Mini-Abstract
A retrospective cohort study was performed to determine whether bariatric surgery is associated with a lower risk of cancer. Patients undergoing bariatric surgery had a 33% lower hazard of developing any cancer during follow-up compared to a matched sample of control patients with severe obesity. In this large multisite cohort of patients with severe obesity, undergoing bariatric surgery was associated with a lower risk of incident cancer than not having surgery.
Abstract
Objective:
To determine whether bariatric surgery is associated with a lower risk of cancer.
Summary Background Data:
Obesity is strongly associated with many types of cancer. Few studies have examined the relationship between bariatric surgery and cancer risk.
Methods:
We conducted a retrospective cohort study of patients undergoing bariatric surgery between 2005 and 2012 with follow-up through 2014 using data from a large integrated health insurance and care delivery systems with five study sites. The study included 22,198 subjects who had bariatric surgery and 66,427 non-surgical subjects matched on sex, age, study site, BMI and Elixhauser comorbidity index. Multivariable Cox proportional hazards models were used to examine incident cancer up to 10 years after bariatric surgery compared to the matched non-surgical patients.
Results:
After a mean follow-up of 3.5 years, we identified 2,543 incident cancers. Patients undergoing bariatric surgery had a 33% lower hazard of developing any cancer during follow-up (HR 0.67, 95% C.I. 0.60, 0.74, p<0.001) compared to matched patients with severe obesity who did not undergo bariatric surgery, and results were even stronger when the outcome was restricted to obesity-associated cancers (HR 0.59, 95% C.I. 0.51, 0.69, p<0.001). Among the obesity-associated cancers, the risk of postmenopausal breast (HR 0.58, 95% C.I. 0.44, 0.77, p<0.001), colon (HR 0.59, 95% C.I. 0.36, 0.97, p=0.04), endometrial (HR 0.50, 95% C.I. 0.37, 0.67, p<0.001), and pancreatic cancer (HR 0.46, 95% C.I. 0.22, 0.97, p=0.04) were each statistically significantly lower among those who had undergone bariatric surgery compared to matched non-surgical patients.
Conclusions:
In this large, multisite cohort of patients with severe obesity, bariatric surgery was associated with a lower risk of incident cancer, particularly obesity-associated cancers, such as post-menopausal breast, endometrial, and colon cancer. More research is needed to clarify the specific mechanisms through which bariatric surgery lowers cancer risk.
Introduction
Obesity is strongly associated with an increased risk of several types of cancer including postmenopausal breast, endometrial, colon, liver, pancreatic, and ovarian cancer1, 2 contributing to an estimated 6% of all cancers3 and 15–20% of all cancer deaths in the U.S.4.
In the United States alone over one-third of the adult population is considered obese with a body mass index (BMI) ≥ 30 kg/m2.5, 6. The prevalence of severe obesity (BMI ≥40 kg/m2) has increased as well going from 0.8% in 1960 to 7.7% in 20146–10.
Bariatric surgery is currently the most effective intervention for weight loss and long-term weight maintenance. This has been consistently demonstrated in numerous randomized controlled trials and cohort studies11–16 and bariatric surgery appears to have a beneficial long term impact on the risk of mortality, including deaths due to cancer17–19. Following bariatric surgery, obesity-related health conditions that are also risk factors for cancer incidence, such as diabetes and metabolic syndrome, are often improved or resolved13, 15.
A limited number of previous observational cohort studies have reported that the risk of cancer is reduced following bariatric surgery17, 20–23. However, these studies had several methodological limitations including lack of ability to match bariatric cases and controls on important risk factors that impact cancer risk, small sample sizes resulting in inadequate power to examine differences in risk by cancer type, lack of generalizability to diverse populations, and inclusion of bariatric procedures that are no longer performed. Due to these limitations, we assembled a large, geographically diverse cohort, using high-quality data sources, contemporary procedure types and robust analytic techniques to better understand the relationship between bariatric surgery and cancer risk.
The aim of this study was to determine whether bariatric surgery was associated with a lower rate of incident cancer compared to a matched cohort of patients with severe obesity. By carefully matching bariatric surgical cases to controls who did not receive surgery, we sought to limit the potential for selection bias and confounding that was present in many of the previous studies.
Methods
Study Design and Study Population
We conducted a retrospective observational cohort study using data from two large integrated health insurance and care delivery systems with five study sites: Kaiser Permanente (regions of Southern California, Northern California, Northwest (Oregon), and Colorado and Group Health Cooperative (Washington). Data covering January 1, 2004 through December 31, 2014 were collected from existing electronic health record databases and registries. Institutional review board approval, including waiver of informed consent, was obtained at Kaiser Permanente Colorado and all other sites ceded IRB review to the KP Colorado IRB.
