Abstract
Short-term plasticity enables synaptic strength to be dynamically regulated by input timing. Excitatory synapses arising from the same axon can have profoundly different presynaptic forms of short-term plasticity onto inhibitory and excitatory neurons. We previously showed that Schaffer collateral synapses onto most hippocampal CA1 stratum radiatum interneurons have less paired-pulse facilitation than synapses onto CA1 pyramidal cells, but little difference in steady-state short-term depression. However, less is known about how synapses onto interneurons respond to temporally complex patterns that occur in vivo. Here we compared Schaffer collateral synapses onto stratum radiatum interneurons and pyramidal cells in acute hippocampal slices in response to physiologically-derived spike trains. We find that synapses onto interneurons have less short-term facilitation than synapses onto pyramidal cells, and a subset expresses only short-term depression. Mathematical modeling predicts this target-cell specific short-term plasticity occurs through differences in initial release probability. All three groups have more short-term facilitation during physiologically-derived train stimulation than during constant-frequency stimulation at the same frequency, indicating that variability in stimulus timing is important. These target-cell specific differences in short-term plasticity reduce the strength of excitatory input onto interneurons relative to pyramidal cells, and of depression interneurons relative to facilitation interneurons, during high frequency portions of the train. This occurs to a similar extent at 25°C and at 33°C, and is even greater at physiological extracellular calcium. Target-cell specific differences in short-term plasticity enable synapses to have different temporal filtering characteristics, which may help to dynamically regulate the balance of inhibition and excitation in CA1.
Keywords: hippocampus, Schaffer collateral, short-term facilitation, short-term depression, short-term plasticity, synaptic dynamics
Introduction
Short-term plasticity refers to transient, activity-dependent changes in synaptic strength that occur on the time scale of milliseconds to tens of seconds (Zucker, 1999; Zucker and Regehr, 2002). Presynaptic forms of short-term plasticity are often target cell specific, in that synapses made by the same type of presynaptic axon onto distinct postsynaptic targets can have differences in short term plasticity (Pelkey and McBain, 2007; Éltes et al., 2017). Because CA1 interneurons and pyramidal cells receive the same excitatory input via Schaffer collateral axons of CA3 pyramidal cells (Freund and Buzsáki, 1996), differences in short-term plasticity will be important for regulating the relative strengths of Schaffer collateral input to these inhibitory and excitatory neurons. Multiple forms of both short-term facilitation and short-term depression exist that have different temporal characteristics (Zucker and Regehr, 2002; Lefort and Petersen, 2017), enabling synaptic strength to vary greatly as a function of input frequency. Even though it is likely to be important for determining cell output, relatively little is known how short-term plasticity affects the frequency-dependence of excitatory inputs to CA1 interneurons compared to pyramidal cells, particularly during complex input patterns such as these synapses receive in vivo.
We have previously shown that the strength and dynamics of Schaffer collateral synapses are target-cell specific and differ between Schaffer collateral synapses onto CA1 interneurons in stratum radiatum and CA1 pyramidal cells (Sun et al., 2005; Sun and Dobrunz, 2006). In addition, stratum radiatum interneurons express heterogeneity in the short-term plasticity of their Schaffer collateral inputs (Sun et al., 2005; Sun and Dobrunz, 2006; Li et al., 2017). The majority of stratum radiatum interneurons (approximately 85% in our previous study) had moderate paired pulse facilitation (Sun et al., 2005); we refer to these cells as facilitation interneurons. A small subset (approximately 15%) showed paired pulse depression (Sun et al., 2005; Li et al., 2017) and we classified these cells as depression interneurons. We found that short-term plasticity was very different between Schaffer collateral synapses onto pyramidal cells, facilitation interneurons, and depression interneurons in response to paired pulse and five pulse constant frequency stimulation (Sun et al., 2005). In contrast, steady-state high frequency depression in response to longer trains of constant frequency stimulation was almost identical between synapses onto pyramidal cells and facilitation interneurons, and only slightly greater at synapses onto depression interneurons (Sun et al., 2005). However, these simple stimulus patterns are not the types of input these synapses receive in vivo. In vivo patterns of action potential activity have been obtained from extracellular recordings of hippocampal place cells in awake, freely moving rats. These patterns are highly variable in their timing and contain a wide mixture of frequencies (Fenton and Muller, 1998). The effects of short-term plasticity are often nonlinear, making it difficult to predict the response of synapses to temporally complex patterns based on the responses to simple patterns.
Previous studies using physiologically derived input patterns have shown that short-term plasticity causes the strength of Schaffer collateral synapses onto CA1 pyramidal cells to be modulated over a wide dynamic range (Dobrunz and Stevens, 1999; Dekay et al., 2006; Klyachko and Stevens, 2006a). However, less is known about how Schaffer collateral synapses onto stratum radiatum interneurons respond to temporally complex stimulus patterns (Sun et al., 2009; Li et al., 2017), or what effects these target-cell specific differences in short-term plasticity have in regulating the frequency dependence and dynamic range over which Schaffer collateral synapses operate. Short-term plasticity has also been shown to be dependent upon recording conditions, including extracellular calcium concentration and temperature (Sippy et al., 2003; Watanabe et al., 2005; Klyachko and Stevens, 2006b; Schlüter et al., 2006). It is not known whether synapses onto interneurons and pyramidal cells respond similarly or differently to changes in calcium and temperature during complex stimulation patterns. Because stratum radiatum interneurons play very different roles from CA1 pyramidal cells in the hippocampal circuit, yet they receive the same excitatory input, the frequency dependence of their Schaffer collateral synapses is likely to be an important factor governing the balance between excitation and inhibition during physiologically relevant patterns of activation.
Here we measure short-term plasticity of Schaffer collateral synapses onto CA1 pyramidal cells and stratum radiatum interneurons in acute slices from juvenile rats, and compare their responses to a temporally complex spike train that is derived from in vivo recordings. We find that the target-cell specific differences in short-term plasticity that are seen in response to simple stimulus patterns also occur in response to physiologically-derived spike trains (PSTs). In addition, these experiments show that short-term facilitation of Schaffer collateral inputs to interneurons is smaller than that of synapses onto pyramidal cells. As a result, synapses onto pyramidal cells have a wider dynamic range. This causes a frequency-dependent decrease in the relative strength of the excitatory input to interneurons vs. pyramidal cells, and of input to depression interneurons vs. facilitation interneurons, during bursts of stimulation. The magnitude of this effect is similar at 25°C and at 33 °C, but is much greater at lower (more physiological) extracellular calcium. Our previous model of short-term plasticity showed that differences in the responses of Schaffer collateral synapses onto pyramidal cells, facilitation interneurons, and depression interneurons to simple input patterns could be accounted for by differences in the initial release probability. Here we extend this model to fit the responses to complex input patterns, and find that the differences in short-term plasticity between Schaffer collateral synapses onto pyramidal cells and interneurons can still be accounted for by differences in the initial release probability. Together, these results show that target-cell specific differences in short-term plasticity are important for dynamically regulating the relative strength of excitatory inputs onto inhibitory vs. excitatory neurons in CA1.
Experimental Procedures
Slice preparation
The University of Alabama at Birmingham Institutional Animal Care and Use Committee provided ethical approval for all experimental protocols performed. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the U.S. National Institute of Health. Acute 400 μm thick hippocampal slices were prepared from adolescent Long Evans rats (12–18 days old). Animals were deeply anesthetized by inhalation of the volatile anesthetic Halothane (2-Bromo-2-chloro-1,1,1-trifluoroethane, 0.2 – 0.4 ml in a 2L container) and then decapitated using a guillotine. Slices were prepared using previously published methods (Sun et al., 2005).
Electrophysiology
Slices were placed in a submersion recording chamber and perfused with external recording solution containing (in mM): NaCl, 120; KCl, 3.5; MgCl2, 1.3; NaH2PO4, 1.25; NaHCO3, 26; and glucose, 10. The solution contained 2.5 mM CaCl2, except in Figure 5A-E, where it contained 1.0 mM CaCl2. Carbogen (95% O2/5% CO2) was used to bubbled the solution and maintain the pH between 7.35 to 7.45. Inhibitory synaptic responses mediated by GABAA receptors was blocked with the addition of 100 μM picrotoxin. Additionally, 100 μM APV (D-2-amino-5-phosphonopentanoic acid) was added to prevent postsynaptic short-term plasticity through NMDA receptors and prevent long-term potentiation (LTP) and long-term depression (LTD). During slicing, the CA3 region of hippocampus was removed to prevent recurrent excitation. Most experiments were performed between 24 °C and 25 °C; except experiments in Figures 4-5 that were between 32 °C and 33 °C. Picrotoxin was obtained from Tocris Bioscience (Ellisville, MO). All other chemicals were obtained from Fischer Scientific (Hampton, NH) or Sigma-Aldrich Corp. (St. Louis, MO).
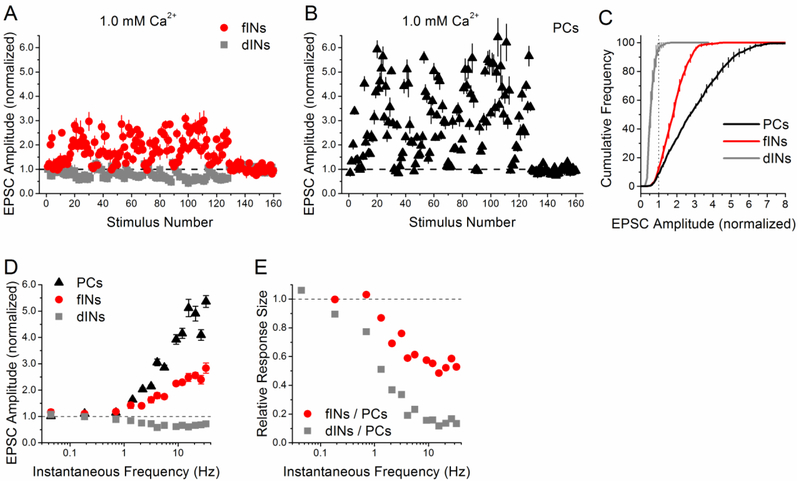
Figure 5. Differences in between interneuron and pyramidal cell responses to the PST are even greater at lower calcium.
