Abstract
Purpose
Effective biomarkers for malignant mesothelioma (MM) are needed for clinical management and the development of mesothelin-targeted therapies. We evaluated serum megakaryocyte potentiating factor (MPF) as a biomarker predictive of treatment outcome in patients with MM and for developing mesothelin-targeted therapies.
Materials and Methods
Serial serum samples from patients with MM in two clinical trials of an antimesothelin immunotoxin were tested with our clinically validated MPF assay. Correlative studies were performed to determine the test effectiveness in treatment monitoring and outcome prediction. MPF was further evaluated for an association with response to an antimesothelin therapy and for disease monitoring.
Results
There was a significant reduction of serum MPF in patients with elevated baseline and radiologic response, with an average change from −52% to −78% after one to six cycles. Using a −50% change as the cutoff, patients with MM with positive MPF response had significantly improved progression-free survival (P < .001), with the median extended from 1.9 to 11.3 months. These patients with MPF response further exhibited improved overall survival (P = .004), with the median extended from 8.8 to 22.3 months. In patients with refractory MM, there was an association between elevated pretreatment serum MPF and radiologic response to an antimesothelin therapy (P = .033). Furthermore, in these response patients, serum MPF was monitored between 32.2 and 63.8 months and was found to reflect treatment response and disease progression.
Conclusion
At a cutoff of −50% change after receiving systemic therapies, a reduction in MPF was associated with improved clinical outcome, both progression-free survival and overall survival. An elevated baseline serum MPF was associated with a response to an antimesothelin therapy in patients with refractory MM; however, this finding needs to be confirmed in another study.
INTRODUCTION
Malignant mesothelioma (MM) is a highly aggressive disease with a poor prognosis.1 In patients with unresectable disease, the standard first-line therapy is pemetrexed and cisplatin, which is associated with a median overall survival (OS) of 12.1 months.2 For patients who have experienced treatment failure with first-line therapy, there is no approved second-line therapy, and responses to other treatments are rare.3 There is an urgent need to develop better therapies, and thus, a high degree of interest in targeting cell surface protein mesothelin.4-7 The antimesothelin immunotoxin SS1P was the first shown to be active in patients with refractory mesothelioma.8 There are multiple mesothelin-targeted agents in clinical development, including chimeric antibody,9 antibody-drug conjugate,10 chimeric antigen receptor T-cell therapy,11 and mesothelin-based vaccine.12 Noninvasive biomarkers for selecting patients, assessing treatment, and evaluating resistance are critical for their development.
There is no effective biomarker test for treatment assessment and outcome prediction in patients with MM to date. Previous work described potential biomarkers for mesothelioma13,14; however, it was difficult to demonstrate their clinical utility for an intended clinical use. The mesothelin gene encodes a precursor protein that is cleaved into a soluble megakaryocyte potentiating factor (MPF) and membrane-bound mesothelin.15,16 Previous studies showed correlations between the computed tomography (CT) response and arbitrarily chosen 10% to 25% reduced serum mesothelin after chemotherapy.17-21 Such low cutoff values are incompatible with a 30% reduction in tumor determined with CT, would contribute to high degrees of false signals, and would make it more difficult to correlate with clinical outcomes.
The currently available MesoMark test (Fujirebio, Malvern, PA) detects serum mesothelin, which is subject to the interference by mesothelin-targeted antibody-based agents. However, as the cleavage product generated during mesothelin maturation,15,16 MPF does not react with mesothelin-targeted agents. In addition to serving as a tumor antigen for MM tumor burden, it would also inform the expression of the drug target mesothelin in tumors. We developed and validated an assay for MPF and further showed that elevated MPF is a worse prognostic biomarker in patients with MM.22 Its effectiveness for monitoring therapies is not known. Thus, it is necessary to assess the MPF assay to evaluate treatment and to predict the outcome of systemic and targeted therapies. We analyzed serial serum samples collected from patients with MM in two trials: (1) treatment-naïve patients treated with the first-line agents pemetrexed and cisplatin in combination with SS1P19; and (2) refractory patients treated with SS1P in combination with the immune suppression agents pentostatin and cyclophosphamide.8
MATERIALS AND METHODS
Clinical Trials and Patient Samples
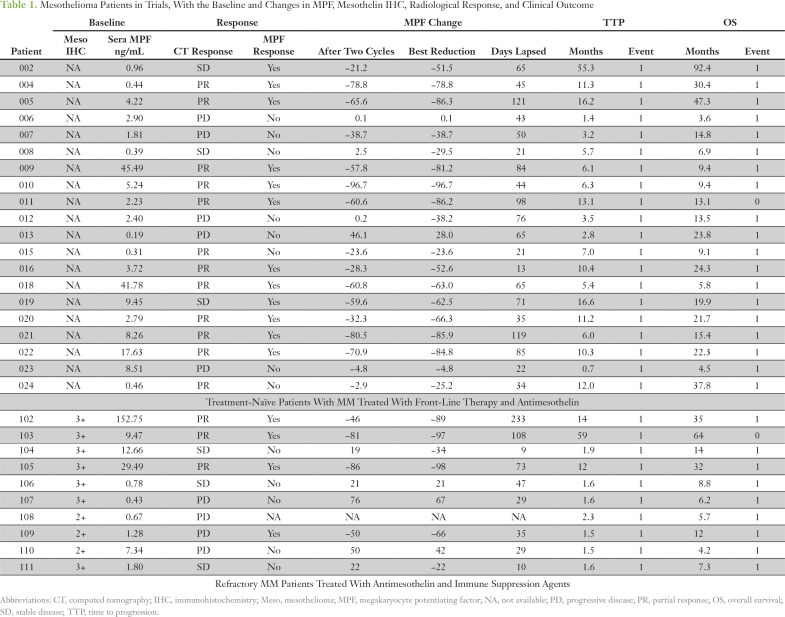
Serum samples were collected at pretreatment and at the end of each treatment cycle from patients enrolled in two antimesothelin trials. The first trial was a phase I study with a standard dose of pemetrexed and cisplatin, and the antimesothelin immunotoxin SS1P (NCT01445392) in chemotherapy-naïve patients with pleural mesothelioma.19 Twenty patients were evaluable, 12 with partial response (PR), three with stable disease (SD), and five with progressive disease (PD). A total of 168 serum samples were collected between October 2008 and January 2013, with the longest sample follow-up of 55.3 months.
