Abstract
Objective:
The current study aimed to 1) identify the occurrence of comorbidities among Chinese- and Korean-American breast cancer survivors (BCS), 2) examine whether health-related quality of life (HRQOL) scores varied with the occurrence of specific comorbidities, and 3) investigate the mediating effect of comorbidities on the relationship between life stress and HRQOL.
Design.
Data were drawn from the parent study, a cross-sectional study investigating HRQOL in 86 Chinese- and 71 Korean-American BCS in Southern California. Two comorbidity-related variables, the occurrence of the specific comorbidity and the total number of comorbidities, were used to comprehensively reflect the characteristics of comorbidity.
Results:
Approximately 60% of participants had at least one comorbid disease, and osteoporosis was the most prevalent comorbidity. HRQOL differences based on the occurrence of a specific comorbidity were evident for arthritis, eye/vision problems, dental and gum problems, lymphedema, and psychological difficulties. Structural equation modeling demonstrated that the nature of the outcome variable, either physical or mental HRQOL, influenced the overall patterns of the findings. For example, life stress was significantly associated with the total number of comorbidities and in turn influenced physical HRQOL. In terms of MCS, arthritis, dental and gum problems, chronic pain, heart disease, lymphedema, and psychological difficulties mediated the relationship between life stress and mental HRQOL.
Conclusion:
The current study adds to the existing literature by examining the mediating effects of comorbidity on the relationship between life stress and HRQOL. The findings support the need for health care professionals to clearly assess physical and psychological comorbidities when providing survivorship care for cancer survivors.
Keywords: Chinese-Americans, Breast Cancer Survivors, Comorbidity, Health-related Quality of Life, Korean-Americans, Life Stress
Introduction
Asian-Americans are the fastest growing ethnic group in the United States (US), increasing by 43% in the 2000–2010 period (U.S. Census Bureau 2011). More specifically, Chinese-Americans are the largest Asian-American subpopulation, comprising 25.9% of the Asian-American population as of 2010, and Korean-Americans are one of the fastest growing ethnic groups in the US, increasing 500% since 1970 (U.S. Census Bureau 2011). Breast cancer is the most prevalent type of cancer in Chinese- (74.4 per 100,000) and Korean-American women (68.0 per 100,000) (American Cancer Society 2016). With advances in cancer care and enhanced treatment regimens, however, the breast cancer mortality rates of Chinese- and Korean-Americans decreased by 16% from 1990 to 2012, and the breast cancer survival rates for all Americans have increased significantly. Now, the 5-year relative survival rates of Chinese- and Korean-Americans are 93% and 92%, respectively. Thus, the number of long-term Chinese- and Korean-American breast cancer survivors (BCS) is rapidly increasing (American Cancer Society 2016).
Although cancer survivors are living longer than ever before, they are at risk of serious physical and psychosocial symptoms (Quesnel, Savard, and Ivers 2009). For example, the most commonly reported adverse effects of BCS at 2- and 3-year follow-ups after cancer treatment were found to be physical and functional difficulties with the shoulder girdle and abdomen (34 % reported difficulties most or all of the time), dissatisfaction with the reconstructed breast (40 %), and general pain and psychosocial difficulties (15–40%) (Jeevan et al. 2009; Winters et al. 2016). Such long-term and late effects during the survivorship phase require post-treatment surveillance care for cancer survivors, and guidelines on cancer survivorship care plans have recently been designed to address such issues among cancer survivors (Institute of Medicine 2006). Survivorship care is expected to provide coordination for post-treatment care focusing on cancer surveillance, general health and wellness counseling, care for comorbid conditions, psychosocial care, and the monitoring and management of long-term and late effects (Institute of Medicine 2006). Several studies have demonstrated that survivorship care can improve health outcomes for cancer survivors. McCollum and colleagues (2014) reported that participating in a cancer survivorship program improved perceived quality of life and reduced distress related to the initial cancer diagnosis and family distress. Stan et al. (2016) also found that exercise interventions improved cancer-related fatigue and health-related quality of life (HRQOL).
Because 68–85% of adult cancer patients have at least one comorbid condition and the overall symptoms and psychological burden increase with the number of comorbidities (Mao et al. 2007), appropriate care for comorbidities is required for cancer survivors. Generally, comorbidity refers to the co-existence of diseases or disorders in addition to a primary disease such as cancer (Sarfati et al. 2009). Several studies have demonstrated that the existence of comorbid conditions increases the risk of poor survival, all-cause mortality, impaired functional status, and low HRQOL in BCS (Ashing-Giwa et al. 2014; Sarfati et al. 2009). For example, BCS with comorbidities such as arthritis, diabetes, and lymphedema have poorer physical and mental health outcomes than those without these comorbidities (Bellury et al. 2012). In addition, a greater number of comorbidities is associated with poor functional ability and HRQOL, suggesting that the combined impact of different diseases should be considered (Bellury et al. 2013).
According to Gijsen’s model describing comorbidity and its causes and consequences (Gijsen et al. 2001), lifestyle factors (i.e., smoking, drinking, nutrition, physical activity), biological risk factors (i.e., cholesterol, obesity), and environmental factors (i.e., air pollution, social environment) can influence comorbidity, and in turn are associated with mortality, HRQOL, and health care. Although Gijsen’s model was developed to identify and summarize the existing information on the causes and consequences of comorbidity, much work has been conducted not on the causes but on the consequences of comorbidity; only four articles were carried out to search for causes of comorbidity. Thus, the current study considered life stress as its cause, given that life stress is related to burdens associated with various aspects of life including family, functional, and environmental factors (Ashing-Giwa and Lim 2010). Several studies have demonstrated that stress lead to dysfunction in the hypothalamic-pituitary-adrenal axis, suggesting that stress may be causative of various types of comorbidity including chronic pain, depression, and cholesterol (Swanson et al. 2013; Bjorntorp 2000). Few studies, however, have focused on stress as a risk factor for comorbidities for cancer survivors. Thus, an investigation of how life stress is associated with comorbidity and which comorbidities are vulnerable to stress is necessary to better manage comorbidities for cancer survivors.
Little is known about the occurrence, causes and consequences of comorbidity for Chinese- and Korean-American BCS. Some studies have reported that ethnic minority women experience more comorbidities than European-Americans and that comorbidities negatively influence their overall health status and HRQOL (Tammemagi 2005; Napoles-Springer 2011). A qualitative study indicated that Chinese- and Korean-Americans are more likely to indicate life stress as a major cause of cancer or other comorbidities, and to believe that their family situation and cultural background play a role in their health (Lim et al. 2009). Other studies found that Chinese- and Korean-American BCS expressed a lower HRQOL than European-American BCS (Ashing-Giwa et al. 2007). However, evidence regarding specific factors that influence HRQOL for Chinese- and Korean-Americans is lacking, although findings that life stress and social support are associated with HRQOL exist (Paek and Lim 2015). Despite the increased attention to comorbidities and their influence on HRQOL, there is no research on the causes and consequences of comorbidities for Chinese- and Korean-American BCS. It is important to better understand the impact of comorbidity on the relationship between life stress and HRQOL for Chinese- and Korean-Americans to propose an ethnically tailored approach to care for comorbid conditions.
