Abstract
Background:
Prescribing practice patterns and factors associated with treatment changes in older patients initiating antipsychotic treatment for the behavioral and psychological symptoms of dementia is not well known.
Objectives:
The objective of this study is to study 90-day prescribing practice patterns across the three most commonly prescribed antipsychotics.
Methods:
This is a retrospective study using national data from the US Department of Veterans Affairs (VA). The study included patients older than 65 years diagnosed with dementia who began outpatient treatment with an antipsychotic medication between 2005 and 2008. Patients were followed for 90 days from their antipsychotic start. The primary event of interest was changing to another psychotropic medication. Cumulative incidence of treatment change was determined with antipsychotic discontinuation and death as competing risks. Covariate-adjusted hazard ratios for treatment change were determined using competing risk regression models.
Results:
During the study period, 15,435 patients initiated an atypical antipsychotic; 14,791 started olanzapine, quetiapine, or risperidone. Over half (55%) of the patients discontinued index treatment within 90 days, 36% continued, 3% died while on index treatment, and 6% changed to another psychotropic medication. Compared with quetiapine, the adjusted hazard of treatment change was higher by 43% (p = 0.005) for olanzapine and by 12% (p = 0.08) for risperidone.
Conclusion:
The higher hazard of treatment change with olanzapine suggests patients either responded worse to or experienced more adverse events with olanzapine compared with quetiapine.
Keywords: atypical antipsychotics, treatment change, dementia, older patients
Introduction
The risk of dementia increases with age, and more than 80% of dementia patients present with neuropsychiatric symptoms (NPS) of dementia (also commonly referred to as behavioral and psychological symptoms of dementia) including agitation, aggression, delusions, and hallucinations (Lyketsos et al., 2002). No medication is approved by the Food and Drug Administration (FDA) for treatment of NPS of dementia. Nevertheless, antipsychotics have long been used to treat NPS. In the Spring of 2005, the FDA issued a warning that the “treatment of behavioral disorders in older patients with dementia with second generation antipsychotic medications is associated with increased mortality.” Use of second generation antipsychotics began to decline significantly in 2003, and the FDA advisory was temporally associated with a significant acceleration in the decline (Kales et al., 2007). Nonantipsychotic medications used to treat NPS include antidepressants, mood stabilizers, and benzodiazepines, but there is little clinical trial data to support their use (Sink et al., 2005). Clinicians treating older patients with NPS are confronted with few pharmacologic alternatives.
Although antipsychotics are often used as a first-line treatment for NPS, there is limited information about the course of treatment and frequencies of discontinuing or changing to other psychotropic medications. A change in treatment can occur for several reasons, including symptom worsening, nonresponse, nonadherence, or side-effects. Treating older patients is particularly challenging because of comorbid illnesses, polypharmacy, and other common factors in the aging population, including changes in drug response and inability to metabolize drugs (Lau et al., 2010; Solomons and Geiger, 2000; Sajatovic et al., 2000). These factors may cause the older patients to be more susceptible to treatment-related or treatment-emergent adverse events (Sajatovic et al., 2000; Jeste et al., 1999; Jeste et al., 2008). Expert consensus is that second generation antipsychotics are not a homogenous class (Davis et al., 2003). For example, in patients with Parkinson’s disease, olanzapine and risperidone are both associated with worsened motor function, while quetiapine is not (Fernandez et al., 2003).
The Veterans Health Administration (VHA) provides an ideal context to examine the patterns of medication use for NPS for a number of reasons, including significant numbers of older patients receiving antipsychotic medications and high-quality pharmacy and health services data, linkable to other critical large-scale data resources. Although there is no guideline pertaining to treatment duration for an antipsychotic medication, a 90-day treatment period is considered a standard treatment duration to evaluate effectiveness (Schneider et al., 2006a). The goal of this retrospective cohort study was to describe and compare prescribing practice patterns in the 90 days after newly starting one of the three most commonly prescribed antipsychotic treatment for NPS in older patients and to identify factors associated with changing treatment. This information has the potential to inform clinicians in deciding which medications to use in older patients with NPS.
Methods
Study cohort
Data came from national VA registries maintained by the Serious Mental Illness Treatment, Resource, and Evaluation Center in Ann Arbor, Michigan, for veterans who received a dementia diagnosis (International Classification of Diseases, Ninth Revision diagnoses 290.0, 290.1×, 290.2×, 290.3, 290.4×, 291.2, 294.10, 294.11, 331.0, 331.1, and 331.82) in a VA inpatient or outpatient setting. Inclusion criteria were as follows: (i) a dementia diagnosis; (ii) new start (after a 12-month clean period of no antipsychotic use) of one of three most commonly used antipsychotics for NPS (olanzapine, quetiapine, and risperidone) as outpatient prescription fills either as regular medication or as PRN (“as needed”) between 1 May 2005 and 30 September 2008; and (iii) age 65 years or older at the time of the new start. This study was approved by the VA Ann Arbor Healthcare System Institutional Review Board.
Follow-up time to changing index medication
Follow-up began on the date of the start of an antipsychotic agent and ended on the date of changing from the index medication to another psychotropic, discontinuation of the index medication (at least 7 days of gap), death, or 90 days, whichever came first. Changing the index medication within 90 days included changing to another antipsychotic (including others in this class beyond the three most commonly prescribed) or other psychotropic medication (e.g., anxiolytic and mood stabilizer) within 7 days of the end of the last supply from a continued exposure. Augmenting the index antipsychotic with another agent was not considered a treatment change. Continued exposure to the index antipsychotic was defined by evaluating the medication fills and the number of days’ supply of medication with any gaps in fills of less than 7 days considered as continued exposures. Mortality data were obtained from the US National Death Index (National Center for Health Statistics, Hyattsville, MD).
Primary predictor and other variables
Primary predictors were usage of the three antipsychotics: olanzapine, quetiapine, and risperidone. Other variables included those known to be or potentially associated with differential use of antipsychotics. They included age, gender, ethnicity, marital status, and a number of psychiatric and medical comorbidities (Charlson et al., 1987), including delirium diagnosis based on a coding scheme for acute confusional states in the year prior to a new medication start, developed for a prior study (Kales et al., 2011). Time since first dementia diagnosis and type of dementia were also included. Calendar year of the new medication start was included to control for potential changes in health care, particularly given the impact of the black box warning in the spring of 2005 and the increased use of quetiapine during the study period (Kim et al., 2011). The model also included health care utilization variables as a proxy for illness severity, including inpatient and nursing home days in the year prior to index medication start and whether the patient had a visit to a psychiatrist in prior 30 days. Exposure to benzodiazepines, valproic acid, carbamazepine, opiates, other anticonvulsants, and antidepressants during 1 year prior to initiating the antipsychotic was also obtained. Facility characteristics included facility size, urban location, region (Northeast, Midwest, West, or South), and academic affiliation of the facility where the medication was prescribed.
