Abstract
The production of emotional tears appears to be uniquely present in Homo Sapiens. Despite the ubiquity of this human behavior, research is only just beginning to uncover the neurobiological underpinnings of human emotional crying. In this paper, we review the current state of the literature investigating the neurobiological aspects of this uniquely human behavior, including neuroanatomical, neurochemical, and psychophysiological findings. To set the context for this review, we first provide a brief overview of the evolutionary background and functions of tearful crying. Despite an accumulating understanding of the neurobiology of human emotional crying, the primary sources of information are currently from animal studies and observations in neurological patients suffering from pathological crying. Currently, most of the research on the neurobiology of crying in humans has focused on autonomic physiological processes underlying tearful crying, which may yield essential clues regarding the neural substrates of the production of crying behavior and its effects on the crier. Further challenges in elucidating the neurobiology of crying involve the complexity of crying behavior, which includes vocalizations, tear production, the involvement of facial musculature, subjective emotional experience, emotion regulatory behaviors, and social behaviors. Future research is needed to comprehensively characterize the neurobiology of this intriguing and complex human behavior.
Introduction
Evolutionary origins of human emotional crying and the adaptive functions of tears as a social signal
Whereas producing emotional tears appears to be a uniquely human behavior, human infants share the production of distress calls with the young of most other mammals and birds, which are typically displayed when they are separated from their mothers [1]. This reaction to separation is typically immediate (i.e., reflex-like), appears to require no previous learning, and it is relatively consistent across different mammal and bird species. The similarity of the acoustic structure of cries across most primates, together with some other specific features of crying, suggests either that this characteristic acoustic structure arose early in primate evolution and had changed very little, or that there has been a considerable degree of convergent evolution toward an acoustic structure that is highly adaptive in a variety of habitats and social settings [2].
Emotional crying in humans seems to have its evolutionary basis in these animal distress calls, which is evident in its solicitation of help-provisioning and nurturing behavior. Gračanin and colleagues [1] postulate that the connection between tears and vocal crying might have developed when, in human newborns, the strong contractions of the orbicularis oculi muscle during the production of distress vocalizations stimulated the sensitive corneal sensory nerves that then trigger the release of tears by the lacrimal gland (which is comparable with the production of non-emotional tears during yawning). This coupling at least suggests that humans at some point in their evolution were confronted with unique challenges for which the shedding of tears proved to be advantageous.
Despite the similarities, humans differ in their crying behavior in two aspects from other animal species that exhibit distress calls. First, in the vast majority of animal species, this behavior is predominantly displayed during infancy, whereas among adult members it is only exceptionally observed (with dogs as a notable exception, among others). Second, whereas in animals crying is limited to vocal forms of expression (i.e., distress vocalizations, separation calls), in humans the shedding of visible tears is an essential additional feature (beginning in infants at approximately 4-8 weeks of age). The production of visible tears appears to gain relevance with increasing age, while the vocal component seems to lose significance. In particular, being moved, a complex emotional response mostly absent in children, is typically associated with silent tear production, occasionally in combination with chills or goosebumps (see later).
Gračanin and colleagues [1] propose that there is a link between visible tear production and the unique prolonged childhood of humans. In this period of prolonged development, children have fully developed motoric skills but are still very much dependent on the protection, nurturance, and guidance of adults. In particular, during this developmental phase, a silent strategy to elicit needed support from specific individuals most likely to provide the desired assistance must have been superior to an acoustical signal that attracts the attention of not just caregivers, but also strangers and possible predators. Consequently, Gračanin et al. conclude that the principal function of crying is to promote social bonding and mutual pro-social behavior.
Another significant development in human crying behavior includes age-related changes in antecedents that evoke the crying response, as well as the development of the gender differential (with adult women crying on average 4-5 times and adult men 0-1 times per month; [3,4]). Adult humans shed their emotional tears typically at the most important events of their lives, including positive events like weddings and the birth of children, as well as negative events such as those involving separation and loss. However, given the frequency of emotional crying in adult humans, it must be concluded that we most often cry in relatively mundane situations. These include common daily occurrences such as conflicts and minor frustrations, as well as reactions to movies and music [4].
Regarding the functions of emotional crying, until recently the focus has been on the intra-personal effects of this behavior (i.e., the effects of crying on the crying individual him or herself). However, research strongly suggests that the possible mood benefits of crying for the crier depend to a great extent on how observers react to the tears [5,6]. Perhaps the direct effects of crying itself, if any, are very limited at best (see, however, [7]). More recently, the focus of research has turned to the effects of tears on observers in the social environment. This research is still in an early phase of development, but the results thus far have yielded evidence that visible tears impact how others perceive the crier’s emotional state, personality, and behavioral intentions (see [1], for a review). More precisely, crying individuals tend to be seen as more warm and friendly, more sincere and honest, but also as likely to be more emotionally unstable, incompetent, and manipulative. Generally, people tend to react to criers (vs non-criers) with a greater willingness to provide help, although several factors likely moderate this reaction in specific functional ways [4].
It is important to highlight that the production of tears represents only one of several important aspects of human emotional crying. Although tears and distress vocalizations are the most characteristic aspects of crying, there is more going on in the face when an individual cries. Specifically, crying typically involves the activation of several facial muscles [4]. In addition, there is the specific psychological state which is associated with the emotional behavior (both as a precursor and a consequence). As we will see below, this implies connections with several specific brain structures and nerves that orchestrate the involvement of facial musculature and neural circuits supporting emotional responding and regulatory behaviors.
In what follows, we review the current literature relevant to the understanding of the neurobiology of human emotional crying. We will first briefly discuss what is known about the anatomy of the vocal system and the lacrimal gland, as well as how these are innervated by specific brain structures. Given the relative lack of human research, we will then summarize the relevant animal literature on the neurobiology of distress vocalizations, as the distress calls of other species likely share similar neural mechanisms. However, we also acknowledge that the production of emotional tears (and some related specific facial expression characteristics of human tearful crying) may have different functional characteristics and neurobiological underpinnings in humans. Therefore, where available, we discuss findings specific for human emotional tears, particularly those pertaining to the motor aspects of vocal crying as well as autonomic nervous system activity associated with the crying process. The animal work additionally provided some relevant information on the neural underpinnings of specific emotional states that are related to crying. However, we should also point out that human (tearful) crying can be associated with a mixture of feelings (not just sadness and helplessness, but also being touched, among others) of which we currently do not yet know the precise neurobiological accompaniments. Finally, we summarize what is now known about the neurobiology of human emotional crying, what remains unknown, and important directions for future research.