The Purpose of the Study
The aims of the present study were the following: (1) to identify the occurrence of comorbidities among Chinese- and Korean-American BCS; (2) to determine whether HRQOL scores vary by the occurrence of the specific comorbidity; and (3) to investigate the mediating effect of comorbidities (i.e., the occurrence of the specific comorbidity and the total number of comorbidities) in the relationship between life stress and HRQOL. Based on Gijsen’s model describing comorbidity and its causes and consequences (Gijsen et al. 2001) and on previous studies (Swanson et al. 2013; Bjorntorp 2000), the current study hypothesized that the occurrence of a specific comorbidity and/or the total number of comorbidities mediates the relationship between life stress and HRQOL.
Methods
Data Source and Participants
Data were drawn from the parent study, the Family Communication Study (FCS), a cross-sectional study that investigated the role of family communication in HRQOL for Chinese- and Korean-American BCS in Southern California from October 2009 to April 2011 (Lim and Paek 2015). The FCS was approved by the Institutional Review Board and the California Cancer Surveillance Program (CSP). Eligibility criteria included the following: 1) self-identification as Chinese or Korean; 2) within 1–5 years of a breast cancer diagnosis (stages I-III); 3) completion of active treatment (i.e., surgery, chemotherapy, or radiation therapy); 4) 18 years of age or older; and 5) able to speak Chinese, Korean, or English. Survivors of stage IV disease, those with other cancer diagnoses, and men with breast cancer were excluded due to significant differences in disease progression and prognosis.
Data Collection Procedures
The methodological details, such as the sampling, recruitment procedures, instrument development, and translation procedure have been reported elsewhere (Lim and Paek 2015). In brief, participants were first identified from the California CSP and from local LA hospital cancer registries. Next, investigators mailed invitation letters to potential participants whose contact information was obtained from the CSP and local hospital registries. The research assistants made follow-up calls to confirm interest and eligibility and subsequently conducted screening over the phone. Eligible participants were mailed a questionnaire and consent form and were asked to return them within 3 weeks. The data collection procedures employed a culturally responsive model for ethnic minority inclusion to maximize Asian-American samples (Ashing-Giwa et al. 2004). For example, all materials were provided in both English and Chinese/Korean due to participants’ limited English proficiency. Trained ethnically and linguistically matched research assistants recruited potential participants. Additionally, community-based recruitment approaches, such as community organizations, support groups, or Korean or Chinese doctors’ offices, were employed to identify more Chinese- and Korean-American BCS.
Instruments
A rigorous “forward-backward” translation procedure was used to create Chinese and Korean versions of the questionnaires whose contents were equivalent to those of the English version. Pilot testing to check the validity and reliability of the translated instruments was also performed with a small convenience sample. Previous studies have demonstrated that no major differences in demographic and medical characteristics or outcomes existed as a result of the language of administration (Lim and Paek 2015).
Health-related quality of life
The Medical Outcome Study (MOS) SF-36, an internally consistent and reliable self-report HRQOL, was used to assess physical and mental HRQOL (Ware et al. 1993). The 36-item scale contains 8 sub-scales including 1) physical functioning (10-item), 2) physical limitation (4-item), 3) pain (2-item), 4) general health perception (5-item), 5) vitality (4-item), 6) social functioning (2-item), 7) emotional limitation (3-item), and 8) mental health (5-item). Sub-scale scores were calculated by summing the items and transforming the raw scale scores into standardized scores. Two summary scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS), were obtained from the 8 sub-scales and through averaging the corresponding subscales. Higher scores represented better physical and mental HRQOL. In the current study, Cronbach’s alpha coefficient for the 8 subscales ranged from 0.81 to 0.91. Cronbach’s alpha coefficients of the PCS and MCS were also 0.84 and 0.88, respectively.
Comorbidity
Two comorbidity-related variables (i.e., the occurrence of a specific comorbidity and the total number of comorbidities) were assessed using a 15-item comorbidity checklist (Ashing-Giwa et al. 2004). The listed comorbidities were 1) allergies, 2) arthritis, 3) high cholesterol, 4) diabetes, 5) eye/vision problems, 6) dental or gum problems, 7) hearing problems, 8) digestive problems, 9) chronic pain, 10) heart disease, 11) high blood pressure, 12) osteoporosis, 13) lymphedema, 14) thyroid problems, and 15) psychological difficulties (e.g., depressive symptoms or anxiety). Here, comorbidities were defined as co-existent diseases in addition to breast cancer. The comorbidity checklist asked whether the participants currently have or did not have each condition listed in the past year, to which they responded “yes” or “no.” Based on the comorbidity checklist, each comorbidity question was considered to separately examine the impact of the occurrence of a specific comorbidity on outcomes. Second, the total number of comorbidities was included by summing the self-reported medical conditions from the comorbidity checklist; thus, the combined impact of different diseases on outcomes was considered.
Life stress
To assess life stress, the 18 items of the Life Stress Scale, which was developed to examine the level of burden associated with various aspects of life during the past 1 year, were used (Ashing-Giwa et al. 2004). This scale comprises 15 items regarding family (e.g., death of family members, raising children), functional (e.g., money, housing) and environmental stress (e.g., neighborhood environment, transportation). Items are rated from 1 to 5, and a higher score indicates higher stress. A total score was obtained by averaging the items. Cronbach’s alpha coefficient of this scale was 0.91.
Demographic and Medical Characteristics
Demographic (e.g., age, education, language) and medical characteristics (e.g., treatment type, cancer stage) were included to describe the sample’s characteristics.
Data Analyses
Exploratory data analyses were conducted to describe the demographic and medical characteristics of the participants. As preliminary analyses, chi-square and independent sample t-tests were used to investigate the relationships and differences in predictors and outcomes by ethnicity. A univariate general linear model was conducted to examine HRQOL differences by the occurrence of specific comorbidity, after controlling for covariates. SPSS 20.0 was used to analyze the data.