Data analysis
We reported proportions of different prescribing practice patterns in the first 90 days after initiating the antipsychotics. The event of interest was changing the index treatment to another psychotropic medication, and we considered death or discontinuing the index treatment as competing events that impede the primary event of interest. We analyzed the cause-specific hazard of experiencing a particular treatment event at any particular time and used competing risks regression to produce cumulative incidence function of changing treatment to another psychotropic medication until 90 days following the start of antipsychotics and to compare the hazard of changing treatment among the three antipsychotics while adjusting for other covariates (Fine and Gray, 1999). The crude and covariate-adjusted effects of olanzapine and risperidone relative to quetiapine were each estimated as cause-specific subhazard ratio (called HR from herein). Continuous covariates were examined for their functional relationship to treatment change and categorized appropriately. Initial prescribed doses varied by antipsychotics, and although initial and last dose may be predictive of treatment change, it was also likely associated dually with efficacy as well as adverse events. We, therefore, explored how dose might be related with treatment change to interpret the main findings. In addition, because previous studies have shown that quetiapine is the preferred antipsychotic in patients with Parkinson’s disease, we repeated the analysis after excluding patients with Parkinson’s diagnoses (Weintraub et al., 2011; Fernandez et al., 2003; Rabey et al., 2007). All statistical analyses were performed using Stata 13.1 (College Station, TX).
Results
During the study period, 14,791 VHA patients with dementia newly started one of the three aforementioned antipsychotics; 50.8% were started on quetiapine, followed by 39.5% on risperidone and 9.6% olanzapine. The patients were 97.7% male with a mean age of 80.6 years. Index dementia diagnoses were made concurrent with the index fill in 12.8% of the patients, with no difference among the three antipsychotics (p = 0.19). In the remainder of the study sample, dementia diagnoses preceded the index antipsychotic fill.
More than half of the study population (54.6%) discontinued the antipsychotic treatment within 90 days, 36.0% continued beyond 90 days, 3.3% died while on the index treatment, and 6.2% changed to another psychotropic medication with a median of 21 days to change, while a more broadly defined treatment changes occurred (defined as change or stop in the index medication treatment, death or 90 days, whichever is earlier) at a median of 52 days (Table 1). Changing the index treatment occurred least frequently with quetiapine (5.7%), followed by risperidone (6.5%) and olanzapine (7.3%). Conversely, continuing the index treatment beyond 90 days was highest with quetiapine (37.1%), followed by olanzapine (35.5%) and risperidone (34.7%).
Table 1.
Distribution of four competing treatment status in 90 days of the new start of an antipsychotic medication in the VA patients older than 65 years and with dementia after black box warning, from May 2005 to September 2008, by the three most commonly prescribed antipsychotics
| Olanzapine N = 1424 | Quetiapine N = 7520 | Risperidone N = 5847 | Total N = 14791 | |
|---|---|---|---|---|
| Treatment status in 90 days, N (%) | ||||
| Changeda index medication | 104 (7.3) | 432 (5.7) | 378 (6.5) | 914 (6.2) |
| Continued treatment ≥90 days | 506 (35.5) | 2787 (37.1) | 2026 (34.7) | 5319 (36.0) |
| Stoppedb index medication | 762 (53.5) | 4078 (54.2) | 3229 (55.2) | 8069 (54.6) |
| Died during index medication | 52 (3.7) | 223 (3.0) | 214 (3.7) | 489 (3.3) |
| Days to treatment status changec | 52 (60) | 54 (60) | 52 (60) | 52 (60) |
| Daily dose in mg/day | ||||
| Initial dose | 5.0 (2.5, 5.3) | 25.0 (25.0, 56.3) | 0.5 (0.5, 0.8) | |
| Last dose | 5.0 (2.5, 5.6) | 50.0 (50.0, 62.8) | 0.5 (0.5, 0.9) | |
| Daily haloperidol equivalent dose | ||||
| Initial prescribed dose | 2.0 (1.1, 2.2) | 0.2 (0.3, 0.7) | 0.6 (0.7, 1.0) | |
| Last dose | 2.0 (1.1, 2.4) | 0.5 (0.6, 0.8) | 0.6 (0.7, 1.2) | |
Cell values are median (interquartile range) for days to treatment status change and median (interquartile range, mean) for daily doses, unless otherwise specified.
Includes 35 (3.8%) deaths after changing treatment but within 90 days.
Includes 373 (4.6%) deaths after stopping treatment but within 90 days.
Treatment change is defined as change or stop in the index medication treatment, death, or 90 days, whichever is earlier.
Among the 914 patients who changed the initial antipsychotic treatment, the most common change was to another antipsychotic agent (26.8%, Table 2). In changes to a psychotropic medication other than an antipsychotic, changes to an acetylcholinesterase inhibitor were most common in quetiapine and risperidone patients, while changes to an antidepressant were most common in olanzapine patients.
Table 2.
Distribution of psychotropic medication class the 914 patients who changed the index antipsychotic medication treatment changed to.
| Medication class changed to | Olanzapine |
Quetiapine |
Risperidone |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Another atypical antipsychotica | 30 | (28.9) | 98 | (22.7) | 117 | (31.0) | 245 | (26.8) |
| Conventional antipsychoticb | 5 | (4.8) | 21 | (4.9) | 16 | (4.2) | 42 | (4.6) |
| Acetylcholinesterase inhibitorc | 14 | (13.5) | 100 | (23.2) | 88 | (23.3) | 202 | (22.1) |
| Antidepressants, not tricyclic antidepressantd | 24 | (23.1) | 70 | (16.2) | 59 | (15.6) | 153 | (16.7) |
| Tricyclic antidepressant | 0 | (0.0) | 5 | (1.2) | 0 | (0.0) | 5 | (0.6) |
| Memantine | 13 | (12.5) | 63 | (14.6) | 38 | (10.1) | 114 | (12.5) |
| Antianxiety medicatione | 8 | (7.7) | 41 | (9.5) | 35 | (9.3) | 84 | (9.2) |
| Anticonvulsantf | 10 | (9.6) | 34 | (7.9) | 25 | (6.6) | 69 | (7.6) |
| Total | 104 | 432 | 378 | 914 | ||||
Includes 40 patients who also added antidepressants, antianxiety, anticholinesterase, anticonvulsant, memantine, conventional antipsychotics, or two atypical antipsychotics.
Includes six patients who also added antidepressant, antianxiety, anticonvulsant, or memantine.
Includes 16 patients who also added memantine.
Includes 12 patients who also added antianxiety, anticholinesterase, anticonvulsant, or memantine.
Includes five patients who also added anticholinesterase or anticonvulsant.
Includes one patient who also added memeatine.
Various baseline patient and facility variables showed many patient and facility characteristics to be predictive of choice of antipsychotics (Table A1). Similarly, baseline characteristics were associated with the treatment status, which included treatment change, the primary event of our interest (Table A2). In short, patients who died tended to be older and sicker, and patients who discontinued but did not change medication tended to be sicker than those who changed or continued the index medication as indicated by higher number of comorbidities and health service utilizations in prior year.
Crude cause-specific HR of treatment change was 1.28 (p = 0.03) with olanzapine and 1.13 (p = 0.07) with risperidone compared with quetiapine (Table 3). The hazard of treatment change also showed the hazard decreased quickly after 30 days and was no longer different across the three antipsychotics (Figure 1(a)). In parallel, the cumulative incidence function describing the probability of changing the index treatment was highest for olanzapine, followed by risperidone and quetiapine during the 90-day follow-up (Figure 1(b)). Hispanic patients were more likely to change than to continue the index treatment, and of the clinical diagnoses, other anxiety disorder diagnosis was associated with treatment change. Having had at least one psychiatric outpatient encounter in prior 30 days was associated with treatment change. Patients with longer years since dementia diagnosis were less likely to change the index treatment.
