stout, highly muscular forelimbs with skeletal modifications that permit large output lever force (Warburton et al., 2013).
Although less pronounced than interspecies variation, human musculoskeletal morphology may likewise mirror locomotor function. It has been demonstrated that sprint athletes have smaller Achilles tendon moment arms, longer toes, and longer fascicles in the quadriceps and plantarflexor muscle compared to non-sprinters (Abe et al., 2000; Baxter et al., 2012; Lee and Piazza, 2009). Muscle fascicle length, in particular, appears to be associated with sprinting ability, correlating with performance among competitive sprinters (Abe et al., 2001; Kumagai et al., 2000). In contrast, endurance marathon runners, who do not routinely undergo high accelerations, have shorter plantarflexor fascicles compared to those of non-runners (Abe et al., 2000), further suggesting that longer fascicles are well suited to movements, like sprinting, that require high acceleration and power. Longer fascicles may enhance sprint performance by altering the operating range of muscles on their force-length and force-velocity curves. Longer fascicles will have more sarcomeres in series over which the overall shortening is distributed, reducing sarcomere shortening and shortening velocity during concentric contractions and increasing muscle force-generating capacity (Lee and Piazza, 2009).
Differences in fascicle length between sprinters and non-sprinters raise the question of whether longer fascicles in sprinters are a result of genetic endowment or if they are a plastic response to high-acceleration training. While fascicle length is associated with titin isoforms and, therefore, has some genetic basis (Stebbings et al., 2018), muscle is also highly adaptable to loading and lengthening stimuli. For example, fascicle lengths have been shown to increase in response to chronic stretch (Koh and Herzog, 1998), intermittent stretching (De Jaeger et al., 2015) and several modes of exercise training (Blazevich et al., 2003; Franchi et al., 2014). While these studies show that fibers lengths can exhibit plasticity, they do not specifically address the influence of high acceleration training.
Further, while there is evidence of muscle lengths adapting to load in adults, we know less about such responses across the growth span. The musculoskeletal system is known be plastic throughout an individual’s lifetime, but the growth period may be critical to the development of the adult phenotype (Johnston, 2006). While muscle plasticity induced by exercise has been demonstrated in growing mice by Rabey et al. (2015), their experimental protocol did not distinguish high and low acceleration training, leaving open the question of how the body adapts muscle architecture to sprint training, in particular.
A second question is whether adaptation to high-acceleration training has similar effects on muscles with different muscle-tendon architectures and mechanical functions. It has been proposed that muscles with long fibers and minimal external tendon are specialized for high power and work production whereas pennate muscles with short fibers and long external tendons are better suited for isometric force and energy recycling in tendon (Biewener, 2016, 1998; Biewener and Roberts, 2000; Roberts et al., 1997). Studies demonstrating that the hip, actuated by strap-like long-fibered muscles, becomes increasingly important in generating positive work in high acceleration running compared to the ankle, actuated by short-fibered pennate muscles, point to such a division of labor (Pires et al., 2018; Williams et al., 2009). It follows that long-fibered muscles might exhibit a more pronounced adaptation to high acceleration training if they are utilized to a greater extent in these activities.
The purposes of this study were 1) to investigate how muscle morphology, and in particular optimal muscle fascicle length, is influenced by high-acceleration training during growth, and 2) to compare these adaptations between two muscles of contrasting architecture and locomotor mechanics. We adopted an avian bipedal model (guinea fowl) that permits performance of controlled longitudinal growth studies not feasible in humans. We hypothesized that a growth period marked by high-acceleration training in guinea fowl will lead to longer muscle fascicles. We also hypothesized that this training effect would be greater in a long-fibered, strap-like muscle (iliotibialis lateralis pars postacetabularis) compared to a short-fibered, long-tendon pennate muscle (lateral gastrocnemius) because it has been proposed that these muscles have distinct locomotor mechanics and energetics.
METHODS
Animals
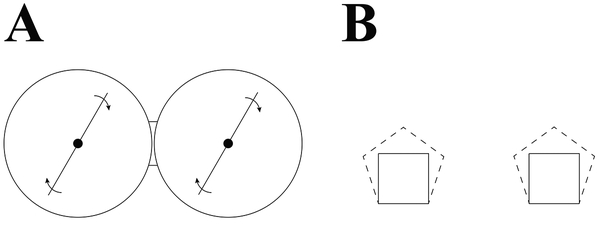
Thirty helmeted guinea fowl (Numida meleagris) were obtained as 1 day-old keats (GuineaFarm, IA). After a 4-week brooding period, guinea fowl were split evenly into two groups: exercise (EXE) and sedentary (SED). EXE birds were housed in a large pen with ample space for running and contained hurdles and perches that further promoted accelerative movements (e.g., jump to perch; see Fig. 1A). SED birds were group-reared in small pens that did not permit running (Fig. 1B). Guinea fowl were provided food and water ad libitum and were raised in a 12h:12h light:dark cycle. The experimental protocol was approved by the Pennsylvania State Institutional Animal Care and Use Committee (IACUC Ref #46435).
Figure 1.
Experimental housing set-up. EXE birds were housed in large, dual-circle pen (A) with ~0.35 m2 of space per bird and the ability to undertake spontaneous running exercise. Each circle contained a rotating boom that turned on for 10 minutes per hour during the lighted hours. EXE pen also contained perches and hurdles that promoted acceleration movements. SED birds were initially housed in small, square pens (B, solid lines) with ~0.05 m2 of space per bird. SED birds were split between the two pens. At week 6 of the protocol, SED pens were expanded (dashed lines) with ~0.10 m2 of space per bird, in accordance with Animal Care regulations.