Power Analysis
For power calculations in structural equation modeling (SEM) research, Mueller (1997) recommended a 10:1 ratio of the number of people to the number of measured or observed variables. Because a total of 12 observed variables were included in this study, at least 120 subjects were required to satisfy the SEM requirements. The following factors must also be considered in computing the power for the goodness-of-fit test statistic: sample size (N), selected significance level (α), degree of freedom (df), test statistics for the null hypothesis (H0), and test statistics for the alternative hypothesis (Ha) (MacCallum 1996). In this study, the hypothesis-testing framework of the root mean square error of approximation (RMSEA) was used as a vehicle to estimate the power of SEM (MacCallum 1996). Given α = 0.05, a null hypothesis RMSEA of 0.05, an alternative hypothesis RMSEA of 0.10, and a df ranging from 30 to 50, the range of statistical power given a sample size of 160 is between 0.87 and 0.97. Thus, the inclusion in the study sample of 157 subjects approximated the recommended sample size for SEM.
Structural Equation Modeling
SEM was used to examine the associations among life stress, comorbidity, and HRQOL using AMOS 20.0. First, confirmatory factor analysis (CFA) was conducted to determine the adequacy of latent constructs. CFA found that the factor loadings for each variable on PCS and MCS latent factors were statistically significant at the 1% level; however, the fit indices were not sufficiently good. Thus, covariances between physical limitation and emotional limitation and between emotional well-being and energy were added, based on theoretical meanings and previous studies (Maratia, Cedillo, and Rejas 2016). The resulting model had a good fit to the data: χ2(17)=32.03, p=0.015; RMSEA=0.07; CFI=0.98. Hence, the revised measurement model of HRQOL, comprising PCS and MCS latent factors was selected and provided the foundation for evaluating the substantive structural model.
Next, the hypothetical model based on the conceptual model was created using two latent outcome variables (PCS and MCS), two mediators (occurrence of the specific comorbidity and the total number of comorbidities), one predictor (life stress), and covariates. Here, PCS and MCS latent factors were used as unit-weighted observed composites for indicators to reduce the number of parameter estimations. The age variable, which is significantly associated with comorbidities, was included as a covariate (Sogaard et al. 2013). Finally, covariances between variables were added because relationships between the occurrence of a specific comorbidity and the total number of comorbidities (Geraci et al. 2005) and between the PCS and the MCS (Mishra, Hockey, and Dobson 2014) were expected to be significant. To examine the effect of the occurrence of a specific comorbidity, a total of 11 different comorbidity variables that exhibited significant HRQOL differences in the preliminary analyses were separately entered in the place of the ‘occurrence of the specific comorbidity’ variable in the hypothetical model; thus, a total of 11 analyses were conducted separately.
Goodness-of-fit indices, including chi-square statistics (p> 0.05 acceptable fit) or discrepancy function, the ratio of the discrepancy function to the degrees of freedom, the root mean square error of approximation (RMSEA; acceptable fit≤.08) (Steiger 1990), and the comparative fit index (CFI; acceptable model fit≥.9), were used to evaluate the hypothetical model (Bentler 1990). Although the chi-square is presented, it was not used to assess model fit because it tends to be stringent (Wu, Li, and Zumbo 2007). The model parameters were considered statistically significant at p<0.05 (Bentler 2007).
Results
Sample Characteristics
Data from a total of 157 Chinese- (n=86) and Korean-Americans (n=71) were used in the analysis. The mean age of the participants was approximately 55 years (SD=9.7), and the mean number of years since cancer diagnosis was 3.5(SD=1.6). Participants were highly educated, were married, and used their own language. Years since cancer diagnosis, education, cancer stage, and axillary node dissection differed significantly by ethnicity. Chinese-Americans had a shorter period after cancer diagnosis in comparison to Korean-Americans. Korean-Americans were more likely than Chinese-Americans to have higher levels of education and to be diagnosed with stage I, and they were less likely to undergo axillary node dissection (Table 1).
Table 1.
Demographic and medical characteristics of Chinese- and Korean-Americans
| Characteristics | Total | Chinese (n=86) | Korean (n=71) | t |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age | 55.3 (9.7) | 55.2 (9.7) | 55.5 (9.7) | −0.19 |
| Length of stay in the US | 24.0 (11.7) | 23.5 (12.2) | 23.9 (9.9) | −0.48 |
| Years since diagnosis | 3.5 (1.6) | 3.2 (1.8) | 3.9 (1.4) | −2.60* |
| Life stress | 1.8 (0.7) | 1.9 (0.7) | 1.7 (0.6) | 1.40 |
| n (%) | x2 | |||
| Education | ||||
| <High school | 18 (11.5) | 16 (18.6) | 2 (2.8) | 10.05** |
| High school graduate | 25 (15.9) | 11 (12.8) | 14 (19.7) | |
| >High school | 114 (72.6) | 59 (68.6) | 55 (77.5) | |
| Household income | ||||
| <25K | 57 (39.3) | 35 (40.7) | 22 (33.8) | 1.65 |
| 25K-45K | 25 (17.2) | 13 (15.1) | 12 (18.5) | |
| 45K-75K | 26 (17.9) | 14 (16.3) | 12 (18.5) | |
| >75K | 37 (25.5) | 18 (20.9) | 19 (29.2) | |
| Current employment status | ||||
| Unemployed/homemaker | 70 (45.5) | 57 (66.3) | 53 (74.6) | 1.30 |
| Employed | 84 (54.5) | 29 (33.7) | 18 (25.4) | |
| Marital status | ||||
| Married | 125 (79.6) | 69 (80.2) | 56 (78.9) | 0.04 |
| Others | 32 (20.4) | 17 (19.8) | 15 (21.1) | |
| Health insurance | ||||
| Private | 65 (47.1) | 38 (48.7) | 27 (45.0) | 1.17 |
| Public (Medicare/Medicaid) | 61 (44.2) | 35 (44.9) | 26 (43.3) | |
| No insurance | 12 (8.7) | 5 (6.4) | 7 (11.7) | |
| Primary language | ||||
| Own language | 142 (90.4) | 76 (88.4) | 66 (93.0) | 0.95 |
| English | 15 (9.6) | 10 (11.6) | 5 (7.0) | |
| Cancer stage | ||||
| 0 | 11 (7.1) | 10 (11.6) | 1 (1.4) | 16.01** |
| I | 56 (35.9) | 22 (25.6) | 34 (48.6) | |
| II | 68 (43.6) | 45 (52.3) | 23 (32.9) | |
| III | 21 (13.5) | 9 (10.5) | 12 (17.1) | |
| Surgery (yes)a | ||||
| Axillary node dissection | 61 (38.9) | 39 (45.3) | 22 (31.0) | 3.38* |
| Lumpectomy | 82 (52.2) | 45 (52.3) | 37 (52.1) | 0.00 |
| Mastectomy | 83 (52.9) | 48 (55.8) | 35 (49.3) | 0.66 |
| Radiation (yes) | 86 (57.0) | 45 (55.6) | 41 (58.6) | 0.14 |
| Chemotherapy (yes) | 105 (67.7) | 61 (72.6) | 44 (62.0) | 2.00 |
| Hormonal therapy (yes) | 99 (63.9) | 58 (68.2) | 41 (58.6) | 1.55 |
Note.
p<0.05;
p<0.01;
***p<0.001;
Participants could select more than one response.