The second trial was a pilot phase II study of SS1P with the addition of a pentostatin and cyclophosphamide immune depletion regimen to decrease antibody response to SS1P in patients with refractory MM (NCT01362790). The patients had received one to six (and an average of three) previous therapies.8 All patients were evaluated for responses: three with PR, three with SD, and four with PD. However, one patient (No. 108) was excluded from the analyses because of the lack of any post-therapy sample, except where only the baseline data were needed. A total of 77 serum samples were collected between December 2011 and December 2016, with the longest follow-up being 59.2 months.
All serum samples were collected using institutional review board–approved protocols at the US National Institutes of Health with informed consent. Patient samples were de-identified, tested, and analyzed in an unbiased and blinded fashion (Table 1). Tumor responses were determined on the basis of CT and calculated in accordance to modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria for mesothelioma.
Table 1.
Mesothelioma Patients in Trials, With the Baseline and Changes in MPF, Mesothelin IHC, Radiological Response, and Clinical Outcome
Statistical Analyses
Prism, Version 7.0 (GraphPad, La Jolla, CA) was used for statistical analysis. For determining the association between changes in MPF levels and CT response, unpaired t tests were performed. A paired t test was performed to compare the changes in MPF in patients with PR during the six-cycle therapy. Pearson correlation analysis was performed for the correlation between best MPF reduction and best CT response within the six cycles of treatment. Linear regression analysis was used to determine the line slope and the change in MPF corresponding to a −30% change in total tumor size.
Kaplan-Meier survival analyses were performed to determine log-rank hazard ratio (HR) and P value of MPF response or CT response patients on PFS or OS. Mann-Whitney test was performed to examine the association between baseline MPF and the response to antimesothelin SS1P and immune therapy. Because it was of interest to compare the duration of survival according to whether a patient was a responder or not, the landmark method was used.23 Because all but one responding patient experienced a response by 91 days, that one patient was considered to be a nonresponder; the analyses began at 91 days after the in-study date.
RESULTS
Systemic Therapies Lead to a Reduction of Serum MPF in Patients With MM With Elevated Baseline and Objective Responses
We assessed the effectiveness of serum MPF in monitoring the treatment response of patients with MM from two clinical trials (Table 1).8,19 The objective tumor responses of the 30 evaluable patients were 15 with PR, six with SD, and nine with PD. MPF values were determined in serial samples from them. The results showed a highly significant reduction of serum MPF in patients with PR compared with those with either SD or PD (Fig. 1A). There was a further correlation between the best MPF reduction and best CT response for treatment-naïve patients19 (Fig 1B). On the basis of the linear regression analysis of corresponding changes in CT and MPF in these 20 patients with MM, a −30% change in CT was interpolated to a −47% change in MPF level (Fig 1B). Thus, a rounded −50% change in serum MPF was selected as the cutoff for MPF response, which corresponded to approximately −30% change in CT using mRECIST.
Fig 1.
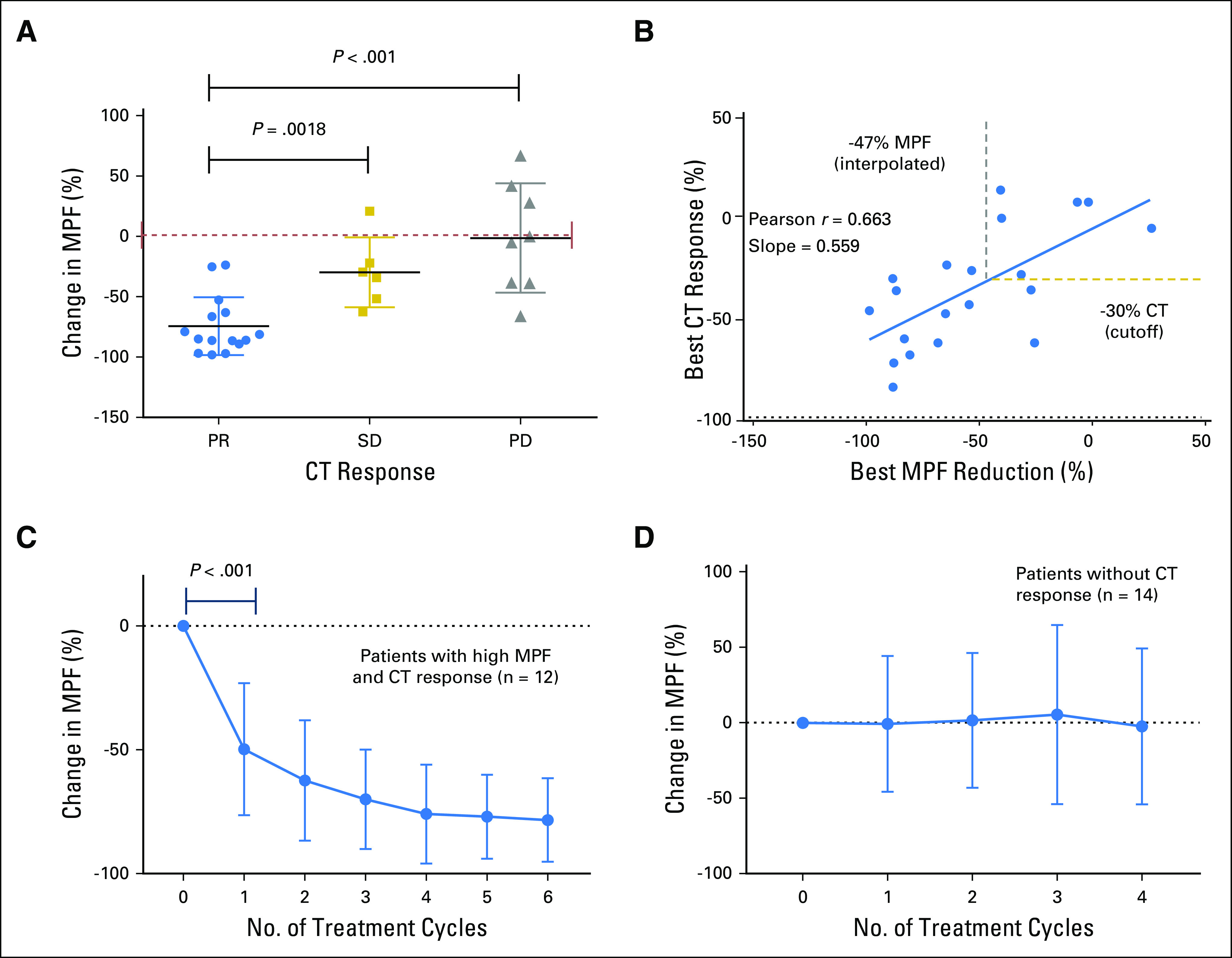
Reduction of megakaryocyte potentiating factor (MPF) associated with clinical response in malignant mesothelioma patients. (A) Analysis of the changes in serum MPF in patients with partial response (PR), stable disease (SD), and progressive disease (PD). The best reduction in MPF after therapies were used for data analysis with details listed in Table 1. A t test showed a highly significant reduction of serum MPF in patients with PR compared with those with either SD or PD (P = .0018 or P < .001). (B) Correlative analysis was performed to examine the association between the best MPF reduction and the best computed tomography (CT) response after the therapy. The analysis of treatment-naïve patients receiving immunotoxin in combination with pemetrexed and cisplatin showed strong correlation between the best MPF reduction and best CT response for (Pearson r = 0.663; P = .0014; n = 20). The slope was generated on the basis of linear regression analysis. Lines indicating −30% CT response (horizontal) and −47% MPF response (vertical) are also shown. (C) Changes in MPF for patients with malignant mesothelioma with elevated baseline MPF and PR. The P value of a t test (n = 12) between the baseline and the end of cycle 1 is shown. (D) Relative changes in MPF for patients with malignant mesothelioma with SD and PD (n = 14). All data are shown as mean ± standard deviation.