Exercise Protocol
From age 4 to 14 weeks, EXE birds were trained via two interventions. First, they were housed in a circular pen that contained rotating booms that operated for 10 minutes per hour every day during the lighted hours (Fig. 1A). Wide strips of plastic hung from the booms, creating a curtain that motivated the birds to run and to jump over radially oriented hurdles in their path. Second, EXE birds were subjected to regular manual high-acceleration exercise 4 times per week. In each exercise period, birds were herded without contact around the pen. Animals were run in this manner for 3-minute intervals with 2-minute rest breaks for a total of 30 minutes (running direction was reversed between intervals). Rather than running continuously during these periods, the birds would use bursts of acceleration to run and jump over hurdles. The total distance traveled during a high-acceleration training was ~500–750 m. High-acceleration movements were also apparent in the spontaneous movements of EXE birds when they jumped to perch or ran in the pens. The SED birds did not receive any exercise stimulus. At 14 weeks-old, the birds were sacrificed (intravenously pentobarbital, >160 mg/kg). Animals were immediately placed into a −20 °C freezer for subsequent morphological analyses.
Body Composition and Bone Measures
To assess adaptations at the muscle level relative to gross anatomical modifications, we measured overall body mass, body composition and limb lengths. Birds were weighed every two weeks throughout the length of the protocol. Lower limb bone lengths (femur, tibiotarsus, and tarsometatarsus) were measured from planar radiographs (13 MP, Fischer Imaging Corp., Wheat Ridge, CO) using OsiriX Lite software (Pixmeo, Bernex, Switzerland). These three lengths were summed to obtain a combined lower limb length for each bird.
Dual X-ray absorptiometry (DXA; Horizon-W, Hologic, Marlborough, MA) was used to assess body composition in intact bird specimens. Birds were placed on the DXA table in a supine position with wings spread.
Specimen Muscle Architecture Preparation
The pelvic limb was separated from the upper body and the left and right legs were then split by sectioning the pelvis at the midline while avoiding muscle attachments. Right limbs were formalin-fixed while left legs were kept fresh/frozen (−20 °C). Right limbs were positioned with joints angles similar to those occurring at mid-swing during running (Rubenson and Marsh, 2009) (hip: 30°, knee: 80°, ankle: 125°, within ± 2°). Right limbs were placed into neutral buffered formalin for fixation for at least two weeks, and subsequently washed and stored in a solution of 0.1M phosphate-buffered saline. Joint angles were confirmed for the fixed limbs using photographs made with a digital camera (Canon EOS550D; Surrey, United Kingdom) and analyzed with ImageJ (National Institutes of Health, Bethesda, MD). Left limbs were kept fresh/frozen for muscle mass measures.
Muscle Analyses
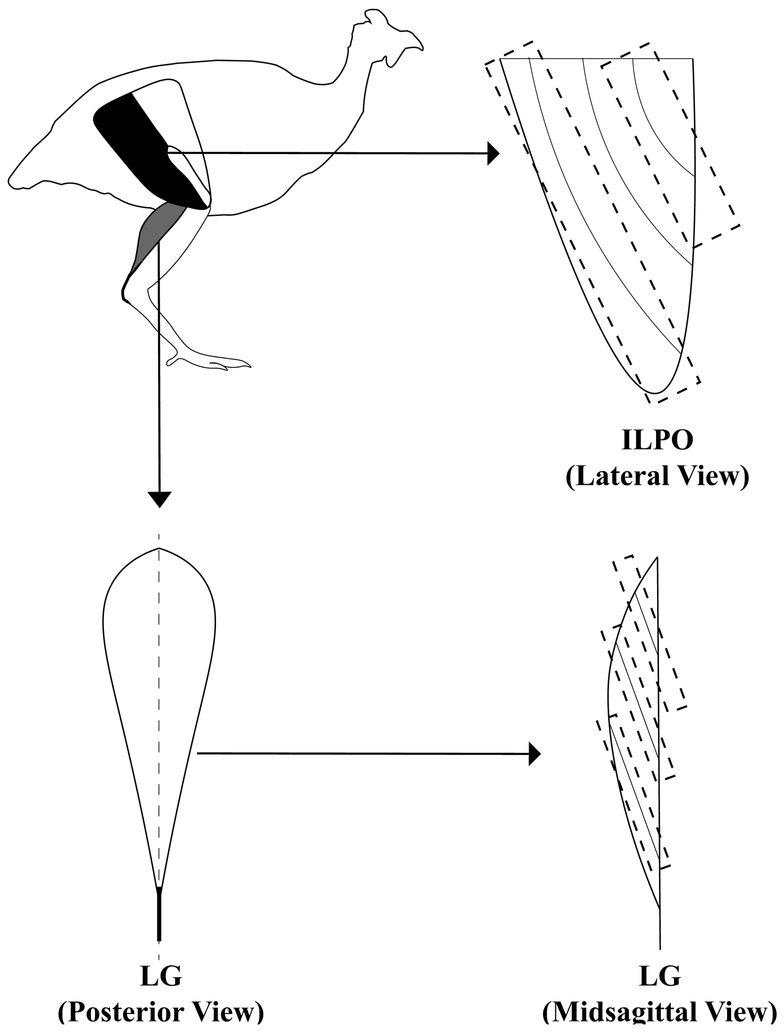
We made measurements of two functionally and architecturally distinct muscles: the iliotibialis lateralis pars postacetabularis (ILPO), a long-fibered biarticular (hip and knee) muscle, and the lateral gastrocnemius (LG), a short-fibered pennate biarticular (knee and ankle) muscle possessing a large external tendon (Figure 2). A detailed justification for investigating these two muscles is presented in the Supplementary Material. ILPO and LG were dissected from the fixed limbs for fascicle length, pennation angle, and sarcomere analysis. LG was first split longitudinally through the mid-belly in order to view fascicle arrangement (Figure 2). Photographs of whole ILPO and split LG made with a digital camera (Canon EOS550D) were imported into ImageJ for measurement of the pennation angle between muscle fascicles and their insertions on the aponeurosis. Due to the expected within-muscle heterogeneity of strain (Azizi and Deslauriers, 2014), each muscle was divided into sections. ILPO was split into anterior and posterior fascicles (Carr et al., 2011c) and LG was split into proximal, middle, and distal sections, each spanning one-third the length of the muscle belly (Figure 2). Average pennation angle was found for each section by taking the mean of three angle measurements.