In terms of HRQOL, the mean PCS score of Korean-Americans was slightly higher than that of Chinese-Americans, 63.0(SD=25.5) and 60.4(SD=23.0), respectively. The mean MCS score exhibited a similar pattern, 63.5(SD=22.4) for Chinese-Americans and 66.7(SD=23.8) for Korean-Americans, respectively. After controlling for covariates, however, Chinese- and Korean-Americans did not exhibit significant differences in the PCS and MCS scores. The two groups also did not exhibit significant differences in life stress.
Prevalence of Comorbidity
Approximately 60% of BCS had at least one comorbid disease. Approximately 15% of the participants had three or more comorbid diseases. The mean total number of comorbidities before cancer diagnosis was 1.97(SD=2.26), while the mean total number of current comorbidities was 3.45(SD=3.33). Among the participants, 59.3% responded that they currently have more comorbidities than they did in the past.
Osteoporosis (n=46) was the most prevalent comorbid disease in BCS, followed by eye/vision problems (n=42), arthritis (n=33), and dental or gum problems (n=33). For Chinese-Americans, osteoporosis (n=30) was the most prevalent comorbid disease, whereas chronic pain (n=31) was the most prevalent comorbidity for Korean-Americans. The occurrence of a specific comorbidity was not significantly associated with ethnicity (Table 2). Thus, ethnicity was combined in the consequent analyses.
Table 2.
Occurrence of current comorbidities by ethnic group
| Comorbidities | Total (n=157) | Chinese-American (n=86) | Korean-Americans (n=71) | t | |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Total number of comorbidities before a cancer diagnosis | 2.0 (2.3) | 2.1 (2.3) | 1.8 (2.3) | 0.94 | |
| Total number of current comorbidities | 3.4 (3.3) | 3.8 (3.2) | 3.0 (3.4) | 1.48 | |
| N (%) | X2 | ||||
| Allergies | No Yes |
129 (82.1) 28 (17.9) |
65 (76.5) 20 (23.5) |
63 (88.7) 8 (11.3) |
3.95 |
| Arthritis | No Yes |
124 (79.0) 33 (21.0) |
66 (76.7) 20 (23.3) |
58 (81.7) 13 (18.3) |
0.57 |
| High cholesterol | No Yes |
128 (81.5) 29 (18.5) |
68 (79.1) 18 (20.9) |
60 (84.5) 11 (15.5) |
0.76 |
| Diabetes | No Yes |
146 (93.0) 11 (7.0) |
81 (94.2) 5 (5.8) |
65 (91.5) 6 (8.5) |
0.42 |
| Eye/vision problems | No Yes |
115 (73.2) 42 (26.8) |
58 (67.4) 28 (32.6) |
57 (80.3) 14 (19.7) |
3.27 |
| Dental or gum problems | No Yes |
124 (79.0) 33 (21.0) |
65 (75.6) 21 (24.4) |
59 (83.1) 12 (16.9) |
1.32 |
| Hearing problems | No Yes |
138 (87.9) 19 (12.1) |
78 (90.7) 8 (9.3) |
60 (84.5) 11 (15.5) |
1.40 |
| Digestive problems | No Yes |
130 (82.8) 27 (17.2) |
73 (84.9) 13 (15.1) |
57 (80.3) 14 (19.7) |
0.58 |
| Chronic pain | No Yes |
126 (80.3) 31 (19.7) |
72 (83.7) 14 (16.3) |
54 (76.1) 17 (23.9) |
1.44 |
| Heart disease | No Yes |
146 (93.0) 11 (7.0) |
78 (90.7) 8 (9.3) |
68 (95.8) 3 (4.2) |
1.54 |
| High blood pressure | No Yes |
134 (85.4) 23 (14.6) |
72 (83.7) 14 (16.3) |
62 (87.3) 9 (12.7) |
0.40 |
| Osteoporosis | No Yes |
111 (70.7) 46 (29.3) |
56 (65.1) 30 (34.9) |
55 (77.5) 16 (22.5) |
2.86 |
| Lymphedema | No Yes |
132 (84.1) 25 (15.9) |
71 (82.6) 15 (17.4) |
61 (85.9) 10 (14.1) |
0.33 |
| Thyroid problems | No Yes |
139 (88.5) 18 (11.5) |
75 (87.2) 11 (12.8) |
64 (90.1) 7 (9.9) |
0.33 |
| Psychological difficulties | No Yes |
134 (85.4) 23 (14.6) |
72 (83.7) 14 (16.3) |
62 (87.3) 9 (12.7) |
0.40 |
Note. None of statistical tests were significant.
Differences in HRQOL by Occurrence of Specific Comorbidity
In terms of PCS, allergies, arthritis, eye/vision problems, dental or gum problems, digestive problems, chronic pain, heart disease, osteoporosis, lymphedema, thyroid problems, and psychological difficulties exhibited significant differences among BCS who reported ‘yes’ or ‘no’ to each comorbidity question. Regarding the MCS, arthritis, eye/vision problems, dental or gum problems, chronic pain, heart disease, lymphedema, thyroid problems, and psychological difficulties differed significantly between the two groups (“yes” vs. “no”). Although the PCS and the MCS showed similar patterns, allergies, digestive problems, and osteoporosis were more likely to be sensitive to the PCS rather than the MCS, while psychological difficulties were sensitive to the MCS. Of all comorbidities, arthritis, eye/vision problems, dental and gum problems, lymphedema, and psychological difficulties exhibited significant differences in the PCS and/or the MCS by BCS who reported ‘yes’ or ‘no’ (Table 3).
Table 3.