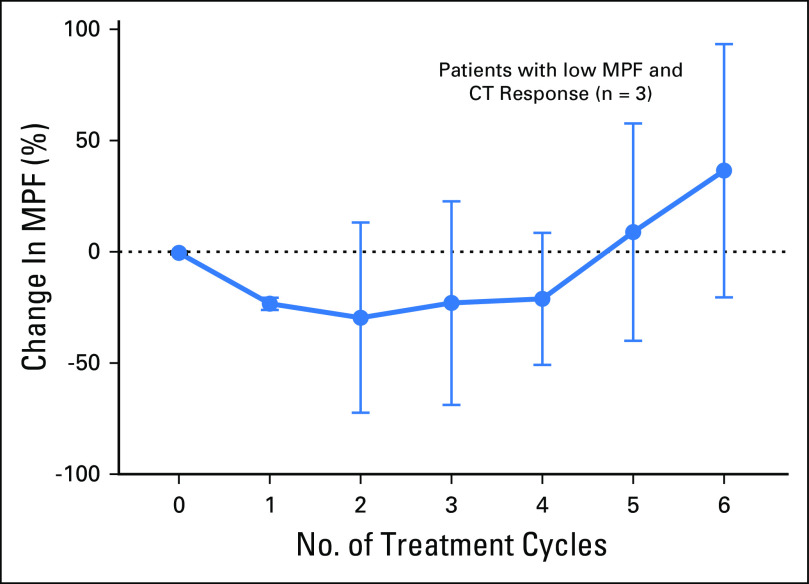
The serum MPF test enables continuous and multiple measurements during treatment. Of the 15 patients who exhibited PR, 12 had elevated baseline MPF. Among PR patients with an elevated MPF using the upper limit of normal at 1.2 ng/mL,22 there was an average of −52% change in MPF after one cycle (P < .001; n = 12; Fig 1C). The decline continued with each treatment cycle to −78% after six cycles. In contrast, for the patients who did not have a CT response, there was no significant reduction of MPF (n = 14; Fig 1D). Serum MPF analysis seems to be only effective in patients with an elevated baseline MPF because there is no trend of decreasing MPF in patients with PR and normal baseline MPF (n = 3; Fig. A1). Thus, the treatment-induced reduction of serum MPF may be an informative biomarker of objective response in patients with MM with elevated baseline MPF.
Association Between the MPF Response and Better PFS
Kaplan-Meier analyses were performed to compare PFS and OS of both trials. Results indicate similarities of both trials on PFS and OS (Appendix Fig. A2). Thus, patients from both studies were combined for the subsequent analysis. The MPF biomarker response (−50% change) in the patients with MM was evaluated for an association with clinical outcome using Kaplan-Meier survival analysis. The results showed that the median time to progression was 11.3 and 1.9 months for the MPF responders and nonresponders (P < .001; Fig 2A), with a log-rank HR (response to nonresponse) of 0.279 (95% CI, 0.109 to 0.713). This result is at least comparable to that of the CT response. The median time to progression values were 11.3 and 1.8 months for the CT responders and nonresponders (P = .022), with an HR of 0.457 (95% CI, 0.208 to 1.017; Fig 2C). Thus, the data suggest that a −50% change in MPF after systemic therapies is strongly associated with improved PFS.
Fig 2.