Figure 2.
Muscle dissection protocol. Anatomical location of ILPO (black) and LG (gray) muscles (top left). ILPO was divided into anterior and posterior sections (top right). LG was split longitudinally (bottom left) and then divided into proximal, middle, and distal sections (bottom right).
Each of the sections (anterior and posterior for ILPO; proximal, middle, distal for LG) was placed into a petri dish of 30% nitric acid for at least 12 hours after which they were rinsed in phosphate buffered saline and placed into glycerol. Muscles were allowed to digest until small bundles of fascicles could be teased out and isolated. Long fascicles were photographed directly in the petri dish under macro magnification. From the digital images, fascicle lengths were measured using the multi-point tool in ImageJ to trace the fascicles, taking into account their curvature. A custom-written MATLAB (Mathworks, Natick, MA) routine was used to compute fascicle lengths from the coordinates of the digitized points. A minimum of three fascicle length measurements were taken from each muscle section. Fascicles were then mounted on microscope slides for laser diffraction analysis (He-Ne laser, 15 mW, 632.8 nm, CVI Melles Griot, Carlsbad, CA) to measure sarcomere lengths (Lieber et al., 1984). The laser diffraction system was calibrated using diffraction gradients of 1.20, 1.67, and 3.33 μm. A minimum of three sarcomere length measurements were taken from each muscle fascicle bundle and averaged to get a mean measured sarcomere length. Optimal fascicle length (L0) was calculated multiplying the length of the fascicle by the ratio of optimal sarcomere length of guinea fowl muscle (2.36 μm; Carr et al., 2011b) to the average measured sarcomere length. L0 were also normalized to total limb length to assess whether adaptations occur independent of a general size effect, since longer muscles can simply be associated with longer bones (Legerlotz et al., 2016).
ILPO and LG were dissected from the fresh-frozen left limbs and weighed to the nearest milligram. Muscle mass was used to obtain physiological cross-sectional area (PCSA) using Equation 1 (Lieber and Fridén, 2000):
| (1) |
where m is muscle mass (in g), is the average pennation angle across muscle sections, ρ is the density of muscle (0.00106 g/mm3), and is the average L0 across muscle sections.
Statistical Analyses
We employed a blocked ANOVA to test the effects of high-acceleration training on L0, limb length-normalized L0, sarcomere length, and pennation angle for each of ILPO and LG. The design treated each section of muscle as a “block” to account for natural anatomic changes in L0 across the muscle. This was especially important in ILPO, where fascicle lengths increase from anterior to posterior regions of the muscle (Carr et al., 2011c). A mixed-model ANOVA using blocks as random effects was run in R (R Core Team, 2016), using the “sjstats” (Lüdecke, 2017) and “psych” (Revelle, 2017) packages. Differences in body mass were tested for using a two-factor (group and time point) repeated-measures ANOVA in SAS (SAS Institute, Cary, NC). Two-tailed independent t-tests with Bonferroni corrections were used to determine if post-sacrifice body composition, bone mineral density, limb length, muscle mass, and PCSA differed between groups. The level of significance was set at α = 0.05 a priori. Cohen’s D (t-tests) and Cohen’s f2 (ANOVAs) were calculated for effect size (ES). Bonferroni corrections to account for multiple t-tests comparisons were applied separately for muscle (p < 0.0125) and bone (p < 0.025) variables.
