Abstract
Using a population-based birth cohort in upstate New York (2008–2010), we examined the determinants of Brain Derived Neurotrophic Factor (BDNF) measured in newborn dried blood spots (n=2637). We also examined the association between neonatal BDNF and children’s development. The cohort was initially designed to examine the influence of infertility treatment on child development but found no impact. Mothers rated children’s development in five domains repeatedly through age 3 years. Socioeconomic and maternal lifestyle determinants of BDNF were examined using multivariable linear regression models. Generalized linear mixed models estimated odds ratios for neonatal BDNF in relation to failing a developmental domain. Smoking and drinking in pregnancy, nulliparity, non-white ethnicity/race, and pre-pregnancy obesity were associated with lower neonatal BDNF. Neonatal BDNF was not associated with failure for developmental domains; however, there was an interaction between BDNF and preterm birth. In preterm infants, a higher BDNF was associated with lower odds of failing any developmental domains, after adjusting for confounders and infertility treatment. This result was particularly significant for failure in communication. Our findings suggest that BDNF levels in neonates may be impacted by maternal lifestyle characteristics. More specifically, lower neonatal BDNF might be an early marker of aberrant neurodevelopment in preterm infants.
Keywords: Brain Derived Neurotrophic Factor, neonates, prospective, development
Introduction
Brain Derived Neurotrophic Factor (BDNF) is a member of the neurotrophin family of growth factors, particularly involved in the growth and differentiation of neurons and the formation and elimination of synapses (Barde, 1990). BDNF exists in the central nervous system and, with a high correlation, in the periphery (Karege, Schwald, & Cisse, 2002). Several animal studies have examined prenatal risk factors that are associated with BDNF levels in newborns. For example, in rats, exposure to nicotine during gestation elevated BDNF levels in the striatum, frontal cortex, and the hippocampus of the offspring (Harrod et al., 2011). In a study with mice, exposure to cigarette smoke during pregnancy decreased BDNF levels in the striatal structures of the offspring’s brain (Yochum et al., 2014). Compared to mice with a normal diet, mice fed a diet to induce maternal obesity had offspring with lower BDNF levels in the hippocampus that resulted in impairments in dendritic arborization of the hippocampal neurons (Tozuka et al., 2010). In addition, rats exposed to prenatal stress had reduced BDNF in the olfactory bulbs and the hippocampus one to five days after birth (Van den Hove et al., 2006). In humans, evidence on maternal lifestyle and environmental factors that could potentially influence neonatal BDNF is sparse. In a cohort study of 395 term singletons, maternal self-report of smoking during pregnancy was associated with higher cord serum levels of BDNF but only among girls (Spulber et al., 2010). In 120 mother-child pairs, it was reported that maternal mental disorders were associated with lower BDNF levels in umbilical cord blood (Flock et al., 2015). Despite a growing body of literature suggesting the impact of maternal adiposity and weight on children’s neurodevelopment (Jo et al., 2015; Van Lieshout, 2013), the effect of maternal obesity on children’s BDNF has not been investigated in humans.
As BDNF has been implicated in several neuropsychiatric disorders such as depression (Molendijk et al., 2014), autism spectrum disorder (ASD) (Nickl-Jockschat & Michel, 2011), epilepsy (Connolly et al., 2006), and schizophrenia (Weickert et al., 2003), the longitudinal association of neonatal BDNF levels and children’s neurodevelopmental outcomes is of interest. For example, children with ASD are consistently shown to have higher levels of BDNF compared to typically developing children (Qin et al., 2016). However, studies of children with diagnosed psychopathology cannot provide insights on the stage of development; therefore longitudinal studies linking BDNF, development, and psychopathology are warranted. Among the few studies that investigated the longitudinal relationship between neonatal BDNF and ASD, results remained inconsistent (Abdallah et al., 2013; Croen et al., 2008; K. B. Nelson et al., 2001; P. G. Nelson et al., 2006). In a retrospective analysis of neonatal dried blood spots (DBSs), Nelson et al. showed an overexpression of BDNF in children with diagnosis of ASD compared to typically developing children (K. B. Nelson et al., 2001). Contradicting their previous findings after using a different laboratory method of detection, Nelson et al. reported no differences in BDNF levels measured in newborn blood spots between children with ASD and healthy controls (P. G. Nelson et al., 2006). Croen et al. also showed no association between neonatal BDNF and ASD diagnosis (Croen et al., 2008). In a case-control study using a National Biobank in Denmark, serum blood samples from neonates later diagnosed with ASD were reported to have lower levels of BDNF than those of controls (Abdallah et al., 2013). High levels of heterogeneity in the previous studies, different methodology for assessment of BDNF, and differences in trajectories of BDNF levels among children with psychopathology and typically developing children are suggested to explain the inconsistencies in previous research (Nickl-Jockschat & Michel, 2011). Considering this heterogeneity, a recent meta-analysis found no prospective association between neonatal BDNF and ASD among four existing studies on neonatal BDNF and ASD (Qin et al., 2016).