The Anatomy and Neural Innervation of the Lacrimal Apparatus and Associated Structures
The vocal system
The production of distress vocalizations is a complex process, which includes laryngeal activity, respiratory movements, and supralaryngeal (articulatory) activity. Mainly based on work with squirrel monkeys, Jürgens [8] found strong support that the motor neurons involved in nonverbal (human) emotional vocalizations are distributed along the neuraxis from the level of the pontine brainstem down to the lumbar spinal cord. As we will see later, in particular, the involvement of the PAG and cerebellum seems evident.
The involved facial musculature
The facial muscles involved in crying are the M. frontalis, the M. corrugator, the M. orbicularis oculi, as well as the M. zygomaticus, the M. depressor anguli oris, and the M. mentalis [4]. The lower and upper facial muscles involved in emotional expressions are innervated via the facial nucleus by the supplementary motor cortex and the rostral cingulate motor cortex, as well as the primary motor cortex, the ventral lateral premotor cortex, and the caudal cingulate motor cortex [9].
The lacrimal gland and its innervation
The lacrimal glands, located in the upper lateral quadrant of the ocular orbits, are responsible for both our reflexive and emotional tears. In contrast, basal tears, which are essential for continuous protection and nourishment of the eye, are produced by the accessory lacrimal glands, located under the eyelids. The lacrimal glands consist of two lobes, the lower of which is situated under the levator aponeurosis and connects to the lateral superior conjunctival fornix by approximately 12 excretory ducts. The lacrimal gland functional unit consists of sensory afferent nerves from the cornea and conjunctiva, efferent parasympathetic and sympathetic nerves that innervate the lacrimal gland, the lacrimal gland secretory cells, and the lacrimal gland excretory ducts. For an extensive description of the neural innervation of the lacrimal glad, we refer to Dartt [10]. Here we limit ourselves to the most important features.
The fluid secreted by the lacrimal glands consists predominantly of water, electrolytes, and proteins. The appropriate amount and composition of lacrimal gland fluid are critical for a healthy, intact ocular surface, and the concentrations of these components are modified by the cells of the duct system [10]. The chief neurotransmitters that regulate the secretions of the secretory cells in the lacrimal gland are the parasympathetic neurotransmitters acetylcholine and vasoactive intestinal peptide, as well as the sympathetic neurotransmitters norepinephrine and neuropeptide Y. In addition, the enkephalins, which are two naturally occurring peptides in the brain with pain relieving properties, provide an inhibitory pathway that blocks lacrimal gland secretion by blocking delta opioid receptors [10,11]. Acetylcholine stimulates the release of electrolytes, water, proteins, and mucins into the tear film. While the precise mechanisms of secretion of these substances from the lacrimal gland differ, they are all under neural control. This allows for a rapid response to meet the needs of the cells of the ocular surface dependent on the environmental conditions, including temperature, humidity, mechanical, chemical, or pathogenic condition and the requirements of the surface epithelia (e.g., growth control, wound healing, electrolyte transport, maintenance of the tear/aqueous humor barrier, and shedding of surface proteins).
Reflex lacrimation occurs when the sensory nerve endings on the ocular surface respond to changes in the environment resulting in a rapid secretion of fluid by the lacrimal gland to wash away and chemically neutralize potential threats to the tear film. Stimulation of corneal sensory nerves facilitates the secretion of fluid by the lacrimal gland and promotes vasodilation, which may result in a quick production of reflex tears (there is no storage of tears that can be shed immediately). This stimulation of the lacrimal gland, mediated by the facial nerve, is referred to as a trigeminal–parasympathetic reflex. However, this is not a simple reflex, because the sensory input from the cornea and conjunctiva is processed in the lacrimal nuclei, a group of cells in the superior salivatory nucleus involved in lacrimal functions (sometimes referred to as the “lacrimal nucleus” e.g., [10,12] although it is not a true distinct nucleus, which also process input from other centers (including the prefrontal cortex and limbic structures, such as the cingulate gyrus), which modulates the output. This modulation implies that low levels of sensory nerve stimulation produce enough lacrimal gland fluid to adequately supply the ocular surface, whereas more intense stimulation causes increased lacrimal gland fluid secretion to wash away deleterious compounds on the ocular surface and produce overflow tears. However, the secretion of tears can also be facilitated by input from the central autonomic network [13], without the involvement of the sensory nerves.
The lacrimal glands are innervated by parasympathetic and sympathetic nerves, but the parasympathetic system predominates, both anatomically and functionally [10,14]. The stimulation of parasympathetic fibers results in a clear increase in tear secretion, whereas a loss of parasympathetic innervation suppresses lacrimal gland function [10]. On the other hand, stimulation of sympathetic fibers appears to have little effect on tear secretion, but this pathway is indirectly involved through the regulation of the blood supply of the main lacrimal gland.
The parasympathetic innervation of the lacrimal glands originates in the lacrimal nuclei located in the superior salivary nucleus in the pontine tegmentum and passes the geniculate ganglion without synapsing, and then emerges as the greater superficial petrosal nerve, which joins the deep petrosal nerve to form the vidian nerve, which terminates in the pterygopalatine or sphenopalatine ganglion [10]. The postganglionic axons that are the output of this ganglion project to the lacrimal gland and supply its parasympathetic innervation (see Figure 1). Dysfunctions in producing (different kinds of) tears may thus be due to malfunctioning of different parts of this system.
Figure 1.