RESULTS
Body Mass and Composition Measurements
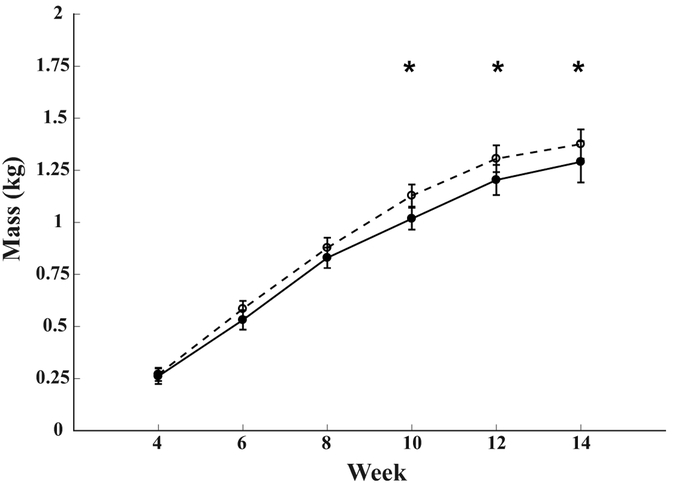
Body mass measured after the brooding period was not different between groups (p = 0.52, Figure 3). By 2 weeks into the experimental protocol (age 6 weeks), EXE birds had significantly lower mass than the SED birds (p = 0.004, ES = −1.23, Figure 2). EXE birds maintained a lower body mass throughout the rest of the protocol. SED animals were 6% heavier at the end of the protocol (p = 0.013, ES = −0.98) (Table 1), but there was no significant difference in body composition (p = 0.300, ES = −0.43). SED birds were also found to have 3% longer overall limb lengths compared to the EXE group (p = 0.019, ES = −0.92). Bone mineral density was not different between the two groups (p = 0.725; ES = −0.14).
Figure 3.
Line graph showing changes in body mass throughout the protocol. Mean ± SD body mass for each group. EXE: solid line / filled circles. SED: dashed line / open circles. * p < 0.05 between groups.
Table 1.
Gross anatomical measures. Values reported as mean ± standard deviation.
| EXE | SED | |
|---|---|---|
| Final Body Mass (kg) | 1.29 ± 0.10† | 1.38 ± 0.07 |
| Body Composition (% fat) | 10.1 ± 1.1 | 10.7 ± 1.7 |
| Bone Mineral Density (g/cm2) | 0.13 ± 0.01 | 0.14 ± 0.01 |
| Limb Length (cm) | 26.4 ± 0.8* | 27.2 ± 0.9 |
indicates statistical significance (p < 0.05).
indicates statistical significance between groups after post-hoc analysis for bone (p < 0.025).
Muscle Architecture
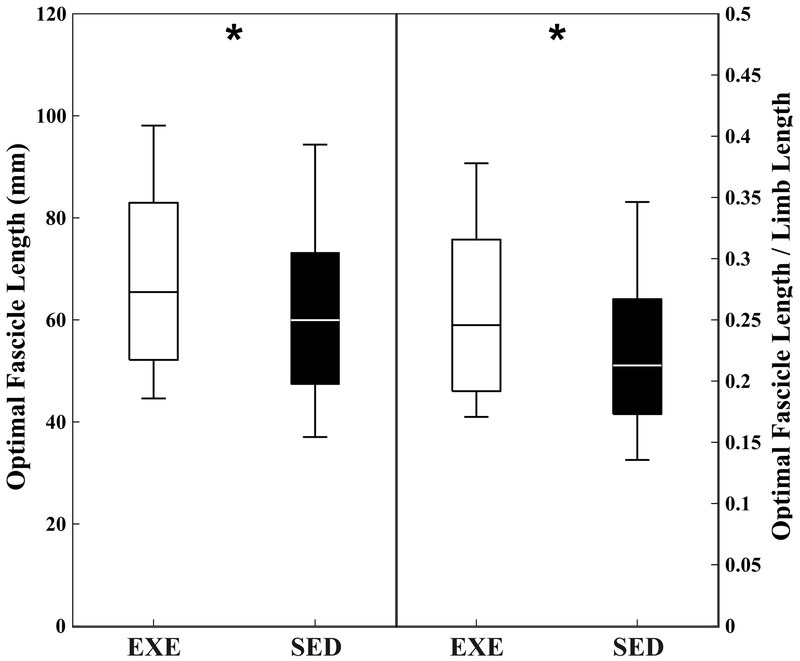
Optimal fascicle length in ILPO was found to be 12% longer in the EXE birds compared to the SED birds (p = 0.007, ES = 0.38) (Table 2). This difference was greater (15%) when L0 were normalized to limb length (p = 0.002, ES = 0.47). In LG, L0 were 14% longer in the EXE birds, but this difference was not statistically significant (p = 0.068). However, when normalized to limb length, LG L0 were significantly longer in the EXE group (p = 0.025, ES = 0.57). Sarcomere lengths were shorter in the EXE group, but these differences were statistically significant only in LG (ILPO: p = 0.051, ES = 0.27 LG: p < 0.001, ES = 0.40). PCSA for ILPO and LG were 16% and 12% smaller in the EXE, though these differences were not statistically significant (ILPO: p = 0.123, ES = −0.70; LG: p= 0.117, ES = −0.80). There were no differences in wet muscle mass, body-mass normalized muscle mass, or pennation angles between muscles or treatments (Table 2).
Table 2.
ILPO morphological measurements. Values reported as mean ± SD.
| EXE | SED | ||
|---|---|---|---|
| Muscle Mass (g) | 12.3 ± 2.1 | 12.6 ± 1.9 | |
| PCSA (mm2) | 151 ± 27 | 181 ± 53 | |
| Sarcomere Length (μm) | Anterior | 2.11 ± 0.18 | 2.16 ± 0.19 |
| Posterior | 2.10 ± 0.14 | 2.24 ± 0.19 | |
| Pennation Angle | Anterior | 15.7 ± 3.3 | 18.0 ± 2.7 |
| Posterior | 29.5 ± 3.9 | 29.0 ± 4.1 | |
| Sarcomere Number | Anterior | 22859 ± 2778* | 20749 ± 3281 |
| Posterior | 35592 ± 2872* | 30019 ± 6372 | |
| L0 (mm) | Anterior | 53.23 ± 6.53* | 49.33 ± 7.33 |
| Posterior | 83.04 ± 6.66* | 72.61 ± 14.13 | |
| Limb length Normalized L0 | Anterior | 0.20 ± 0.02* | 0.18 ± 0.03 |
| Posterior | 0.32 ± 0.03* | 0.27 ± 0.06 |
indicates statistical significance between groups from blocked ANOVA after post-hoc analysis.