Whether BDNF levels in infancy predict children’s normal trajectory of development is also less clear. A handful of studies that investigated cord blood or neonatal BDNF with child development showed a positive association with personal-social skills in children (Yu et al., 2016), no association with cerebral palsy, and a positive association with Down Syndrome (K. B. Nelson et al., 2001). Impairments in several domains of development are shown to precede psychopathology in childhood (Skovgaard et al., 2008). For example, retrospective assessment of children with ASD and prospective follow-up of children at risk confirms that children with ASD diagnosis showed symptoms of delays in motor development, expressive language, and social development already in the ‘prodrome’ phase (Landa, Stuart, Gross, & Faherty, 2013; Yirmiya & Charman, 2010). Therefore, it is possible that neonatal BDNF levels are associated with broad developmental outcomes in young children before the emergence of psychopathology.
As such, in a large population-based U.S. cohort, we examined socioeconomic and maternal lifestyle determinants of neonatal BDNF levels as measured using DBS. Based on evidence from animal models, we hypothesized that prenatal exposures would be associated with lower neonatal BDNF levels. We also explored whether neonatal BDNF was associated with children’s development through age three years in a large population-based sample, specifically with the domains of fine and gross motor, communication, personal-social functioning, and problem-solving ability. Because of its role in the growth and differentiation of neurons and the formation and elimination of synapses, lower neonatal BDNF levels were hypothesized to be associated with delays in these five domains of development through age three years. DBSs (five drops of whole blood collected on filter paper from a heel prick) are a rich resource with the potential to measure circulating biomarkers and early exposures of neonates (McDade, Williams, & Snodgrass, 2007). Previously, it is shown that DBS provides a robust and convenient sample for immunoassay analysis of inflammatory markers (including BDNF) in whole blood, especially if DBS is stored at low temperature (Skogstrand et al., 2008).
Methods
Participants
This study used data from the Upstate KIDS Study, a population-based birth cohort which was originally designed to evaluate the long-term impact of infertility treatment on child development (Buck Louis et al., 2014). Recruitment, which was based on birth certificate indication of infertility treatment and plurality, occurred in New York State (excluding New York City) from 2008 to 2010. Infertility treatments included two broad categories: (1) assisted reproductive technology-specific treatment including in vitro fertilization (with or without intracytoplasmic sperm injection), and (2) ovulation induction via oral or injectable medications with or without intrauterine insemination (Buck Louis et al., 2014). All live births conceived with infertility treatment and all multiple gestations (regardless of mode of conception) were recruited. Singletons conceived by treatment were also recruited at a 1:3 ratio to those not conceived by treatment, while also frequency matching on region of birth. There were 3905 mothers of singletons and 1084 mothers of twins who were enrolled in Upstate KIDS. We previously showed that infertility treatment was not associated with children’s development after accounting for plurality (Yeung, et al., 2016c). In these analyses, mother-child pairs with consent on the use of residual newborn DBS and information on BDNF were included (n=3145, 2095 singletons and 1050 twins). Among them, characteristics between parents, who consented for DBS use and those who did not, were found to be very similar (Yeung, 2016b).