A-E) Responses at 33 °C and 1.0 mM [Ca2+]. Group data for facilitation interneurons (n=5 cells), depression interneurons (n=3 cells), and pyramidal cells (n=5 cells). A, B) Normalized EPSC amplitude vs. stimulus number during the PST (first 128 points), normalized by the average amplitude during the end of the control period (0.1 Hz constant frequency, last 32 points). C) Cumulative histograms of the normalized EPSC amplitudes during the PST. Dotted vertical line at 1 indicates separation between facilitation and depression. D) Normalized EPSC amplitude vs. instantaneous frequency. E) Ratio of PST responses from facilitation interneurons vs. pyramidal cells and depression interneurons vs. pyramidal cells, shown as a function of instantaneous frequency.
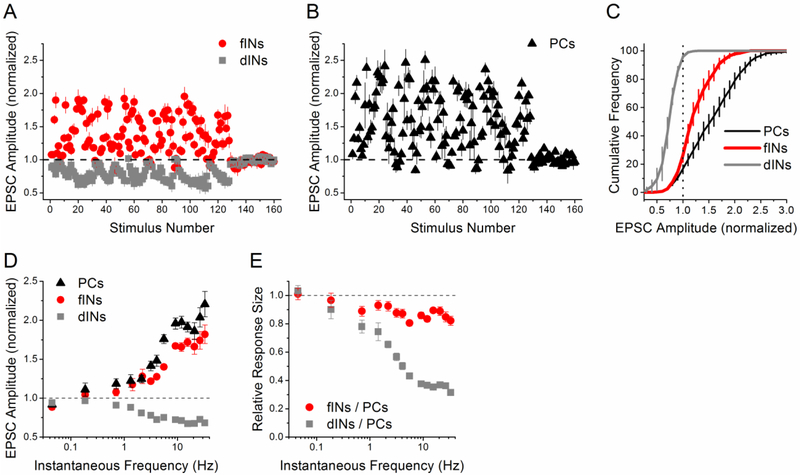
Figure 4. Differences in between interneuron and pyramidal cell responses to the PST are also found at warmer temperature.
A-E) Responses at 33 °C and 2.5 mM [Ca2+]. Group data for facilitation interneurons (n=6 cells), depression interneurons (n=3 cells), and pyramidal cells (n=5 cells). A, B) Normalized EPSC amplitude vs. stimulus number during the PST (first 128 points), normalized by the average amplitude during the end of the control period (0.1 Hz constant frequency, last 32 points). C) Cumulative histograms of the normalized EPSC amplitudes during the PST. Dotted vertical line at 1 indicates separation between facilitation and depression. D) Normalized EPSC amplitude vs. instantaneous frequency. E) Ratio of PST responses from facilitation interneurons vs. pyramidal cells and depression interneurons vs. pyramidal cells, shown as a function of instantaneous frequency.
Visually identified pyramidal cells in stratum pyramidale of CA1 and interneurons in stratum radiatum of CA1 were recording using previously described methods (Sun et al., 2005). Voltage-clamp recordings were made in at a holding potential at −60 mV. Patch electrodes (3 – 4.5 MΩ) were filled with internal solution containing (in mM): Cs-Gluconate, 100; EGTA, 0.6; MgCl2, 5.0; HEPES, 10; pH was adjusted to 7.2 with CsOH. In order to prevent interneuron LTP and LTD (Laezza et al., 1999) and postsynaptic short-term plasticity, the internal solution also contained 10 mM BAPTA; QX-314 (5 mM) was used to improve space clamp; 10 mM ATP was used to chelate intracellular polyamines and prevent possible postsynaptic short-term plasticity at calcium-permeable AMPA receptors (Bähring et al., 1997). In a subset of experiments, 0.5% biocytin was added to the internal to enable post hoc morphological analysis of neurons recorded.
Excitatory postsynaptic currents (EPSCs) were generated in response to extracellular stimulation of Schaffer collateral axons. Stimulation was obtained from a Master-8-cp stimulator (A.M.P.I, Jerusalem, Israel) and applied with a BSI-2 biphasic stimulus isolator (BAK Electronics, Mount Airy, MD) through a bipolar tungsten microelectrode (FHC, Bowdoinham, ME) that was placed in stratum radiatum. The strength of stimulation was adjusted (10 to 50 μA for a 100 μs pulse) to produce a single peak EPSC with fixed latency.
Physiologically-derived Spike Train
The methods for the Physiologically-derived Spike Train (PST) experiments are described in deKay et al (2006) and Dobrunz and Stevens (1999). The PST was derived from the timing of in vivo action potentials of hippocampal place cells recorded in awake and freely moving rats. In vivo timing patterns are courtesy of Dr. Robert Muller; additional details of recording methods are in Fenton and Muller (1998). The applied stimulus train contained 128 points (the PST) followed by 32 points at 0.1 Hz constant frequency (the control period) for normalization. Each repetition (PST + control period) takes over 9 minutes to complete. The interstimulus intervals in the PST vary over 3 orders of magnitude, from 30 ms to greater than 30 seconds. The median interstimulus interval is 228 ms, corresponding to a frequency of 4.4 Hz, which is much higher than the average frequency of the entire pattern (0.52 Hz). The majority of the interstimulus intervals (77%) are between 30-500 ms.
For each cell, EPSCs were averaged across 3-5 repetitions of the pattern for each point in the PST pattern, and normalized by the mean response size at the end of the control period (0.1 Hz). This controls for possible variability in the initial EPSC size; the normalized responses therefore represent short-term plasticity. In order to visualize the full pattern, the normalized EPSC amplitudes are plotted versus stimulus number because the stimuli come in clusters separated by long timing intervals, making it difficult to see individual responses when plotted against time (Dobrunz and Stevens, 1999). Group data for each cell type are obtained from individual cells and presented as mean ± SE for each point within the pattern, with n numbers indicating numbers of cells.
For plots of normalized EPSC amplitude vs. frequency in response to the PST, responses were binned by frequency for each individual cell, then averaged for each group. Responses are shown mean ± standard error. For plots of relative response sizes (ratio of two cell types) vs. frequency, the average responses for each cell group are binned by frequency and the ratio is computed for each frequency.
Histology
In a subset of experiments, slices that contained biocytin filled neurons were stored overnight in a solution of 4% paraformaldehyde made in 0.1 M phosphate buffer. Visualization of labelled neurons was obtained through an avidin–HRP reaction followed by a peroxidase reaction requiring the use of diaminobenzidine (DAB). Briefly, the slices were incubated in ABC complex (Elite Vectastain ABC Kit, Vector Laboratories, Inc. Burlingame, CA, USA) for 4 hours, rinsed with phosphate buffer, and then transferred to DAB (Peroxidase Substrate Kit, Vector Laboratories, Inc.). The reaction was stopped after 1–5 min by washing the slices twice in phosphate buffer or water, dehydrated, and mounted on microscope slides. The slices were examined under a microscope using a 40x objective and images were taken of biocytin filled cells. Neurolucida software (MBF Bioscience, Williston, VT, USA) was used to trace the cells.
Statistical analysis
Data are presented as mean ± standard error (s.e.). One-way ANOVA is used for statistical comparisons, with P < 0.05 considered significant.
Mathematical Modeling
We have previously used a mathematical description of short-term plasticity that we developed (Sun et al, 2005) to fit the data in response to simple stimulus patterns (Sun et al., 2005; Walters et al., 2014). Here we extended that model by adding a second, slower component of facilitation. To do this, we incorporated changes to several of kinetic equations as described below. All of the other kinetic equations are the same. See Table 1 for definitions of the terms in the equations.
Table I.
Model parameters held constant in all simulations
| Symbol | Definition | Value | Unit |
|---|---|---|---|
| τin | Time constant for entry into refractory state | 3* | ms |
| k0 | Baseline recovery rate from the refractory state | 2* | s−1 |
| kmax | Maximum recovery rate from the refractory state | 30* | s−1 |
| KD | Dissociation constant of CaXD | 2* | N/A |
| ΔD | Incremental increase in CaXD after a stimulus | 12.8 | (normalized) |
| KF | Dissociation constant of CaXF | 4 | N/A |
Parameters held constant in the model simulations for all cell types and stimulus protocols.
Parameters are the same as used in our previous paper Sun et al. 2005.
Previously, facilitation had one component, which was used to modify the release probability per vesicle α(t) as follows:
We modified this to include a second component of facilitation which is smaller and longer lasting, corresponding to the form of short-term facilitation called augmentation (Stevens and Wesseling, 1999; Zucker and Regehr, 2002). The resulting equations are:
The equation for release probability per vesicle remained the same:
This adds two additional parameters to the model, τF2 and ΔF2.
All model fitting was done by minimizing the sum of the least squares error between the model and the data. The model was initially fit to the pyramidal cell group data in Figure 2. In addition to α1 and nT, the model parameters ΔD, τD, ΔF1, τF1, ΔF2, τF2, and R were allowed to change during the initial optimization of the model to fit the pyramidal cell group data. For model fitting to facilitation interneuron and depression interneuron group data, all parameter values were the same as those in the simulation of pyramidal cell group data except α1 and nT, which were allowed to vary in order to fit the data. The model was also fit to each individual experimental data set for all three cell groups with only α1 and nT allowed to vary; the average parameters are given in Figure 3G-I along with the parameter values from the fits to the group data.
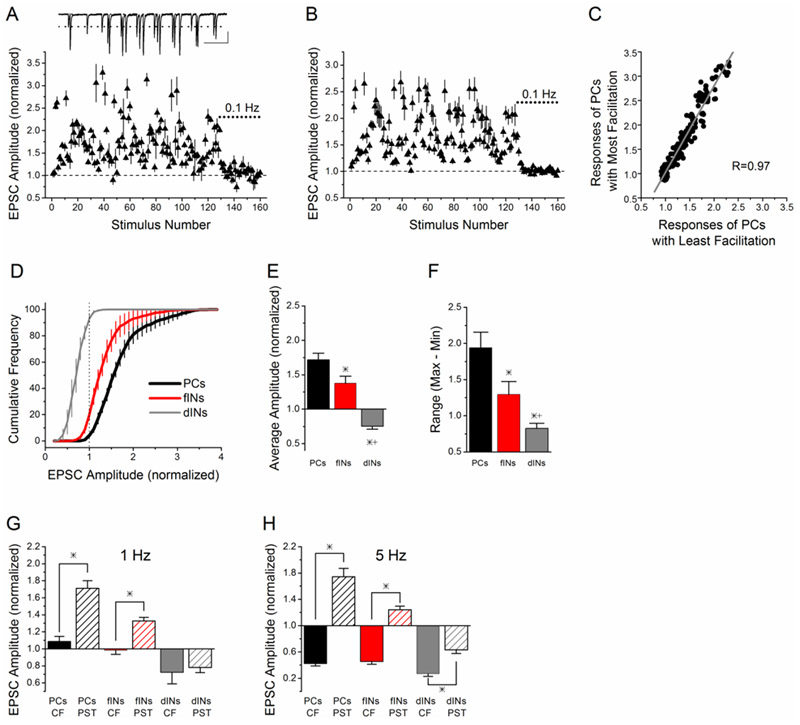
Figure 2. Schaffer collateral synapses onto interneurons have less facilitation and narrower dynamic range compared to pyramidal cells.