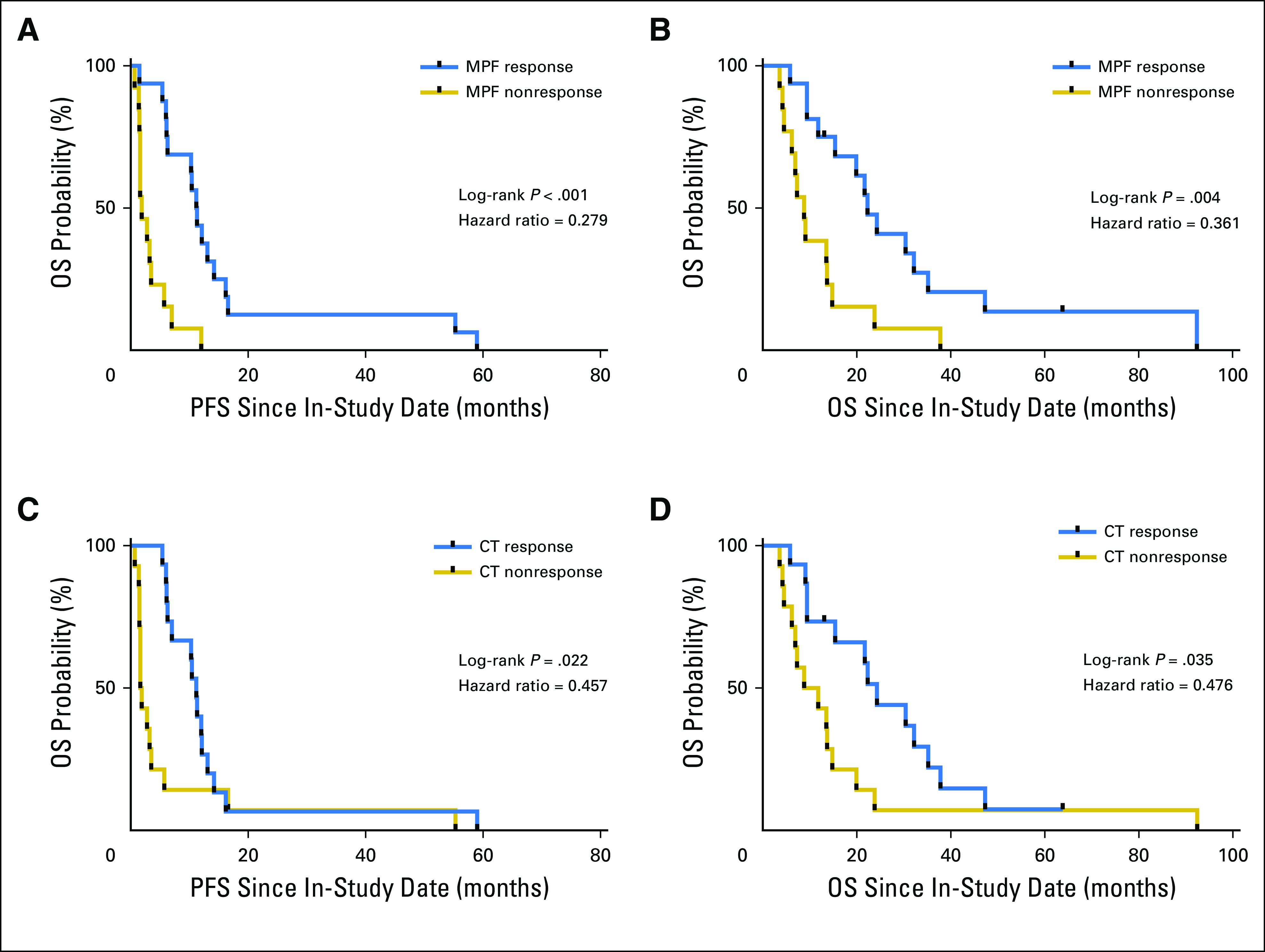
Association of megakaryocyte potentiating factor (MPF) response and improved clinical outcome. (A) Progression-free survival (PFS) and (B) overall survival (OS) using MPF response at a threshold of −50% or more after therapies. (C) Progression-free survival and (D) OS for patients with malignant mesothelioma using computed tomography (CT) response on the basis of modified Response Evaluation Criteria in Solid Tumors. The in-study date was used as date 0.
Reduction of MPF After Systemic Treatment as a Potential Biomarker for Better OS
The MPF response was further correlated with OS. Kaplan-Meier analysis shows that the median survival is 22.3 and 8.8 months for the MPF responders and nonresponders, (P = .004), with a log-rank HR (response to nonresponse) of 0.361 (95% CI, 0.151 to 0.861; Fig 2B). The MPF response is at least comparable to that of the CT response. The median survival time was 24.3 and 10.3 months for the CT responders and nonresponders, respectively (P = .035), with an HR of 0.476 (95% CI, 0.214 to 1.055; Fig 2D). Landmark analysis of OS for MPF and CT response was performed, with a landmark date at 91 days, at which time all patients had experienced a response but one. The results further confirmed that MPF response after systemic therapies was associated with better OS (P = .031) and compared favorably with the CT response (P = .056; Appendix Fig A3).
Elevated Baseline Serum MPF in Patients With Refractory MM Who Responded to an Effective Antimesothelin Therapy
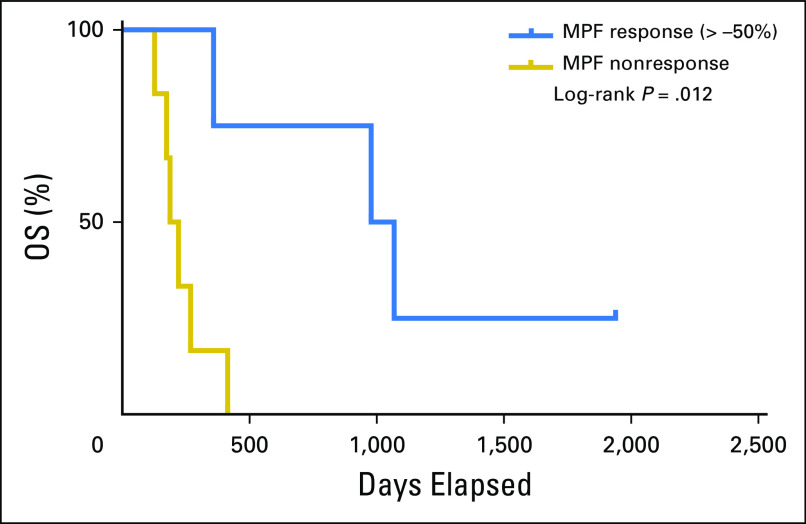
In the second trial of 10 patients with refractory MM with immunotoxin SS1P and an immune depletion regimen of pentostatin and cyclophosphamide, major cancer regressions were observed and were attributed primarily to the activities of SS1P.8 The nonparametric Mann-Whitney test showed that the patients with PR (by CT) had elevated baseline serum MPF levels, which were higher than those in the nonresponse group (P = .033; Fig 3A). Thus, data suggest that elevated baseline serum MPF may be associated with the response to an effective antimesothelin therapy in patients with refractory MM, whereas the MPF response is associated with improved survival in these patients (P = .012; Appendix Fig A4). Thus, the results suggest an association between elevated serum MPF and a response to antimesothelin SS1P, with evidence of MPF reduction associated with improved clinical outcome in patients with refractory MM.
Fig 3.