Bariatric surgery cases were identified using CPT-4 and ICD-9 codes for surgery between January 1, 2005 and December 31, 2012. Subjects were 18 to 79 years of age at the time of surgery and were excluded if they had a history of any cancer prior to surgery identified by an ICD-9 diagnosis code or by a tumor registry entry, did not have one year of enrollment in the health plan prior to surgery extending to at least six months post-surgery, had a prior bariatric procedure, no documented BMI of at least 35 kg/m2 within one year prior to surgery, or had a pre-surgery diagnosis of ascites or peptic ulcer disease (Figure 1). Of the 33,378 potential surgical cases that were initially identified in the 5 sites, 22,198 patients who underwent bariatric surgery were included in the final analysis.
Figure 1.
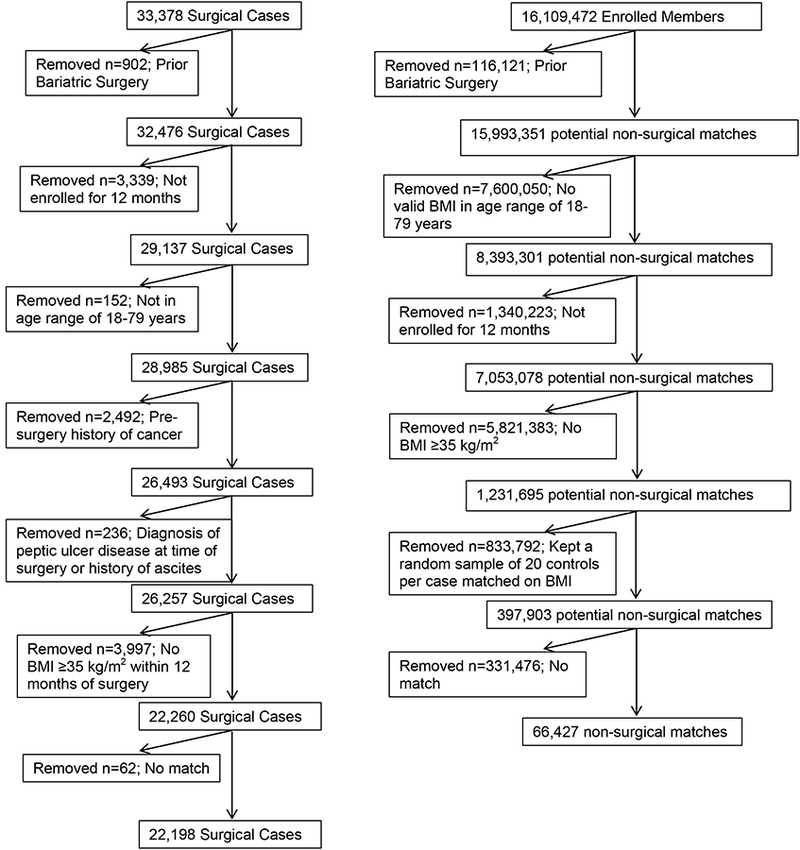
Cohort consort for bariatric surgery patients and the matched non-surgical controls.
Control patients were identified from the same electronic health record databases and matched to surgical cases using a two-phase matching process. Eligible controls had at least one BMI measurement ≥35 kg/m2 and the sampling was weighted such that the pool of potential controls had a distribution of BMIs similar to that of the surgery cases. Potential controls were then matched to each surgical case using site, sex, and birthdate within one year, allowing controls to be provisionally matched to multiple cases. The index date was the date of surgery for the bariatric surgery and the corresponding date for the matched controls. We then excluded potential controls with a cancer prior to the index date, BMI < 35, < 6 months post-index date enrollment, and those with ascites or peptic ulcers. Remaining potential controls were further restricted to those with a BMI within 5% of a surgical case. Finally, up to three controls were selected for each case based on the smallest differences in Elixhauser comorbidity index24 score. The Elixhauser comorbidity index score is a method for quantifying patient comorbidity based upon administrative data. Controls with an Elixhauser difference of 3 or greater were removed from the matched set and returned to the pool of potential matches. At the end of phase 1, there were 265 cases that remained without a match. In the second phase of the matching process, the BMI matching window was extended to +/−10% of the case and the remaining potential controls were again matched with cases lacking three controls based on the smallest Elixhauser comorbidity index score difference. After this second matching process, 99.65% of cases had three matched controls, with 0.25% having two matched controls and 0.1% having one matched control. The second phase of matching process yielded 2.85% of the final matches. After the second matching iteration, only 0.8% of matches had an Elixhauser score difference of 3 or greater, and only 62 bariatric cases remained without a match.