A) Example of short-term plasticity in response to stimulation with a PST of Schaffer collateral synapses onto a CA1 pyramidal cell. Normalized EPSC amplitude vs. stimulus number during the PST, as in Figure 1. Inset: example trace of EPSCs recorded a burst of the PST. Scale bars: 500 ms, 35 pA. B) Group data (mean ± s.e.) for pyramidal cells (n=8 cells). C) Point-by-point comparison of the average responses of the 4 pyramidal cells with most facilitation to the 4 pyramidal cells with least facilitation shows strong correlation. D) The cumulative histograms (mean ± s.e.) of the normalized EPSC amplitudes show differences in the frequency of responses with short-term facilitation (>1.0) and short-term depression (<1.0) between facilitation interneurons (n=9 cells), depression interneurons (n=4 cells), and pyramidal cells (n=8 cells). Dotted vertical line at 1 indicates separation between facilitation and depression. E) The average amplitude of the responses to the 128 point PST is different between the three cell groups (p<0.05). F) The dynamic range over which the synapses operate (Max-Min, mean ± s.e.) is smaller at Schaffer collateral synapses onto both facilitation interneurons and depression interneurons, and different between all three groups (p<0.05). G) Average amplitude (normalized to control value at 0.1 Hz constant frequency) for PST responses for stimuli 1-31 of the pattern (average frequency 1.1 Hz, hatched bars) vs. constant frequency stimulation at 1 Hz (solid bars). H) Average amplitude (normalized to control value at 0.1 Hz constant frequency) for PST responses for stimuli 91-113 of the pattern (average frequency 5 Hz, hatched bars) vs. constant frequency stimulation at 5 Hz (solid bars).
For the warm temperature data (33 °C) at 2.5 mM calcium, the model was initially fit to the pyramidal cell group data, with τD, τF1, τF2, and R allowed to vary, in addition to α1 and nT. The model was then fit to the facilitation interneuron group data, depression interneuron group data, and all of the individual data sets, with only α1 and nT as variable. For the warm temperature data at 1.0 mM calcium, the model was initially fit to the pyramidal cell group data, with parameter values for τD, τF1, τF2, and R as determined for the warm temperature fits at 2.5 mM calcium, and parameter values for ΔF and ΔF2 allowed to vary, in addition to α1 and nT. The model was then fit to the facilitation interneuron group data, depression interneuron group data, and all of the individual data sets, with only α1 and nT as variable.
Parameter values that were held constant between all three cell groups are given in Table 1. Parameter values that were different at the two temperatures are given in Table II. Parameter values that were different at the two calcium concentrations are given in Table III. Parameter values that were variable between the three cell groups in all simulations are given in Table IV.
Table II.
Model parameters changed in simulations at 33 °C
| Symbol | Value at 25 °C |
Value at 33 °C |
Unit | |
|---|---|---|---|---|
| R | Refilling rate of readily releasable vesicle pool | 0.300 | 0.322 | s−1 |
| τD | Decay time constant of CaXD after a stimulus | 15.9 | 23.5 | ms |
| τF1 | Decay constant of CaXF1 | 130.0 | 129.1 | ms |
| τF2 | Decay constant of CaXF2 | 10,700 | 5,891 | ms |
Table III.
Model parameters changed in simulations at 1.0 mM Ca2+
| Symbol | Value at 2.5 mM |
Value at 1.0 mM |
Unit | |
|---|---|---|---|---|
| ΔF1 | Incremental increase in CaXF1 after a stimulus | 0.600 | .0227 | (normalized) |
| ΔF2 | Incremental increase in CaXF2 after a stimulus | 0.0218 | .00104 | (normalized) |
Table IV.
Model parameters that varied between cell groups
| Temperature | 25 °C | 33 °C | 33 °C | ||
|---|---|---|---|---|---|
| [Ca2+] | 2.5 mM | 2.5 mM | 1.0 mM | ||
| α1 | Pyramidal cells |
Group fit | 0.0311 | 0.037 | 0.00183 |
| Individual fits | 0.0316 ±0.0023 | 0.0374 ± 0.0043 | 0.00182 ± 0.00011 | ||
| Facilitation interneurons |
Group fit | 0.0400 | 0.0430 | 0.00422 | |
| Individual fits | 0.0420 ± 0.0041* | 0.0433 ± 0.0016 | 0.0043 ± 0.00027* | ||
| Depression interneurons |
Group fit | 0.141 | 0.151 | 0.0723 | |
| Individual fits | 0.136 ±0.018*+ | 0.177 ± 0.031*+ | 0.0936 ± 0.023*+ | ||
| nT | Pyramidal cells |
Group fit | 6.41 | 6.30 | 6.50 |
| Individual fits | 6.36 ±0.73 | 6.57 ± 1.25 | 6.46 ± 1.00 | ||
| Facilitation interneurons |
Group fit | 8.67 | 8.01 | 7.36 | |
| Individual fits | 8.89 ±0.91 | 8.04 ±0.35 | 7.87 ±0.83 | ||
| Depression interneurons |
Group fit | 6.70 | 7.52 | 7.01 | |
| Individual fits | 8.10 ± 1.42 | 7.26 ± 1.23 | 5.77 ±0.76 | ||
Significantly different from pyramidal cells.
Significantly different from facilitation interneurons.
Model goodness-of-fit was assessed by two measures, Pearson’s R and RMSSD (root mean squared scaled deviation) (Schunn and Wallach, 2005). Pearson’s R measures the correlation between model fits and experimental data, with values closest to 1 indicating best correlation. Because the model fit can be correlated with the data without being at the same location as the data (i.e. if the model result for each point was twice the experimental data value), we also used RMSSD. RMSSD is a scale-invariant measure of how much the model results diverge from the exact values of the experimental data points, with lower numbers indicating better fits. The deviations are scaled relative to the standard error of the experimental data; RMSSD is therefore scale invariant, which facilitates comparisons between fits to data sets of different magnitude and range. For example, a value of 1.5 indicates that the average deviation of the model from the data values is 1.5 standard error units. RMSSD is defined as
where i is the stimulus number, mi is the model result for stimulus i, di is the data mean for stimulus i, si is the standard deviation for each data i, ni is the number of cells, and k is the total number of stimuli.
Results
Spiking patterns obtained from in vivo recordings, which have a high degree of temporal complexity, have been shown to be useful in studying the complex interplay of multiple forms of short-term plasticity in vitro (Dobrunz and Stevens, 1999; Frerking et al., 2005; Dekay et al., 2006; Frerking and Ohliger-Frerking, 2006; Klyachko and Stevens, 2006a; Speed and Dobrunz, 2008, 2009; Li et al., 2017). We refer to these patterns as Physiologically-derived Spike Trains (PSTs) because they come from recordings in intact animals (under normal physiological conditions); these patterns provide input that is more representative of the temporally complex input patterns that occur at hippocampal synapses in vivo. The timing of these patterns is very irregular (Dobrunz and Stevens, 1999); they consist of bursts of stimuli at short interstimulus intervals separated by long periods with little or no activity. In addition, the stimulus timing is also variable within the bursts. Here we compared the responses of Schaffer collateral synapses onto CA1 stratum radiatum interneurons and pyramidal cells in response to a PST.
Short-term plasticity is target-cell specific during the Physiologically-derived Spike Train
Interneurons in stratum radiatum of CA1 have differences in short-term plasticity of their Schaffer collateral inputs in response to the PST. While the majority of interneurons have almost exclusively short-term facilitation, a subset has primarily short-term depression. Figure 1 shows examples of the responses from an interneuron with short-term facilitation (Figure 1A), and one with short-term depression (Figure 1B). The amplitudes are shown as mean ± s.e. for 5 repetitions of the pattern, and normalized by the average response of the control period (0.1 Hz) applied between repetitions. While both cells show variability in the responses during the PST, the patterns of responses are clearly different (facilitation vs. depression).
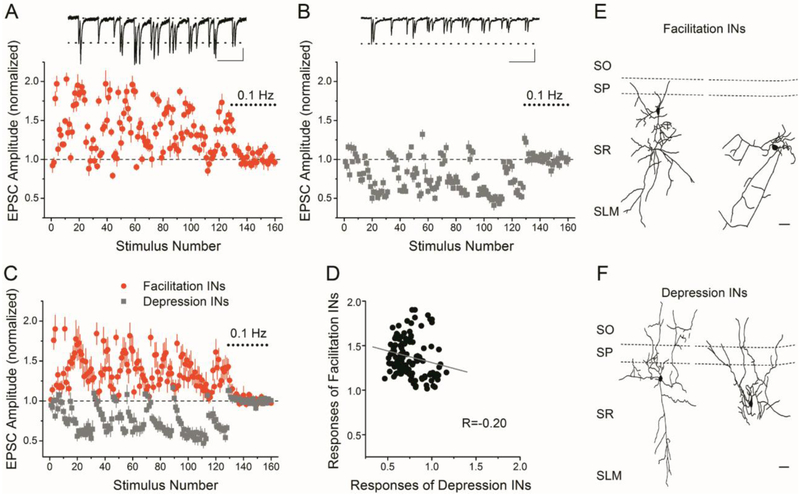
Figure 1. Stratum radiatum interneurons are heterogeneous with respect to the short-term plasticity of their Schaffer collateral synapses in response to a PST.
A and B) Example of short-term plasticity in response to stimulation with a PST of Schaffer collateral synapses onto an stratum radiatum interneuron that shows short-term facilitation (A) and an stratum radiatum interneuron that shows short-term depression (B). Normalized EPSC amplitude vs. stimulus number during the PST (first 128 points), normalized by the average amplitude during the end of the control period (0.1 Hz constant frequency, last 32 points, dotted bar). Responses are shown as mean ± s.e. for 5 repetitions of the pattern. Insets show example EPSC traces recorded during a 3.5 second burst of the PST. Scale bars: A: 500 ms, 30 pA; B: 500 ms, 20 pA. C) Group data (mean ± s.e.) for facilitation interneurons (n=9 cells) and depression interneurons (n=5 cells). D) Point-by point comparison of the responses of facilitation interneurons vs. depression interneurons shows poor correlation. E, F) Morphological reconstruction of two facilitation interneurons (E) and two depression interneurons (F). Scale bars: 100 μm.