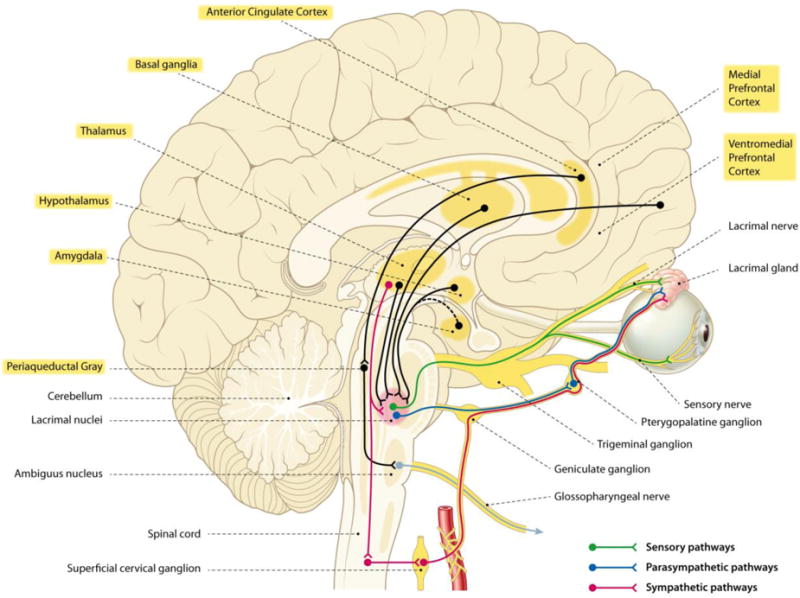
Anatomical schematic of the neural innervation of the lacrimal gland and the neurobiological structures involved in vocal emotional crying. Note that the lacrimal nuclei are located in the superior salivatory nucleus (not represented in relative true size). These latter cells modulate the connection between the sensory nerves and the parasympathetic efferents to the lacrimal gland. For the sake of simplicity, the further pathways involved in (voluntary) crying and the possible influences of the cerebellum are not indicated. The yellow marked labels refer to structures which are part of the central autonomic network (CAN).
For example, the substantial reduction in the secretion of reflex tears often observed in patients with familial dysautonomia, a rare neurodevelopmental genetic disorder, is likely due to a selective impairment of the sensory neurons involved in the tear reflex [15]. The lack of corneal afferent sensation in familial dysautonomia patients may explain their impairments in reflex and basal tearing. But it is much harder to assume that these afferent mechanisms also play a role in impairments in the production of emotional tears, which are also characteristic for these patients. Instead, dysfunction of structures that would have a role in top-down mechanisms of emotional tearing seems much more likely. A possible future direction of research on the neurological underpinnings of tearful crying might be inspired by recent findings suggesting that there is a significant reduction in the number of Van Economo or spindle neurons of the insular cortex in patients with familial dysautonomia [15,16]. The possible role of insular spindle neurons also seems plausible because tearful crying and these specific neural units both appeared relatively recently in human evolution. In addition, these neurons are much more abundant in humans than in great apes, our closest living ancestors, and they are also found in brains of some whale and elephant species, which points to convergent evolution that is most likely a consequence of the adaptation to large brains [17]. However, findings suggesting their specific involvement in empathic responses, social awareness, and self-control in humans (e.g., [18]) may point to their possible role in emotional crying as well.
In the next section, we describe the involvement of the autonomic nervous system in crying in detail.
Crying and the Autonomic Nervous System
In the previous section, we reviewed the importance of parasympathetic innervation of the lacrimal gland in tear production. The parasympathetic nervous system conserves energy and slows heart rate and is important for processes related to rest, recovery, and relaxation. According to Porges’ polyvagal theory [19,20], the mammalian vagus nerve (10th cranial nerve; responsible for parasympathetic innervation of multiple organs) can quickly alter cardiac activity to support engagement and disengagement with the environment. Specifically, during periods of rest, the vagus has an inhibitory influence on the heart, acting as a “brake,” whereas, during time of stress, this influence of the vagus on the heart can be quickly withdrawn, resulting in an increase in physiological arousal to prepare the organism for engaging with the stressor. Respiratory sinus arrhythmia (RSA), often assessed as the high-frequency component of heart rate variability (HF HRV) reflecting variations in heart rate linked to the respiratory cycle, is a commonly used index of resting vagal tone and thought to be responsible for top-down emotion regulatory processes via specific neural circuits (e.g., nucleus ambiguous, mPFC; [21,20]), and has been associated with the ability to regulate responses to emotion or stress (e.g., [22–25]). Importantly, the vagus nerve is also linked to the cranial nerves that regulate social engagement via facial expression and vocalization [26]. Since the parasympathetic innervation of the lacrimal gland occurs via seventh cranial nerve, a possible co-activation of the vagus nerve with the production of emotional tears is likely the consequence of the activity of higher brain centers stimulating parasympathetic fibers in both of these nerves.
Recently, theorists have attempted to stake out two seemingly opposing views of human crying: (1) crying as an arousing behavior that typically accompanies distress, or (2) crying as a soothing behavior that promotes the reduction of arousal after distress. However, unpacking the peripheral psychophysiology of crying is a complex issue given that it is a complex behavior with multiple components. Methodological differences in empirical studies to date, such as in timing of stimuli or measurement duration of psychophysiological responses, also make it difficult to compare research findings. First, it is difficult to determine the precise moment of onset of the tear response, and even more difficult to determine the precise offset. A second consideration is the possible confounding influences of sobbing and other respiratory influences which influence physiological measures, particularly RSA. In addition, other accompanying physical or behavioral reactions, such as chills and changes in facial expressions associated with crying may also have an impact on autonomic responses.
A handful of laboratory studies have attempted to investigate the psychophysiology of tearful crying in adults. The majority of these have used emotional films to elicit crying. In the earliest work, Kraemer and Hastrup [27] found an increase in sympathetic activity, as reflected in increased heart rate and skin conductance, for criers relative to non-criers just before the onset of crying. Gross and colleage [28], found increases in sympathetic activity (also assessed by increased heart rate and skin conductance) during crying for criers vs. non-criers, but found no difference for the 1-minute period just prior to crying onset which continued through the onset of crying.
While these earlier studies focused on changes in sympathetic activity, more recent studies have also assessed parasympathetic activity using RSA. Sakuragi and colleagues’ [29] findings corroborate Gross and colleagues’ observations in that tearful crying is associated with increases in sympathetic activity, as indicated by increases in heart rate and electrodermal activity, but they also observed decreases in parasympathetic activity (as indicated by decreases in RSA) during crying. In the post crying period, the balance of sympathetic to parasympathetic activation returned to baseline levels. Similar to prior findings, Rottenberg et al. [30] demonstrated that nondepressed criers showed greater sympathetic activation during the sad film than non-criers (i.e., increased heart rate and skin conductance), with less activation in a depressed group. In a follow-up study, Rottenberg and colleagues [31] found that nondepressed criers showed the expected increases in RSA during resolution of crying, whereas this physiological reaction was absent in depressed persons, suggesting that the physiological self-regulatory mechanisms invoked by tearful crying apparently may be compromised in depression. Hendriks et al. [32] also found that tearful participants exhibited heart rate increases that rapidly subsided after crying onset (as determined by a button press of the participant). The onset of the production of tears was also associated with increased RSA and slowed breathing, while there were no significant changes in sympathetic activity (as indexed by pre-ejection period, PEP). These results thus suggest increases in both sympathetic and parasympathetic activity just before the onset of crying. Crucially, parasympathetic activity remained increased for a longer duration after the crying onset in criers relative to non-criers, whereas sympathetic activity returned to baseline after the onset of tearing. It is plausible that this effect is driven by changes in respiratory rate, which was slowed in criers.