Outcomes
Incident cancers were identified from the tumor registries at each site. We included all types of cancers in our analysis and then separately considered obesity associated cancers. Cancers were considered to be obesity associated if they were one of the 14 types described by the International Agency for Research on Cancer (IARC) working group as having sufficient evidence for an association2. These include esophageal adenocarcinoma, postmenopausal breast cancer and cancers of the kidney, colon, rectum, gastric cardia, liver, gallbladder, pancreas, ovary, corpus uteri, thyroid, multiple myeloma and meningioma. We defined postmenopausal breast cancer as those diagnosed at age ≥55 years, consistent with previous studies using electronic data sources25. In our primary analyses, we only considered cancers that occurred more than 6 months after the index date to minimize the impact of preexisting cancers. In sensitivity analyses, we included all cancers after the index date.
Covariates
Covariates were identified using a combination of ICD-9 codes and CPT codes from inpatient and outpatient visit records, laboratory data, and pharmacy data in the year prior to the index date.
Follow-up time
The follow-up time was calculated from the date of study inclusion (bariatric surgery or index date for matched controls) until the first occurrence of one of the following events: diagnosis of cancer, the end of health care coverage or a break of > 92 days in health care coverage, death, or the end of follow-up on December 31, 2014.
Statistical Analysis
We calculated means, medians, and frequencies for variables to characterize the study sample. Subjects with a history of bariatric surgery were compared to the matched controls using standardized differences. Kaplan-Meier curves were generated for each outcome of interest. We estimated Cox proportional hazard models to compare the development of any cancer, non-obesity associated cancer, obesity associated cancer, and individual obesity-associated cancers between the two groups. Matching was accounted for by using robust sandwich estimators. These estimators were chosen over estimators based on treating the matched sets as strata because the sandwich estimators make more complete use of the data in the presence of covariates or when examining some subgroups. To assess the extent to which our results were sensitive to this choice of methods, we compared results from the two methods for our main outcomes. For each outcome, we created both unadjusted models and non-parsimonious models adjusted with the covariates that potentially impact cancer risk. We tested the assumption of constant proportional hazards over time by testing the surgery by time linear interaction. The alpha for all tests was a two-tailed p=0.05, unadjusted for multiple tests, and all analyses were performed using SAS v9.4 (Cary, NC).
Results
In our final matched cohort, we had 22,211 bariatric surgical cases matched to 66,481 control subjects who did not receive surgery. Over 80% were female. Due to the matching process, surgery cases and controls were comparable on most demographic and clinical characteristics including the presence of diabetes and hypertension (Table 1). Subjects with a history of bariatric surgery were more likely to have several important cancer related risk factors including a slightly higher BMI, higher prevalence of smoking, slightly greater use of hormone replacement therapy, and slightly higher baseline rates of non-alcoholic steatohepatitis. The use of screening mammograms was slightly higher amongst the control group in the year prior to the index date. The average follow-up time was longer in the bariatric surgery cases (47 months) than the controls (41 months; P<0.001). The bariatric surgery group developed 488 incident cases of cancer over 87,071 person-years of follow-up while the nonsurgical group developed 2,055 incident cases of cancer over 228,010 person-years of follow-up.
Table 1.