Other cells tested showed responses similar to one of these two patterns, with the majority of interneurons having short-term facilitation. We therefore divided the interneurons into two groups for further analysis. These are functional groupings based on the short-term plasticity of their Schaffer collateral synapses during the PST; there may be heterogeneity of morphological and/or neurochemical interneuron subtypes within each group. Of the 14 stratum radiatum interneurons recorded, 9 had facilitation patterns (facilitation interneurons) and 5 had depression patterns (depression interneurons). While there was variability in the magnitude of facilitation or depression within each group, the classification of cells into the facilitation interneuron group or depression interneuron group was unambiguous. The same grouping was made, with 9 facilitation interneurons (average amplitude > 1.10), 5 depression interneurons (average amplitude< 0.90), whether the criteria was based on the overall average amplitude (all 128 points), or the average amplitude for a subset of high frequency points (e.g. interstimulus intervals ≤ 30 ms, 5 points). Figure 1C shows the average responses (mean ± s.e.) for the facilitation interneurons (n=9) and depression interneurons (n=5). The responses were similar from cell-to-cell within each group, as indicated by the small error bars in Figure 1C. However, it is clear that the pattern of responses are different between cell groups. This is also illustrated by the poor correlation of the responses when plotted against each other (Figure 1D, R=−0.20). Figures 1E and 1F show examples of the morphology of facilitation interneurons and depression interneurons. The morphology of the cells was variable within each group and did not appear to differ between facilitation interneuron and depression interneurons.
Schaffer collateral synapses onto interneurons have less facilitation and narrower range of responses than Schaffer collateral synapses onto pyramidal cells
We next compared the responses of Schaffer collateral onto stratum radiatum interneurons with the responses of Schaffer collateral synapses onto CA1 pyramidal cells (pyramidal cells). In contrast to the heterogeneity observed among interneurons, all CA1 pyramidal cells (pyramidal cells) tested showed a similar pattern of short-term facilitation in response to the same PST (Figure 2A single example, Figure 2B, group results, n=8). To verify this, we compared the responses of the 4 pyramidal cells with the greatest facilitation to the 4 pyramidal cells with least facilitation and found that the responses were highly correlated (R=0.97, Figure 2C). We therefore considered all pyramidal cells in a single group for further analysis. The responses of pyramidal cells were correlated with those of facilitation interneurons (R=0.92), but showed little correlation with those of depression interneurons (R=−0.36), when responses from the different cell types were plotted against each other (data not shown).
Schaffer collateral synapses onto both groups of interneurons had less short-term facilitation than Schaffer collateral synapses onto pyramidal cells (Figure 1C, Figure 2B). This was also observed in response to other PSTs (data not shown), indicating the effect is not limited to a specific pattern. We quantified the effect in Figure 2D, which shows the cumulative frequency plots of EPSC amplitudes during the PST for the three different cell types; the cumulative frequency distribution was calculated individually for each cell and then averaged for each group. For depression interneurons, nearly 100% of the stimuli during the PST caused short-term depression (normalized amplitudes < 1). In contrast, almost all stimuli of the PST caused short-term facilitation in pyramidal cells, and some responses were as much as 3-fold larger than the control (0.1 Hz constant frequency) responses. For facilitation interneurons, the majority of stimuli (around 80%) caused facilitation, but the magnitude of the facilitation was less than that in pyramidal cells, as seen by the leftward shift of the cumulative frequency plot. As a result, Schaffer collateral synapses onto both facilitation interneurons and depression interneurons had lower average amplitudes (across the whole PST) than Schaffer collateral synapses onto pyramidal cells (Figure 2E, n=9 facilitation interneurons, n= 5 depression interneurons, n=8 pyramidal cells, p<0.05). In addition, Schaffer collateral synapses onto facilitation interneurons and depression interneurons operate over narrower dynamic ranges than Schaffer collateral synapses onto pyramidal cells (Figure 2D, 2F). Figure 2F shows the average range (maximum – minimum) of responses during the PST, which was calculated individually for each cell and then averaged for each group. This indicates that the smaller dynamic range for interneurons is a property of the Schaffer collateral synapses onto individual interneurons, and not a result of averaging together the responses from heterogeneous populations of cells.
The responses of Schaffer collateral synapses onto both types of interneurons had low trial-to-trial variability when the same pattern was repeated within the same cell (e.g. Figure 1A, Figure 1B). This was also true for Schaffer collateral synapses onto pyramidal cells, as has previously been shown (Dobrunz and Stevens, 1999; Dekay et al., 2006). We quantified this by computing the trial-to-trial coefficient of variation (CV = standard deviation/mean) for each stimulus and averaging these values for all points in the PST. The average trial-to-trial CV is not different among the three cell groups (0.18 ± 0.01, n= 9 facilitation interneurons; 0.16 ± 0.03, n=5 depression interneurons; 0.14 ± 0.01, n=8 pyramidal cells, P>0.05). This indicates that Schaffer collateral synapses onto interneurons, like those onto pyramidal cells, are able to modulate their synaptic strength with high precision in response to temporally complex inputs.
Variability in the stimulus timing is important
We next compared the PST responses to data from constant frequency stimulation at the same (or very similar) frequency, in order to investigate the importance of temporal variability on short-term plasticity at Schaffer collateral synapses. Figure 2G compares the average response amplitudes from the first 31 points of the PST, for which the average frequency was 1.18 Hz, with data recorded during 1 Hz constant frequency stimulation, taken from our previous study (Sun et al., 2005). Stimulation with the PST resulted in a higher average response for both Schaffer collateral synapses onto pyramidal cells and facilitation interneurons (P<0.05). Figure 2H compares the average amplitude of the responses from the PST for stimuli 91-113, which had an average frequency of 5.04 Hz, with the responses to constant frequency stimulation at 5 Hz, taken from our previous study (Sun et al., 2005). The average amplitude during this section of the PST was significantly greater for all cell types. This is very different from the steady-state responses to constant frequency stimulation over the same frequency range, in which pyramidal cells and facilitation interneurons showed similar levels of short-term depression. This indicates that the temporal variability is important in determining the overall amount of short-term facilitation, and thus governing the average strength of the excitatory input onto both pyramidal cells and feed-forward interneurons.
Frequency-dependence of responses differs between interneurons and pyramidal cells
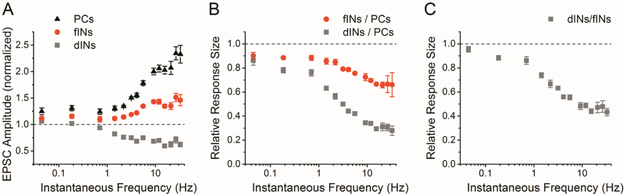
To investigate the frequency dependence of the cell type specific differences in short-term plasticity, we examined the short-term plasticity as a function of instantaneous frequency (the reciprocal of the interstimulus interval). Figure 3A shows response amplitudes plotted against frequency for pyramidal cells, facilitation interneurons and depression interneurons. Responses are binned and averaged based on frequency, and shown as mean ± s.e. There were differences in the relationship between the response amplitude (amount of short-term facilitation or depression) and the input frequency for the three cell groups. The responses of pyramidal cells and facilitation interneurons had a similar shape, in that facilitation increased linearly between about 1-10 Hz, and at higher frequencies was largely independent of frequency, although the maximum facilitation is greater for pyramidal cells. The frequency response of Schaffer collateral synapses onto depression interneurons during the PST is the mirror image to that of pyramidal cells and facilitation interneurons, in that the amount of short-term depression increases with frequency between about 1-5 Hz and then levels out at higher frequencies (~65% of control).
Figure 3. Relative strength of Schaffer collateral inputs to interneurons decreases during high frequency stimuli of PST.
A) Normalized EPSC amplitude vs. instantaneous frequency shows different relationships for pyramidal cells, facilitation interneurons, and depression interneurons. B) Ratio of PST responses from facilitation interneurons vs. pyramidal cells and depression interneurons vs. pyramidal cells, shown as a function of instantaneous frequency. C) Ratio of PST responses from depression interneurons vs. facilitation interneurons, shown as a function of instantaneous frequency.
These target-cell specific differences in short-term plasticity of Schaffer collateral synapses will result in history-dependent changes in the relative strengths of the excitatory input onto interneurons vs. pyramidal cells during temporally complex stimulation such as the PST. To investigate the frequency-dependence of this effect, we calculated the ratio of the responses of the different cell types during the PST. Figure 3B shows the ratio of interneuron responses to pyramidal cell responses; results are binned and averaged based on frequency, and shown as mean ± s.e. Because Schaffer collateral synapses onto facilitation interneurons have less short-term facilitation than those onto pyramidal cells at all frequencies, all of the relative responses are less than 1.0. This indicates that the relative synaptic strength of Schaffer collateral synapses onto facilitation interneurons is reduced compared to Schaffer collateral synapses onto pyramidal cells during the PST. The effect is dependent upon the frequency of inputs, with more reduction occurring at higher frequencies. This also occurs for Schaffer collateral synapses onto depression interneurons vs. pyramidal cells, and the magnitude of the effect is larger. For frequencies higher than 5Hz (which comprise 46% of the stimuli in the pattern), the average size of the facilitation interneuron/pyramidal cell response is 69% ± 2 %, while the average size of the depression interneuron/pyramidal cell response is 33% ± 1%.
The relative strengths of the Schaffer collateral input to facilitation interneurons compared to depression interneurons also change dynamically during the PST, as shown in Figure 3C. For frequency higher than 5 Hz, the relative response of Schaffer collateral synapses onto depression interneurons is 48% ± 1% of the response of Schaffer collateral synapses onto facilitation interneurons. The ratio of responses of interneurons to pyramidal cells, and between the two types of interneurons, is insensitive to changes in frequency at higher frequencies (above ~10 Hz), and also at very low frequencies (below ~0.8 Hz) where little or no short-term plasticity occurs. This shows that the target-cell specific differences in short-term plasticity result in a large reduction in the relative strength of the Schaffer collateral input to interneurons vs. pyramidal cells, and depression interneurons vs. facilitation interneurons, during the high frequency portions of the PST.