Differences in HRQOL by the occurrence of current comorbidities
| Comorbidities | SF-36 PCS | SF-36 MCS | ||||
|---|---|---|---|---|---|---|
| Reported? | F | Reported? | F | |||
| Yes | No | Yes | No | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Allergies | 52.2 (25.8) | 64.2 (23.0) | 5.67* | 58.9 (27.1) | 66.7 (22.0) | 2.14 |
| Arthritis | 45.2 (23.1) | 66.3 (22.1) | 15.03*** | 52.0 (25.1) | 68.7 (21.3) | 8.76** |
| High cholesterol | 58.9 (26.8) | 62.8 (23.2) | 0.33 | 59.6 (25.5) | 66.6 (22.3) | 1.37 |
| Diabetes | 58.3 (22.4) | 62.4 (23.9) | 0.19 | 59.0 (25.5) | 65.8 (22.8) | 0.64 |
| Eye/vision problems | 47.8 (21.4) | 67.6 (22.4) | 15.80*** | 54.2 (24.2) | 69.6 (21.1) | 8.49** |
| Dental problems | 46.7 (23.7) | 66.3 (22.1) | 16.37*** | 48.9 (25.1) | 69.8 (20.4) | 20.14*** |
| Hearing problems | 55.1 (22.1) | 63.0 (23.9) | 0.85 | 60.7 (23.6) | 66.0 (22.9) | 0.36 |
| Digestive problems | 49.5 (25.6) | 64.5 (22.8) | 4.43* | 56.2 (26.1) | 67.1 (22.1) | 2.01 |
| Chronic pain | 46.8 (27.8) | 65.6 (21.5) | 12.12** | 52.7 (27.5) | 68.2 (21.0) | 8.29** |
| Heart disease | 39.0 (18.6) | 63.9 (23.3) | 8.02** | 39.7 (19.5) | 67.3 (22.1) | 11.21** |
| High blood pressure | 65.0 (25.5) | 61.7 (23.6) | 0.87 | 64.9 (25.8) | 65.5 (22.6) | 0.06 |
| Osteoporosis | 54.1 (23.6) | 65.5 (23.1) | 4.69* | 61.3 (23.2) | 67.1 (22.8) | 0.63 |
| Lymphedema | 43.1 (24.0) | 66.2 (21.8) | 19.95*** | 45.3 (26.7) | 69.7 (19.7) | 25.6*** |
| Thyroid | 51.6 (16.3) | 63.7 (24.3) | 5.88* | 52.4 (19.9) | 67.3 (22.9) | 8.89** |
| Psychological difficulties | 47.4 (26.6) | 64.8 (22.3) | 5.45* | 41.8 (22.8) | 69.7 (20.3) | 25.43*** |
Note. A univariate general linear model was conducted, controlling for income, ethnicity, cancer stage, and hormonal therapy; PCS=Physical Component Summary; MCS=Mental Component Summary
p<0.05;
p<0.01;
p<0.001.
The Mediating Effect of Comorbidities on the Relationship between Life Stress and HRQOL
The hypothetical model was evaluated to investigate the mediating effect of comorbidities on the relationship between life stress and HRQOL (Figure 1). A total of 11 analyses were conducted to confirm similarities/differences in the associations among variables based on the occurrence of the specific comorbidity that was entered into the model. Overall, the hypothetical model produced a moderate fit (CFI≥0.947, RMSEA≤0.081). Life stress was significantly associated with both the PCS (β=−0.46~−0.52, p<0.001) and the MCS (β=−0.48~−0.58, p<0.001). Age was directly related to the total number of comorbidities (β=0.44, p<0.001) (Table 4).
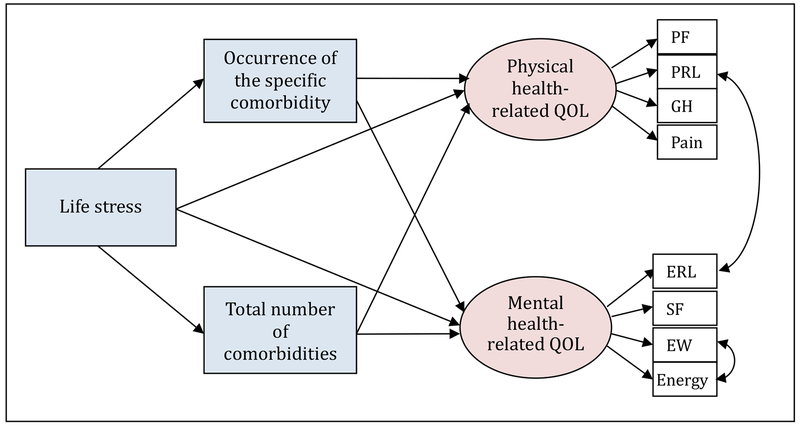
Figure 1.
Hypothetical Model: the Mediating Effect of Comorbidity
Note. PF=physical functioning; PRL=physical role limitation; GH=general health; ERL=emotional role limitation; SF=social functioning; EW=emotional wellbeing; Age is controlled.
Table 4.
Factor loadings and path coefficients by the occurrence of the specific comorbidity
| Occurrence of the specific comorbidity (A): Standardized beta coefficients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergy | Arthritis | Eye | Dental | Digestive | Pain | Heart | Osteoporosis | Lymph-edema | Thyroid | Psycho | |
| Factor loadings | |||||||||||
| PCS → PF | 0.66*** | 0.67*** | 0.66*** | 0.67*** | 0.66*** | 0.67*** | 0.67*** | 0.67*** | 0.67*** | 0.67*** | 0.67*** |
| PCS → PRL | 0.74*** | 0.73*** | 0.75*** | 0.74*** | 0.74*** | 0.74*** | 0.74*** | 0.74*** | 0.74*** | 0.74*** | 0.74*** |
| PCS → GH | 0.72*** | 0.71*** | 0.72*** | 0.72*** | 0.73*** | 0.72*** | 0.72*** | 0.72*** | 0.72*** | 0.72*** | 0.72*** |
| PCS → Pain | 0.79*** | 0.80*** | 0.79*** | 0.79*** | 0.79*** | 0.79*** | 0.79*** | 0.78*** | 0.79*** | 0.79*** | 0.79*** |
| MCS → ERL | 0.71*** | 0.71*** | 0.71*** | 0.71*** | 0.71*** | 0.71*** | 0.71*** | 0.72*** | 0.72*** | 0.71*** | 0.71*** |
| MCS → SF | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.85*** | 0.86*** |
| MCS → EW | 0.78*** | 0.78*** | 0.78*** | 0.78*** | 0.78*** | 0.78*** | 0.77*** | 0.78*** | 0.77*** | 0.78*** | 0.77*** |
| MCS → Energy | 0.76*** | 0.76*** | 0.76*** | 0.77*** | 0.76*** | 0.76*** | 0.77*** | 0.76*** | 0.76*** | 0.76*** | 0.76*** |
| Path Coefficients | |||||||||||
| Stress → A | 0.13 | 0.20** | 0.20* | 0.33*** | 0.11 | 0.23** | 0.22** | 0.17* | 0.33*** | 0.15* | 0.35*** |
| Stress → total # | 0.19** | 0.19** | 0.19* | 0.19** | 0.19** | 0.19** | 0.19** | 0.19** | 0.19** | 0.19** | 0.19** |
| Stress → PCS | −0.52*** | −0.49*** | −0.49*** | −0.48*** | −0.51*** | −0.50*** | −0.51*** | −0.51*** | −0.46*** | −0.52*** | −0.50*** |
| Stress → MCS | −0.57*** | −0.55*** | −0.55*** | −0.51*** | −0.57*** | −0.55*** | −0.54*** | −0.58*** | −0.50*** | −0.56*** | −0.48*** |
| A → PCS | −0.11 | −0.26*** | −0.23** | −0.17* | −0.18* | −0.19* | −0.12 | −0.12 | −0.20** | −0.07 | −0.07 |
| A → MCS | −0.03 | −0.21** | −0.14 | −0.24** | −0.10 | −0.15* | −0.19** | −0.01 | −0.25*** | −0.11 | −0.29*** |
| Total # → PCS | −0.25*** | −0.20** | −0.26*** | −0.24** | −0.28*** | −0.23** | −0.26*** | −0.26*** | −0.28*** | −0.28*** | −0.27*** |
| Total # → MCS | −0.15* | −0.10 | −0.15* | −0.11 | −0.16* | −0.12 | −0.13 | −0.16* | −0.16* | −0.16* | −0.14 |
| Age → A | 0.05 | 0.26*** | 0.17* | 0.07 | 0.13 | 0.19* | 0.14 | 0.18* | 0.05 | −0.15 | 0.08 |
| Age → total # | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** | 0.44*** |
| Fit Indices | |||||||||||
| X2 (df=46) | 83.078 | 85.056 | 89.530 | 84.577 | 82.883 | 85.379 | 84.521 | 88.155 | 85.196 | 84.676 | 84.465 |
| CFI | 0.954 | 0.953 | 0.947 | 0.953 | 0.954 | 0.952 | 0.953 | 0.949 | 0.953 | 0.952 | 0.954 |
| RMSEA | 0.075 | 0.077 | 0.081 | 0.077 | 0.075 | 0.078 | 0.077 | 0.080 | 0.077 | 0.077 | 0.077 |
| Variance in PCS | 0.424 | 0.479 | 0.467 | 0.438 | 0.446 | 0.444 | 0.427 | 0.426 | 0.450 | 0.417 | 0.417 |
| Variance in MCS | 0.393 | 0.433 | 0.411 | 0.437 | 0.403 | 0.412 | 0.426 | 0.393 | 0.448 | 0.402 | 0.466 |