DISCUSSION
High-acceleration training during growth increases L0
Here we provide evidence that longer optimal fascicle lengths are an adaptation to activity during growth. Our results using an avian bipedal model support our hypothesis that high-acceleration training during growth results in longer L0 in the muscles of the lower limb than those observed following a sedentary growth period. This difference offers a theoretical functional advantage in terms of force-generating capacity: Using the operating ranges for length and velocity of ILPO reported during fast incline running in guinea fowl (2.0 m/s, 20% incline; Carr et al., 2011a), a 15% increase in L0 (as we report for the EXE group) would result in a ~10 % higher force-generating capacity (based on idealized force-length and force-velocity curves; Zajac, 1989). Thus, the longer fascicles seen in the EXE group are likely more beneficial in movements requiring high mechanical work and power production. Accurate muscle strain and velocity data for high power movements do not exist for guinea fowl LG, but the above example indicates that substantial benefits to force, power, and work production are possible as a consequence of the observed increase in L0.
Numerous studies have demonstrated that muscle adapts to its environmental conditions (for reviews see Garland and Kelly, 2006; Johnston, 2006). For example, classic experiments have shown muscles adapt to aerobic training by increasing the proportion of slow, glycolytic muscle fibers, as well as accompanying enzymatic and physiological adaptations making them better suited to high aerobic demand (Wilson et al., 2012). Other environmental stimuli, such as temperature, have likewise been shown to elicit advantageous modifications in muscle phenotype (Johnston et al., 2003). Here we provide evidence that muscle fascicle length similarly exhibits a plastic response during growth that facilitates function, possibly conveying an advantage aiding in survival for adult animals in fluctuating environmental and ecological conditions.
While this study is, to the best of our knowledge, the first to assess muscle fascicle length changes following high-acceleration training during growth, our results are generally consistent with previous experiments assessing the effect of exercise or stretch perturbations on fascicle length. Rabey et al. (2015) also found activity-dependent differences in fascicle length in murine shoulder muscles during growth, but did not normalize these differences by limb length. Such normalization is potentially important because fascicle lengths may simply increase along with bone length in larger animals (Legerlotz et al., 2016). Our finding that muscle length increases despite a decrease in bone length with exercise is strong evidence that this is an adaptation that enhances muscle function during high-acceleration movement, and not an effect following from size differences. Sprint-training in adult humans has likewise been found to have an influence on muscle length (Blazevich et al., 2003). In that study, 5 weeks of sprint-training increased fascicle length in the proximal and distal portions of the vastus lateralis and rectus femoris substantially (3–7 cm). While these findings and those of Rabey et al. (2015) are similar to the results of the present study, the length increases (~25–80%) reported by Blazevich et al. (2003) are considerably greater and were observed only in certain regions of the muscle. The length differences we observed between EXE and SED are comparable to those of previous studies showing increases in fascicle length with retinaculum release (~11–19%), intermittent stretching (~9%), and eccentric training (~12%), all of which induce a lengthening perturbation on the muscle (De Jaeger et al., 2015; Franchi et al., 2014; Koh and Herzog, 1998).
Increases in muscle length are independent of muscle architecture
Our finding of similar differences between groups for both ILPO and LG fascicle lengths was not in agreement with our second hypothesis, and instead indicate that fascicle length adaptations to high-acceleration training during growth occur independent of muscle architecture. This could have several possible explanations. First, it is possible the signal for altered muscle length is generalized across locomotor muscles, although analysis of other muscles would be needed to test this hypothesis. These results could also be explained if both ILPO and LG perform similar mechanical functions during running acceleration, and thus undergo a similar remodeling in response to acceleration training. Data on muscle mechanics (Daley and Biewener, 2003) and muscle blood flow (Rubenson et al., 2006) show that LG contributes relatively little to the high energy cost of incline running, suggesting it has a limited role in the production of mechanical work, but its mechanical role during running accelerations may be different. The increased reliance on the hip joint (actuated by long-fibered muscles) for generating the positive mechanical work in high acceleration running that has been observed in some species does not support this interpretation (Pires et al., 2018; Williams et al., 2009). It should be noted, however, that although the hip increases its contribution to total positive work production in these studies, the ankle’s positive work production (actuated by short-fibered, pennate muscles) was also high (Pires et al., 2018; Williams et al., 2009). In accelerating turkeys, the net ankle and hip work has also been found to be similar (Roberts and Scales, 2004), although positive joint work, which is closer associated with muscle fiber work (Sasaki et al., 2009), was not assessed. More direct evidence that short-fibered, pennate muscles can contribute significantly to work production can be seen in the pennate and short-fibered LG and peroneus ankle extensor muscles in turkeys that are capable of producing substantial mechanical work during uphill running (Gabaldón et al., 2004). It remains possible, therefore, that LG underwent increases in mechanical power and work production that were sufficient to elicit the same remodeling response as that of ILPO. Finally, it is possible that other signals common to both muscles are driving the muscle length remodeling (e.g., passive stretch; De Jaeger et al., 2015; Koh and Herzog, 1998).