Target-cell specific differences in short-term plasticity are enhanced at near physiological conditions
These results were obtained at 24-25 °C, and a higher extracellular calcium concentration (2.5 mM) than the physiological level in vivo (1 to 1.5 mM) (Silver and Erecińska, 1990; Jeong et al., 2006). We next tested whether these target-cell specific differences in the responses to the PST also are seen at more physiological conditions. Figures 4A-4E show the results for recordings done at higher temperature (32-33 °C) and the same Ca2+ concentration (2.5 mM). Interneurons were again divided into facilitation and depression interneuron groups, and the results were very similar to those obtained at room temperature (n=5 pyramidal cells, n=6 facilitation interneurons, n=4 depression interneurons). At high frequencies the average of the facilitation interneuron/pyramidal cell responses decreased to ~75%, and the average of the depression interneuron/pyramidal cell responses decreased to ~40% (Figure 4E). This indicates that recording at closer to physiological temperature did not alter our findings and had only minor effects on the magnitude of the effects.
Lastly, we tested the effects of reducing extracellular calcium to a more physiological level (1.0 mM), keeping the temperature between 32 °C to 33 °C; the results are shown in Figures 5A-5E (n=5 pyramidal cells, n=5 facilitation interneurons, n=3 depression interneurons). The basic findings are again the same, although the magnitude of the differences between the three groups is greater. The difference in the amount of short-term facilitation between pyramidal cells and facilitation interneurons is markedly enhanced. A subset of interneurons still has clear short-term depression instead of facilitation, and the amount of short-term depression at high frequencies is still large (~60%). The average of the facilitation interneuron/pyramidal cell responses decreased to ~60% at high frequencies, and the average of the depression interneuron/pyramidal cell responses decreased to ~15%. Together, these results demonstrate that the differences in short-term plasticity between Schaffer collateral synapses onto interneurons and pyramidal cells also occur, and are in fact enhanced, at conditions more closely resembling in vivo conditions.
Mathematical model fits PST data with differences in initial release probability between cell types
We previously developed a mathematical model of short-term plasticity at Schaffer collateral synapses that incorporated the effects of the initial release probability (number of readily releasable vesicles and release probability per vesicle), a single component of facilitation, vesicle depletion and refilling, inactivation of synapses after release, and a calcium-dependent recovery from inactivation (Sun et al., 2005). The model predicted that target-cell specific differences in short-term plasticity of Schaffer collateral synapses in response paired pulse, five pulse, and constant frequency trains could be accounted for by differences in the initial release probability at Schaffer collateral synapses onto different target cells. The model predicted that synapses onto pyramidal cells have lower release probability than those onto interneurons, which we confirmed experimentally (Sun et al., 2005). However, the model and its prediction were created for synaptic responses in response to simple, artificial input patterns, and it has not yet been tested for more complex stimulus patterns such as the PST.
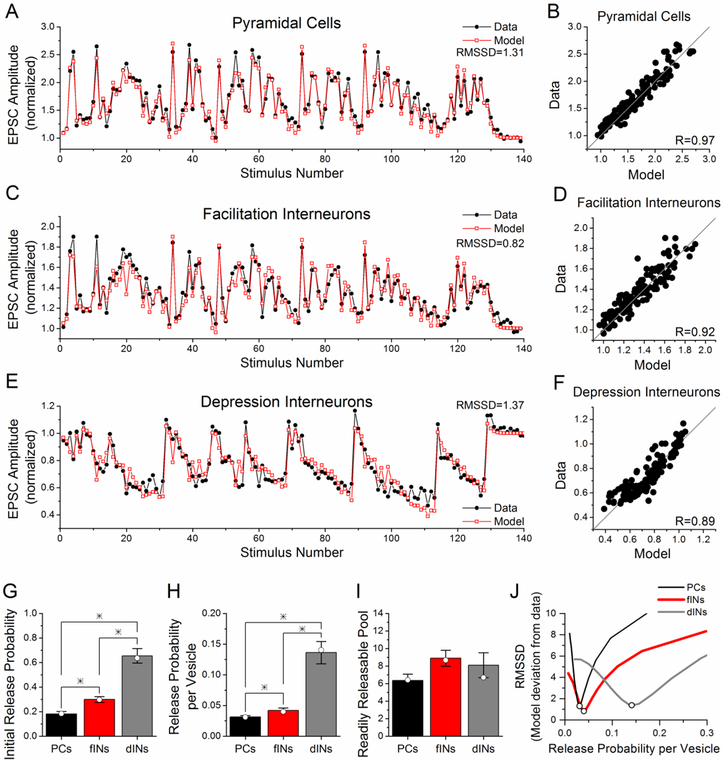
Here we simulated the experimental data in response to the PST using the model. The model was first fit to the average response for the pyramidal cells for the data at 24-25 °C and 2.5 mM Ca2+ (from Figure 2B). In order to enable the model to fit the PST data, we needed to add a second component of facilitation, which is smaller and decays more slowly than the first facilitation component, corresponding to the form of short-term facilitation called augmentation (Stevens and Wesseling, 1999). Figure 6A shows the results of model fit to the group data for the responses to the PST of Schaffer collateral synapses onto pyramidal cells; the fit is excellent, with a correlation coefficient R=0.97 and RMSSD = 1.31 (Figure 6B). RMSSD is a more rigorous measurement of the goodness of fit than the correlation coefficient, with lower values indicating closer agreement between model results and experimental data (Schunn and Wallach, 2005). We next fit the model to the group data for the responses of facilitation interneurons and depression interneurons with only a change in the initial release probability per vesicle (μ1) and the initial readily releasable pool size (nT), which together determine the initial release probability (P1). All other parameters were kept the same for all three cell groups (Table 1). The model was able to provide excellent fits to the average responses of the facilitation interneurons (Figure 6C) and depression interneurons (Figure 6E); the correlation between model responses and experimental data was high and the RMSSD was low (Figures 6D, 6F). The model fits to the average group data for facilitation interneurons and depression interneurons had only a change in the initial release probability (Figure 6G, open symbols), caused by changes in the release probability per vesicle (Figure 6H, open symbols) and small changes in the readily releasable pool size (Figure 6I, open symbols). The model simulations predict that the release probability is lowest for synapses onto pyramidal cells and highest for synapses onto depression interneurons, consistent with what was observed using simple stimulus patterns.
Figure 6. Model fits PST data from all three cell group with differences only in the initial release probability.
A-F) Model fits (gray, open squares) to experimental data (black, closed circles) for pyramidal cells (A, data from Figure 2B), facilitation interneurons (C, data from Figure 1C) and depression interneurons (E, data from Figure 1C). The only two model parameters that were variable between the three cell groups are the initial release probability per vesicle (μ1) and the initial readily releasable pool size (nT), which result in differences in the initial release probability (P1). Values of all other model parameters, which are the same for all three cell groups, are given in Table 1. B, D, F) Correlations between experimental data and model responses. G, H, I) Average parameter values from model fits to the responses of each individual cell for the initial release probability per vesicle (H) and initial readily releasable pool size (I), and the resulting initial release probability (G), shown as mean ± s.e. The average values from the individual model fits are very similar to the parameters from model fits to the group data, which are indicated by open circles. * indicates statistically different (p<0.05). (J). Model simulations were done where initial release probability was held constant and readily releasable pool size was varied from 1 to 50, causing corresponding decreases in release probability per vesicle. Initial release probability is 0.184 for pyramidal cells, 0.298 for facilitation interneurons, and 0.637 for depression interneurons. RMSSD is plotted as a function of release probability per vesicle for comparisons of model simulations to experimental data.
In addition, we fit the model to the responses of each individual cell, and the parameter values (mean ± s.e.) for each of the cell types are given in the bar graphs in Figures 6G-6I. The average values from the individual fits are very similar to the values obtained from the model fits to the group data. The differences in the initial release probability were significant among all three groups (Figure 6G, p<0.05), as were the differences in the release probability per vesicle (Figure 6H, p<0.05). There was a trend towards a higher readily releasable pool size in the facilitation interneurons as compared to the pyramidal cells, but the difference was not significant (Figure 6I). The modeling results show that changes in the initial release probability are sufficient to explain the differences in the responses to the PST between the three cell groups, although it does not rule out possible cell-type specific changes in other parameters.
We wondered whether the model fit was dependent only on the initial release probability, or whether the combination of release probability per vesicle and readily releasable pool size (that determined the initial release probability) were also important. To test this, we ran a series of model simulations with the initial release probability held constant and varying readily releasable pool size (1-50), which caused changes in release probability per vesicle. This resulted in a different range of release probability per vesicle values for the three cell types (pyramidal cells 0.004 - 0.184, facilitation interneurons 0.007 - 0.298, depression interneurons 0.020 - 0.639). Then for each simulation, we calculated the RMSSD that determines the goodness of fit between the model result and the experimental data and then plotted the result as a function of release probability per vesicle (Figure 6J). For each of the three cell types, the minimum value of RMSSD was at the release probability per vesicle value obtained in the previous model fitting (values in Table IV), and increased greatly for values above or below that value.
We next fit the model to the group data for responses at the higher temperature and 2.5 mM Ca2+; the model provided an excellent fit to the pyramidal cell data (R=0.93, RMSSD = 1.49), with a large decrease in τF2 and small changes in τD and R (Table II) as compared to the values used at 25 °C. The model could fit the group data for the facilitation interneurons (R=0.88, RMSSD = 1.83) and depression interneurons (R=0.84, RMSSD = 1.50) using the same parameters, except with α1 and nT, and thus P1, allowed to vary compared to pyramidal cells. There was essentially no change in α1, nT, or P1 with the increase in temperature for any cell type; the model parameters for fits to group data are given in Table V. In addition, we fit the model to the responses of each individual cell for all three groups, and the parameter values (mean ± s.e.) for each of the cell types are given in Table V. As at room temperature, the average values from the individual fits to the data at warmer temperature are very similar to the values obtained from the model fits to the group data. The differences in the initial release probability were significantly different between all three cell groups (p<0.05). The differences in release probability per vesicle were significantly different between depression interneurons and both pyramidal cells and facilitation interneurons (p<0.05), but not between pyramidal cells and facilitation interneurons, and there was no difference in the readily releasable pool size among the groups.
Table V.