The New York State Department of Health (NYSDOH) and the University at Albany Institutional Review Board (IRB) approved the study (NYSDOH IRB #07–097; UAlbany #08–179) and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. Parents provided written informed consent on behalf of their children.
Measurements
BDNF levels (ng/ml).
In the State of New York, DBSs are collected as part of the Newborn Screening Program. Parental consent for using residual DBSs (minus one reserved for future NYSDOH needs) were requested when the children were 8 months old. DBS cards of those with consent were retrieved from cold storage (4°C). Punches of the residual spots were extracted in a similar manner as previously described for other analytes (Yeung, et al., 2016b). Eluants from the extraction of each 3.2 mm punch were frozen at −80°C until analysis. The BDNF levels were measured as part of Millipore’s Neurodegenerative Panel III (EMD Millipore, Billerica, MA, USA) using a Luminex100 analyzer with xPONENT 3.1 software (Luminex System, Austin, TX, USA). Based on 3658 replicate measurements, the intra-assay coefficient of variation was 12.5%. Instrument reported values for those below the limit of detection (including samples where the instrument could not detect the presence of the analyte) were used without censoring to prevent potential bias (Schisterman, Vexler, Whitcomb, & Liu, 2006). Batch effects in the BDNF values were removed using COMBAT, a statistical program which is commonly used to remove batch to batch measurement error (Johnson, Li, & Rabinovic, 2007; Leek, Johnson, Parker, Jaffe, & Storey, 2012).
Children’s development.
Using the Ages & Stages Questionnaire© (ASQ), mothers reported on their children’s development at ages 4–6, 8, 12, 18, 24, 30, and 36 months of age (corrected for gestational age). The ASQ is a validated screening instrument designed for the early detection of developmental delays (Guevara et al., 2013). The ASQ encourages parents to participate in activities with their children to obtain an accurate developmental assessment and then respond to questions assessing five developmental domains (i.e., fine motor, gross motor, communication, personal-social functioning, and problem-solving ability) (Jane Squires & Bricker, 2009). In Upstate KIDS, we first implemented the ASQ-2nd edition for screening at ages 4 to 12 months and the 3rd edition from ages 18 months onwards as ASQ-3 became available in 2009 after recruitment began. Each questionnaire item was scored (“yes”=10 points, “sometimes”=5 points, “not yet”=0 points) and summed for each domain (0–60 points) (J Squires, Potter, & Bricker, 1999).
Study personnel called parents to follow-up when the child failed any of the five domains or parental concern was noted. Trained specialists implemented an age-appropriate follow-up ASQ for children failing any of the domains or for children whose parent reported concerns. We defined age at failing as the time of the initial screen fail (regardless of the time of the follow-up call). When follow-up was incomplete, the child remained as having failed for that age-appropriate ASQ. Screening instruments were considered valid only if they were completed in the specified age windows. We previously used data from the New York State Early Intervention Program (EIP) to confirm findings from ASQ screening (Yeung, et al., 2016c).
The ASQ is a validated instrument that has been shown to identify early developmental delays in children between 0–5 years of age (Jane Squires & Bricker, 2009). As a screener, the ASQ has adequate psychometric properties (75% sensitivity and 81% specificity) and modest agreement with other instruments (Gollenberg, Lynch, Jackson, McGuinness, & Msall, 2010; Schonhaut, Armijo, Schonstedt, Alvarez, & Cordero, 2013). Due to the instrument’s properties and a continuous raw score with a very skewed distribution, we used ASQ as a binary outcome (fail/pass status) in all analyses. Domain specific fails were defined as scores below 2 standard deviations of the mean for the child’s age based on normative data (Jane Squires & Bricker, 2009).
Covariates.