Baseline Characteristics of Bariatric Surgical Patients and Matched Non-Surgical Patients
| Surgical Patients (n=22198) | Matched Non-Surgical Patients (n=66427) | Standardized Difference | |
|---|---|---|---|
| Female (%) | 81.08 | 81.13 | a |
| Age, mean (SD) | 45.02 (11.08) | 45.10 (11.05 | 0.01 |
| Body Mass Index, mean (SD), kg/m2 | 44.84 (6.71) | 44.37 (6.24) | 0.07 |
| Follow-up, mean (SD), months | 47.07 (23.08) | 41.19 (24.42) | 0.24 |
| Race/ethnicity | |||
| Non-hispanic white (%) | 48.5 | 41.34 | |
| Hispanic (%) | 30.55 | 32.22 | |
| African-American (%) | 16.02 | 15.74 | |
| Asian (%) | 1.42 | 2.36 | |
| Other (%) | 3.5 | 8.35 | |
| Site (%) | a | ||
| Group Health Cooperative | 4.65 | 4.63 | |
| Kaiser Permanente Southern Cal. | 58.74 | 58.78 | |
| Kaiser Permanente Nouthern Cal. | 27.61 | 27.65 | |
| Kaiser Permanente Northwest | 2.03 | 2.02 | |
| Kaiser Permanente Colorado | 6.97 | 6.92 | |
| Clinical Characteristicsb | |||
| Diabetes (%) | 34.47 | 35.12 | 0.01 |
| % of Patients with Diabetes on Insulin | 30.78 | 29.01 | 0.03 |
| % of Patients with Diabetes on Metformin | 60.02 | 63.53 | 0.07 |
| Hypertension (%) | 60.28 | 60.06 | 0.005 |
| Hyperlipidemia (%) | 42.96 | 36.38 | 0.13 |
| % of Patients with Hyperlipidemia on Statins | 71.95 | 82.6 | 0.27 |
| Coronary Artery Disease (%) | 2.04 | 1.92 | 0.08 |
| Smoker, ever (%)c | 32.1 | 25.46 | 0.15 |
| Nonalcoholic steatohepatitis (%) | 2.93 | 1.32 | 0.12 |
| Alcohol Abuse (%) | 1.32 | 2.09 | 0.06 |
| Peripheral Vascular Disease (%) | 0.98 | 1.56 | 0.05 |
| Cerebral Vascular Disease (%) | 0.76 | 1.1 | 0.03 |
| Use of Hormone Replacement Therapy | |||
| Estrogen Only (% of women) | 3.12 | 2.08 | |
| Progesterone Only (% of women) | 2.83 | 2.52 | |
| Combination (% of women) | 1.83 | 1.26 | |
| Elixhauser, mean (SD) | 1.72 (1.55) | 1.61 (1.48) | 0.07 |