Table 3.
Patient and facility characteristics at the time of the newly filled antipsychotic medication by whether patients changed the index antipsychotics treatment (N = 914) or continued the index treatment (N = 5319), and cause-specific unadjusted hazard ratios of treatment change with antipsychotic discontinuation and deaths as competing risks
| Changed treatment N (%) |
Continued treatment N (%) |
|||||
|---|---|---|---|---|---|---|
| Characteristics | 914 | (6.2) | 5319 | (36.0) | Hazard ratio (95% CI) | p-value |
| Initial antipsychotics | ||||||
| Quetiapine | 432 | (5.7) | 2787 | (37.1) | 1.0 | Ref |
| Olanzapine | 104 | (7.3) | 506 | (35.5) | 1.28 (1.02, 0.60) | 0.03 |
| Risperidone | 378 | (6.5) | 2026 | (34.7) | 1.13 (0.99, 1.28) | 0.07 |
| Initial haloperidol dose (mg/day), mean ± SD | 1.1 | ±1.5 | 0.9 | ±1.3 | 1.04 (1.00, 1.07) | 0.03 |
| Age on index fill date (years), mean ± SD | 80.5 | ±6.1 | 80.6 | ±5.9 | 1.00 (0.98, 1.01) | 0.42 |
| Male | 890 | (6.2) | 5195 | (36.0) | 1.12 (0.75, 1.67) | 0.57 |
| Race | ||||||
| White | 706 | (6.3) | 4111 | (36.6) | 1.00 | Ref |
| Black | 85 | (5.8) | 352 | (24.0) | 0.92 (0.72, 1.18) | 0.51 |
| Other | 17 | (7.2) | 86 | (36.6) | 1.15 (0.76, 1.75) | 0.50 |
| Unknown | 106 | (5.7) | 770 | (41.7) | 0.91 (0.74, 1.12) | 0.37 |
| Hispanic ethnicity | 85 | (8.6) | 234 | (23.5) | 1.40 (1.14, 1.71) | 0.001 |
| Married | 582 | (6.2) | 3534 | (37.6) | 1.01 (0.90, 1.14) | 0.84 |
| Dementia types: Alzheimer’s | 799 | (6.3) | 4635 | (36.4) | 1.13 (0.94, 1.38) | 0.20 |
| Dementia types: vascular dementia | 198 | (5.8) | 1099 | (32.1) | 0.91 (0.78, 1.07) | 0.26 |
| Dementia types: DLBD/PDD | 37 | (4.7) | 284 | (36.1) | 0.74 (0.53, 1.05) | 0.09 |
| Prior medication use | ||||||
| Antidepressant | 451 | (5.5) | 3071 | (37.2) | 0.76 (0.66, 0.88) | <0.001 |
| Opioid | 311 | (6.3) | 1694 | (34.5) | 1.04 (0.91, 1.18) | 0.57 |
| Clinical diagnoses | ||||||
| Alcohol abuse/dependence | 30 | (6.6) | 126 | (27.9) | 1.08 (0.73, 1.60) | 0.71 |
| Drug abuse/dependence | 30 | (7.2) | 140 | (33.4) | 1.16 (0.78, 1.72) | 0.45 |
| PTSD | 58 | (6.7) | 292 | (33.6) | 1.09 (0.87, 1.36) | 0.44 |
| Other anxiety disorder | 107 | (7.3) | 482 | (33.0) | 1.22 (1.02, 1.45) | 0.03 |
| Delirium | 465 | (6.4) | 2391 | (33.1) | 1.09 (0.94, 1.25) | 0.25 |
| Depression | 299 | (6.4) | 1655 | (35.5) | 1.06 (0.92, 1.22) | 0.45 |
| Schizophrenia/affective | 15 | (4.8) | 87 | (27.7) | 0.76 (0.49, 1.19) | 0.23 |
| Other psychoses | 176 | (6.0) | 977 | (33.5) | 0.97 (0.83, 1.13) | 0.67 |
| Parkinson’s diseasea | 74 | (4.9) | 520 | (34.4) | 0.77 (0.58, 1.02) | 0.07 |
| Bipolar 1a | 27 | (8.4) | 110 | (34.3) | 1.39 (0.90, 2.13) | 0.14 |
| Bipolar 2a | 6 | (3.4) | 57 | (32.2) | 0.54 (0.25, 1.16) | 0.11 |
| Charlson comorbiditiesb, mean ± SD | 1.5 | ±1.8 | 1.4 | ±1.7 | 1.00 (0.97, 1.03) | 0.90 |
| Prior treatment | ||||||
| ≥1 psych visit in 30 days | 381 | (6.8) | 1873 | (33.4) | 1.17 (1.02, 1.35) | 0.03 |
| No. any inpatient days, mean ± SD | 3.5 | ±15.3 | 3.7 | ±18.6 | 0.99 (0.99, 1.00) | 0.12 |
| No. nursing home days, mean ± SD | 1.2 | ±10.1 | 1.8 | ±16.7 | 0.99 (0.99, 1.00) | 0.07 |
| Years since dementia diagnosis,mean ± SD | 1.8 | ±2.2 | 1.9 | ±2.1 | 0.95 (0.92, 0.97) | <0.001 |
| Facility characteristics | ||||||
| Urban facility | 834 | (91.3) | 4640 | (87.2) | 1.26 (0.97, 1.63) | 0.09 |
| Fiscal year of medication start | ||||||
| 2005 | 185 | (6.0) | 1137 | (36.6) | 1.0 | Ref |
| 2006 | 271 | (5.8) | 1723 | (37.1) | 0.98 (0.82, 1.17) | 0.82 |
| 2007 | 245 | (6.0) | 1463 | (35.9) | 1.01 (0.85, 1.19) | 0.94 |
| 2008 | 213 | (7.2) | 996 | (33.6) | 1.22 (1.00, 1.47) | 0.05 |
| Region | ||||||
| Northeast | 172 | (5.9) | 1078 | (37.0) | 1.0 | Ref |
| Midwest | 181 | (5.4) | 1408 | (41.6) | 0.90 (0.71, 1.16) | 0.43 |
| West | 157 | (6.7) | 885 | (37.9) | 1.15 (0.90, 1.45) | 0.26 |
| South | 404 | (6.6) | 1948 | (31.6) | 1.11 (0.89, 1.39) | 0.34 |
Unless otherwise stated, all variables are defined based on data during 1 year prior to the index medication fill date and are expressed as N (%). Abbreviations: CI, confidence interval; PTSD, post-traumatic stress disorder; DLBD, diffuse Lewy body disease; PDD, Parkinson’s disease dementia.
Parkinson’s disease includes ICD 9 of 3320, 3330, and 33390; Bipolar 1 includes ICD 9 of 2960, 2961, 2964, 2965, 2966, and 2967; bipolar 2 includes ICD 9 of 2968.
Charlson weighted diagnoses score, excluding dementia, can range from 0 to 32.
Figure 1.