Information on maternal age, parity, plurality, infant’s gender, gestational age, and birth size was obtained from vital records. Mothers reported at four months postpartum on socio-demographic characteristics –such as highest acquired education level and race/ethnicity– and history of smoking (never, stopped when pregnancy was known, and continued in pregnancy) and pregnancy alcohol consumption (yes/no), pre-pregnancy weight and height, infertility treatment of any type, and use of fish oil or multivitamin supplement. We used the International Code for Disease 9th Revision (ICD-9) codes to identify maternal history of depressive disorders in pregnancy as registered in hospital discharge data in the Statewide Planning and Research Cooperative System (SPARCS). SPARCS is a comprehensive reporting system capturing longitudinal inpatient and outpatient hospital discharge data from New York State including details on patient characteristics, diagnoses, treatments, services, and charges. Maternal BMI was calculated using pre-pregnancy weight and height as provided in the vital records and in the maternal questionnaire at four months postpartum (if missing in vital record) and categorized as thin or normal weight (<25 kg/m2), overweight (25–29.99 kg/m2), or obese (>30 kg/m2).
Statistical analyses
Analyses were performed in the primary cohort of the study, which included all singletons and a random twin from each pair (n=2637). We used multivariable linear regression models to investigate the associations of maternal age, maternal race, maternal educational levels, parity, infertility treatment, having private insurance, maternal categories of BMI, maternal history of drinking and smoking in pregnancy, maternal history of depressive disorders in pregnancy, preterm delivery, and plurality with neonatal BDNF levels. First, models were run univariately for each of these variables. A parsimonious model including factors that were statistically significant (p<0.1) from these analyses was built to estimate the association of examined socioeconomic and maternal lifestyle determinants with neonatal BDNF (Rothman, Greenland, & Lash, 2008). BDNF levels were log-transformed for normality.
Next, we used generalized linear mixed models with logit function and random effect to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of the association between BDNF levels and failing the five ASQ domains over time (up to age three years). We also examined the same association with any ASQ fail, defined as failing any of the five domains at each assessment, up to 7 times. All models included an infant-level random intercept to account for repeated measures for each infant and to model the child-specific variable effect. These models utilize children’s repeated ASQ pass/fail information over time. The random intercept variance and Bayesian Information Criterion (BIC) for these models were reported. We examined interactions between preterm birth and BDNF as well as plurality and BDNF in relation to overall ASQ failure. In the presence of an interaction, stratified analyses were performed. We also categorized BDNF levels in quartiles to examine a nonlinear association between BDNF and odds of ASQ failure. To examine whether effects may have been more subtle and independent of cut-off choice, we ran the analyses using a lower cut-off (1.5 SD of the scores in the study population).
We selected confounders on the basis of the associations between maternal lifestyle factors, neonatal BDNF levels, and children’s neurodevelopment in previous literature (Abdallah et al., 2013; Spulber et al., 2010). Also, we included the socioeconomic and maternal lifestyle factors that were associated with neonatal BDNF in our analyses. All models were adjusted for child gender, preterm birth, plurality, parity, infertility treatment, maternal age, race, education, history of smoking and drinking, pre-pregnancy BMI, and having private insurance. We did not adjust the models for a child’s age, because ASQ fails were age-specific. Models included the discrete ‘time’ variable indicating the wave of ASQ assessment. In all analyses, sampling weights were used to account for the study’s design of oversampling infants conceived with infertility treatment and twins as well as having consent to use DBS for assessment of BDNF (Buck Louis et al., 2014). Weights were derived using New York State birth certificate data on infertility treatment, plurality and region of birth for all infants born during the period of recruitment (Buck Louis et al., 2014). Missing covariate data were less than 5% and therefore imputation was not conducted. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Characteristics of study participants are summarized in Table 1. Given that the cohort was originally designed to track children conceived by infertility treatment, 32% of the current analytic sample was conceived by treatment. As such, the cohort was highly educated (with 31% having graduate level education) and predominantly Non-Hispanic White (83%). In addition, 45% of mothers were nulliparous, 79% had private insurance, 11% smoked in pregnancy, and 13% reported history of any alcohol drinking in pregnancy. In 2637 children included in the study, 820 children (31%) had ASQ assessments at all seven time points and 525 children (20%) had data on six ASQ assessments. Only 50 children (1.9%) had one ASQ from all seven assessment waves. From ages 4 to 36 months, 2–4% of children failed the screen in each of the five domains of fine motor, gross motor, communication, personal-social and problem solving (Table A.1). The percentage of children failing in at least one of the five developmental domains (any ASQ fail) when viewed altogether were 7% at ages 4–6 months, 11% at age 8 months, 9% at 12 months, 6% at 18 months, 10% at 24 months, 7% at 30 months, and 6% at 36 months.