Most recently, other researchers have focused on the physiology of the combination of tears and chills (goosebumps), both characteristic expressions of being moved, in response to participant-selected film clips [33,34]. Specifically, Wassiliwizky et al. [33] observed increased sympathetic activity (e.g., increased skin conductance and heart rate) in periods of tearful crying, which always followed rather than preceded goosebumps. Mori and Iwagana [34] also showed that chills and tears developed during emotional music that elicited increases in arousal. However, there was some differentiation in responses as well—around the peak onset, chills were accompanied by increased electrodermal activity, whereas tears were accompanied by decreased electrodermal activity, indicating a reduction in psychophysiological arousal for tears and increased arousal for chills.
In sum, while our knowledge of the peripheral psychophysiology of tearful crying is still modest, some consistent patterns have emerged. Researchers have consistently found increases in sympathetic activity associated with crying. Findings for parasympathetic activation are somewhat more mixed, but there is some suggestion that the resolution of crying is associated with increases in parasympathetic activity, perhaps suggesting a recovery process associated with crying. The overall pattern suggests that the production of tears is both an arousing distress signal and a means to restore physiological balance (and perhaps also psychological), depending on how and when this complex behavior is displayed. Further research will be needed to investigate more precisely the time course of crying and the role of specific physiological mechanisms in crying onset and subsequent changes and its relationship with emotion (regulation) and stress processes. A methodological issue that needs future attention is the precise determination of the onset and offset of tearful crying. Both obervational and self-report methods have been used, but we do not yet know the validity and reliability of these different methods.
The Neural Circuitry Underlying Human Emotional Crying
Newman [2] provides a detailed review of the neural basis of cry vocalizations in animals and humans. Here we focus on human emotional crying (including tear production) and draw upon findings specific to vocalizations where relevant. The neural circuits involved in the different components of emotional crying (i.e., muscular activity, vocalization, tear production, emotional experience) appear to primarily include structures that are part of the central autonomic network (CAN) [13]. The CAN is involved in visceromotor, neuroendocrine, complex motor, and pain modulating control mechanisms essential for the maintenance of homeostasis, emotional expression, and responses to stress, and, as such, it is crucial for adaptation and survival. The CAN relies on the activity of several neurotransmitters, including amino acids, acetylcholine, monoamines, and neuropeptides.
CAN consists of a network of interconnected brain areas, such as the telencephalon, diencephalon, and brainstem, which control preganglionic sympathetic and parasympathetic visceromotor outputs. The specific components of the CAN generally include the following: (l) the insular and medial prefrontal cortices, (2) the central nucleus of the amygdala and the bed nucleus of the stria terminalis, (3) the hypothalamus, (4) the periaqueductal gray matter in the midbrain, (5) the parabrachial Kolliker-Fuse region in the pons, (6) the nucleus of the solitary tract (tractus solitarii), and (7) the medullary intermediate reticular zone, particularly the ventrolateral medulla [13]. In some cases, the cerebellum has also been considered part of the CAN [35]. As already said, the lacrimal nuclei, which are under the influence of the limbic system and/or CAN, are cells that directly modulate the secretion of tear fluid by the lacrimal gland (see Figure 1). The main output of the CAN is mediated through the preganglionic sympathetic and parasympathetic neurons. Preganglionic sympathetic neurons, located in the intermediolateral cell column and extending from the C8 to L2 segments of the spinal cord, are organized into separate functional units that innervate vasomotor, sudomotor, pilomotor, and visceromotor effectors.
As there is currently a dearth of neuroimaging research on tearful, emotional crying in adult humans, the limited neuroanatomical knowledge about emotional crying in humans so far is mainly based on animal studies (in particular of the brain structures involved in emotional vocalizations) and case studies with neurological patients suffering from pathological crying and extreme tearfulness, as well as occasional observations with very specific and rare patients groups (e.g., anencephalic children). These case studies further allow us to learn more about the neural circuitry of crying in the absence of the typically associated emotional responses. For example, case studies have demonstrated that involuntary emotional expressions may occur after particular lesions in certain subcortical brain areas [9]. Notably, anencephalic infants who lack a telencephalon are still able to cry vocally and tearfully, which is a strong indication that structures rostral to the midbrain are not essential to display this behavior. This additionally implies no essential role for the cortex and other structures within the cerebral hemispheres (at least in reflexive crying without an emotional component). Hence, this has been referred to as the “brainstem model” [2].
Newman [2] aptly reviews relevant research on animal brain stimulation, animal lesion studies, and the effects of administering different pharmaceutical agents on distress calls to better understand the neural circuits involved in vocal crying (see also [36]). The (rostral) periaqueductal gray (PAG) of the midbrain has been relatively frequently studied compared to other areas, with rather consistent results thus far. Electrical and chemical stimulation of this structure in infant guinea pigs and other animals results in the production of distress vocalizations, whereas PAG lesion studies demonstrate a long-lasting elimination or substantial reduction of distress vocalizations in adult squirrel monkeys (see also [37]). On the other hand, direct stimulation of the PAG or adjacent tegmentum in adult mammals generally does not result in the production of crying, even in species in which distress calls continue into adulthood (e.g., the squirrel monkey).
The PAG also receives extensive input from the amygdala and other limbic nuclei. The coordinated activity of these structures enables an individual (human or animal) to laugh, cry, or howl. When PAG is activated by impulses from the limbic system or neocortex (or via spinal nuclei involved in transmitting noxious stimuli), it activates the appropriate motor program and subsequently organizes and coordinates the oral-laryngeal and respiratory muscles so that the appropriate sounds can be produced [38]. Presumably, PAG stores these neural motor programs. Similar to laughing, crying is also dependent on the functional integrity of the PAG, as well as the pons, medulla, and cranial nerves 12, 10, and 9, 5 and 7. It is also important to note that authentic emotional expressions of subcortical origin are typically more synchronized, smooth, and symmetrical, relative to voluntary “fake” expressions, which typically are less smooth and have more variable dynamics. Voluntary control of vocalizations requires the forebrain, in particular, the mediofrontal cortex (including anterior cingulate gyrus and supplementary as well as pre-supplementary motor area) and the motor cortex via pyramidal/corticobulbar as well as extrapyramidal pathways [8,39].