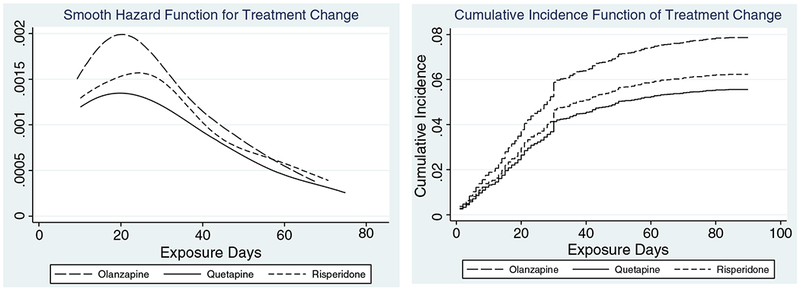
(1a) Cause-specific hazards for changing treatment over the initial 90 days of newly initiating an antipsychotic agent. (1b) Cumulative incidence function of changing to other psychotropic medication within 90 days of the newly initiating an antipsychotic agent based on competing risk regression model.
After adjusting for baseline variables, the hazard of treatment change was 1.43 times higher (p = 0.005) for olanzapine than quetiapine and 1.12 times higher (p = 0.08) for risperidone (Table 4). Hispanic patients remained as 1.45 times more likely to change treatment. Prior year antidepressant use and depression diagnoses were grouped into four mutually exclusive variables based on presence of either or both. Those with antidepressant use, depression diagnosis, or both were all associated with lower risk of treatment change compared with those who neither filled an antidepressant nor received a depression diagnosis in prior year. Interestingly, patients with other anxiety disorder remained more likely to change than to continue the treatment. Generally, patients with variables associated with greater severity of dementia and NPS (including recent antidepressant fills, having five or longer inpatient hospitalization days in the prior year, and longer years since first dementia diagnosis) were less likely to change treatments. Changing the index treatment was more likely in 2008 than earlier years even after adjusting for other variables.
Table 4.
Cause-specific adjusted hazard ratios associated with the different antipsychotic agents used initially and patient and facility characteristics on the cumulative incidence of changing treatment to another psychotropic medication in 90 days based on competing risk regression, adjusted for clustering within 128 facilities
| Characteristics | HR | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Initial antipsychotics | ||||
| Quetiapine | 1.00 | Ref | ||
| Olanzapine | 1.43 | 0.18 | 0.005 | (1.03–1.61) |
| Risperidone | 1.12 | 0.08 | 0.08 | (0.98–1.28) |
| Race | ||||
| White | 1.00 | Ref | ||
| Black | 0.91 | 0.12 | 0.50 | (0.70–1.19) |
| Other race | 1.04 | 0.25 | 0.86 | (0.66–1.65) |
| Unknown race | 0.96 | 0.17 | 0.79 | (0.68–1.35) |
| Hispanics (ref = non-Hispanics) | 1.45 | 0.14 | <0.001 | (1.19–1.76) |
| Alzheimer’s (ref = all other dementia types) | 1.18 | 0.12 | 0.10 | (0.97–1.43) |
| At least one psych visit in 30 days | 1.09 | 0.09 | 0.26 | (0.94–1.28) |
| Depression diagnosis and prior antidepressant fill | ||||
| Neither diagnosis nor antidepressant fill | 1.00 | Ref | ||
| Prior year antidepressants fill | 0.61 | 0.07 | <0.001 | (0.50–0.76) |
| Depression diagnosis | 0.92 | 0.18 | 0.68 | (0.63–1.36) |
| Prior year antidepressant use and diagnosis | 0.85 | 0.08 | 0.09 | (0.70–1.03) |
| PTSD | 1.08 | 0.13 | 0.40 | (0.46–1.36) |
| Other anxiety disorder | 1.30 | 0.14 | 0.01 | (1.06–1.60) |
| Schizophrenia/affective | 0.65 | 0.17 | 0.09 | (0.39–1.07) |
| Parkinson’s disease | 0.89 | 0.12 | 0.37 | (0.68–1.16) |
| Bipolar 1 | 1.64 | 0.41 | 0.05 | (1.00–2.66) |
| Bipolar 2 | 0.47 | 0.22 | 0.11 | (0.19–1.17) |
| At least one comorbid illness in addition to dementiaa | 1.11 | 0.07 | 0.13 | (0.97–1.26) |
| No. of inpatient days (ref = 0): | ||||
| 1–5 | 1.23 | 0.17 | 0.13 | (0.94–1.60) |
| ≥5 | 0.79 | 0.09 | 0.05 | (0.62–1.00) |
| No. of years since dementia | 0.94 | 0.01 | <0.001 | (0.92–0.97) |
| Urban (ref = rural) | 1.20 | 0.14 | 0.12 | (0.95–1.52) |
| Year (ref = 2005): | ||||
| 2006 | 0.98 | 0.09 | 0.86 | (0.82–1.19) |
| 2007 | 1.03 | 0.10 | 0.74 | (0.85–1.25) |
| 2008 | 1.30 | 0.13 | 0.01 | (1.06–1.59) |
| Region (ref = south): | ||||
| North east | 0.93 | 0.11 | 0.54 | (0.73–1.18) |
| Midwest | 0.89 | 0.10 | 0.28 | (0.72–1.10) |
| West | 0.97 | 0.12 | 0.79 | (0.76–1.23) |
The model was also adjusted for age, marital status, alcohol abuse/dependence, other substance abuse/dependence, prior year benzodiazepine use, prior year opioid use, personality disorder, delirium, other psychoses, having ≥1 nursing home days, and whether the facility has high academic affiliation and whether the facility has more than 400 beds, but they were not significantly associated with changing treatment.
Abbreviation: HR is hazard ratio.
Based on Charlson diagnoses.
Analysis of dose
Prescribing antipsychotics only as PRN was less common with olanzapine (10%) than quetiapine (17%) or risperidone (16%, p < 0.001). In those with dose data available, median initial prescribed doses were 5.0 mg/day for olanzapine, 25.0 mg/day for quetiapine, and 0.5 mg/day for risperidone. Based on dose standardized to haloperidol equivalent dose (Andreasen et al., 2010), initial dose was highest for olanzapine and lowest for quetiapine. Similarly, the last prescribed haloperidol equivalent dose among patients who changed medication was highest in olanzapine and lowest for quetiapine patients.
Secondary exploratory analyses
We assessed if being prescribed the dosage in the adverse event range for an antipsychotic was associated with treatment change, as we expected it would suggest intolerability as a potential driver of treatment change for that antipsychotic. For each of the three antipsychotics, a substantial percentage of patients were prescribed a dose in the range of adverse events (Rossum et al., 2010; Schneider et al. 2006b). Those who were prescribed their last dosing in the adverse event range was 62% in olanzapine patients (adverse event range was defined as ≥5 mg per day), 21% in quetiapine patients (≥100 mg per day), and 42% in risperidone patients (≥1 mg per day, Table 5). We did not find last dosage being in adverse event dosing range to be associated with hazard of treatment change in any antipsychotics.
Table 5.