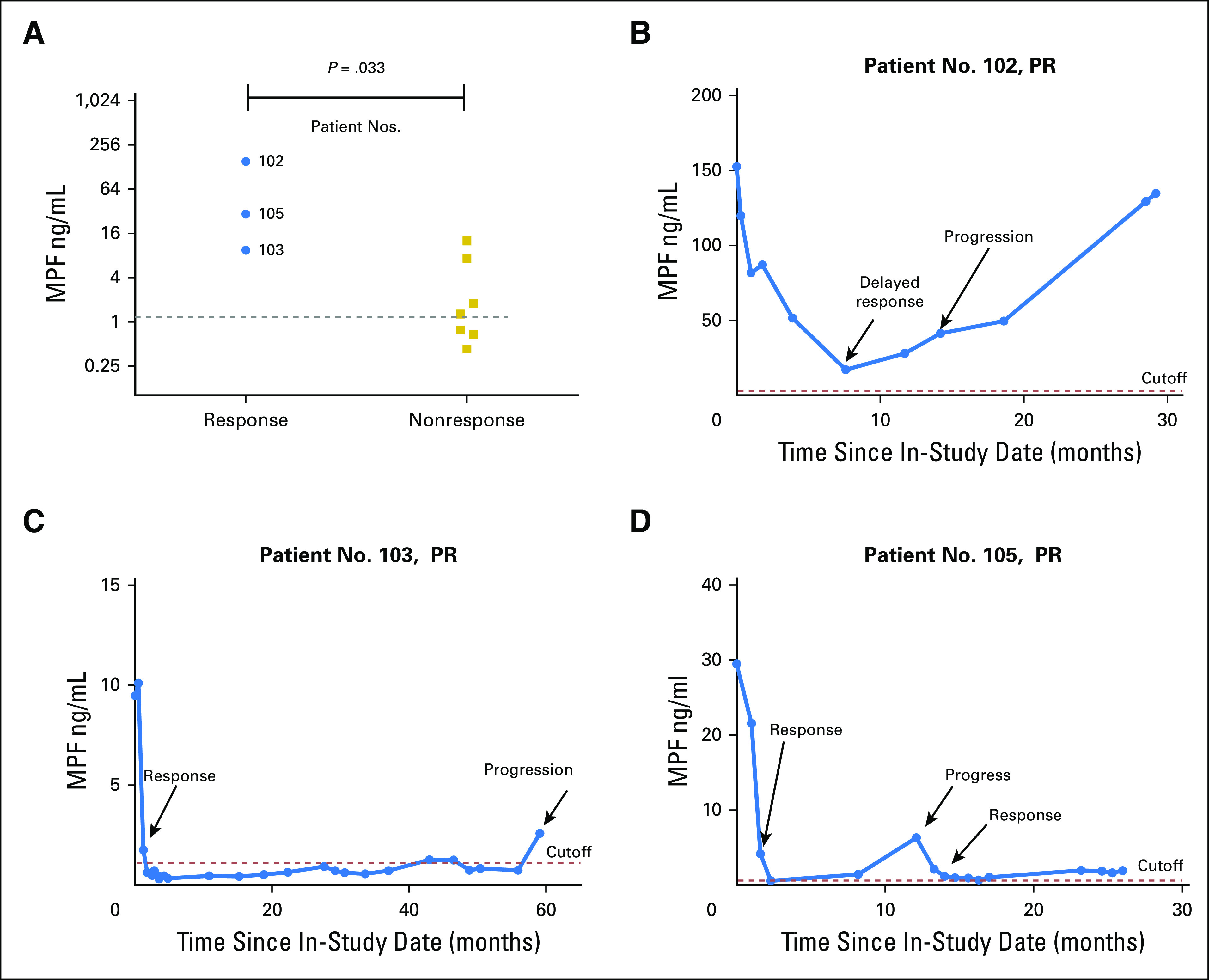
Elevated baseline megakaryocyte potentiating factor (MPF) associated with response and long-term follow-up of individual patients with major tumor regression after antimesothelin and immune suppression. (A) Association between elevated baseline MPF and the clinical response (computed tomography to an effective antimesothelin therapy with immune suppression in treatment-refractory malignant mesotheliomoa. Mann-Whitney (Wilcoxon rank sum) test was performed to generate the P value. (B) Patient No. 102 was treated with two cycles of SS1P before the development of antidrug antibody. He presented with delayed computed tomography response only after 7 months in therapy, which was maintained until 14 months. (C) Patient No. 103 was treated with six cycles of SS1P as originally planned. She experienced a 97% decrease in serum MPF, which stayed at or within the reference interval for 5 years. (D) Patient No. 105 was treated with four cycles of SS1P. He experienced a 98% decrease in serum MPF. He had disease progression at 1 year, which responded to another chemotherapy treatment. The dotted line is the upper limit of the reference interval for healthy individuals. PR, partial response.
Serum MPF Test for Long-Term Follow-Up of Patients With MM With a Major Cancer Response
We explored the use of the MPF test in monitoring the first three patients with MM who experienced major tumor regression to an effective antimesothelin therapy and correlated the MPF levels with tumor response and progression. Patient No. 102 was a 50-year-old man with extensive peritoneal involvement and received two cycles of SS1P because of SS1P antibody development.8 He had a delayed response with substantial tumor shrinkage at 7 months. The response continued to 14 months. The MPF level was reduced by 46% after two cycles and by 89%, to reach its lowest point at 7 months (Fig 3B). Despite a nearly 90% reduction in serum MPF, the level at 7 months was still highly elevated (17.3 ng/mL). The PR continued until 14 months, at which time, the MPF had more than doubled from its lowest point at 7 months, to 41.2 ng/mL. This patient progressed at 14 months, and the MPF level eventually rose to the pretreatment level by 29 months.
Patient No. 103, a 56-year-old woman with pleural mesothelioma, had rapid disease progression before treatment and was the only patient who completed all six cycles of therapy. Radiologically, she had 74% tumor shrinkage, which was maintained for 59 months. MPF analysis showed that the MPF fall to the reference interval after three cycles was rapid (Fig 3C). By the end of six cycles of therapy, the MPF level was decreased by 97%, to 0.31 ng/mL, lower than the reference level of 1.2 ng/mL. Her MPF levels remained low and were normalized for nearly 5 years until the last testing, at which time her MPF level was elevated to 2.6 ng/mL and she had signs of tumor progression.
Patient No. 105 was a 51-year-old man with peritoneal mesothelioma who had four cycles of SS1P and 70% tumor shrinkage at 5 months. His tumors progressed at 12 months and were subsequently treated with additional chemotherapy, which produced another PR. The results of blood MPF agreed with his CT-based clinical evaluation. The MPF level was decreased by 98% to 0.6 ng/Ml after three cycles, below the reference value (Fig 3D). His MPF level rose to 6.3 ng/mL at 12 months when the tumor progressed and was subsequently reduced to near-normal levels after additional successful chemotherapy.
Taken together, all three patients with MM with responses to an effective antimesothelin therapy were found to show an approximately ≥ –90% change in serum MPF after the initial treatment. The subsequent clinical behavior of the tumors was well correlated with changes in serum MPF in the follow-up of 29 to 60 months. There was evidence of increased serum MPF at the time of disease progression for all.