Release probability
| Temperature | 25 °C | 33 °C | 33 °C | ||
|---|---|---|---|---|---|
| [Ca2+] | 2.5 mM | 2.5 mM | 1.0 mM | ||
| P1 | Pyramidal cells |
Group fit | 0.184 | 0.212 | .015 |
| Individual fits | 0.181 ± 0.020 | 0.206 ± 0.019 | 0.012 ± 0.002 | ||
| Facilitation interneurons |
Group fit | 0.298 | 0.297 | 0.048 | |
| Individual fits | 0.30 ± 0.02* | 0.300 ± 0.018* | 0.0331±0.004* | ||
| Depression interneurons |
Group fit | 0.637 | 0.708 | 0.409 | |
| Individual fits | 0.655 ± 0.059*+ | 0.717 ± 0.075*+ | 0.426 ± 0.114*+ | ||
Significantly different from pyramidal cells.
Significantly different from facilitation interneurons.
Finally, we fit the model to the group data at the lower calcium concentration (1.0 mM Ca2+ and 33 °C). The model could fit the pyramidal cell data (R=0.98 RMSSD = 1.41), facilitation interneuron data (R=0.94, RMSSD = 1.19), and depression interneuron data (R=0.84, RMSSD 1.55) with only a decrease (as compared to the parameter values at higher temperature and 2.5 mM calcium) in α1, ΔF1 and ΔF2, each of which would be expected to be calcium-dependent (Tables III, IV). Not surprisingly, this caused a large reduction in the release probability (Table V). The model values for initial release probability are again different between the three cell groups Together, the modeling results show that changes in the initial release probability are sufficient to explain the differences in the responses to the PST between the three cell groups across a range of experimental conditions, including near physiological conditions (warmer temperature, lower calcium).
Discussion
Here we provide a detailed analysis of the frequency-dependence of excitatory synapses onto inhibitory interneurons during a physiologically-derived stimulus pattern. We find two distinct patterns of responses from Schaffer collateral synapses onto stratum radiatum interneurons, as previously seen with simple stimulus patterns (Sun et al., 2005). The majority of interneurons have Schaffer collateral inputs that express short-term facilitation, although the amount of facilitation is less than that of Schaffer collateral synapses onto pyramidal cells. A subset of interneurons has Schaffer collateral inputs with short-term depression and no short-term facilitation. This is also observed at warmer temperatures and lower (more physiological) levels of calcium, both conditions which normally increase short-term facilitation and reduce short-term depression (Sippy et al., 2003; Watanabe et al., 2005; Klyachko and Stevens, 2006b; Schlüter et al., 2006), suggesting that it is likely to occur in vivo. Mathematical modeling indicates that these differences in short-term plasticity can be caused by differences in the initial release probability at these synapses. Target-cell specific regulation of initial release probability is thereby a mechanism that can determine not only the baseline strength of excitation, but also differentially modulate the synaptic dynamics, which are important for information transmission (Rotman et al., 2011) and for modulating the balance of excitation /inhibition and circuit function (Bartley and Dobrunz, 2015).
An important finding of this study is that the target-cell specific differences in short-term plasticity that occur during physiologically-relevant stimulation alter the relative strength of excitatory inputs onto facilitation interneurons, depression interneurons, and pyramidal cells in a frequency-dependent manner. This was observed under all recording conditions, with the magnitude of the effect being largest at near physiological conditions. Short-term facilitation of excitatory inputs has been shown to cause facilitation of spiking in both pyramidal cells and interneurons (Bartley and Dobrunz, 2015; Tominaga and Tominaga, 2016; Li et al., 2017), while short-term depression can causes depression of spiking (Li et al., 2017). The reduction in excitatory input to interneurons during temporally complex firing patterns would therefore be expected to decrease the firing of interneurons compared to pyramidal cells during high frequency inputs. Similarly, the differences in short-term plasticity between Schaffer collateral synapses onto facilitation and depression interneurons may shift the relative balance of firing between different types of interneurons, depending upon frequency input. Consistent with this, in vivo studies have shown that specific subtypes of interneurons fire preferentially during particular oscillation frequencies (Lapray et al., 2012; Lasztóczi and Klausberger, 2014; Müller and Remy, 2014; Allen and Monyer, 2015). This diversity in firing patterns helps to modulate the balance of excitation and inhibition onto pyramidal cells, which has been shown to be dependent upon short-term plasticity (Bartley and Dobrunz, 2015; Bartley et al., 2015). The dynamics of the excitation to inhibition balance will also be governed by the activity dependent properties of the inhibitory synapses, which primarily show short-term depression (Galarreta and Hestrin, 1998; Varela et al., 1999; Klyachko and Stevens, 2006a). The overall effect of these target-cell and synapse-specific differences in short-term plasticity could be important in releasing the CA1 pyramidal cells from feed-forward inhibition and allowing them to fire action potentials, thus enable the successful transmission of relevant signals (Klyachko and Stevens, 2006a) and generation of oscillation patterns (Keeley et al., 2017). In contrast, excitatory synapses onto fast spiking interneurons versus pyramidal cells in layer V of cortex showed the same amount of steady-state high frequency depression in response to constant frequency trains (Galarreta and Hestrin, 1998), which is thought to be important for stabilizing the circuit.
Our relatively simple mathematical model is able to provide excellent fits to the PST responses for all three cell groups, despite the high degree of temporal variability in the stimulation pattern and the complexity of the resulting short-term plasticity. However, the fit required the addition of a second component of facilitation, which is smaller and decays more slowly than the first facilitation component, to our previous model (Sun et al., 2005). Surprisingly, the model is able to fit the PST responses for all three cell groups with only the initial release probability being different between the different cell types. This indicates that a difference in the initial release probability is sufficient to cause the large differences in short-term plasticity observed between Schaffer collateral synapses onto stratum radiatum interneurons and pyramidal cells. While the modeling does not rule out a possible contribution of other mechanisms, it predicts that a difference in the initial release probability between Schaffer collateral synapses onto interneurons versus pyramidal cells is likely to be a major mechanism responsible for differences in short-term plasticity during the PST.
The model simulations predict that Schaffer collateral synapses onto CA1 pyramidal cells have a low estimated release probability, and that the release probability is likely to be higher at Schaffer collateral synapses onto both groups of interneurons. The specific values of release probability that the model predicts might not match the actual values for each of the cells and each of the conditions, as there are multiple model parameters that cannot be determined experimentally. However, the model prediction that Schaffer collateral synapses onto facilitation interneurons have a higher initial release probability than these synapses onto pyramidal cells is consistent with our previous study, which demonstrated this experimentally using the MK-801 method (Sun et al., 2005). The model also predicts that Schaffer collateral synapses onto depression interneurons have an even larger initial release probability than synapses onto facilitation interneurons, which remains to be confirmed experimentally. Large target-cell specific differences in initial release probability have also been observed from cortical layer 2/3 pyramidal cells onto pyramidal cells and two subtypes of interneurons (Koester and Johnston, 2005).
The modeling suggests that the differences in synaptic release probability are caused by variations in the initial release probability per vesicle, but not in the readily releasable pool size. A correlation between readily releasable vesicles and release probability per vesicle has previously been shown experimentally across a population of individual Schaffer collateral synapses onto CA1 pyramidal cells (Dobrunz, 2002), where higher values of both parameters contribute to higher release probability. However, our data here suggest that the correlation does not hold for Schaffer collateral synapses across cell types, where there are large differences in release probability per vesicle between pyramidal cell and interneuron inputs, without changes in pool size. Large variability in both readily releasable pool size and release probability per vesicle have also been shown for synapses in culture, including synapses made by the same axon (Ariel et al., 2012).
Differences in the release probability per vesicle could be caused by differences in the amount of calcium influx (Koester and Johnston, 2005; Éltes et al., 2017) and/or the sensitivity of the release machinery to calcium. Many possible mechanisms could cause differences in calcium influx at different synapses, including differences in the density or subtype of calcium channels (Miyazaki et al., 2005; Éltes et al., 2017), or variation in the duration of the action potential (Ali et al., 2007). However, one study showed that although synapses could have large differences in the relative contributions of N and P/Q type calcium channels, this did not correlate with differences in synaptic efficacy (Ariel et al., 2012). In addition, some synapses have been shown to have presynaptic receptors that modulate calcium influx that are tonically activated by ambient levels of neurotransmitters, such as glutamate, (Lauri et al., 2005; Wang et al., 2005) or adenosine (Yang et al., 2007; Kerr et al., 2013). The mechanisms by which synapses regulate release probability differentially based on the identity of the target neurons are only beginning to be discovered (Ermolyuk et al., 2012; Sylwestrak and Ghosh, 2012; Blackman et al., 2013).
We find that increasing the recording temperature from 25 °C to 33 °C had only small effects on the magnitude of short-term plasticity and the frequency-dependence of responses to the PST for Schaffer collateral synapses onto all three cell groups. In accord with our data, a previous study on developmental changes in short-term plasticity at Schaffer collateral synapses onto pyramidal cells found only minor increases in the amount of short-term facilitation at warmer temperatures (Speed and Dobrunz, 2008). This suggests that any temperature dependent changes in short-term plasticity affect Schaffer collateral synapses onto different cell types, and at different ages, to a similar extent. Fitting the model to the data at the warmer temperature required a large decrease in the slow time constant of facilitation, τF2, which most likely corresponds to an accelerated decay of augmentation, as has been previously observed (Klyachko and Stevens, 2006b). However, model fits to the individual cells showed no difference in the average values between the two temperatures for the readily releasable pool size, release probability per vesicle, or initial release probability for any of the three cell groups. Although the dynamics of short-term plasticity are modulated by temperature, release probability remains unchanged in this temperature range.
A much greater effect was caused by lowering the extracellular calcium to 1 mM, which increased the maximal short-term facilitation for both pyramidal cells and facilitation interneurons. However, there were still interneurons that had short-term depression in response to the PST even at 33 °C and 1 mM calcium, and the frequency-dependence and magnitude of the depression was similar to that observed at 25 °C and 2.5 mM calcium. The cell-type specific differences in short-term plasticity of Schaffer collateral synapses in response to the PST were observed under all conditions, and the differences were greatest at 33 °C and 1 mM calcium, indicating that these effects are likely to be as large, or possibly even larger, in vivo. Decreasing calcium to the more physiological value of 1 mM caused a large decrease in the average release probability per vesicle for all three cell groups, as expected, with no significant changes in the readily releasable pool sizes. Fitting the data at 1 mM calcium also required large reductions in the parameters that controlled the magnitude of facilitation, ΔF and ΔF2. This is also not surprising, since facilitation and augmentation are both known to be calcium dependent (Stevens and Wesseling, 1999; Zucker and Regehr, 2002; Garcia-Perez and Wesseling, 2008; Blackman et al., 2013). However, the differences in release probability between the three cell groups were observed at both higher and lower calcium levels. Our experiments show that lowering calcium had a much larger effect to increase facilitation in pyramidal cells than in facilitation interneurons, and had relatively little overall effect on short-term plasticity in depression cells. Consistent with this, the modeling predicted that lowering calcium has a much larger effect to decrease initial release probability at synapses onto pyramidal cells compared to facilitation interneurons, and an even smaller effect at synapses onto depression interneurons. Previous studies have shown 3-4 fold changes in Schaffer Collateral field potential responses over a similar range of changes in extracellular calcium (Mulkeen et al,1988; Muller and Lynch, 1989). The decrease in release probability predicted by our model for synapses onto pyramidal cells when calcium is reduced from 2.5 mM to 1 mM is considerably larger than this. Some of this discrepancy could be due to differences in experimental conditions, however it suggests that additional model parameters may be calcium dependent. It is possible that some parameters might be differentially calcium sensitive between the three cell groups, thereby contributing to the larger magnitude of the target-cell specific at low calcium. Taken together, our results suggest that cell-type specific differences in release probability enable diversity in dynamics of these synapses across a range of experimental conditions, including near-physiological conditions.