Number (%) of patients by the last prescribed dosage levels and treatment status in patients whose dosage is available, that is, not PRN
| Treatment status | |||||
|---|---|---|---|---|---|
| Changed | Stayed | Discontinued | Died | Total | |
| Olanzapine (mg/day) | |||||
| <2.5 | 1 (5.3) | 9 (47.4) | 9 (47.4) | 0 (0) | 19 (1.5) |
| 2.5–5 | 32 (7.0) | 173 (37.7) | 240 (52.3) | 14 (3.1) | 459 (36.2) |
| ≥5 | 58 (7.3) | 292 (37.0) | 411 (52.0) | 29 (3.7) | 790 (62.3) |
| Quetiapine (mg/day) | |||||
| <50 | 160 (5.5) | 1044 (36.1) | 1603 (55.4) | 86 (3.0) | 2893 (47.0) |
| 50–100 | 107 (5.4) | 775 (39.1) | 1038 (52.3) | 63 (3.2) | 1983 (32.2) |
| ≥100 | 81 (6.3) | 586 (45.7) | 578 (45.1) | 37 (2.9) | 1282 (20.8) |
| Risperidone (mg/day) | |||||
| <0.5 | 48 (5.2) | 338 (36.4) | 515 (55.5) | 27 (2.9) | 928 (19.1) |
| 0.5–1 | 127 (6.7) | 677 (35.9) | 1023 (54.3) | 57 (3.0) | 1884 (38.8) |
| ≥1 | 128 (6.3) | 778 (38.2) | 1044 (51.2) | 89 (4.4) | 2039 (42.0) |
For each antipsychotic, the highest dosage level corresponds to adverse event dosage range.
Note: Percentages add to 100% for each row within each dosage level of each antipsychotic, except the last total column, which adds to 100% within each antipsychotics.
Although we considered treatment change as a more clearly directed decision by a provider than treatment discontinuation, we also compared treatment discontinuation across the three antipsychotics and found, unlike treatment change, no difference in the cause-specific hazard of treatment discontinuation across the three antipsychotics (hazard ratio [HR] of 1.01 for olanzapine versus quetiapine and 1.02 for risperidone versus quetiapine). However, further inspection of last prescribed dosage showed that in quetiapine and risperidone patients, lower percentage of those with last dosage in adverse event dosing range discontinued compared with those prescribed lower dosing range (Table 5). In fact, last dosage being in adverse event dosing range was associated with a significantly lower hazard of treatment discontinuation in quetiapine patients ((HR = 0.78, p < 0.001) and in risperidone patients ((HR = 0.90, p = 0.009) but not in olanzapine patients (HR = 0.97, p = 0.68).
We tried to gain insight into the finding of higher hazard of treatment change in those of Hispanic ethnicity. First, in non-Hispanic subgroup, hazard of treatment change remained higher in olanzapine (HR = 1.52; p = .001) than quetiapine patients. Hispanic patients were significantly different from non-Hispanic patients in various demographic, health, and facility characteristics. To name a few, Hispanic patients tended to be of facilities in urban areas, high academic affiliation, and south region. Even after adjusting for baseline differences, patients of Hispanic ethnicity, compared with non-Hispanic patients, were 2.8 times (p < 0.001) more likely to initiate quetiapine than olanzapine and 1.5 times (p = 0.03) more likely to initiate quetiapine than risperidone. In the subgroup of patients of Hispanic ethnicity, although no difference was found among the three antipsychotics, quetiapine patients tended to change treatment more than olanzapine patients. After excluding patients with Parkinson’s disease, the hazard of treatment change was 1.43 (p = 0.008) for olanzapine relative to quetiapine patients.
Discussion
Quetiapine was prescribed in half of our study population of older patients with dementia. For all three antipsychotics, within 90 days of newly initiating an antipsychotic, the majority (54.6%) discontinued the antipsychotic without changing to a different agent, while 36.0% continued the treatment beyond 90 days. The one-third of patients who continued on the medication may reflect the efficacy of these medications in a subgroup of patients or the natural course of the problematic behaviors being treated (Devanand et al., 2012). The larger proportion of patients who discontinued the medication and were not changed to another psychotropic may reflect patients whose symptoms resolved. Behavioral and psychological symptoms of dementia in many patients may be transient (Gitlin et al., 2012), and the symptom that prompted the initial antipsychotic prescription may resolve and not be judged to require further treatment. Another subgroup of those who discontinued was more ill as indicated by greater illness severity in prior year of index treatment in those who discontinued (Table A2) and higher 90-day mortality seen in those who discontinued (4.6%) than those who changed treatment (3.8%, Table 1).
Our data also showed that providers quickly changed the index treatment in a small but substantial group of the patients (6.2%). Although olanzapine was prescribed in only 10% of the patients, the hazard of treatment change was highest in olanzapine patients, followed by risperidone and quetiapine. Covariate-adjusted hazard of treatment change remained significantly higher with olanzapine than with quetiapine. The hazard of treatment change was high in the first few weeks during which the hazard of treatment change differed across the three antipsychotics; but beyond the first few weeks, no difference in the hazard of treatment change was seen.
Our results are in contrast to the highest percentage of treatment discontinuation for any reason seen in patients randomly assigned to quetiapine in a clinical trial comparing three antipsychotics and placebo (Schneider et al., 2006a); our results, however, are consistent with the lowest percentage of discontinuation because of intolerability, adverse events, or death seen in quetiapine patients of the same study. In our data, quetiapine patients also showed the highest percentage of continuation of the index treatment beyond 90 days, which indicates that quetiapine is likely more tolerable. Examination of dose also suggested that tolerability may be better in quetiapine or risperidone patients. Although for all three antipsychotic agents, the hazard of treatment change was slightly elevated in those who received dosing in the adverse event range than lower, no difference was found in any antipsychotics, and this suggests that intolerability was not the major driver of treatment change in these agents. However, the hazard of treatment discontinuation was significantly lower for those who received adverse event dosing range than not for risperidone (HR = 0.90) and in particular for quetiapine (HR = 0.78), suggesting that tolerability may indeed be better in these agents.
In our previous study, we reported lower covariate-adjusted 180-day mortality in those exposed to quetiapine than to risperidone or olanzapine based on the data from patients newly treated between 1999 and 2008 that was inclusive of the study period of the data used in this study (Kales et al., 2011). Our study also showed the crude 90-day mortality risk associated with quetiapine to be lower than risperidone or olanzapine. Trial data have often suggested no benefit in older patients to quetiapine treatment (Schneider et al., 2006b; Ballard et al., 2005). Assuming that most common reasons for changing medication are due either to adverse events or lack of response, the lowest percentage of treatment change and highest percentage continuing the treatment beyond 90 days associated with quetiapine may suggest that patients experienced fewer adverse events or possibly responded better with quetiapine than with the other antipsychotics, particularly olanzapine. The greater stability in treatment even after adjusting for baseline differences seen here may support the greater real-world tolerability. And coupled with lowest mortality and lowest percentage of patients being prescribed a dose in an adverse event range at their last prescription among those who discontinued treatment, it is possible that patients experienced natural improvement in symptoms at lower dose with quetiapine than with olanzapine.
We found the risk of treatment change not to be associated with demographic variables, other than Hispanic ethnicity. Although many of the baseline variables, mostly reflective of illness severity, were found to be associated with treatment change, none showed a statistically significant hazard estimate with a large magnitude, that is, >2.0 or <0.5. The finding that variables indicating illness severity were associated with lower hazard of treatment change suggests that more severe patients are likely more willing to persist with their treatment or their doctors are more reluctant to make changes. As this study was conducted after the FDA’s black box warning, changes following the black box warning were of interest; however, we found the hazard of treatment change to be higher only in year 2008. It is notable that although the risk of treatment change was higher in Hispanic patients, which is consistent with greater nonadherence reported with antidepressants and antipsychotics (Gilmer et al., 2009; Ayalon et al., 2005), no difference in treatment change was found among the antipsychotics in the subgroup of Hispanic patients.