DISCUSSION
Our study indicates that the serum MPF test is effective in monitoring systemic therapies for patients with MM, because the patients with MPF responses have much better PFS and OS than those without, regardless of baseline levels. The data further suggest a potential association between elevated baseline MPF and clinical responses to an effective antimesothelin therapy in patients with refractory MM. Finally, an MPF test is feasible for monitoring patients with MM undergoing mesothelin-targeted therapy, with the longest follow-up of 5 years, providing information on treatment response, disease stabilization, and tumor progression and associated drug-target mesothelin expression.
Our results show that in patients with MM with elevated MPF baseline values, MPF is reduced in patients with PR during systemic therapies. In these responders, there is an average of a –52% change in serum MPF after one treatment cycle, which is steadily decreased to –78% after six cycles. In MM, RECIST needs to be modified because one-dimensional measurement of the nonspherical growth pattern was difficult.24 The mRECIST requires repeated total tumor measurements on two occasions 4 weeks apart for PR determination.25 The quantitative changes of serum MPF may provide an alternative, low-cost, and nonradiative assessment of therapy response in patients with MM with elevated MPF. Thus, changes in serum MPF may assist the CT-based response assessment.
The data further indicate that the serum MPF test results are informative of patient outcome. We used a more stringent cutoff value of a –50% change in MPF to define the biomarker response compared with the previous reports17-21; this cutoff was supported by our correlative analysis of CT and MPF responses in patients with MM. Such a stringent cutoff correlates with the mRECIST criterion of a 30% reduction in the sum for all target lesions and thus leads to a significant reduction of false-positive reports during monitoring. Our study shows that there is an association between MPF response and clinical outcome. Patients with MM with MPF responses have better PFS and OS, which is at least comparable to those with CT responses, regardless of their baseline MPF levels. Thus, in addition to confirming the association between changes in MPF and patients’ response to systemic therapy,18 our results further showed that an MPF biomarker response is associated with improved PFS and OS.
As the cleavage product during mesothelin maturation with a short half-life in blood,22,26 serum MPF levels reflect the expression of target antigen mesothelin on tumor cells, without being bound by the therapeutic agents. In fact, in untreated mesothelioma patients, there is a strong correlation between MPF and soluble mesothelin.22,27 The MPF test could be valuable for the clinical development of mesothelin-targeted therapies and for monitoring targeted therapies against MM. The results show that the MPF test is feasible for continued disease assessment for up to 5 years in patients with refractory MM experiencing PR. The long-term follow-up study shows that tumor progression is accompanied with re-elevated MPF. Therefore, serum MPF could facilitate long-term disease monitoring and provide a noninvasive assessment of the drug-target mesothelin expression in MM at the time of disease progression, which can be critical for evaluating alternative therapies.
One of the most important issues in the development of targeted therapies is the stratification of the intended patient population. Mesothelin is ubiquitously detected in epithelioid MM by immunohistochemistry (Table 1); therefore, mesothelin immunohistochemistry is not predictive of response to mesothelin-targeted therapies in MM, and a different biomarker test is required. Currently, there is no companion diagnostic on the basis of a serum tumor antigen. There are many challenges for a serum-based test for patient selection, including the expression of the tumor antigen by nontumor cells and the variations in shedding of it by tumor cells. In addition, it is possible that serum MPF may be influenced by renal dysfunction in patients receiving nephrotoxic chemotherapies because prior reports showed elevated serum MPF in patients with renal disease.28,29 However, the patients described in our study had normal renal functions at the time of enrollment. Our study provides the first evidence that elevated baseline MPF may be associated with radiologic response of patients with refractory MM to an antimesothelin therapy. Although our previous data suggested that patients with MM with elevated MPF have a worse prognosis,22 the results here suggest that the reduction of serum MPF in patients with refractory MM is associated with improved clinical outcome. We speculate that the ability to normalize the elevated serum MPF in patients could be a key finding for the mesothelin-targeted therapy. Because many mesothelin-targeted agents are in clinical development, we believe that the association between elevated baseline MPF and clinical outcome need to be verified for mesothelioma.
There is an even stronger need to evaluate the use of elevated MPF for the development of antimesothelin agents in other cancer types, including lung, pancreas, and ovarian,6,7 where both the prevalence of MPF-positive patients and response rates are expected to be much lower.
In conclusion, the findings of this study suggest that an MPF assay may be effective in assessing treatment and in predicting clinical outcome in patients with epithelioid MM. Limitations of the study include its retrospective design and the small number of patients with MM in the outcome study. There is unique and exploratory evidence of an association between the elevated baseline serum MPF and clinical response to an antimesothelin agent, with a rationale for drug-mediated elimination of MPF-producing tumor cells, a finding that needs to be further investigated in a larger trial.
ACKNOWLEDGMENT
We are grateful to all patients and blood donors for participating in the clinical studies at the National Cancer Institute, Seth Steinberg and Yuxin Zhang for statistical analyses and advice, Betsy Morrow for assistance with samples and clinical data search, and Konrad Huppi for critique of the manuscript.
Appendix
Materials and Methods: MPF test
We developed the serum megakaryocyte potentiating factor (MPF) test and conducted comprehensive preclinical and clinical validation of the test (for details, see article). Briefly, the MPF assay uses capture antibody MPF49 (γ2a, κ), which binds to MPF topographic epitope 3, and detection antibody MPF25 (γ1, κ), which binds to epitope 1.26 MPF49 was biotinylated, and MPF25 was conjugated with Sulfo-Tag NHS-Ester (Meso-Scale Diagnostics, Rockville, MD). The assay was then optimized on a 96-well strepavidin plate.
All tests were performed according to our standard operating procedures under good laboratory practice. Briefly, the strepavidin assay plates were blocked with blocking buffer for 1 hour at room temperature with constant shaking. The biotinylated Capture Antibody Solution was added to the microplates. After incubation for 1 hour at room temperature, plates were washed, and the serially diluted MPF calibrator, 1:10 diluted sample serum, and reference samples were added and further incubated for 1 hour. After a wash step, the Sulfo-Tag Detection Antibody Solution was added and incubated for an additional hour. After washing, a 2× Read Buffer was added to the plates, which was read with a QuickPlex instrument (Meso-Scale Diagnostic) within 20 minutes. The data were analyzed with WorkBench 4.0 software (Meso-Scale Diagnostic) for determining the concentration of MPF in serum samples using the 4 Parameter Logistic nonlinear regression model. The MPF test was validated both analytically and clinically (for details, see article). A reference interval (0 to 1.2 ng/mL) was determined before this study, with higher levers outside of it considered elevated.