Note. ‘A’ refers to the specific comorbidity entered as the “occurrence of the specific comorbidity” variable; PF=physical functioning, PRL=physical role limitation, GH=general health perception, ERL=emotional role limitation, SF=social functioning, EW=emotional wellbeing, PCS=physical component summary, MCS=mental component summary; Age is controlled.
p<0.05;
p<0.01;
p<0.001.
In terms of the mediating effect, first, the total number of comorbidities mediated the relationship between life stress and PCS, even after the occurrence of the specific comorbidity was entered. This result indicates that lower life stress was significantly associated with fewer comorbidities and, consequently, a better PCS. However, the mediating effect of the total number of comorbidities on the relationship between life stress and MCS appeared in the models only when allergy, eye/vision problems, digestive problems, osteoporosis, lymphedema, or thyroid problems were entered. When other comorbidities (i.e., arthritis, dental and gum problems, chronic pain, heart disease, or psychological difficulties) were entered, the total number of comorbidities was no longer significantly associated with the MCS.
Second, of 11 comorbidity variables entered into the ‘occurrence of the specific comorbidity,’ arthritis, eye/vision problems, dental and gum problems, chronic pain, and lymphedema mediated the relationship between life stress and PCS. This result indicates that those who had higher life stress were more likely to report having a specific comorbidity such as arthritis, eye/vision problems, dental and gum problems, chronic pain, or lymphedema and, consequently, have worse physical HRQOL. In terms of MCS, arthritis, dental and gum problems, chronic pain, heart disease, lymphedema, and psychological difficulties mediated the relationship between life stress and mental HRQOL. Each model by comorbidity predicted 0.417 to 0.479 of the variance in PCS and 0.393 to 0.466 of the variance in MCS (Table 4).
Discussion
The issue of care for comorbid conditions in Chinese- and Korean-American cancer survivors is not fully understood. As survivors live longer after a cancer diagnosis, appropriate survivorship care, including care for psychosocial and comorbid conditions, takes on greater importance. The current study intended to identify the occurrence of comorbidities, examine HRQOL differences by the occurrence of specific comorbidity, and investigate the mediating effect of comorbidities on the relationships between life stress and HRQOL for Chinese- and Korean-American BCS. Specifically, this study utilized two comorbidity-related variables to comprehensively consider the combined effects of different diseases and the impact of the occurrence of specific comorbidities on outcomes.
First, the current study revealed that approximately 60% of Chinese- and Korean-American BCS had at least one comorbidity. These levels are lower than those reported for other ethnicities in previous studies (68% to 85%) (Ashing-Giwa et al. 2014). Because previous studies mostly focused on the elderly population and the risk of comorbid conditions increases with age (Sogaard et al. 2013), the current study finding seems to be reasonable because the average age of our study sample was 55 years. Additionally, the findings showed that osteoporosis was the most prevalent comorbid disease for both Chinese- and Korean-American BCS, followed by eye/vision problems and then arthritis. In Ashing-Giwa et al.’s (2014) study, African-Americans were most likely to report having high blood pressure, while Latinas were most likely to have arthritis or psychological difficulties. Future studies need to further investigate whether there are similarities or differences in terms of the occurrence of specific comorbidities by ethnic subgroup. Meanwhile, the types of comorbidities that were common among Chinese- and Korean-Americans seemed to be associated with the aging process, which is consistent with Ashing-Giwa’s study. Because comorbidities negatively influence cancer treatment and survivorship care and breast cancer primarily occurs after age 50, this finding suggests that it is important to thoroughly manage diseases that are involved in the aging process in the initial stage. Promoting healthy behaviors at a younger age can delay the aging process, reducing risk factors associated with disease and mortality (Loef and Walach 2012). Therefore, the current study suggests that health-promoting lifestyle behaviors such as diet, exercise, and stress- and self-management skills are required.
Third, this study investigated HRQOL differences based on the occurrence of specific comorbidities. Of all types of comorbidities, allergies, digestive problems, and osteoporosis were more likely to be associated with physical HRQOL. Moreover, arthritis, eye/vision problems, dental and gum problems, lymphedema, and psychological difficulties were significantly related to both physical and mental HRQOL at a p<0.01 level. Previous studies have focused on lymphedema in BCS specifically because lymphedema is the most common side effect of breast cancer treatments (Ridner et al. 2016). The current study found that other comorbidities, in addition to lymphedema are significantly associated with HRQOL for BCS, suggesting that the aggressive management of comorbid conditions should be provided after initial cancer treatment. Given that cancer survivors should be considered to have a chronic illness requiring long-term surveillance due to risks for a wide range of late effects (McCorkle et al. 2011), the Chronic Care Model (Wagner, Austin, and Von Korff 1996) may be used for healthcare management for cancer survivors. The Chronic Care Model requires effective chronic illness management programs for optimal care, including the features of a health care system that encourages high-quality care (McCorkle et al. 2011). Future studies are necessary to develop long-term survivorship care plans to enable and empower BCS to engage in the symptomatic management and prevention of comorbidities.