Limitation
Because our study was based on the VHA data, patients are predominantly men and may not represent the older female populations with dementia. Medication exposure duration was calculated based on the recorded days of supply in the administrative data, and we therefore potentially overestimated the time to discontinuation if patients discontinued medication before it ran out. We, however, do not feel it is a significant limitation of this study as our primary interest is in treatment changes made by the provider, which we have accurate data for. Although we were able to include in our analyses many of the administratively available measures of severity, provider characteristics potentially associated with prescribing practice patterns were not available. However, we were able to include several facility characteristics. Our data also do not include symptoms or functional outcome measures, and therefore, the medication trajectories cannot be linked to adverse events, natural resolution, or response to medication. Lastly, we note that quetiapine being prescribed in 50% of patients and olanzapine in 10% indicates a strong preference, experience with, or expectation bias for quetiapine and an avoidance of olanzapine. Despite these limitations, we believe our results provide a valid description of prescribing practice patterns of the three most popular antipsychotics used for NPS in the older patients and provide support for our conclusion that more adverse outcomes may be associated with olanzapine than quetiapine.
Key points.
In older patients with dementia treated with atypical antipsychotics, over half discontinued index treatment within 90 days, 36% continued, 3% died while on index treatment, and 6% changed to another psychotropic medication.
The adjusted hazard of treatment change was higher by 43% (p = 0.005) for olanzapine compared with quetiapine.
Acknowledgements
This research was supported in part by a grant from the National Institute of Mental Health, R01-MH081070. Resources were also contributed by the Serious Mental Illness Treatment, Resource, and Evaluation Center, Ann Arbor, MI. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Sponsor had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of the paper.
Appendix
Table A1.
Patient and facility characteristics at the time of the newly filled antipsychotic medication by initial antipsychotic agent
| Olanzapine N = 1424 |
Quetiapine N = 7520 |
Risperidone N = 5847 |
Total N = 14,791 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % | ||
| Age on index fill date‡ | 80.8 | ±6.2 | 80.4 | ±6.1 | 81.0 | ±6.0 | 80.6 | ±6.1 | ||
| Male† | 1383 | (97.1) | 7366 | (98.0) | 5696 | (97.4) | 14,445 | (97.7) | ||
| Race‡ | ||||||||||
| White | 1074 | (75.4) | 5743 | (76.4) | 4428 | (75.7) | 11,245 | (76.0) | ||
| Black | 130 | (9.1) | 691 | (9.2) | 643 | (11.0) | 1464 | (9.9) | ||
| Other | 20 | (1.4) | 121 | (1.6) | 94 | (1.6) | 235 | (1.6) | ||
| Unknown | 200 | (14.0) | 965 | (12.8) | 682 | (11.7) | 1847 | (12.5) | ||
| Hispanic‡ | 43 | (3.8) | 638 | (10.3) | 313 | (6.5) | 994 | (8.2) | ||
| Married‡ | 834 | (58.6) | 4976 | (66.2) | 3578 | (61.2) | 9388 | (63.5) | ||
| Dementia types | ||||||||||
| Alzheimer’s‡ | 1251 | (87.9) | 6408 | (85.2) | 5065 | (86.6) | 12,724 | (86.0) | ||
| Vascular dementia | 319 | (22.4) | 1725 | (22.9) | 1376 | (23.5) | 3420 | (23.1) | ||
| DLBD/PDD‡ | 43 | (3.0) | 617 | (8.2) | 127 | (2.2) | 787 | (5.3) | ||
| Other dementia | 66 | (4.6) | 333 | (4.4) | 214 | (3.7) | 613 | (4.1) | ||
| Prior medication use | ||||||||||
| Benzodiazapine† | 350 | (24.6) | 1855 | (24.7) | 1327 | (22.7) | 3532 | (23.9) | ||
| Valproic acids‡ | 138 | (9.7) | 476 | (6.3) | 338 | (5.8) | 952 | (6.4) | ||
| Antidepressant‡ | 805 | (56.5) | 4283 | (57.0) | 3169 | (54.2) | 8257 | (55.8) | ||
| Carbamazepine† | 26 | (1.8) | 80 | (1.1) | 65 | (1.1) | 171 | (1.2) | ||
| Other anticonvulsant‡ | 160 | (11.2) | 924 | (12.3) | 559 | (9.6) | 1643 | (11.1) | ||
| Opioid | 472 | (33.2) | 2488 | (33.1) | 1948 | (33.3) | 4908 | (33.2) | ||
| Clinical diagnoses | ||||||||||
| Alcohol abuse/dependence | 46 | (3.2) | 220 | (2.9) | 186 | (3.2) | 452 | (3.1) | ||
| Drug abuse/dependence | 49 | (3.4) | 208 | (2.8) | 162 | (2.8) | 419 | (2.8) | ||
| PTSD | 80 | (5.6) | 465 | (6.2) | 323 | (5.5) | 868 | (5.9) | ||
| Other anxiety disorder | 135 | (9.5) | 755 | (10.0) | 569 | (9.7) | 1459 | (9.9) | ||
| Personality disorders | 10 | (0.7) | 48 | (0.6) | 45 | (0.8) | 103 | (0.7) | ||
| Delirium | 668 | (46.9) | 3664 | (48.7) | 2895 | (49.5) | 7227 | (48.9) | ||
| Depression | 458 | (32.2) | 2401 | (31.9) | 1802 | (30.8) | 4661 | (31.5) | ||
| Schizophrenia/affective‡ | 49 | (3.4) | 122 | (1.6) | 143 | (2.5) | 314 | (2.1) | ||
| Other psychoses† | 287 | (20.2) | 1414 | (18.8) | 1216 | (20.8) | 2917 | (19.7) | ||
| Parkinson’s diseasea,‡ | 93 | (6.5) | 1152 | (15.3) | 267 | (4.6) | 1512 | (10.2) | ||
| Bipolar 1a,‡ | 54 | (3.8) | 168 | (2.2) | 99 | (1.7) | 321 | (2.2) | ||
| Bipolar 2a,‡ | 33 | (2.3) | 95 | (1.3) | 49 | (.8) | 177 | (1.2) | ||
| Number of comorbiditiesb,† | 1.4 | ±1.8 | 1.5 | ±1.8 | 1.6 | ±1.9 | 1.5 | ±1.8 | ||
| Prior treatment | ||||||||||
| ≥1 psych visit in 30 days‡ | 590 | (41.4) | 2755 | (36.6) | 2271 | (38.8) | 5616 | (38.0) | ||
| No. any inpatient days | 5.6 | ±21.2 | 4.4 | ±17.2 | 4.8 | ±20.8 | 4.7 | ±19.1 | ||
| No. nursing home days | 3.1 | ±22.3 | 2.1 | ±16.8 | 2.5 | ±20.0 | 2.4 | ±18.7 | ||
| Years since dementia diagnosis | 2.0 | ±2.3 | 2.0 | ±2.2 | 2.0 | ±2.2 | 2.0 | ±2.2 | ||
| Fiscal year of medication start‡ | ||||||||||
| 2005 | 330 | (23.2) | 1508 | (20.1) | 1268 | (21.7) | 3106 | (21.0) | ||
| 2006 | 467 | (32.8) | 2310 | (30.7) | 1864 | (31.9) | 4641 | (31.4) | ||
| 2007 | 359 | (25.2) | 2083 | (27.7) | 1636 | (28.0) | 4078 | (27.6) | ||
| 2008 | 268 | (18.8) | 1619 | (21.5) | 1079 | (18.5) | 2966 | (20.1) | ||
| Facility characteristics | ||||||||||
| Urban facility‡ | 1198 | (84.1) | 6823 | (90.7) | 5186 | (88.7) | 13,207 | (89.3) | ||
| High academic affiliation‡ | 669 | (47.0) | 3790 | (50.4) | 2700 | (46.2) | 7159 | (48.4) | ||
| Number of beds | 418 | ±277 | 420 | ±264 | 423 | ±282 | 421 | ±273 | ||
| Region‡ | ||||||||||
| Northeast | 305 | (21.4) | 1422 | (18.9) | 1185 | (20.3) | 2912 | (19.7) | ||
| Midwest | 300 | (21.1) | 1577 | (21.0) | 1507 | (25.8) | 3384 | (22.9) | ||
| West | 272 | (19.1) | 1224 | (16.3) | 839 | (14.4) | 2335 | (15.8) | ||
| South | 547 | (38.4) | 3297 | (43.8) | 2316 | (39.6) | 6160 | (41.7) | ||
Unless otherwise stated, all variables are defined based on data during 1 year prior to the index medication fill date.