| Mammogram (%) | 19.81 | 22.94 | 0.08 |
| Bariatric Procedure Type (n,%) | |||
| Gastric Bypass | 13545 (61.0%) | ||
| Sleeve Gastrectomy | 6047 (27.2%) | ||
| Laproscopic adjustable band | 1236 (5.6%) | ||
| Otherd | 15 (0.1%) | ||
| Indeterminatee | 1355 (6.1%) | ||
Cases and Controls matched exactly
All clinical conditions were identified in the year prior to the index date
Smoking was only identified using ICD-9 codes
Other includes biliopancreatic diversion and vertical gastric banding
Indeterminate includes procedures for which more than one procedure type was coded for on the same day
Kaplan Meier curves show that the unadjusted rates of incident cancer differed across the bariatric cases and controls. The K-M plots for each group deviate early and their diverging trajectories continue throughout the follow-up period (Figure 2). K-M estimated cancer-free survival at 3, 5, and 10 years, was 98.45%, 97.2%, and 94.11% for the bariatric surgery patients and 97.34%, 95.56% and 89.25% for the control patients, respectively.
Figure 2.
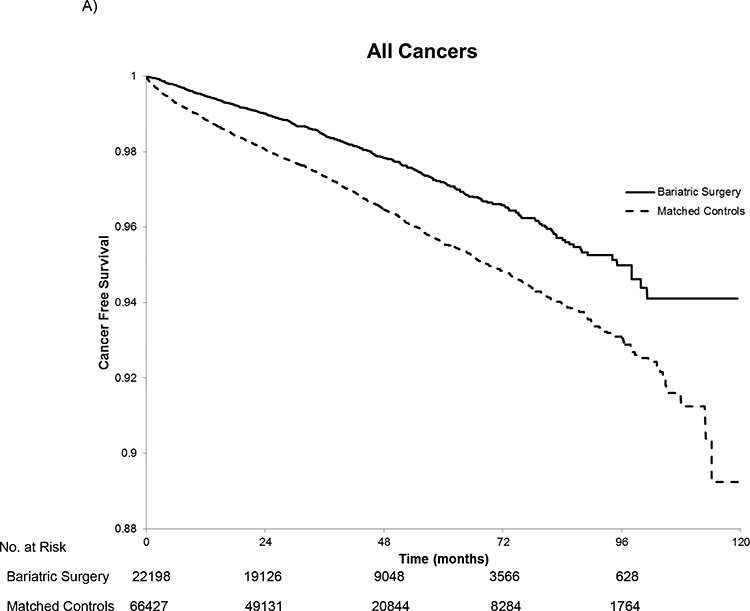
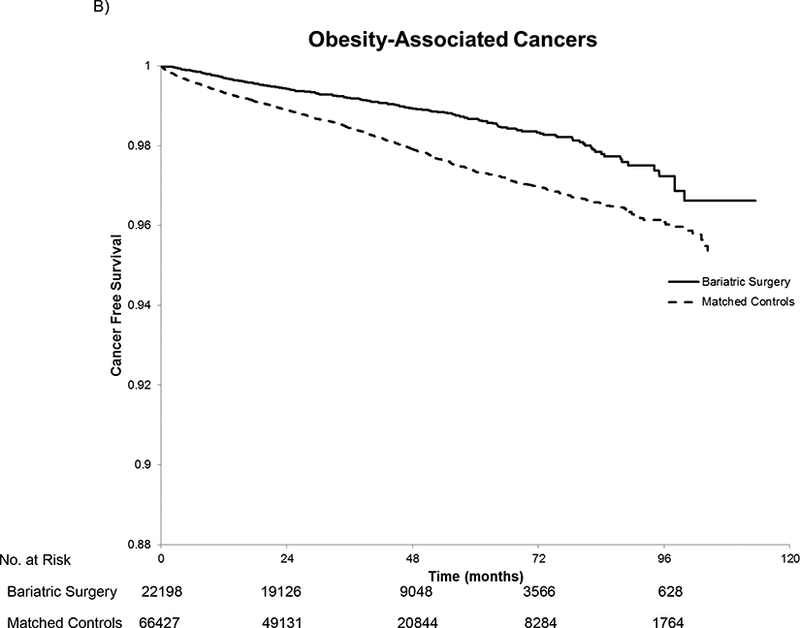
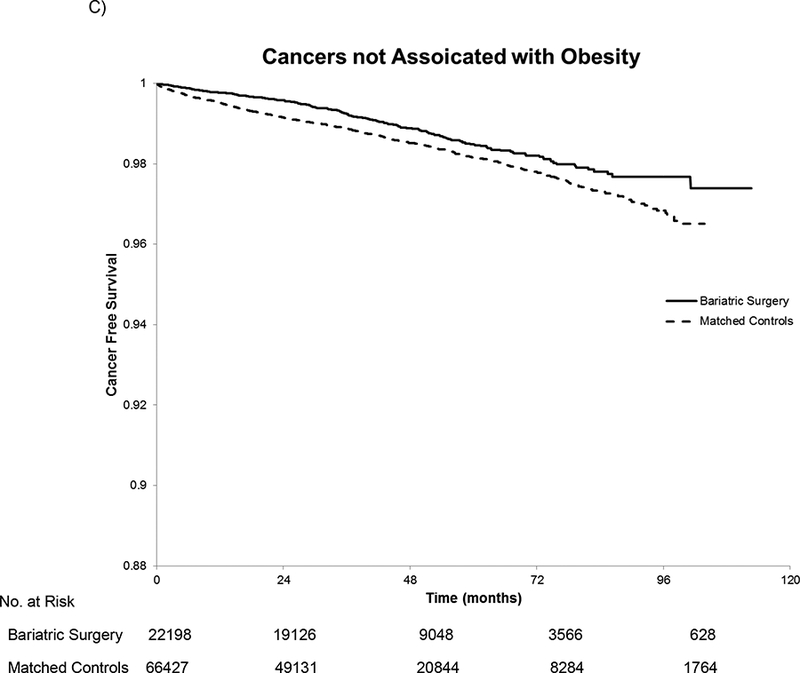
Kaplan-Meier Estimated Cancer-Free Survival for All Cancers (A), Obesity-Associated Cancers (B), and Cancers not Associated with Obesity(C). In panel A, there were 488 cancers in the bariatric surgery group and 2,055 cancers in the matched controls. For the obesity-associated cancers in panel B, there were 246 cancers in the bariatric surgery group and 1,185 in the matched controls. In panel C, there were 242 cancers not associated with obesity in the bariatric surgery group and 872 among the matched controls. The log rank test had a p-value of <0.001 for all three comparisons. The number at risk is the same in each panel because patients were censored at the first cancer regardless of the type.