Fig A1.
Relative changes in megakaryocyte potentiating factor (MPF) in patients with malignant mesothelioma and normal baseline MPF (within the reference interval) and partial response. All results are shown as mean ± standard deviation. CT, computed tomography.
Fig A2.
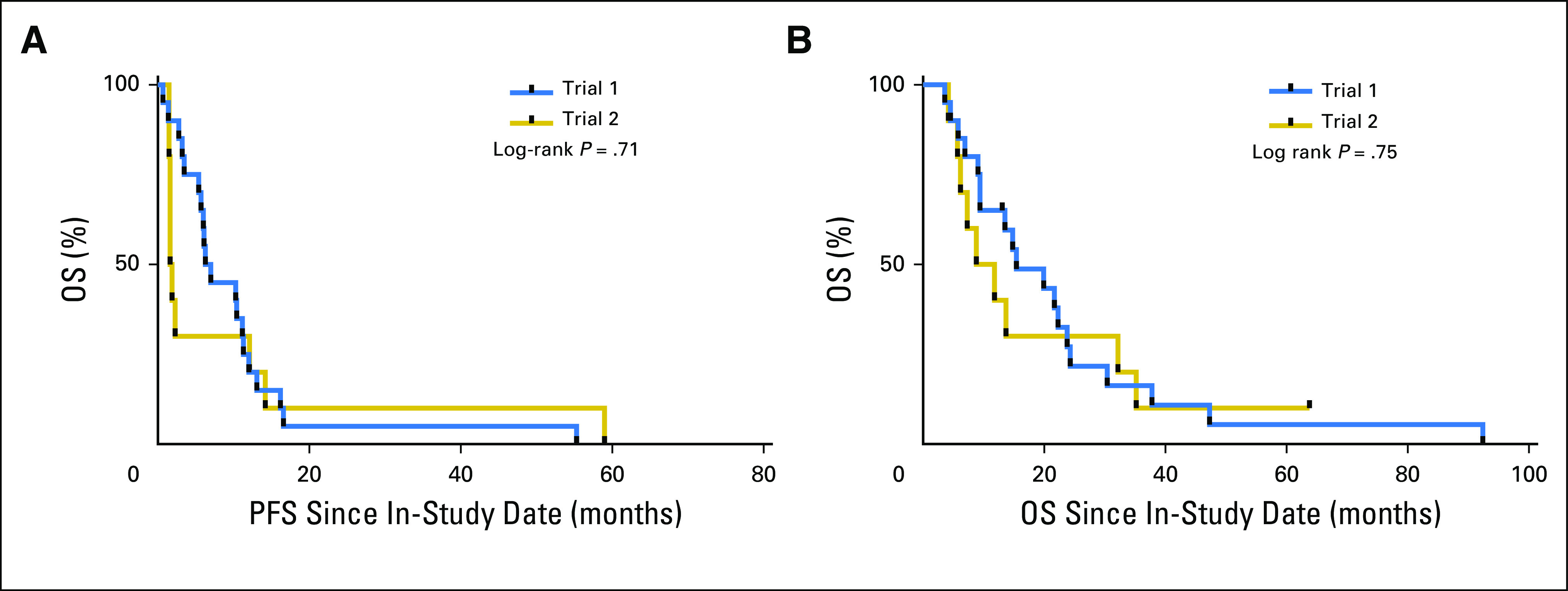
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) between the two trials. (A) PFS analysis of the two trials. (B) OS analysis of the two trials. Trial 1, treatment-naïve patients with malignant mesothelioma treated with pemetrexed and cisplatin, and the antimesothelin immunotoxin SS1P. Trial 2, patients with refractory malignant mesothelioma treated with pentostatin and cyclophosphamide, and SS1P.
Fig A3.
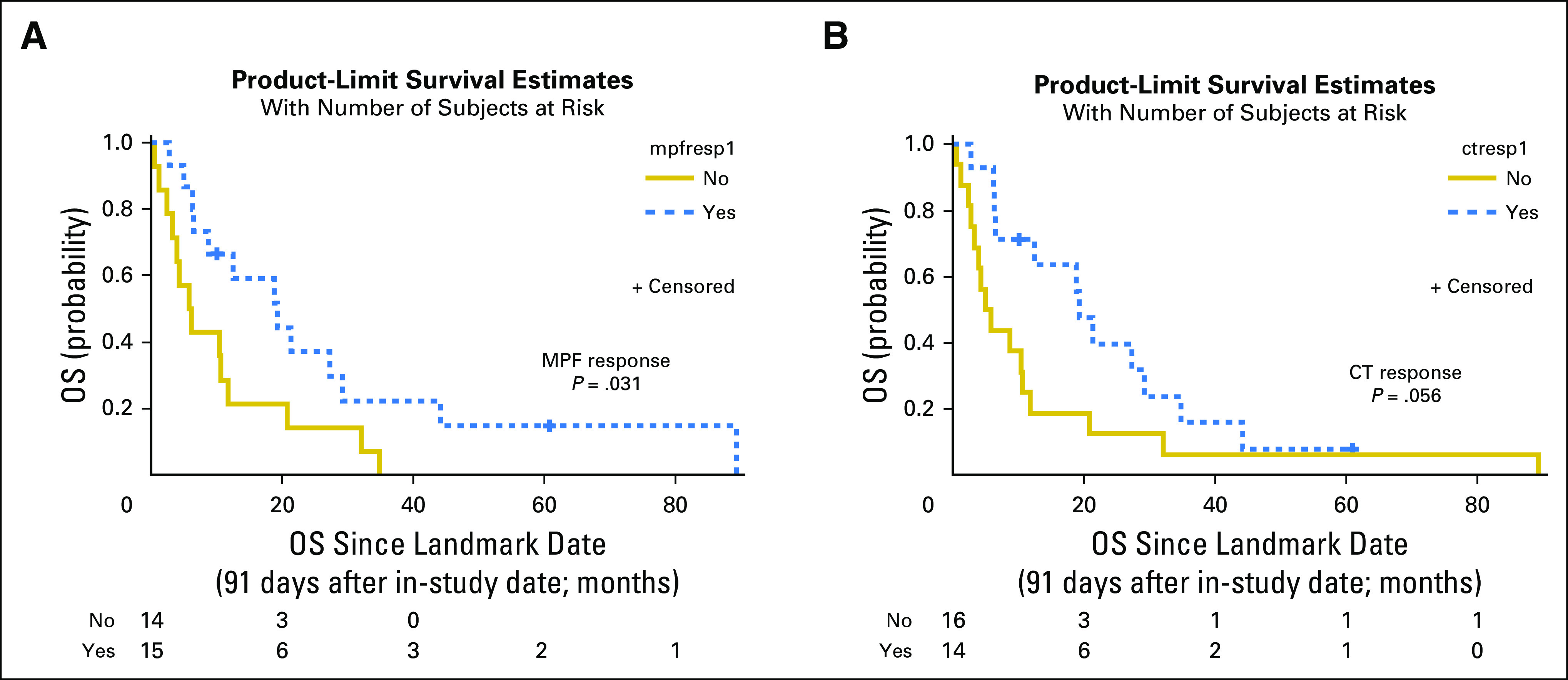
Landmark analysis of overall survival (OS) for megakaryocyte potentiating factor (MPF) and computed tomography (CT) responses. Because all but one responding patient experienced a response by 91 days, the analyses began at 91 days after the in-study date. (A) The median OS for MPF responders at the landmark date was 19.3 months (95% CI, 6.4 to 29.2 months), and for MPF nonresponders, it was 6.0 months (95% CI, 2.5 to 11.8 months). (B) The median OS starting at the landmark date for CT responders was 19.3 months (95% CI, 6.4 to 29.2 months) and was 5.4 months for CT nonresponders (95% CI, 2.7 to 10.6 months). ctresp, CT response; mpfrsp1, MPF response.