As with any other behavior that is under cortical influences, when the PAG and the lower brainstem have been disconnected from forebrain control, normal brainstem activities, e.g., vocalization, become uncontrolled. In the same vein, when the brainstem nuclei mediating facial expression are affected, the facial expression is also out of control. The face then becomes contorted and seemingly expressive of extreme joy or grief although patients deny experiencing these feelings. It goes without saying that these patients may, however, feel severely distressed and embarrassed by this occurrence. The PAG is thus a critical relay of the limbic brain that maintains strong connections to autonomic control circuits located in the brainstem and spinal cord and relays limbic commands to body systems for mediating survival challenge (fight/flight/freeze) and/or emotional expression (vocalization, singing, but in particular also laughing and crying).
Although not a study on crying specifically, a human positron emission tomography (PET) study [40] demonstrated increases in blood flow in the PAG, as well as the cerebellar vermis and parts of the thalamus during voiced speech, which share some laryngeal control mechanisms with crying. This structure thus seems particularly involved in the motoric-vocal aspects of emotional expressions, specifically the coordination of the activity of the laryngeal, oral-facial, and principal and accessory muscles of respiration and inspiration [38]. However, the current knowledge about the role of PAG in human crying is largely limited to vocal utterances (in animals and humans), although recent findings about pathological crying in humans, on which we elaborate next, seem to represent a reasonable argument for the importance of the PAG for the production of emotional tears as well.
Further, the role of the amygdala in crying is clearly apparent in a study demonstrating that ablation of this structure in rhesus monkeys significantly decreased distress vocalizations. In contrast, ablation of the overlying neocortex had only a transient effect on distress calls after separation, whereas crying produced in other contexts, such as feeding, physical restraint, and alarm, appeared to be unaffected. Consistent with these findings, Panksepp [41] also concluded that the circuitry running from the dorsal PAG to the anterior cingulate cortex (ACC) might be regarded as the core neural circuit involved in animal separation-distress reactions. Some of these findings may reflect a decrease in subjective distress, as fMRI findings in humans demonstrate that the amygdala and ACC are strongly activated during self-induced sadness (e.g., [42,43]).
Another important source of knowledge concern clinical observations in neurological patients with damage in specific brain structures, who display pathological crying (and laughing). The connection between the motoric-expressive and the felt aspects of emotion seems particularly dependent on certain cortico-bulbar and fronto-limbic-pontine-medullary pathways. In addition, the cerebellum seems to be involved. These pathways are responsible for conveying the felt aspects of emotional expressions, such as is typically involved in laughter and crying. Disruptions of these interconnections, for example as a consequence of different forms of pathology (e.g., progressive bulbar (pontine-medullary) palsy, brainstem hemorrhage, tumor, amyotrophic lateral sclerosis (ALS), or related injuries to the hypothalamus or areas such as corona radiata, internal capsule, PAG, midbrain tegmentum, as well as bulbar and cerebellar areas), may induce crying (and sometimes also laughing) without the characteristic accompanying feelings. In neurology, these conditions are well-known as pathological crying, or pseudobulbar affect [44–47].
Parvizi et al. [48] and Rabins and Arciniegas [46]; specifically discuss the role of the cerebellum in pathological crying and laughing. Basing themselves on clinical data, they conclude that lesions in these patients are in particular located in the cerebro-ponto-cerebellar pathways such that the cerebellar structures are no longer able to automatically modulate laughing or crying behavior such that it is appropriate to the situational context. In particular, they suggest that a partial deafferentation of the cerebellum, especially when inputs from the left telencephalic structures to the right cerebellar hemisphere are removed, results in a lack of appropriate modulation of crying (and laughing) behavior (both regarding the behavior profiles and in terms of the intensity and duration of the behavior). The cerebellum receives projections from telencephalic structures that convey the relevant contextual information, and cerebellar projections to the brainstem and telencephalic inductor and effector sites allow it to coordinate the responses involved in crying, including the facial movements, laryngopharyngeal and rhythmic clonic diaphragmatic movements, etc.
To the best of our knowledge, only one single investigation specifically focused on human emotional crying and the brain [49]. In this study, only the activity of the medial prefrontal cortex (mPFC) was recorded in a very small sample of 8 individuals who cried in response to an emotional movie. The investigators distinguished three consecutive phases: (1) the pre-tear stage; (2) the tear-triggering stage; and (3) the crying stage. A gradual increase in the activity of the mPFC in the pre-tear stage was observed, followed by a sharp increase when the participants started crying. The investigators suggested that this acute activation might indicate the switch from a sympathetic to a parasympathetic activation, consistent with some psychophysiological findings. However, it is not certain whether the increased mPFC activity actually temporally precedes the onset of tears, which is extremely difficult to determine, or that it rather reflects the awareness of the initiation of crying in the brain. In addition, it is hard to eliminate the influence of other processes, such as self-control efforts [50] that often accompany crying episodes, and which are known to be related to mPFC activity [51]. Indeed, the mPFC is known to be critically involved in emotional processing and regulation more generally [52].
In conclusion, investigation of the specific neural circuits supporting emotional, tearful crying in humans is still in its infancy. Studies which apply neuroimaging techniques such as fMRI during emotional crying are badly needed and would significantly contribute to our understanding of the functional neural circuitry underlying human emotional crying.
The Neurochemistry of Vocal Emotional Crying
Earlier we reviewed the primary neurotransmitters involved in regulating lacrimal secretions. Here we focus on what is known regarding the neurochemistry underlying distress vocalizations associated with crying in animals and humans. Most of the prior work has been done in animals. As early as the 1980’s, Panksepp et al. [53] summarized the rich literature revealing the involvement of opioids in separation-induced crying in several species, including puppies, young guinea pigs, and chicks (for more recent overviews, see Panksepp, [41,54]). According to Panksepp’s reviews, the administration of morphine consistently results in a reversible decrease in distress calls, which can be blocked by the opioid antagonist naloxone. Further, in rhesus macaque infants, naloxone has been demonstrated to significantly increase distress vocalizations compared to a control (no drug) condition. It further has been shown that ligands that are agonists to mu, delta, and kappa opioid receptors all influence the distress calls of rat pups, with mu and delta agonists resulting in suppressed crying, and the kappa agonist stimulating the production of distress vocalizations. These findings suggest an essential role of the opiate system in the production of distress vocalizations; however, it may be that the role of the opioid system in reducing the distress underlying these vocalizations is the primary driving factor.