In matched unadjusted and multivariable adjusted Cox proportional hazards models the proportional hazards assumption was met for all models. Patients undergoing bariatric surgery had a 33% lower hazard of developing any cancer during follow-up (HR 0.67, 95% C.I. 0.60, 0.74, P<0.001) than matched controls (Table 2.). Results were similar when our outcomes were restricted to obesity associated cancers (HR 0.59, 95% C.I. 0.51, 0.69, P<0.001). Although there was no statistically significant interaction between the effect of bariatric surgery on cancer incidence by sex in these models, we had a priori planned to examine results with sex strata. Women who had bariatric surgery were associated with significantly fewer incident cancers (HR 0.64, 95% C.I. 0.57, 0.72, P<0.001), obesity-associated cancers (HR 0.58, 95% C.I. 0.49, 0.67, P<0.001), and cancers not obesity-associated (HR 0.74, 95% C.I. 0.62, 0.89, P=0.001) than in female control patients. For men, there were no statistically significant reductions in cancer risk for any cancer type, although there was a non-significant trend towards fewer obesity-associated cancers. The unadjusted and adjusted hazard ratios were very similar for all analyses. All models accounted for the matching on age, sex, BMI, Elixhauser comorbidity index score and study site and the adjusted models were adjusted for race, diabetes, hyperlipidemia, hypertension, coronary artery disease, peripheral vascular disease, nonalcoholic steatohepatitis, a history of smoking, alcohol use, and use of hormone replacement therapy.
Table 2.
Hazard Ratios for the Risk of Cancer from Cox Regression Modelsa
| Outcomeb | N | Unadjusted HR | 95% CI | P-value | Adjusted HRc | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| All Patients | Any Cancer | 87996 | 0.70 | 0.63–0.77 | <.001 | 0.67 | 0.60–0.74 | <.001 |
| Obesity-Associated Cancer | 87996 | 0.60 | 0.52–0.70 | <.001 | 0.59 | 0.51–0.69 | <.001 | |
| Cancer not Associated with Obesity | 87996 | 0.83 | 0.71–0.96 | 0.01 | 0.77 | 0.66–0.89 | 0.001 | |
| Women | Any Cancer | 71341 | 0.66 | 0.50–0.75 | <.001 | 0.64 | 0.57–0.72 | <.001 |
| Obesity-Associated Cancer | 71341 | 0.58 | 0.50–0.68 | <.001 | 0.58 | 0.49–0.67 | <.001 | |
| Cancer not Associated with Obesity | 71341 | 0.80 | 0.67–0.96 | 0.02 | 0.74 | 0.62–0.89 | 0.001 | |
| Men | Any Cancer | 16655 | 0.85 | 0.68–1.07 | 0.17 | 0.79 | 0.63–1.002 | 0.054 |
| Obesity-Associated Cancer | 16655 | 0.71 | 0.47–1.07 | 0.1 | 0.7 | 0.46–1.07 | 0.1 | |
| Cancer not Associated with Obesity | 16655 | 0.94 | 0.71–1.24 | 0.64 | 0.85 | 0.64–1.12 | 0.25 | |
All models accounted for matching on age, sex, BMI, Elixhauser comorbidity index score and study site
all outcomes start at 6 months after the index date
Models adjusted for race, diabetes, hyperlipidemia, hypertension, coronary artery disease, peripheral vascular disease, nonalcoholic steatohepatitis, a history of smoking, alcohol use, and use of hormone replacement therapy.
Of the obesity-related cancers, postmenopausal breast (HR 0.58, 95% C.I. 0.44, 0.77, P<0.001), colon (HR 0.59, 95% C.I. 0.36, 0.97, P=0.04), endometrial (HR 0.50, 95% C.I. 0.37, 0.67, P<0.001), and pancreatic cancer (HR 0.46, 95% C.I. 0.22, 0.97, P=0.04) were all significantly lower after bariatric surgery compared to controls (Figure 3). For esophageal adenocarcinoma, there were no cases amongst the bariatric surgery group and 16 cases in the control group (p=0.02). Liver, gallbladder, multiple myeloma, ovarian, rectal and thyroid cancer showed no statistically significant reduction in incidence following bariatric surgery compared to controls although all of the hazard ratios were less than one.
Figure 3.
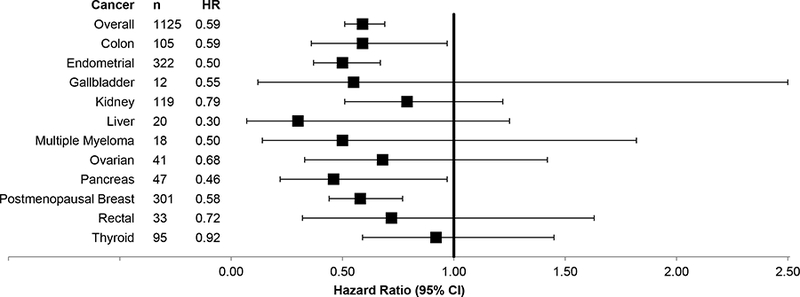
Forest Plot of Multivariable Cox Proportional Hazards models for Obesity-Associated Cancers. The box represents the hazard ratio and the error bars depict the 95% confidence interval. Matching occurred on age, sex, BMI, Elixhauser comorbidity index score and study site. The models are adjusted for race, diabetes, hyperlipidemia, hypertension, coronary artery disease, peripheral vascular disease, nonalcoholic steatohepatitis, a history of smoking, alcohol use, and use of hormone replacement therapy.