In terms of the mediating effects of comorbidities, the current study found that the nature of the outcome variable, i.e., whether it is related to either the physical or mental HRQOL of the survivor, influenced the overall patterns of the findings. That is, with regard to physical HRQOL, life stress was significantly associated with the total number of comorbidities and in turn influenced physical HRQOL, regardless of the occurrence of the specific comorbidity. This result indicates that the total number of comorbidities can vary according to life stress and the number of comorbid diseases survivors are currently experiencing can influence physical QOL specifically. Psychosocial and environmental stresses increase susceptibility to disease and illness as well as the development of somatic symptoms (Beatty, Lee, and Wade 2009). Thus, appropriate physical or psychological interventions are required to control the number of comorbidities for cancer survivors. For example, mindfulness-based interventions may be effective to relieve life stress because meditative practices are believed to promote healthier ways of relating to inner experiences through enhanced awareness, attention regulation, and the acceptance of thoughts, emotions, and states (Burton et al. 2016). Cognitive behavioral therapy may also be a promising and effective treatment to improve psychological and physical functioning (Van Beugen et al. 2014). Additionally, a comprehensive survivorship program that addresses physical, psychosocial and behavioral factors should be developed.
The finding that life stress was directly associated with physical and mental HRQOL for BCS is consistent with other studies (Beatty, Lee, and Wade 2009). Life stress, which refers to experiences and circumstances in daily life, may make it harder physically and emotionally to cope with the added burden of breast cancer (Turner et al. 2005; Ashing-Giwa and Lim 2010). Thus, stress-management interventions might be an important method to reduce negative the consequences of life stress and to increase HRQOL for BCS. Further investigation of the impact of life stress on HRQOL for cancer survivors is clearly warranted.
Recent comorbidity-related studies (Fu et al. 2015; Ording et al. 2013) have utilized validated measures such as the Charlson Comorbidity Index (CCI) in which the severity of individual diseases is reflected by the presence of disease (Charlson et al. 1987). However, Fu and colleagues (2015) demonstrated that results regarding associations between HRQOL and comorbidities were similar when comorbidities were assessed by self-report and by CCI; therefore, our findings seem to be reliable. Nevertheless, given that the reliability of patient self-report measures can vary by educational levels due to the enhanced ability to report comorbidity conditions (Fu et al. 2015), comorbidity-related measures should be used with caution. In addition to the total number of comorbidities, the current study investigated the impact of the occurrence of a specific comorbidity on HRQOL to further explore the combined effect of the disease. Two comorbidity-related measures produced different findings. For example, when heart disease was entered as an “occurrence of the specific comorbidity,” the total number of comorbidities was no longer significant. This indicates that the presence of heart disease can seriously reduce mental HRQOL regardless of how many comorbidities individuals have. Therefore, an assessment of the types of comorbid conditions that survivors are currently experiencing can help health care professionals effectively manage the physical or psychological aspects of HRQOL.
Although the current study has a number of strengths, it also has limitations. Because this study is based on a cross-sectional design, the causal relationships among variables cannot be determined. Another limitation is that the validated measure of comorbidities was not utilized. Thus, the severity of the comorbidities was not considered, and other comorbid conditions were not included. However, we expect that the inclusion of two comorbidity-related variables would be able to contribute to the exploration of which comorbidity variable is more important, controlling the effect of other variables. Additionally, we did not include other Asian subgroups. Other Asian subgroups must be included to comprehensively understand the patterns and role of comorbidities among Asian-Americans. Finally, the current study was based on self-reporting; thus the findings may be influenced by recall bias.
Overall, the findings add to the existing literature by examining the mediating effects of comorbidity on the relationship between life stress and HRQOL. This study demonstrates the importance of examining the effects of comorbidities based on the physical or psychological aspects of HRQOL. These data support the need for health care professionals to clearly assess physical or psychological comorbidities when providing cancer survivorship care. Future research should extend these findings to other Asian subgroups with more specific and validated measures of comorbidities and investigate other psychosocial and environment variables that may be associated with comorbidities for cancer survivors.
Acknowledgements:
This study was funded by the NIH/NCI (Grant number: R03 CA139941). This was also supported by Kangnam University Research Grant.
Footnotes
Conflict of Interest:
The author declares no conflict of interest.
References
- American Cancer Society. 2016. “Cancer Facts & Figures 2016.” In. Atlanta, GA: American Cancer Society. [Google Scholar]
- Ashing-Giwa K, and Lim JW. 2010. “Exploring the association between functional strain and emotional well-being among a population-based sample of breast cancer survivors.” Psycho-Oncology 19:150–9. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa K, Rosales M, Lai L, and Hurria A. 2014. “Occurrence of comorbidities among African-American and Latina breast cancer survivors.” Journal of Cancer Survivorship 8:312–8. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa K, Padilla G, Tejero J, and Kim J. 2004. “Breast cancer survivorship in a multiethnic sample: Challenges in recruitment and measurement.” Cancer 101 (3):450–65. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, and Hellemann G. 2007. “Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma.” Quality of Life Research 16 (3):413–28. [DOI] [PubMed] [Google Scholar]
- Beatty L, Lee C, and Wade TD. 2009. “A prospective examination of perceived stress as a mediator of the relationship between life-events and QOL following breast cancer.” British Journal of Health Psychology 14 (4):789–804. [DOI] [PubMed] [Google Scholar]
- Bellury L, Ellington L, Beck SL, Pett MA, Clark JA, and Stein K. 2013. “Older breast cancer survivors: can interaction analysis identify vulnerable subgroups? A report from the American Cancer Society Studies of Cancer Survivors.” Oncology Nursing Forum 40:325–36. [DOI] [PubMed] [Google Scholar]
- Bellury L, Pett MA, Ellington L, Beck SL, Clark JC, and Stein KD. 2012. “The effect of aging and cancer on the symptom experience and physical function of elderly breast cancer survivors.” Cancer 118:6171–8. [DOI] [PubMed] [Google Scholar]
- Bentler PM 1990. “Comparative fix indexes in structural models “Psychological Bulletin 107 (2):238–46. [DOI] [PubMed] [Google Scholar]
- Bentler PM 2007. “On tests and indices for evaluating structural models.” Personality and Individual Differences 42:825–9. [Google Scholar]
- Bjorntorp P 2000. “Do stress reactions cause abdominal obesity and comorbidities?” Obesity Reviews 2:73–86. [DOI] [PubMed] [Google Scholar]
- Burton A, Burgess C, Dean S, Koutsopoulou GZ, and Hugh-Jones S. 2016. “How effective are mindfulness-based intervention for reducing stress among healthcare professionals? A systematic revew and meta-analysis.” Stress and Health:in press. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, and MacKenzie CR. 1987. “A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation.” Journal of Chronic Diesease 40:373–83. [DOI] [PubMed] [Google Scholar]
- Fu MR, Axelrod D, Guth AA, Cleland CM, Ryan CE, Weaver KR, Qiu JM, et al. 2015. “Comorbidities and quality of life among breast cancer survivors: a prospective study.” Journal of Personalized Medicine 5:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci JM, Escalante CP, Freeman J, and Goodwin JS. 2005. “Comorbid disease and cancer: the need for more relevant conceptual models in health services research.” Journal of Clinical Oncology 23 (30):7399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, and Van Den Bos GA. 2001. “Causes and consequences of comorbidity: a review.” Journal of Clinical Epidemiology 54 (7):661–74. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. 2006. From cancer patient to cancer survivor: Lost in transition Washington DC: The National Academies Press. [Google Scholar]
- Jeevan R, Cromwell D, Browne J, Van Der M, Caddy CM, and Pereira J. 2015. “Second Annual Report of the National Mastectomy and Breast Reconstruction Audit 2009.” NHS Information Centre, Accessed June 8 https://www.rcseng.ac.uk/surgeons/research/surgical-research/docs/national-mastectomy-and-breast-reconstruction-audit-second-report-2009.