The cingulate gyrus and associated connections seem to play a significant role in these vocalizations, which are activated by glutamate and corticotropic-releasing factor (CRF), a polypeptide hormone [41], and interventions with agents that block the activity of glutamate and CRF are effective in the treatment of patients with pathological crying [45,54]. A second neurochemical receptor system implicated in separation calls is the alpha-2 adrenoreceptor system, and administration of its agonist clonidine also results in a reduction of distress calls in adult nonhuman primates in a dose-dependent manner [55]. Since the activation of this system inhibits the activity of the sympathetic nervous system, this seems to suggest that (vocal) crying and increased activity of the sympathetic nervous system are not compatible. Other neurochemical systems that play a role in the distress call production include the benzodiazepine receptor complex, as well as cholinergic and serotonergic pathways (see [2] for animal research; [47] for evidence from pathological crying studies).
The neuropeptides vasopressin, oxytocin, and prolactin, known for their involvement in the regulation of social (attachment) behavior in animals and humans [45], additionally seem to play a role in the production of distress vocalizations. Animal work has demonstrated robust decreases in separation calls after the administration of oxytocin. These neuropeptides are especially active in the amygdala, both with respect to receptors and to the effects of these neuropeptides on specific neuronal populations. Since (infant and adult) crying is considered to be an attachment behavior [1,56,57], with the aim to maintain and restore the bond between the individuals, the involvement of these substances in social bonding processes involving crying is plausible. However, it is unknown whether these systems play a unique role in crying behavior specifically, versus social attachment-related behavior more generally. It is further worth mentioning that oxytocin is involved in the regulation of parasympathetic activity [58], which fits with the findings demonstrating the involvement of parasympathetic system in crying discussed above.
The specific kind of pleasure associated with social contact and strong attachment bonds is also mediated by opioids, as well as other neuropeptides like oxytocin [59,60]. Whether neurotransmitters like opioids and oxytocin also play a role in the hypothesized soothing effects induced by crying mediated by social context remains to be established [61]. Through the actions of these substances, being close to significant others leads to feelings of comfort, security, and pleasure, which may play a role in social influences on the crier. These brain molecules occur very early in our evolutionary history and are thought to have initially evolved for their analgesic and other homeostatic properties. Later, they came also to be associated with social processes over the course of our evolutionary development [60].
More precisely, these peptides signaled to the organism whether it was socially connected or not, with social pain (separation distress) indicating low levels of these critical opioids [60]. This latter affective mechanism, which Panksepp [60] refers to as the PANIC–GRIEF system, is particularly highly developed in social animals (mammals and birds). This system consists of a neuronal network that courses between the anterior cingulate gyrus, various basal forebrain and diencephalic nuclei, and the dorsal PAG. When social attachment bonds are broken through separation or loss, these brain mechanisms that make the sufferer “feel bad” in a particular way, and distress vocalizations are the best indicators of this separation distress [60].
Relatedly, there is some relevant work of Scott and colleagues (see [36] for an overview) who investigated the effects of several psychopharmacological agents on the separation calls in Telomian puppies. Their research revealed that the vast majority of the evaluated psychotropic agents (including chlorpromazine and minor tranquilizers, sedatives, and alcohol) failed to have any effect on this behavior. In contrast, stimulants such as D-amphetamine sulfate seemed to increase distress vocalizations of the puppies in response to separation, whereas the antidepressant imipramine was most effective in reducing these vocalizations. These findings provide support for the notion that increasing brain serotonin levels result in reduced vocalizations. It is also relevant to note that anti-depressants are also quite effective, even in small doses, in the treatment of pathological crying [62]. Moreover, Van der Veen et al. [63] demonstrated that the SSRI paroxetine decreased the crying reactions to emotional films in healthy female students. It is also possible that anti-depressants have an impact on crying by reducing the intensity of emotional reactions. Regarding the effects of alcohol on crying in humans, Van Tilburg et al. exposed 100 female students (66% had consumed alcohol) to an emotional movie [64]. Although the alcohol and non-alcohol group did not differ in their response to the question to which extent the film had emotionally moved them, in contrast to the findings with puppies above, the alcohol group reported more crying episodes. These results suggest that while alcohol may not have an impact on distress calls in animals, alcohol can reduce the crying threshold for emotional tears in humans.
In the lay press, it has frequently been suggested that the difference in crying between adult women and men might be attributed to female sex hormones. Along these lines, Frey [65] claimed a significant role for prolactin. This hypothesis was mainly based on case studies and observations in a certain duck species [66]. However, some of his speculations have been refuted in more recent research. For example, Van Tilburg et al. [64] showed that same age menstruating, and non-menstruating girls did not differ in crying behavior, which was contrary to Frey’s prediction. On the other hand, Frey’s [65] prolactin hypothesis is still worth testing empirically. Somewhat more convincing may be the hypothesis that the male hormone testosterone has an inhibitory influence on crying. In addition to animal work examining the effects of administering testosterone or castrating male animals and evaluating the effects on distress vocalization [60], there are additional supportive observations in men receiving antihormone therapy to block testosterone (i.e., such as in male-to-female gender transition or prostate cancer treatment). Together, these observations suggest that testosterone has an inhibitory effect on (tearful) crying behavior [3].
In sum, not surprisingly, neurotransmitter systems relevant for the experience and regulation of emotion or distress, as well as social functioning and attachment-related behavior, also seem to be involved in human emotional crying. Further research will be needed to disentangle whether any of these processes are unique for crying or more general emotional and social processes. For example, research is needed to compare individuals who cry vs. do not cry in response to the same emotional stimulus (and have the same emotional reaction), which is a major methodological challenge.