For our primary analyses reported above, we excluded cancers occurring within 6 months after the index date. In sensitivity analyses we eliminated this exclusion and included all cancers occurring after the index date. Our results were unchanged but the association between bariatric surgery and lower cancer incidence was stronger (all cancers: HR 0.59, 95% CI 0.54, 0.66, P<0.001; obesity-associated cancers: HR 0.53, 95% CI 0.47, 0.61, P<0.001; cancers not associated with obesity: HR 0.67, 95% CI 0.59, 0.78, P<0.001). In unadjusted models predicting each cancer outcome, the sandwich estimator method and the strata method produced HRs that differed by less than 10%, usually by less than 5%. The surgery by time linear interaction was nonsignificant in all models satisfying the assumption of constant proportional hazards over time.
Discussion
Obesity is a significant risk factor for the development of many types of cancer. In this large multicenter cohort study, we found that bariatric surgery is associated with a lower long-term risk of cancer compared to carefully matched patients with severe obesity who did not get bariatric surgery. The reduction was greatest for cancers that have been shown to be associated with obesity and persisted for the duration of 10 year follow up in this study.
Further, we found that some cancer types appeared to be reduced by bariatric surgery, while others did not. The differing rates of cancer reduction for the various obesity related cancers is not surprising given the multiple mechanisms by which obesity increases the risk of cancer26, 27. Reductions in cancer risk were strongest for post-menopausal breast and endometrial cancers. Both of these cancers are highly sensitive to estrogen levels28, 29 and react rapidly to changes. Weight loss has been shown to reduce levels of circulating estrogen30 thereby decreasing the risk of these cancers. Other obesity-associated cancers, such as thyroid cancer, may not be impacted as much by bariatric surgery due to the alternate mechanisms by which obesity increases their risk.
The risk of cancer was significantly lower among women but not men when the analysis was stratified. There are several potential reasons for this finding. Only women are at risk of developing postmenopausal breast and endometrial cancer, and these are the two most common obesity-associated cancers with breast cancer being the most common cancer among women31. Additionally, over 80% of the surgical cases were women. For men, prostate and lung cancer are the most common cancers, neither of which is associated with obesity2, 31.
Our results are consistent with several prior, smaller studies that examined the risk of cancer after bariatric surgery, including two with long term follow-up. In the Utah Obesity Study of 6,596 patients who had gastric bypass and were followed for a mean of 12.5 years, Adams and colleagues32 found that total cancer incidence was reduced (HR 0.76; 95% CI, 0.65–0.89; P = 0.0006) and obesity associated cancers were also reduced (HR, 0.62; 95% CI, 0.49–0.78; P < 0.0001). These hazard ratios are slightly different than those reported here; however, Adams and colleagues only adjusted for age, sex and BMI and did not account for differences in comorbidity across groups. The Swedish Obese Subjects (SOS) study included 2010 bariatric cases and a well-defined comparison group of 2037 subjects that were matched on 18 clinical characteristics. The SOS study also found fewer cancers in the bariatric surgery cohort compared to the control patients (HR 0.67, 95% CI 0.53–0.85, P=0.0009)18. Similarly to our study, the finding was significant in women but not in men.
In our current study of gastric bypass and sleeve gastrectomy, which are the two main procedures performed in the U.S., bariatric surgery was associated with a reduced risk of esophageal adenocarcinoma. A Swedish study22 including 34,437 bariatric surgery patients found no difference in esophageal adenocarcinoma incidence between bariatric surgery cases and the general obese population. However, this study did not match cases and controls, and, as in the SOS study, this study included bariatric procedures that are now outdated. These older procedures have different effects on weight loss and gastroesophageal reflux disease which may explain the difference in our findings.
Finally, a study involving 15,095 bariatric surgery patients and 62,016 obese control patients identified from Swedish registries found an increased risk of colorectal cancer after bariatric surgery33. This study has many of the same limitations as the other Swedish studies noted above, and importantly, did not distinguish between colon cancer and rectal cancer. It is likely that the impact of bariatric surgery on these two cancers is different given their differing etiology, risk factors, and epigenetic and genetic profiles34 as we found a reduction in the risk of colon cancer but no reduction in the risk of rectal cancer.