Abbreviations: PTSD, post-traumatic stress disorder; DLBD, diffuse Lewy body disease; PDD, Parkinson’s disease dementia.
Parkinson’s disease includes ICD 9 of 3320, 3330, and 33390; bipolar 1 includes ICD 9 of 2960, 2961, 2964, 2965, 2966, and 2967; bipolar 2 includes ICD 9 of 2968.
Weighted Charlson diagnoses score, excluding dementia.
p < 0.05.
p < 0.01 for comparison across the three antipsychotics.
Table A2.
Patient and facility characteristics at the time of the newly filled antipsychotic medication by 90 days treatment status
| Treatment statusa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changed 914 (6.2) |
Continued 5319 (36.0) |
Stopped 8069 (54.6) |
Died 489 (3.3) |
Total 14,791 |
||||||||||||
| Characteristics | N | % | N | % | N | % | N | % | N | % | ||||||
| Age on index fill date‡ | 80.5 | ±6.1 | 80.6 | ±5.9 | 80.6 | ±6.1 | 82.5 | ±5.8 | 80.6 | ±6.1 | ||||||
| Male | 890 | (97.4) | 5195 | (97.7) | 7874 | (97.6) | 486 | (99.4) | 14,445 | (97.7) | ||||||
| Race‡ | ||||||||||||||||
| white | 706 | (77.2) | 4111 | (77.3) | 6051 | (75.0) | 377 | (77.1) | 11,245 | (76.0) | ||||||
| Black | 85 | (9.3) | 352 | (6.6) | 999 | (12.4) | 28 | (5.7) | 1464 | (9.9) | ||||||
| Other | 17 | (1.9) | 86 | (1.6) | 128 | (1.6) | 4 | (0.8) | 235 | (1.6) | ||||||
| Unknown | 106 | (11.6) | 770 | (14.5) | 891 | (11.0) | 80 | (16.4) | 1847 | (12.5) | ||||||
| Hispanic‡ | 85 | (11.0) | 234 | (5.4) | 661 | (9.9) | 14 | (4.0) | 994 | (8.2) | ||||||
| Married‡ | 582 | (63.7) | 3534 | (66.4) | 4981 | (61.7) | 291 | (59.5) | 9388 | (63.5) | ||||||
| Dementia types | ||||||||||||||||
| Alzheimer’s‡ | 799 | (87.4) | 4635 | (87.1) | 6865 | (85.1) | 425 | (86.9) | 12,724 | (86.0) | ||||||
| Vascular dementia‡ | 198 | (21.7) | 1099 | (20.7) | 2011 | (24.9) | 112 | (22.9) | 3420 | (23.1) | ||||||
| DLBD/PDD | 37 | (4.1) | 284 | (5.3) | 437 | (5.4) | 29 | (5.9) | 787 | (5.3) | ||||||
| Other dementia | 39 | (4.3) | 223 | (4.2) | 341 | (4.2) | 10 | (2.0) | 613 | (4.1) | ||||||
| Prior medication use | ||||||||||||||||
| Benzodiazapine | 219 | (24.0) | 1257 | (23.6) | 1921 | (23.8) | 135 | (27.6) | 3532 | (23.9) | ||||||
| Valproic acids | 61 | (6.7) | 337 | (6.3) | 527 | (6.5) | 27 | (5.5) | 952 | (6.4) | ||||||
| Antidepressant‡ | 451 | (49.3) | 3071 | (57.7) | 4486 | (55.6) | 249 | (50.9) | 8257 | (55.8) | ||||||
| Carbamazepine | 13 | (1.4) | 66 | (1.2) | 85 | (1.1) | 7 | (1.4) | 171 | (1.2) | ||||||
| Other anticonvulsant | 94 | (10.3) | 581 | (10.9) | 920 | (11.4) | 48 | (9.8) | 1643 | (11.1) | ||||||
| Opioid | 311 | (34.0) | 1694 | (31.9) | 2709 | (33.6) | 194 | (39.7) | 4908 | (33.2) | ||||||
| Clinical diagnoses | ||||||||||||||||
| Alcohol abuse/dependence‡ | 30 | (3.3) | 126 | (2.4) | 288 | (3.6) | 8 | (1.6) | 452 | (3.1) | ||||||
| Drug abuse/dependence | 30 | (3.3) | 140 | (2.6) | 237 | (2.9) | 12 | (2.5) | 419 | (2.8) | ||||||
| PTSD | 58 | (6.4) | 292 | (5.5) | 505 | (6.3) | 13 | (2.7) | 868 | (5.9) | ||||||
| Other anxiety disorder‡ | 107 | (11.7) | 482 | (9.1) | 817 | (10.1) | 53 | (10.8) | 1459 | (9.9) | ||||||
| Personality disorders | 5 | (0.6) | 35 | (0.7) | 62 | (0.8) | 1 | (0.2) | 103 | (0.7) | ||||||
| Delirium‡ | 465 | (50.9) | 2391 | (45.0) | 4123 | (51.1) | 248 | (50.7) | 7227 | (48.9) | ||||||
| Depression‡ | 299 | (32.7) | 1655 | (31.1) | 2591 | (32.1) | 116 | (23.7) | 4661 | (31.5) | ||||||
| Schizophrenia/affective‡ | 15 | (1.6) | 87 | (1.6) | 204 | (2.5) | 8 | (1.6) | 314 | (2.1) | ||||||
| Other psychoses‡ | 176 | (19.3) | 977 | (18.4) | 1675 | (20.8) | 89 | (18.2) | 2917 | (19.7) | ||||||
| Parkinson’s diseaseb,‡ | 74 | (8.1) | 520 | (9.8) | 858 | (10.6) | 60 | (12.3) | 1512 | (10.2) | ||||||
| Bipolar 1b | 27 | (3.0) | 110 | (2.1) | 180 | (2.2) | 4 | (0.8) | 321 | (2.2) | ||||||
| Bipolar 2b,† | 6 | (0.7) | 57 | (1.1) | 112 | (1.4) | 2 | (0.4) | 177 | (1.2) | ||||||
| Number of comorbiditiesc,‡ | 1.5 | ±1.8 | 1.4 | ±1.7 | 1.6 | ±1.9 | 2.2 | ±2.3 | 1.5 | ±1.8 | ||||||
| Prior treatment | ||||||||||||||||
| ≥1 psych visit in 30 days‡ | 381 | (41.7) | 1873 | (35.2) | 3268 | (40.5) | 94 | (19.2) | 5616 | (38.0) | ||||||
| No. any inpatient days‡ | 3.5 | ±15.3 | 3.7 | ±18.6 | 5.3 | ±20.0 | 6.6 | ±14.7 | 4.7 | ±19.1 | ||||||
| No. nursing home days‡ | 1.2 | ±10.1 | 1.8 | ±16.7 | 2.8 | ±20.3 | 3.2 | ±22.9 | 2.4 | ±18.7 | ||||||
| Years since dementia diagnosis‡ | 1.8 | ±2.2 | 1.9 | ±2.1 | 2.1 | ±2.2 | 1.9 | ±2.1 | 2.0 | ±2.2 | ||||||
| Fiscal year of medication start | ||||||||||||||||
| 2005 | 185 | (20.2) | 1137 | (21.4) | 1673 | (20.7) | 111 | (22.7) | 3106 | (21.0) | ||||||
| 2006 | 271 | (29.7) | 1723 | (32.4) | 2503 | (31.0) | 144 | (29.5) | 4641 | (31.4) | ||||||
| 2007 | 245 | (26.8) | 1463 | (27.5) | 2228 | (27.6) | 142 | (29.0) | 4078 | (27.6) | ||||||
| 2008 | 213 | (23.3) | 996 | (18.7) | 1665 | (20.6) | 92 | (18.8) | 2966 | (20.1) | ||||||
| Facility characteristics | ||||||||||||||||
| Urban facility‡ | 834 | (91.3) | 4640 | (87.2) | 7301 | (90.5) | 432 | (88.3) | 13,207 | (89.3) | ||||||
| High academic affiliation | 454 | (49.7) | 2610 | (49.1) | 3862 | (47.9) | 233 | (47.7) | 7159 | (48.4) | ||||||
| Number of beds‡ | 423 | ±275 | 432 | ±283 | 416 | ±266 | 394 | ±262 | 421 | ±273 | ||||||