A trade-off between muscle power and isometric force?
Our results also highlight a functional tradeoff between maximizing isometric force capacity and minimizing losses in force capacity due to force-length-velocity effects. Although lean mass was slightly lower in the EXE animals, we observed similar ILPO and LG muscle mass between groups. Despite the similar mass, there was a clear effect of training on the architecture of the muscles between the groups. In maintaining the same muscle mass, longer fascicles in the EXE group appear to occur at the expense of a reduction in PCSA. The 12% and 14% increases in L0 in ILPO and LG were accompanied by 16% and 12% reductions (although not significantly different at the α = 0.05 level) in PCSA, respectively. It is possible, therefore, that when muscle mass is conserved, muscles in the EXE group remodeled to maintain an optimal strain profile for dynamic contractions at the expense of reducing isometric force capacity (as indicated by PCSA). This observation supports the argument that muscles will have an architecture that is best suited, given the constraint of constant mass, for either maximum force or shortening velocity (Lieber and Fridén, 2000). Whether a similar tradeoff favoring PCSA would occur in animals subjected to elevated isometric force requirement (e.g., demands on maintaining posture) warrants further investigation.
Limitations
There are several limitations and delimitations to this study that could influence the results and their interpretation. First, to assess body composition, the “infant” setting was used on the DXA machine as the birds were too large for the scan area on the “small animal” setting. Both groups were scanned using the same set-up, and body fat percentages acquired from DXA for our guinea fowl are similar to those found in chickens via chemical analysis (Swennen et al., 2004). For muscle analyses, ILPO was chosen due to its function during steady-state running and its capacity to do work, but it possesses a challenging architecture. The fascicles in ILPO gradually increase from the anterior to posterior portions. Though great care was taken to ensure consistent sectioning of ILPO, fascicles within a section still vary in length due to the natural architecture of the muscle. Our results from LG, where fascicle lengths are more homogenous (within ~2 mm) throughout the muscle, lead us to trust our overall findings. LG and ILPO were chosen due to their differences in architecture and differences in function during steady-state running (Carr et al., 2011a, 2011b; Daley and Biewener, 2003; Rubenson and Marsh, 2009). It is possible, however, that both increased their mechanical work output during acceleration movements in a manner sufficient to elicit a similar remodeling response. Thus, it is possible our results only apply to muscles that function during acceleration, and similar adaptations may not occur in locomotor muscles not important for acceleration tasks. Another limitation is that this study used only one intervention to investigate muscle adaptations to training during the growth period. Our results suggest high-acceleration training can increase fascicle length, but we do not know how this compares to other modes of training (e.g., endurance, resistance, etc.), especially mixed modes of training. Results from Rabey et al. (2015) show fascicle length adaptations can be activity-dependent, but adaptations to various running-based types of training remain unclear.
We also do not know whether a similar loading signal in an adult animal would have a similar remodeling response. Finally, functional interpretations of our results are a lso hindered by a lack of knowledge about compositional and mechanical adaptations by LG and ILPO, for example fiber type composition and specific-tension.
Conclusion
In summary, we conclude that longer optimal fascicle lengths appear to be associated with high-acceleration training during growth. This increase in optimal fascicle length occurred in two muscles with disparate architectures and functional role during locomotion. Our findings suggest that high-acceleration training during the growth period results in muscles that are optimized for reducing sarcomere shortening velocity, enhancing dynamic force generation rather than isometric strength.
Supplementary Material
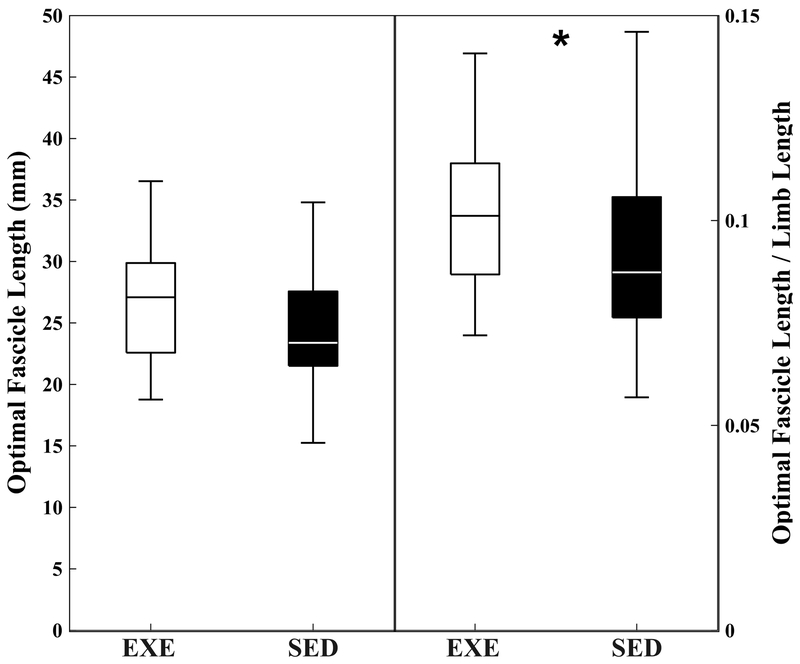
Figure 4.