- Lim JW, Gonzalez P, Wang M, and Ashing-Giwa K. 2009. “Understanding the cultural health belief model influencing health behaviors and health-related quality of life between Latina and Asian-American breast cancer survivors.” Supportive Care in Cancer 17 (9):1137–47. doi: 10.1007/s00520-008-0547-5. [DOI] [PubMed] [Google Scholar]
- Lim JW, and Paek MS. 2015. “Recruiting Chinese- and Korean-Americans in Cancer Survivorship Research: Challenges and Lessons Learned.” Journal of Cancer Education:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M, and Walach H. 2012. “The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis.” Preventive Medicine 55 (3):163–70. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, & Sugawara HM 1996. “Power analysis and determination of sample size for covariance structure modeling.” Psychological Methods 1:130–49. [Google Scholar]
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, and Farrar JT. 2007. “Symptom burden among cancer survivors: impact of age and comorbidity.” Journal of the American Board of Family Medicine 20 (5):434–43. [DOI] [PubMed] [Google Scholar]
- Maratia S, Cedillo S, and Rejas J. 2016. “Assessing health-related quality of life in patients with breast cancer: a systematic and standardized comparison of available using the EMPRO tool.” Quality of Life Research:in press. [DOI] [PubMed] [Google Scholar]
- McCollum KH, Wood FG, and Auriemma K. 2014. “Evaluation of a breast and colon cancer survivorship program.” Clinical Journal of Oncology Nursing 18 (2):231–6. [DOI] [PubMed] [Google Scholar]
- McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, and Wagner EH. 2011. “Self-management: enabling and empowering patients living with cancer as a chronic illness.” CA A Cancer Journal for Clinicians 61 (1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GD, Hockey R, and Dobson AJ. 2014. “A comparison of SF-36 summary measures of physical and mental health for women across the life course.” Quality of Life Research 23:1515–21. [DOI] [PubMed] [Google Scholar]
- Mueller RO 1997. “Structural equation modeling: back to the basics.” Structural Equation Modeling 4:353–69. [Google Scholar]
- Napoles-Springer A 2011. “Coping resources and self-rated health among Latina breast cancer survivors.” Oncology Nursing Forum 38 (5):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ording AG, Garne JP, Nystrom PMW, Froslev T, Sorensen HT, and Lash TL. 2013. “Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis - a Danish Nationawide Matched Cohort Study.” PLoS ONE 8 (10):e76013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek MS, and Lim JW. 2015. “Understanding the stress process of Chinese- and Korean-American breast cancer survivors.” Journal of Immigrant and Minority Health:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel C, Savard J, and Ivers H. 2009. “Cognitive impairments associated with breast cancer treatments: results from a longitudinal study.” Breast Cancer Research and Treatment 116:113–23. [DOI] [PubMed] [Google Scholar]
- Ridner SH, Rhoten BA, Radina ME, Adair M, Bush-Foster S, and Sinclair V. 2016. “Breast cancer survivors’ perspectives of critical lymphedema self-care support needs.” Supportive Care Cancer 24 (6):2743–50. [DOI] [PubMed] [Google Scholar]
- Sarfati D, Hill S, Blakely T, Robson B, Purdie G, Dennett E, Cormack D, and Dew K. 2009. “The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study.” BMC Cancer 9 (116):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, and Norgaard M. 2013. “The impact of comorbidity on cancer survival: a review.” Clinical Epidemiology 5:3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan DL, Croghan KA, Croghan IT, Jenkins SM, Sutherland SJ, Cheville AL, and Pruthi S. 2016. “Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue.” Supportive Care in Cancer:in press. [DOI] [PubMed] [Google Scholar]
- Steiger JH 1990. “Structural model evaluation and modification: An interval estimation approach.” Multivariate Behavioral Research 25:173–80. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Swanson SA, Zeng Y, Weeks M, and Colman I. 2013. “The contribution of stress to the comorbidity of migraine and major depression: results from a prospective cohort study.” BMJ 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammemagi CM 2005. “Comorbidity and survival disparities among Black and White patients with breast cancer.” JAMA 294 (14):1765–72. [DOI] [PubMed] [Google Scholar]
- Turner J, Kelly B, Swanson C, Allison R, and Wetzig N. 2005. “Psychosocial impact of newly diagnosed advanced breast cancer.” Psycho-Oncology 14:396–407. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2011. “2010 American Community Survey.” In available at http://www.census.gov/.
- Van Beugen S, Ferwerda M, Hoeve D, Rovers MM, Spillekom-van Koulil S, and Van Middendorp H. 2014. “Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review.” Journal of Medical Internet Research 16 (3):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, and Von Korff M. 1996. “Organizing care for patients with chronic illness.” Milbank Quarterly 74:511–44. [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, and Gandek B. 1993. SF-36 health survey: manual and interpretation guide. Boston: Health Institute, New England Medical Center. [Google Scholar]
- Winters ZE, Afzal M, Balta V, Freeman J, Llewellyn-Bennett R, Cook RJ, Greenwood R, and King MT. 2016. “Patient-reported outcomes and their predictors at 2- and 3-year follow-up after immediate latissimus dorsi breast reconstruction and adjuvant treatment.” British Journal of Surgery 103:524–36. [DOI] [PubMed] [Google Scholar]
- Wu AD, Li Z, and Zumbo BD. 2007. “Decoding the meaning of factorial invariance and updating the practice of multi-group confirmatory factor analysis: A demonstration with TIMSS data.” Practice Assessment, Research & Evaluation 12 (3):1–26. [Google Scholar]