Conclusion
Human emotional crying is a complex and important behavior that has surprisingly received relatively little attention from scientists, particularly regarding its neurobiological mechanisms. It is only recently that we arrived at some understanding of the evolutionary background and principal functions of tears. Currently, the hypothesis regarding the function of this behavior can be best summarized as: tearful crying facilitates social connections. Based on the results of experiments with animals and observations of patients with neurological disorders who display pathological crying, we have gained some first insights into the neurobiology of human emotional crying. Although the production of tears from the lacrimal glands is a predominantly parasympathetically-mediated reaction, the sympathetic nervous system plays an important role as well in emotional crying. It seems that crying onset is associated with an increase in sympathetic activity, and the resolution of crying may also be associated with increases in parasympathetic activity. Regarding the neural circuits supporting crying, the ACC is closely linked with the state of distress that typically triggers distress vocalizations. The orchestration of these systems in crying seems to depend primarily on the well-coordinated activation of components of the Central Autonomic Network (CAN), which is also implicated in regulated autonomic responses to distress [35]. There is further some suggestive evidence of an involvement of neurochemical systems, including oxytocin, vasopressin, and endogenous opioids, and hormones such as prolactin and testosterone may have an additional influence on an individual’s crying threshold. It remains unclear at this point what neural circuits are specific to emotional crying or emotional responses more generally. Future research will be needed to understand the neurobiological basis of human emotional crying and how this complex behavior fits with overall emotional functioning and related expressive and social behaviors.
Acknowledgments
The authors want to express their gratitude for the input of Ton van Boxtel and Kees Brunia on earlier versions of the figure. The final figure was drawn by Rogier Trompert. The authors also appreciate the feedback from two anonymous reviewers for their helpful feedback on an earlier version of this manuscript. The first author, Lauren M. Bylsma, is supported by a NIMH K01 Award (MH104325).
Footnotes
Conflicts of Interest
On behalf of all authors, the corresponding author states there are no conflicts of interest.
Contributor Information
Lauren M. Bylsma, University of Pittsburgh
Asmir Gračanin, University of Rijeka.
Ad J. J. M. Vingerhoets, Tilburg University
References
- 1.Gračanin A, Bylsma LM, Vingerhoets AJJM. Why only humans shed emotional tears: evolutionary and cultural perspectives. Hum Nat. 2018 doi: 10.1007/s12110-018-9312-8. [DOI] [PubMed]
- 2.Newman JD. Neural circuits underlying crying and cry responding in mammals. Behav Brain Res. 2007;182:155–165. doi: 10.1016/j.bbr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vingerhoets AJJM, Bylsma LM. The riddle of human emotional crying: a challenge for emotion researchers. Emot Rev. 2016;8:207–217. doi: 10.1177/1754073915586226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vingerhoets AJJM. Why only humans weep: unravelling the mysteries of tears. Oxford University Press; Oxford: 2013. [Google Scholar]
- 5.Bylsma LM, Vingerhoets AJJM, Rottenberg J. When is crying cathartic? An international study. J Social Clin Psychol. 2008;27:1165–1187. [Google Scholar]
- 6.Rottenberg J, Bylsma LM, Vingerhoets AJJM. Is crying beneficial? Curr Dir Psychol Sci. 2008;17:400–404. [Google Scholar]
- 7.Gračanin A, Vingerhoets AJJM, Kardum I, Zupčić M, Šantek M, Šimić M. Why crying does and sometimes does not seem to alleviate mood: a quasi-experimental study. Motiv Emot. 2015;39:953–960. doi: 10.1007/s11031-015-9507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jürgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- 10.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- 12.Van Haeringen N. The (neuro)anatomy of the lacrimal system and the biological aspects of crying. In: Vingerhoets AJJM, Cornelius RR, editors. Adult crying: a biopsychosocial approach. Routledge; Hove: 2001. pp. 19–36. [Google Scholar]
- 13.Benarroch EE. The Central Autonomic Network: Functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 14.Kardon R. Anatomy and physiology of the autonomic nervous system. In: Miller NR, Newman NJ, Biousse V, Kerrison JB, editors. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 6th. Vol. 1. Lippincott, Wiliams, & Wilkins; Philadelphia: 2005. pp. 647–671. [Google Scholar]
- 15.Mendoza-Santiesteban CE, Palma J, Norcliffe-Kaufmann L, Kaufmann H. Familial dysautonomia: a disease with hidden tears. J Neurol. 2017;264:1290–1291. doi: 10.1007/s00415-017-8486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hof PR. Von Economo Neurons in FD. 2011 International Familial Dysautonomia Research Conference; New York. 2011. [Google Scholar]
- 17.Allman JM, Tetreault NA, Hakeem AY, et al. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann NY Acad Sci. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeley WW. Selective functional, regional, and neuronal vulnerability in fronto-temporal dementia. Curr Opin Neurol. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 20.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–240. [Google Scholar]
- 23.Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM. Correspondence between physiological and self-report measures of emotion dysregulation: a longitudinal investigation of youth with and without psychopathology. J Child Psychol Psychiatry. 2009;50:1357–1364. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 24.Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. RSA Reactivity in Current and Remitted Major Depressive Disorder. Psychosom Med. 2014;76:66–73. doi: 10.1097/PSY.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74:233–255. doi: 10.1016/j.neubiorev.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Porges SW, Lewis GF. The polyvagal hypothesis: common mechanisms mediating autonomic regulation, vocalizations and listening. In: Brudzynski SM, editor. Handbook of mammalian vocalization: An integrative neuroscience approach. Elsevier; Amsterdam: 2010. pp. 255–264. [Google Scholar]
- 27.Kraemer DL, Hastrup JL. Crying in adults: self-control and autonomic correlates. J Soc Clin Psychol. 1988;6:53–68. [Google Scholar]
- 28.Gross JJ, Fredrickson BL, Levenson RW. The psychophysiology of crying. Psychophysiology. 1994;31:460–468. doi: 10.1111/j.1469-8986.1994.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 29.Sakuragi S, Sugiyama Y, Takeuchi K. Effects of laughing and weeping on mood and heart rate variability. J Physiol Anthropol Appl Hum Sci. 2002;21:159–165. doi: 10.2114/jpa.21.159. [DOI] [PubMed] [Google Scholar]