Fig A4.
Kaplan Meier analysis of overall survival (OS) for patients with refractory malignant mesothelioma receiving SS1P and immune suppression. A change of megakaryocyte potentiating factor (MPF) of more than −50% was used as the cutoff for the patients with refractory patients with malignant melanoma. Kaplan-Meier analysis showed patients with MPF response have better OS (P = .012), with an HR (response to nonresponse) of 0.234 (95% CI, 0.058 to 0.950).8
Footnotes
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
AUTHOR CONTRIBUTIONS
Conception and design: Liang Cao, Raffit Hassan
Provision of study materials or patients: Raffit Hassan, Ira Pastan
Collection and assembly of data: Liang Cao, Yunkai Yu, Anish Thomas, Jingli Zhang, Raffit Hassan
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Liang Cao
No relationship to disclose
Yunkai Yu
No relationship to disclose
Anish Thomas
No relationship to disclose
Jingli Zhang
No relationship to disclose
Masanori Onda
No relationship to disclose
Paul Meltzer
Research Funding: Genzyme (Inst)
Raffit Hassan
Research Funding: Aduro Biotech (Inst), Morphotek (Inst), Bayer (Inst)
Ira Pastan
No relationship to disclose
REFERENCES
- 1.Robinson BW, Lake RA: Advances in malignant mesothelioma. N Engl J Med 353:1591-1603, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. : Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636-2644, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Tsao AS, Wistuba I, Roth JA, et al. : Malignant pleural mesothelioma. J Clin Oncol 27:2081-2090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan R, Bera T, Pastan I: Mesothelin: A new target for immunotherapy. Clin Cancer Res 10:3937-3942, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Pastan I, Hassan R: Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 74:2907-2912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morello A, Sadelain M, Adusumilli PS: Mesothelin-targeted CARs: Driving T cells to solid tumors. Cancer Discov 6:133-146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan R, Thomas A, Alewine C, et al. : Mesothelin immunotherapy for cancer: Ready for prime time? J Clin Oncol 34:4171-4179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Miller AC, Sharon E, et al. : Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 5:208ra147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan R, Cohen SJ, Phillips M, et al. : Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 16:6132-6138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golfier S, Kopitz C, Kahnert A, et al. : Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 13:1537-1548, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Beatty GL, Haas AR, Maus MV, et al. : Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2:112-120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Brockstedt DG, Nir-Paz R, et al. : A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res 18:858-868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pass HI, Levin SM, Harbut MR, et al. : Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 367:1417-1427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pass HI, Lott D, Lonardo F, et al. : Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 353:1564-1573, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chang K, Pastan I: Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 93:136-140, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima T, Oh-eda M, Hattori K, et al. : Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem 270:21984-21990, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Wheatley-Price P, Yang B, Patsios D, et al. : Soluble mesothelin-related peptide and osteopontin as markers of response in malignant mesothelioma. J Clin Oncol 28:3316-3322, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hollevoet K, Nackaerts K, Gosselin R, et al. : Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol 6:1930-1937, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hassan R, Sharon E, Thomas A, et al. : Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 120:3311-3319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creaney J, Francis RJ, Dick IM, et al. : Serum soluble mesothelin concentrations in malignant pleural mesothelioma: Relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res 17:1181-1189, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hooper CE, Lyburn ID, Searle J, et al. : The South West Area Mesothelioma and Pemetrexed trial: A multicentre prospective observational study evaluating novel markers of chemotherapy response and prognostication. Br J Cancer 112:1175-1182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y RB, Thomas A, Morrow B, et al. : Elevated serum megakaryocyte potentiating factor as a predictor of poor survival in patients with mesothelioma and primary lung cancer. J Appl Lab Med 10.1373/jalm.2017.025015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson JR, Cain KC, Gelber RD: Analysis of survival by tumor response. J Clin Oncol 1:710-719, 1983 [DOI] [PubMed] [Google Scholar]
- 24.van Klaveren RJ, Aerts JG, de Bruin H, et al. : Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer 43:63-69, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Byrne MJ, Nowak AK: Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257-260, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Onda M, Nagata S, Ho M, et al. : Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res 12:4225-4231, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Iwahori K, Osaki T, Serada S, et al. : Megakaryocyte potentiating factor as a tumor marker of malignant pleural mesothelioma: Evaluation in comparison with mesothelin. Lung Cancer 62:45-54, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Somers EB, O’Shannessy DJ: Folate receptor alpha, mesothelin and megakaryocyte potentiating factor as potential serum markers of chronic kidney disease. Biomark Insights 9:29-37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creaney J, Sneddon S, Dick IM, et al. : Comparison of the diagnostic accuracy of the MSLN gene products, mesothelin and megakaryocyte potentiating factor, as biomarkers for mesothelioma in pleural effusions and serum. Dis Markers 35:119-127, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]