Our study has several limitations. As in all observational studies, unmeasured differences may exist between the bariatric surgery patients and the control patients. While the matching process and control for covariates attempted to mitigate this, differences may persist, particularly in behavioral risk factors, such as diet and exercise. For example, we found that the control subjects in our study were more likely to get mammograms in the year before surgery, an example of a healthy behavior. However, with our rich resource of detailed data on longitudinal medical risk factors and cancer screening from the electronic medical record, we were able to match on an extensive list of covariates that were not previously considered in other studies of cancer outcomes following bariatric surgery. Another limitation is that we identified a history of smoking through ICD-9 codes, which may misclassify some individuals; however this is unlikely to have a significant impact on our results given that smoking is not a strong risk factor for most of the cancers in this study. Because our study is observational in nature, we cannot draw firm causal conclusions about the relationship between bariatric surgery and incident cancer. However, many of the elements of causality are met, including the large effect size, the consistency of the associations across multiple observational studies, the plausibility of the mechanism of effect (particularly for obesity-associated cancers), and the temporality demonstrated in this long-term follow-up study35. A limitation of this study is that the average follow-up was less than 4 years. Given the natural history of many cancers, this may lead to underestimation of the association between bariatric surgery and cancer as the effect may not be seen for several years. However, the hazards remained proportional throughout follow-up meaning that the association between bariatric surgery and cancer risk did not change over time. Our study also has many strengths. The large sample size, comprehensive data sources, long term follow-up, and matching methods all contribute to the strength of the results.
Conclusions
In this carefully matched retrospective cohort study we have demonstrated that bariatric surgery is associated with a lower the risk of obesity-associated cancers, especially post-menopausal breast, endometrial and colon cancer. We found no significant association between bariatric surgery and cancer risk among men. Promoting intentional weight loss, especially through the use of bariatric surgery, may greatly reduce the risk of cancer amongst patients with severe obesity.
Acknowledgments
Source of Support: NIH/NCI 1R01CA175346–01A1
Footnotes
Conflicts of Interests:
No conflicts of interest to declare.
References
- 1.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine. 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polednak AP. Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev 2008;32:190–9. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- 8.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–50. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 10.Sturm R Increases in morbid obesity in the USA: 2000–2005. Public Health 2007;121:492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med 2005;142:547–59. [DOI] [PubMed] [Google Scholar]
- 12.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:933–49. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of Weight Status following Laparoscopic Gastric Bypass. Obes Surg 2006;16:1227–31. [DOI] [PubMed] [Google Scholar]
- 15.Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes. The New England journal of medicine. 2014;371:682. [DOI] [PubMed] [Google Scholar]
- 16.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA surgery. 2016;151:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring). 2009;17:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–62. [DOI] [PubMed] [Google Scholar]
- 19.Arterburn DE, Eid G, Maciejewski ML. Long-term survival following bariatric surgery in the VA health system--reply. Jama. 2015;313:1474–5. [DOI] [PubMed] [Google Scholar]
- 20.Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis 2008;4:691–5. [DOI] [PubMed] [Google Scholar]
- 21.McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg 2009;208:1093–8. [DOI] [PubMed] [Google Scholar]
- 22.Maret-Ouda J, Tao W, Mattsson F, et al. Esophageal adenocarcinoma after obesity surgery in a population-based cohort study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. The Lancet. Oncology 2009;10:653–62. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Medical care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 25.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas 2010;67:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annual review of medicine. 2010;61:301–16. [DOI] [PubMed] [Google Scholar]
- 27.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews. Cancer 2004;4:579–91. [DOI] [PubMed] [Google Scholar]
- 28.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute. 2003;95:1218–26. [DOI] [PubMed] [Google Scholar]
- 29.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1531–43. [PubMed] [Google Scholar]
- 30.de Waard F, Poortman J, de Pedro-Alvarez Ferrero M, et al. Weight reduction and oestrogen excretion in obese post-menopausal women. Maturitas 1982;4:155–62. [DOI] [PubMed] [Google Scholar]
- 31.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 32.Adams TD, Hunt SC. Cancer and obesity: effect of bariatric surgery. World journal of surgery. 2009;33:2028–33. [DOI] [PubMed] [Google Scholar]
- 33.Derogar M, Hull MA, Kant P, et al. Increased risk of colorectal cancer after obesity surgery. Annals of surgery. 2013;258:983–8. [DOI] [PubMed] [Google Scholar]
- 34.Hong TS, Clark JW, Haigis KM. Cancers of the colon and rectum: identical or fraternal twins? Cancer discovery. 2012;2:117–21. [DOI] [PubMed] [Google Scholar]
- 35.Hill AB. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]