The modeling has shown that differences in initial release probability could potentially account for the observed differences in target-cell specific short-term plasticity during physiologically derived stimulus patterns, which are highly variable in their timing, and under several different recording conditions. The good fit of the model to the data for three different cell types under three different recording conditions, with only two model parameters allowed to vary between cell type, is a stringent test of the model. Because the model error quickly grew large when release probability per vesicle was changed while initial release probability was held constant, the modeling also showed that release probability per vesicle is important for determining short-term plasticity, not simply the overall initial release probability. For simplicity, we have assumed in our modeling that all parameters except readily releasable pool size and release probability per vesicle are the same between the three cell groups. However, our results do not rule out possible contributions from target-cell specific differences in other model parameters. In particular, there may be differences in the model parameters that more directly control facilitation, including the magnitude and/or time course of facilitation. Because facilitation is calcium dependent, and our results show that synapses onto the three cell types are differentially sensitive to calcium, these parameters might also show target-cell specific differences. It is also possible that synapses onto depression interneurons lack the mechanism for facilitation, which has been suggested for other synapses that show short-term depression when initial release probability is reduced by lowering calcium (Markram et al., 1998). There may also be differences in the rate of refilling of the readily releasable pool that contribute to the differences in short-term plasticity between synapse types. However, the modeling did not predict differences in readily releasable pool size between synapses or under any of the conditions, even though it was allowed to vary in all simulations. The study of target cell specific plasticity has begun to identify candidate molecules that can differentially regulate the release properties of the synapse based on the postsynaptic cell (Blackman et al., 2013; Sun & Dobrunz, 2006; Sun et al., 2009). As their role is further evaluated, these mechanisms could also be incorporated into the modeling.
Short-term plasticity helps to determine the dynamic range over which synapses can operate. The dynamic range reflects the ability of synapses to modulate their strength in response to different frequencies and temporal patterns of input. Here we show that the dynamic range of Schaffer collateral synapses is different depending upon the target neuron. Schaffer collateral synapses onto CA1 pyramidal cells operate over a fairly large dynamic range (Dobrunz and Stevens, 1999; Dekay et al., 2006). As a result, Schaffer collateral synapses onto CA1 pyramidal cells have the ability to quickly go from providing weak synaptic input at low frequencies to much stronger synaptic input at higher frequencies, and back. This is likely to be important for their role in the circuit, which is to transmit information. In contrast Schaffer collateral synapses onto both facilitation interneurons and depression interneurons in stratum radiatum have a much smaller dynamic range, which means that the strength of their excitatory synaptic input will vary to a lesser extent during changes in input frequency. This is consistent with the fact that these interneurons play a different role (or roles) in the function of the hippocampal circuit. The narrower dynamic range of their Schaffer collateral excitatory inputs makes the probability of interneuron firing likely to be more consistent, although this will also depend upon other factors such as their inhibitory inputs and the effects of cell-type specific differences in intrinsic excitability. However, the level of excitatory input to these interneurons is less variable with frequency, which may be important for circuit stability. We also find that the trial to trial variability in response to the PST is also low for recordings of EPSC amplitude from individual pyramidal cells and stratum radiatum interneurons. This indicates synaptic strength is modulated with high precision by the timing of the input patterns for Schaffer collateral synapses onto both pyramidal cells and interneurons, despite their differences in the initial synaptic release probability (reliability). This supports the idea that synaptic unreliability, combined with presynaptic short-term plasticity, is actually a powerful tool for regulating the dynamic range of neuronal responses (Smetters and Zador, 1996).
The timing of PST used here is highly variable, and this variability appears to have important functional consequences for Schaffer collateral synapses. We show that stimulus patterns with the same average frequency but different amounts of temporal variability (PST vs. constant frequency) show different average levels of short-term plasticity at Schaffer collateral synapses onto CA1 pyramidal cells. This was also true for Schaffer collateral synapses onto interneurons, although the differences were smaller. The effect was also frequency-dependent, being larger at 5 Hz compared to 1 Hz. This suggests that the variability of the stimulus timing has more of an effect on synaptic strength for Schaffer collateral synapses onto pyramidal cells compared to interneurons. It also indicates that the steady state response to constant frequency stimulation is not always a good predictor for the amount of short-term depression in response to behaviorally relevant stimuli. Consistent with this, our previous study using constant frequency trains showed little or no difference in steady state high frequency depression between Schaffer collateral synapses onto pyramidal cells versus interneurons over a range of frequencies (Sun et al., 2005), whereas experiments here clearly show differences between these types of synapses in response to the PST. In cortex, synaptic responses have been recorded to irregular trains using Poisson stimulus patterns (Markram et al., 1998b; Varela et al., 1999). The firing patterns of cortical input neurons have been shown to be similar to Poisson in their variability (Shadlen and Newsome, 1998). In hippocampus, however, spiking patterns are much more temporally variable (Fenton and Muller, 1998), and cannot be well approximated by the Poisson process. The PST is therefore an important tool for investigating short-term plasticity at hippocampal synapses (Frerking and Ohliger-Frerking, 2006; Klyachko and Stevens, 2006a; Speed and Dobrunz, 2008, 2009; Sun et al., 2009). Our results highlight the importance of using temporally complex stimulus paradigms (rather than constant frequency or Poisson stimulation) to assess differences in short-term plasticity.
Hippocampal interneurons, including those in stratum radiatum, have been shown to be heterogeneous in their morphology and biochemistry, as well as showing variability with respect to nearly every physiological parameter tested (Bezaire and Soltesz, 2013). In particular, stratum radiatum interneurons show considerable heterogeneity in their axonal targeting; while the majority target the pyramidal cell dendrites, some are basket cells providing inhibition to pyramidal cell bodies, and still others provide inhibitory input to other inhibitory interneurons (Freund and Buzsáki, 1996; Bezaire and Soltesz, 2013). Here we show that stratum radiatum interneurons have (at least) two distinct patterns of short-term plasticity of Schaffer collateral inputs in response to temporally complex stimulation. Because there are more than two types of stratum radiatum interneurons as defined by biochemical and morphological differences (Bezaire and Soltesz, 2013), each of our two physiologically defined subgroups is likely to contain multiple interneuron subtypes. Even within a specific biochemically defined subgroup of interneurons there can be distinct patterns of short-term plasticity in response to activation of Schaffer collateral synapses, as has recently been shown for Neuropeptide Y expressing interneurons (Li et al., 2017). Neuropeptide Y ivy cells in stratum radiatum have been shown to have two subtypes, one with short-term facilitation and the other with short-term depression in response to Schaffer collateral stimulation (Li et al., 2017). Although we did not biochemically identify the interneurons studied here, the depression interneurons we recorded are likely to be primarily Neuropeptide Y ivy cells, although there could also be other types of interneurons as well. Because they are the largest interneuron type in stratum radiatum (Bezaire and Soltesz, 2013), Neuropeptide Y ivy cells are likely to also comprise a large part of the recorded facilitation interneurons (Li et al., 2017). The remainder could be cholecystokinin expressing interneurons, which are the next largest interneuron type in stratum radiatum, and/or interneuron-specific interneurons (Bezaire and Soltesz, 2013), whose input plasticity is not yet known. Interestingly, previous studies have shown that trains of constant-frequency stimulation shift the balance between somatic and dendritic inhibition (Pouille and Scanziani, 2004; Gabernet et al., 2005), indicating that differences in short-term plasticity of excitatory inputs to interneuron subtypes are important for circuit function. It remains to be determined whether the short-term plasticity of these two groups of stratum radiatum interneurons correlates with their axonal targeting, and whether physiologically derived input patterns modulate the balance of somatic and dendritic inhibition.
We have previously performed a limited study of the responses to a PST of Schaffer collateral synapses onto a specialized subset of stratum radiatum interneurons containing somatostatin (Sun et al., 2009) using the GIN mice (Oliva et al., 2000). Because they have short-term facilitation that is much larger than that observed at Schaffer collateral synapses onto pyramidal cells (Sun and Dobrunz, 2006; Sun et al., 2009), and they are very sparsely expressed in stratum radiatum (Oliva et al., 2000), we think they are a distinct group of interneurons from the facilitation interneurons studied here. The large short-term facilitation at Schaffer collateral synapses onto somatostatin interneurons is due in part to synaptic activation of presynaptic kainate receptors (Sun and Dobrunz, 2006; Sun et al., 2009), which also contribute large facilitation of excitatory inputs onto other somatostatin interneurons (Sylwestrak and Ghosh, 2012). However, we found no evidence for synaptic activation of kainate receptors that modulate release probability at Schaffer collateral synapses onto non-somatostatin interneurons or pyramidal cells (Sun and Dobrunz, 2006; Sun et al., 2009), indicating that modulation of short-term plasticity by kainate receptors is unlikely to play a role in the responses of the Schaffer collateral synapses onto the neurons studied here.
Stimulation with the PST has also been shown to cause the modulation of short-term facilitation at Schaffer collateral synapses onto CA1 pyramidal cells by a mechanism that involves both mGluR1 receptors and GABAB receptors (Speed and Dobrunz, 2008). This effect occurs primarily in slices from young adult rats, with almost no effects in slices from juvenile rats (Speed and Dobrunz, 2008) such as we used in this study. Future studies will be needed to determine if a similar mechanism modulates Schaffer collateral synapses onto interneurons in either juveniles or young adults, and investigate a possible role for other neuromodulators in regulating short-term plasticity of Schaffer collateral synapses onto other specific subtypes of interneurons.