Table 1.
Baseline characteristics of participants, n=2637.
| Child characteristics | N | Mean (SD)* |
|---|---|---|
| Gender, male, % | 1372 | 52.0 |
| Gestational age, weeks | 2637 | 39.0 (34.0, 41.0) |
| Birth weight, gram | 2637 | 3203 (681) |
| Plurality, singletons, % | 2095 | 79.5 |
|
Maternal Characteristics | ||
| Age, years | 2637 | 31.1 (6.0) |
| Parity, nulliparous, % | 1200 | 45.9 |
| Race/ethnicity, % | ||
| Non-Hispanic White | 2200 | 83.4 |
| Not White or Other | 437 | 16.6 |
| Educational levels, % | ||
| Less than high school | 113 | 4.3 |
| High school equivalent | 288 | 10.9 |
| Some college | 754 | 28.6 |
| College graduate | 646 | 24.5 |
| Graduate/professional school | 836 | 31.7 |
| Private health insurance, % | 2089 | 79.3 |
| Smoking in pregnancy, % | 296 | 11.2 |
| Alcohol consumption during pregnancy, % | 357 | 13.5 |
| Pre-pregnancy body mass index | 2633 | 25.3 (19.3, 41.4) |
| Fish oil supplement during pregnancy, % | 451 | 17.4 |
| Multivitamin supplement during pregnancy, % | 1842 | 71.1 |
| Infertility treatment, % | 843 | 32.0 |
| Diagnosis of depressive disorders in pregnancy, % |
95 | 3.7 |
Numbers are mean (standard deviation) for continuous normally distributed variables, median (90% range) for continuous variables with skewed distribution, and percentage for categorical variables.
Observations counted from singletons and one randomly selected twin of each pair (Primary cohort, n=2637).
Information on maternal age, parity, plurality, infant’s gender, gestational age, and birth size was obtained from vital records. Mothers reported at four months postpartum on socio-demographic characteristics –such as highest acquired education level and race/ethnicity– and history of smoking (never, stopped when pregnancy was known, and continued in pregnancy) and pregnancy alcohol consumption (yes/no), pre-pregnancy weight and height, infertility treatment of any type, and use of fish oil or multivitamin supplement. International Code for Disease 9th Revision (codes was used to identify maternal history of depressive disorders in pregnancy as registered in hospital discharge data in the Statewide Planning and Research Cooperative System.
Table A.1.
Ages and Stages Questionnaire (ASQ) responses up to age 3 years
| Age of assessment, months | |||||||
|---|---|---|---|---|---|---|---|
| n (%) | 4–6 | 8 | 12 | 18 | 24 | 30 | 36 |
| Response rate (of 2637) | 2540 (96.3%) | 2442 (92.6%) | 2050 (77.7%) | 1746 (66.2%) | 1547 (58.7%) | 1558 (59.1%) | 1415 (53.7%) |
| Screen invalid | 119 | 229 | 227 | 274 | 273 | 383 | 206 |
| Absolute valid response | 2421 | 2213 | 1823 | 1472 | 1274 | 1175 | 1209 |
| Any domain fail* | 177 (7%) | 233 (11%) | 157 (9%) | 95 (6%) | 129 (10%) | 85 (7%) | 72 (6%) |
| Fine motor fail* | 84 (3%) | 63 (3%) | 22 (1%) | 31 (2%) | 49 (4%) | 32 (3%) | 42 (3%) |
| Gross motor fail* | 69 (3%) | 58 (3%) | 78 (4%) | 39 (3%) | 38 (3%) | 47 (4%) | 24 (2%) |
| Communication fail* | 40 (2%) | 108 (5%) | 71 (4%) | 41 (3%) | 77 (6%) | 49 (4%) | 35 (3%) |
| Personal-Social fail* | 57 (2%) | 99 (4%) | 43 (2%) | 45 (3%) | 50 (4%) | 35 (3%) | 33 (3%) |
| Problem Solving fail* | 53 (2%) | 50 (2%) | 38 (2%) | 37 (3%) | 32 (3%) | 45 (4%) | 32 (3%) |
Responses were counted from twin-pairs once by random selection of one of the twins (Primary cohort)
Percentage out of valid responses
Table 2 shows the associations between prenatal determinants and neonatal BDNF. When we examined the relationship between prenatal factors and neonatal BDNF levels in a multivariate model, we observed that nulliparity (p<0.001), maternal non-White ethnicity/race (p=0.004), maternal smoking in pregnancy (p=0.02), maternal alcohol consumption in pregnancy (p=0.05), and pre-pregnancy obesity (p<0.001) were associated with lower BDNF levels in neonates. Education and depressive disorders did not remain significant in the multivariate model.