Boxplots for optimal fascicle length (left) and limb length normalized optimal fascicle length (right) in ILPO. Data shown are averaged across both anterior and posterior portions of the muscle. Box represents 25th-75th percentiles. Line within box represents median. * indicates statistical significance between groups from blocked ANOVA.
Figure 5.
Boxplots for optimal fascicle length (left) and limb length normalized optimal fascicle length (right) in LG. Data shown is averaged across proximal, middle, and distal portions of the muscle. Box represents 25th-75th percentiles. Line within box represents median. Open circle represents outlier (>3 mean absolute deviations from the mean). * indicates statistical significance between groups from blocked ANOVA.
Table 3.
LG morphological measurements. Values reported as mean ± SD.
| EXE | SED | ||
|---|---|---|---|
| Muscle Mass (g) | 6.5 ± 1.0 | 6.7 ± 0.8 | |
| PCSA (mm2) | 224 ± 37 | 254 ± 39 | |
| Sarcomere Length (μm) | Proximal | 2.13 ± 0.19* | 2.34 ± 0.30 |
| Middle | 2.07 ± 0.30* | 2.21 ± 0.26 | |
| Distal | 2.07 ± 0.28* | 2.34 ± 0.20 | |
| Pennation Angle | Proximal | 19.8 ± 2.9 | 19.0 ± 3.0 |
| Middle | 19.5 ± 2.9 | 18.2 ± 2.2 | |
| Distal | 15.9 ± 2.2 | 15.6 ± 2.6 | |
| Sarcomere Number | Proximal | 11043 ± 2203 | 10919 ± 2652 |
| Middle | 12359 ± 2241 | 10523 ± 2805 | |
| Distal | 11180 ± 1502 | 10366 ± 2228 | |
| L0 (mm) | Proximal | 25.88 ± 4.78 | 25.09 ± 5.68 |
| Middle | 28.76 ± 5.87 | 24.64 ± 5.92 | |
| Distal | 26.35 ± 3.55 | 24.27 ± 4.61 | |
| Limb length Normalized L0 | Proximal | 0.10 ± 0.02* | 0.09 ± 0.02 |
| Middle | 0.11 ± 0.02* | 0.09 ± 0.02 | |
| Distal | 0.10 ± 0.01* | 0.09 ± 0.02 |
indicates statistical significance between groups from blocked ANOVA after post-hoc analysis.
ACKNOWLEDGMENTS
We would like to thank Justin Csaszar, Josh Remillard, Leighann Warholak, Paige Reynolds, Melissa Minniti, Jillian Butkiewicz, Sina Pooresmaeil, Stephen Blakely, Brandon Stone, and Tyler Faimon for their help with the training, dissections, and movement scores. We would also like to thank Emily Southmayd and Melissa Welker for performing the DXA and x-ray scans, respectively. Finally, we would like to thank the staff of the Penn State Animal Resource Program for the care of our animals during the study.
FUNDING
This work was funded, in part, through a seed grant from the Center for Human Evolution and Diversity, Penn State University and NIH grant AR071558 to J.R. and S.J.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Abe T, Fukashiro S, Harada Y, Kawamoto K, 2001. Relationship between sprint performance and muscle fascicle length in female sprinters. Journal of Physiological Anthropology and Applied Human Science 20, 141–147. [DOI] [PubMed] [Google Scholar]
- Abe T, Kumagai K, Brechue WF, 2000. Fascicle length of leg muscles is greater in sprinters than distance runners. Medicine and Science in Sports and Exercise 32, 1125–1129. [DOI] [PubMed] [Google Scholar]
- Azizi E, Deslauriers AR, 2014. Regional heterogeneity in muscle fiber strain: the role of fiber architecture. Frontiers in Physiology 5:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter JR, Novack TA, Van Werkhoven H, Pennell DR, Piazza SJ, 2012. Ankle joint mechanics and foot proportions differ between human sprinters and non-sprinters. Proceedings of the Royal Society B: Biological Sciences 279, 2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA, 2016. Locomotion as an emergent property of muscle contractile dynamics. Journal of Experimental Biology 219, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA, 1998. Muscle Function in vivo: A Comparison of Muscles used for Elastic Energy Savings versus Muscles Used to Generate Mechanical Power. American Zoologist 703–717. [Google Scholar]
- Biewener AA, Roberts TJ, 2000. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exercise and Sport Sciences Reviews 28, 99–107. [PubMed] [Google Scholar]
- Blazevich AJ, Gill ND, Bronks R, Newton RU, 2003. Training-Specific Muscle Architecture Adaptation after 5-wk Training in Athletes: Medicine & Science in Sports & Exercise 35, 2013–2022. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC, 2007. Quantifying behavior the JWatcher way. Sinauer Associates, Sunderland, Mass. [Google Scholar]
- Carr JA, Ellerby DJ, Marsh RL, 2011a. Function of a large biarticular hip and knee extensor during walking and running in guinea fowl (Numida meleagris). Journal of Experimental Biology 214, 3405–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, Ellerby DJ, Marsh RL, 2011b. Differential segmental strain during active lengthening in a large biarticular thigh muscle during running. The Journal of Experimental Biology 214, 3386–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, Ellerby DJ, Rubenson J, Marsh RL, 2011c. Mechanisms producing coordinated function across the breadth of a large biarticular thigh muscle. Journal of Experimental Biology 214, 3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley MA, Biewener AA, 2003. Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. Journal of Experimental Biology 206, 2941–2958. [DOI] [PubMed] [Google Scholar]
- De Jaeger D, Joumaa V, Herzog W, 2015. Intermittent stretch training of rabbit plantarflexor muscles increases soleus mass and serial sarcomere number. Journal of Applied Physiology 118, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Franchi MV, Atherton PJ, Reeves ND, Flück M, Williams J, Mitchell WK, Selby A, Beltran Valls RM, Narici MV, 2014. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiologica 210, 642–654. [DOI] [PubMed] [Google Scholar]