In summary, our results show that target-cell specific differences in short-term plasticity of Schaffer collateral synapses onto stratum radiatum feed-forward interneurons vs. CA1 pyramidal cells enable these synapses to have different temporal filtering characteristics. This is likely to be an important factor that helps to dynamically regulate the balance of inhibition and excitation, and control CA1circuit function and hippocampal output.
Highlights.
Short-term plasticity is target-cell specific during physiological-derived spike trains.
Target-cell specific short-term plasticity expresses different temporal filtering properties.
Excitatory input is reduced onto CA1 interneurons compared to pyramidal cells during high frequency stimulation.
These differences in short-term plasticity can be explained by changes only in the initial release probability.
Variability in timing is an important feature of physiological spike trains that allows for more short-term facilitation.
Acknowledgements
We would like to thank Dr. Robert Phair for help with the mathematical modeling, and Dr. Smadar Lapidot for help editing the manuscript. We would also like to thank Dr. Robert Muller and Dr. Andre Fenton for providing the in vivo spike timing patterns used in these experiments.
This work has been supported by NIH grants R01/R56 MH065328, R01 MH098534, and R01 MH108342 to L.E.D., a Civitan International Research Center Emerging Scholars Award to H.Y.S., and NIH grants P30HD038985 and P30NS057098 to the University of Alabama at Birmingham.
Abbreviations:
- dINs
depression interneurons
- fINs
facilitation interneurons
- PCs
pyramidal cells
- PST
Physiologically-derived Spike Train
Footnotes
Conflict of Interest:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali AB, Bannister AP, Thomson AM (2007) Robust correlations between action potential duration and the properties of synaptic connections in layer 4 interneurones in neocortical slices from juvenile rats and adult rat and cat. J Physiol (Lond) 580:149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Monyer H (2015) Interneuron control of hippocampal oscillations. Curr Opin Neurobiol 31:81–87. [DOI] [PubMed] [Google Scholar]
- Ariel P, Hoppa MB, Ryan TA (2012) Intrinsic variability in Pv, RRP size, Ca(2+) channel repertoire, and presynaptic potentiation in individual synaptic boutons. Frontiers in synaptic neuroscience 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML (1997) Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Lond) 502 (Pt 3):575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley AF, Dobrunz LE (2015) Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells. Eur J Neurosci 41:1402–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley AF, Lucas EK, Brady LJ, Li Q, Hablitz JJ, Cowell RM, Dobrunz LE (2015) Interneuron Transcriptional Dysregulation Causes Frequency-Dependent Alterations in the Balance of Inhibition and Excitation in Hippocampus. J Neurosci 35:15276–15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezaire MJ, Soltesz I (2013) Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23:751–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman AV, Abrahamsson T, Costa RP, Lalanne T, Sjöström PJ (2013) Target-cell-specific short-term plasticity in local circuits. Frontiers in synaptic neuroscience 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekay JGT, Chang TC, Mills N, Speed HE, Dobrunz LE (2006) Responses of excitatory hippocampal synapses to natural stimulus patterns reveal a decrease in short-term facilitation and increase in short-term depression during postnatal development. Hippocampus 16:66–79. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE (2002) Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci 20:225–236. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF (1999) Response of hippocampal synapses to natural stimulation patterns. Neuron 22:157–166. [DOI] [PubMed] [Google Scholar]
- Éltes T, Kirizs T, Nusser Z, Holderith N (2017) Target Cell Type-Dependent Differences in Ca(2+) Channel Function Underlie Distinct Release Probabilities at Hippocampal Glutamatergic Terminals. J Neurosci 37:1910–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolyuk YS, Alder FG, Henneberger C, Rusakov DA, Kullmann DM, Volynski KE (2012) Independent regulation of basal neurotransmitter release efficacy by variable Ca2+ influx and bouton size at small central synapses. PLoS Biol 10:e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Muller RU (1998) Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc Natl Acad Sci U S A 95:3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P (2006) Functional consequences of presynaptic inhibition during behaviorally relevant activity. J Neurophysiol 96:2139–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schulte J, Wiebe SP, Stäubli U (2005) Spike timing in CA3 pyramidal cells during behavior: implications for synaptic transmission. J Neurophysiol 94:1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M (2005) Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48:315–327. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S (1998) Frequency-dependent synaptic depression and the balance of excitation and inhibition in the rat neocortex. Nature Neurosci 1:587–594 Available at: http://www.ncbi.nlm.nih.gov/pubmed/10196566. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez E, Wesseling JF (2008) Augmentation controls the fast rebound from depression at excitatory hippocampal synapses. J Neurophysiol 99:1770–1786. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Hahm KD, Shin JW, Leem JG, Lee C, Han SM (2006) Changes in magnesium concentration in the serum and cerebrospinal fluid of neuropathic rats. Acta Anaesthesiol Scand 50:211–216. [DOI] [PubMed] [Google Scholar]
- Keeley S, Fenton AA, Rinzel J (2017) Modeling fast and slow gamma oscillations with interneurons of different subtype. J Neurophysiol 117:950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MI, Wall MJ, Richardson MJE (2013) Adenosine A1 receptor activation mediates the developmental shift at layer 5 pyramidal cell synapses and is a determinant of mature synaptic strength. J Physiol (Lond) 591:3371–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF (2006a) Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains. PLoS Biol 4:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF (2006b) Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses. J Neurosci 26:6945–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D (2005) Target cell-dependent normalization of transmitter release at neocortical synapses. Science 308:863–866. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R (1999) Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285:1411–1414. [DOI] [PubMed] [Google Scholar]
- Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T (2012) Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci 15:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóczi B, Klausberger T (2014) Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron 81:1126–1139. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Segerstråle M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JTR, Taira T (2005) Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci 25:4473–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S, Petersen CCH (2017) Layer-Dependent Short-Term Synaptic Plasticity Between Excitatory Neurons in the C2 Barrel Column of Mouse Primary Somatosensory Cortex. Cereb Cortex: 1–10. [DOI] [PubMed] [Google Scholar]
- Li Q, Bartley AF, Dobrunz LE (2017) Endogenously Released Neuropeptide Y Suppresses Hippocampal Short-Term Facilitation and Is Impaired by Stress-Induced Anxiety. J Neurosci 37:23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Pikus D, Gupta A, Tsodyks M (1998a) Potential for multiple mechanisms, phenomena and algorithms for synaptic plasticity at single synapses. Neuropharmacol 37:489–500. [DOI] [PubMed] [Google Scholar]
- Markram H, Gupta A, Uziel A, Wang Y, Tsodyks M (1998b) Information processing with frequency-dependent synaptic connections. Neurobiol Learn Mem 70:101–112. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Ishizuka T, Yawo H (2005) Synapse-to-synapse variation of calcium channel subtype contributions in large mossy fiber terminals of mouse hippocampus. Neuroscience 136:1003–1014. [DOI] [PubMed] [Google Scholar]
- Mulkeen D, Anwyl R, and Rowan M (1988) The effects of external calcium on long-term potentiation in the rat hippocampal slice. Brain Res 447, 234–238. [DOI] [PubMed] [Google Scholar]
- Muller D, and Lynch G (1989) Rate-limiting step for transmission at excitatory synapses in hippocampus. Synapse 3, 67–73. [DOI] [PubMed] [Google Scholar]
- Müller C, Remy S (2014) Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Frontiers in synaptic neuroscience 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jiang M, Lam T, Smith KL, Swann JW (2000) Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20:3354–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ (2007) Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol 17:366–373. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M (2004) Routing of spike series by dynamic circuits in the hippocampus. Nature 429:717–723. [DOI] [PubMed] [Google Scholar]
- Rotman Z, Deng P-Y, Klyachko VA (2011) Short-term plasticity optimizes synaptic information transmission. J Neurosci 31:14800–14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter OM, Basu J, Südhof TC, Rosenmund C (2006) Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci 26:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunn CD, Wallach D (2005) Evaluating goodness-of-fit in comparison of models to data. Available at: http://www.lrdc.pitt.edu/schunn/gof/.
- Shadlen MN, Newsome WT (1998) The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18:3870–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecińska M (1990) Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol 95:837–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy T, Cruz-Martín A, Jeromin A, Schweizer FE (2003) Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor-1. Nat Neurosci 6:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetters DK, Zador A (1996) Synaptic transmission: noisy synapses and noisy neurons. Curr Biol 6:1217–1218. [DOI] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE (2008) Developmental decrease in short-term facilitation at Schaffer collateral synapses in hippocampus is mGluR1 sensitive. J Neurophysiol 99:799–813. [DOI] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE (2009) Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus 19:187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Wesseling JF (1999) Augmentation is a potentiation of the exocytotic process. Neuron 22:139–146. [DOI] [PubMed] [Google Scholar]
- Sun HY, Bartley AF, Dobrunz LE (2009) Calcium-permeable presynaptic kainate receptors involved in excitatory short-term facilitation onto somatostatin interneurons during natural stimulus patterns. J Neurophysiol 101:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Dobrunz LE (2006) Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci 26:10796–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Lyons SA, Dobrunz LE (2005) Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats. J Physiol (Lond) 568:815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, Ghosh A (2012) Elfin1 regulates target-specific release probability at CA1-interneuron synapses. Science 338:536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Tominaga Y (2016) Paired Burst Stimulation Causes GABAA Receptor-Dependent Spike Firing Facilitation in CA1 of Rat Hippocampal Slices. Front Cell Neurosci 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB (1999) Differential depression at excitatory and inhibitory synapses in visual cortex. J Neurosci 19:4293–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Hallengren JJ, Theile CS, Ploegh HL, Wilson SM, Dobrunz LE (2014) A catalytic independent function of the deubiquitinating enzyme USP14 regulates hippocampal synaptic short-term plasticity and vesicle number. J Physiol (Lond) 592:571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kitai ST, Xiang Z (2005) Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta. J Neurosci Res 82:778–787. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Rozov A, Wollmuth LP (2005) Target-specific regulation of synaptic amplitudes in the neocortex. J Neurosci 25:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S-C, Chiu T-H, Yang H-W, Min M-Y (2007) Presynaptic adenosine A1 receptors modulate excitatory synaptic transmission in the posterior piriform cortex in rats. Brain Res 1156:67–79. [DOI] [PubMed] [Google Scholar]
- Zucker RS (1999) Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 9:305–313. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405. [DOI] [PubMed] [Google Scholar]