| Region‡ | ||||||||||||||||
| Northeast | 172 | (18.8) | 1078 | (20.3) | 1582 | (19.6) | 80 | (16.4) | 2912 | (19.7) | ||||||
| Midwest | 181 | (19.8) | 1408 | (26.5) | 1675 | (20.8) | 120 | (24.5) | 3384 | (22.9) | ||||||
| West | 157 | (17.2) | 885 | (16.6) | 1206 | (15.0) | 87 | (17.8) | 2335 | (15.8) | ||||||
| South | 404 | (44.2) | 1948 | (36.6) | 3606 | (44.7) | 202 | (41.3) | 6160 | (41.7) | ||||||
Unless otherwise stated, all variables are defined based on data during 1 year prior to the index medication fill date.
Abbreviations: PTSD, post-traumatic stress disorder; DLBD, diffuse Lewy body disease; PDD, Parkinson’s disease dementia.
Parkinson’s disease includes ICD 9 of 3320, 3330, and 33390; bipolar 1 includes ICD 9 of 2960, 2961, 2964, 2965, 2966, and 2967; bipolar 2 includes ICD 9 of 2968.
Weighted Charlson diagnoses score, excluding dementia.
p < 0.05.
p < 0.01 for comparison across the four 90 days treatment status groups.
Footnotes
Conflict of interest
None declared.
References
- Andreasen N, Pressler M, Nopoulos P, et al. 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 67(3): 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon L, Area’n PA, Alvidrez J 2005. Adherence to antidepressant medications in Black and Latino elderly patients. Am J Geriatr Psychiatry 13(7): 572–80. [DOI] [PubMed] [Google Scholar]
- Ballard C, Margallo-Lana M, Juszczak E, et al. 2005. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomized double blind placebo controlled trial. BMJ 330: 874–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5): 373–83. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N, Glick ID. 2003. A meta-analysis of the efficacy of secondgeneration antipsychotics. Arch Gen Psychiatry 60(6): 553–64. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Mintzer J, Schultz SK, et al. 2012. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med 367(25): 1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HH, Triesxhmann ME, Friedman JH. 2003. Treatment of psychosis in Parkinson’s disease: safety considerations. Drug Saf 26: 643–9. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Trieschmann ME, Burke MA, et al. 2003. Long-term outcome of quetiapine use for psychosis among Parkinsonian patients. Mov Disord 18(5): 510–4. [DOI] [PubMed] [Google Scholar]
- Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509. [Google Scholar]
- Gitlin LN, Kales HC, Lyketsos CG. 2012. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 308(19): 2020–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer TP, Ojeda VD, Barrio C, et al. 2009. Adherence to antipsychotics among Latinos and Asians with schizophrenia and limited English proficiency. Psychiatr Serv 60(2): 175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Rockwell E, Harris MJ, et al. 1999. Conventional vs. newer antipsychotics in elderly patients. Am J Geriatr Psychiatry 7(1): 70–6. [PubMed] [Google Scholar]
- Jeste DV, Blazer D, Casey D, et al. 2008. ACNP white paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 33(5): 957–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Valenstein M, Kim HM, et al. 2007. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 164(10): 1568–76 [DOI] [PubMed] [Google Scholar]
- Kales HC, Zivin K, Kim HM, et al. 2011. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry 68(2): 190–7. [DOI] [PubMed] [Google Scholar]
- Kim HM, Chiang C, Kales HC. 2011. After the black box warning: predictors of psychotropic treatment choices for older patients with dementia. Psychiatr Serv 62(10): 1207–14. [DOI] [PubMed] [Google Scholar]
- Lau DT, Mercaldo ND, Harris AT, et al. 2010. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord 24(1): 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, et al. 2002. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA 288(12): 1475–83. [DOI] [PubMed] [Google Scholar]
- Rabey JM, Prokhorov T, Miniovitz A, et al. 2007. Effect of quetiapine in psychotic Parkinson’s disease patients: a double-blind labeled study of 3 months’ duration. Mov Disord 22(3): 313–8. [DOI] [PubMed] [Google Scholar]
- Rossum RC, Rector TS, Lederle FA, et al. 2010. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc 58(6): 1027–34. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Madhusoodanan S, Buckley P. 2000. Schizophrenia in the elderly: guidelines for management. CNS Drugs 13(2): 103–15. [Google Scholar]
- Schneider LS, Dagerman K, Insel PS. 2006a. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 14: 191–210. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, et al. 2006b. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 355(15): 1525–38. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffee K. 2005. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 293(5): 596–608. [DOI] [PubMed] [Google Scholar]
- Solomons K, Geiger O. 2000. Olanzapine use in the elderly: a retrospective analysis. Can J Psychiatry 45(2): 151–5. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Chen P, Ignacio RV, et al. 2011. Patterns and trends in antipsychotic prescribing for Parkinson disease psychosis. Arch Neurol 68(7): 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]