- 30.Rottenberg J, Gross JJ, Wilhelm FH, Najmi S, Gotlib IH. Crying threshold and intensity in major depressive disorder. J Abnorm Psychol. 2002;111:302–312. [PubMed] [Google Scholar]
- 31.Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40:1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- 32.Hendriks MCP, Rottenberg J, Vingerhoets AJJM. Can the distress signal and arousal-reduction view be reconciled? Evidence from the cardiovascular system. Emotion. 2007;7:458–463. doi: 10.1037/1528-3542.7.2.458. [DOI] [PubMed] [Google Scholar]
- 33.Wassiliwizky E, Jacobsen T, Heinrich J, Schneiderbauer M, Menninghaus W. Tears falling on goosebumps: co-occurrence of emotional lacrimation and emotional piloerection indicates a psychophysiological climax in emotional arousal. Front Psychol. 2017 doi: 10.3389/fpsyg.2017.00041. [DOI] [PMC free article] [PubMed]
- 34.Mori K, Iwanaga M. Two types of peak emotional responses to music: the psychophysiology of chills and tears. Sci Rep. 2017 doi: 10.1038/srep46063. [DOI] [PMC free article] [PubMed]
- 35.De Morree HM, Szabó BM, Rutten GJ, Kop WJ. Central nervous system involvement in the autonomic responses to psychological distress. Neth Heart J. 2013;21:64–69. doi: 10.1007/s12471-012-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JP. Effects of psychotropic drugs on separation distress in dogs. In Proceedings of IX Congress of the Collegium International Neuropsychopharmacologicum. Int Congr Ser- Excerpta Med. 1974;359:735–745. [Google Scholar]
- 37.Gruber-Dujardin E. Role of the periaqueductal gray in expressing vocalization. In: Brudzynski SM, editor. Handbook of mammalian vocalization: an integrative neuroscience approach. Elsevier; Amsterdam: 2010. pp. 313–327. [Google Scholar]
- 38.Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: the role of the midbrain periaqueductal gray. J Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]
- 39.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 40.Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cereb Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- 41.Panksepp J. The neurobiology of social loss in animals: some keys to the puzzle of psychic pain in humans. In: Jensen-Campbell LA, MacDonald G, editors. Social pain: Neuropsychological and health implications of loss and exclusion. American Psychological Association; Washington: 2011. pp. 11–52. [Google Scholar]
- 42.Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. NeuroImage. 2003;18:760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 43.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion: Activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 44.Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of Pseudobulbar Affect (PBA): distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectrum. 2005;10:1–14. doi: 10.1017/s1092852900026602. [DOI] [PubMed] [Google Scholar]
- 45.Miller A, Pratt H, Schiffer RB. Pseudobulbar affect: The spectrum of clinical presentations, etiologies, and treatments. Expert Rev Neurother. 2011;11:1077–1088. doi: 10.1586/ern.11.68. [DOI] [PubMed] [Google Scholar]
- 46.Rabins PV, Arciniegas DB. Pathophysiology of involuntary emotional expression disorder. CNS Spectrums. 2007;12(4 suppl):17–22. doi: 10.1017/s1092852900025979. [DOI] [PubMed] [Google Scholar]
- 47.Wortzel HS, Oster TJ, Anderson CA, Arciniegas DB. Pathological laughing and crying: Epidemiology, pathophysiology, and treatment. CNS Drugs. 2008;22:531–545. doi: 10.2165/00023210-200822070-00001. [DOI] [PubMed] [Google Scholar]
- 48.Parvizi J, Coburn KL, Shillcutt SD, Coffey CE, Lauterbach EC, Mendez MF. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci. 2009;21:75–87. doi: 10.1176/jnp.2009.21.1.75. [DOI] [PubMed] [Google Scholar]
- 49.Sato-Suzuki I, Fumoto M, Seki Y, et al. Activation of the medial prefrontal cortex during crying with emotional tears: near-infrared spectroscopy study. Auton Neurosci. 2007;135:128–137. [Google Scholar]
- 50.Znoj H. When remembering the lost spouse hurts too much: first results with a newly developed observer measure for tears and crying related coping behavior. In: Vingerhoets AJJM, van Bussel FJ, Boelhouwer AJW, editors. The (non)expression of emotions in health and disease. Tilburg University Press; Tilburg: 1997. pp. 337–352. [Google Scholar]
- 51.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panksepp J, Meeker R, Bean NJ. The neurochemical control of crying. Pharmacol Biochem Be. 1980;12:437–443. doi: 10.1016/0091-3057(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 54.Panksepp J. Emotional causes and consequences of social-affective vocalization. In: Brudzynski SM, editor. Handbook of mammalian vocalization: an integrative neuroscience approach. Elsevier; Amsterdam: 2010. pp. 201–208. [Google Scholar]
- 55.Harris JC, Newman JD. Mediation of separation distress by α2-adrenergic mechanisms in a non-human primate. Brain Res. 1987;410:353–356. doi: 10.1016/0006-8993(87)90337-4. [DOI] [PubMed] [Google Scholar]
- 56.Bowlby J. Attachment. Basic Books; New York: 1969. [Google Scholar]
- 57.Nelson JK. Seeing through tears: crying and attachment. Routledge; New York: 2005. [Google Scholar]
- 58.Snowdon CT, Ziegler TE. Reproductive hormones. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd. Cambridge University Press; Cambridge: 2004. pp. 368–396. [Google Scholar]
- 59.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 60.Panksepp J. Affective neuroscience: the foundations of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- 61.Gračanin A, Bylsma LM, Vingerhoets AJJM. Is crying a self-soothing behaviour? Front Psychol. 2014 doi: 10.3389/fpsyg.2014.00502. [DOI] [PMC free article] [PubMed]
- 62.Hackett ML, Yang M, Anderson CS, Horrocks JA, House A. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database of Systematic Reviews 2. 2010 doi: 10.1002/146551858.CD003690.pub3. Art. No.: CD003690. [DOI] [PubMed]
- 63.Van der Veen FM, Jorritsma J, Krijger C, Vingerhoets AJJM. Paroxetine reduces crying in young women watching emotional movies. Psychopharmacology. 2012;220:303–308. doi: 10.1007/s00213-011-2477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Tilburg MAL, Unterberg M, Vingerhoets AJJM. Crying during adolescence: the role of gender, menarche, and empathy. Br J Dev Psychol. 2002;20:77–87. [Google Scholar]
- 65.Frey WH. The mystery of tears. Winston Press; Minneapolis: 1985. [Google Scholar]
- 66.Eugster A, Horsten M, Vingerhoets AJJM. Menstrual cycle, pregnancy, and crying. In: Vingerhoets AJJM, Cornelius RR, editors. Adult crying: A biopsychosocial approach. Brunner-Routledge; Hove: 2001. pp. 177–198. [Google Scholar]