- Gabaldón AM, Nelson FE, Roberts TJ, 2004. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. The Journal of Experimental Biology 207, 2277–2288. [DOI] [PubMed] [Google Scholar]
- Garland T, Kelly SA, 2006. Phenotypic plasticity and experimental evolution. Journal of Experimental Biology 209, 2344–2361. [DOI] [PubMed] [Google Scholar]
- Hedrick TL, 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspiration & Biomimetics 3, 34001. [DOI] [PubMed] [Google Scholar]
- Henry HT, Ellerby DJ, Marsh RL, 2005. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. The Journal of Experimental Biology 208, 3293–3302. [DOI] [PubMed] [Google Scholar]
- Johnston IA, 2006. Environment and plasticity of myogenesis in teleost fish. Journal of Experimental Biology 209, 2249–2264. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Manthri S, Alderson R, Smart A, Campbell P, Nickell D, Robertson B, Paxton CGM, Burt ML, 2003. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar L.). The Journal of Experimental Biology 206, 1337–1351. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Herzog W, 1998. Increasing the moment arm of the tibialis anterior induces structural and functional adaptation: implications for tendon transfer. Journal of Biomechanics 31, 593–599. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Abe T, Brechue WF, Ryushi T, Takano S, Mizuno M, 2000. Sprint performance is related to muscle fascicle length in male 100-m sprinters. Journal of Applied Physiology 88, 811–816. [DOI] [PubMed] [Google Scholar]
- Lee SSM, Piazza SJ, 2009. Built for speed: musculoskeletal structure and sprinting ability. Journal of Experimental Biology 212, 3700–3707. [DOI] [PubMed] [Google Scholar]
- Legerlotz K, Marzilger R, Bohm S, Arampatzis A, 2016. Physiological adaptations following resistance training in youth athletes—A narrative review. Pediatric Exercise Science 28, 501–520. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fridén J, 2000. Functional and clinical significance of skeletal muscle architecture. Muscle & Nerve 23, 1647–1666. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Yeh Y, Baskin RJ, 1984. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophysical Journal 45, 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D, 2017. sjstats: Statistical Functions for Regression Models.
- Pires NJ, Lay BS, Rubenson J, 2018. Modulation of joint and limb mechanical work in walk-to-run transition steps in humans. The Journal of Experimental Biology [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rabey KN, Green DJ, Taylor AB, Begun DR, Richmond BG, McFarlin SC, 2015. Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. Journal of Human Evolution 78, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienne, Austria. [Google Scholar]
- Revelle W, 2017. psych: Procedures for Personality and Psychological Research. Northwestern University, Evanston, IL. [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR, 1997. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Scales JA, 2004. Adjusting muscle function to demand: joint work during acceleration in wild turkeys. The Journal of Experimental Biology 207, 4165–4174. [DOI] [PubMed] [Google Scholar]
- Rubenson J, Henry HT, Dimoulas PM, Marsh RL, 2006. The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris). Journal of Experimental Biology 209, 2395–2408. [DOI] [PubMed] [Google Scholar]
- Rubenson J, Marsh RL, 2009. Mechanical efficiency of limb swing during walking and running in guinea fowl (Numida meleagris). Journal of Applied Physiology 106, 1618–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Neptune RR, Kautz SA, 2009. The relationships between muscle, external, internal and joint mechanical work during normal walking. Journal of Experimental Biology 212, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings GK, Williams AG, Herbert AJ, Lockey SJ, Heffernan SM, Erskine RM, Morse CI, Day SH, 2018. TTN genotype is associated with fascicle length and marathon running performance. Scandinavian Journal of Medicine & Science in Sports 28, 400–406. [DOI] [PubMed] [Google Scholar]
- Swennen Q, Janssens GPJ, Geers R, Decuypere E, Buyse J, 2004. Validation of dual-energy x-ray absorptiometry for determining in vivo body composition of chickens. Poultry Science 83, 1348–1357. [DOI] [PubMed] [Google Scholar]
- Warburton NM, Grégoire L, Jacques S, Flandrin C, 2013. Adaptations for digging in the forelimb muscle anatomy of the southern brown bandicoot (Isoodon obesulus) and bilby (Macrotis lagotis). Australian Journal of Zoology 61, 402. [Google Scholar]
- Williams SB, Usherwood JR, Jespers K, Channon AJ, Wilson AM, 2009. Exploring the mechanical basis for acceleration: pelvic limb locomotor function during accelerations in racing greyhounds (Canis familiaris). The Journal of Experimental Biology 212, 550–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, McGuigan MP, Su A, van Den Bogert AJ, 2001. Horses damp the spring in their step. Nature 414, 895–899. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim J-S, 2012. The effects of endurance, strength, and power training on muscle fiber type shifting. Journal of Strength and Conditioning Research 26, 1724–1729. [DOI] [PubMed] [Google Scholar]
- Zajac FE, 1989. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering 17, 359–411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.