Table 2.
Prenatal determinants of Brain Derived Neurotrophic Factor (BDNF) in neonates.
| Neonatal BDNF |
||
|---|---|---|
| Univariate model | Multivariable model | |
| B (95%CI) | B (95%CI) | |
| Maternal age | −0.001 (−0.003, 0.002) | - |
| Nulliparous, yes | −0.05 (−0.07, −0.02)** | −0.04 (−0.07, −0.02)** |
| Race/ethnicity | ||
| Non-Hispanic White | Reference | Reference |
| Not White or Other | −0.05 (−0.09, −0.02)** | −0.05 (−0.09, −0.02)** |
| Educational levels | ||
| Less than high school | −0.04 (−0.10, 0.02) | 0.004 (−0.07, 0.06) |
| High school equivalent | 0.01 (−0.03, 0.06) | 0.03 (−0.01, 0.08) |
| Some college | −0.05 (−0.08, −0.01)** | −0.03 (−0.07, 0.003)* |
| College graduate | −0.02 (−0.05, 0.02) | −0.02 (−0.06, 0.02) |
| Graduate/professional school | Reference | Reference |
| Having private health insurance | 0.02 (−0.01, 0.05) | - |
| Smoking in pregnancy, yes | −0.04 (−0.08, −0.003)** | −0.05 (−0.08, −0.01)** |
| Alcohol consumption during pregnancy, yes | −0.03 (−0.07, 0.004)* | −0.04 (−0.08, −0.003)** |
| Diagnosis of depressive disorders in pregnancy, yes | 0.06 (−0.01, 0.13)* | 0.07 (−0.01, 0.14) |
| Infertility treatment, yes | 0.04 (−0.01, 0.09) | - |
| Fish oil supplement during pregnancy | −0.02 (−0.06, 0.02) | - |
| Multivitamin supplement during pregnancy | 0.01 (−0.02, 0.03) | - |
| Pre-pregnancy body mass index | ||
| Thin or normal weight (<25 kg/m2) | Reference | Reference |
| Overweight (25–29.99 kg/m2) | −0.02 (−0.06, 0.01) | −0.03 (−0.06, 0.01) |
| Obese (>30 kg/m2) | −0.07 (−0.10, −0.04)** | −0.07 (−0.10, −0.04)** |
| Preterm delivery | −0.07 (−0.12, −0.02)** | −0.06 (−0.12, −0.01)** |
| Plurality | −0.08 (−0.16, 0.01)* | −0.04 (−0.13, 0.05) |
Analyses were performed in the primary cohort including singletons and one randomly selected twin of each pair (n=2637). First, maternal age, maternal race, maternal educational levels, parity, infertility treatment, having private insurance, maternal categories of BMI, maternal history of drinking and smoking in pregnancy and maternal history of depressive disorders in pregnancy were included in the model of association with BDNF separately (Univariate model). Next, a parsimonious model including factors that were statistically significant (p<0.1) from these analyses was built to estimate the association of determinants with neonatal BDNF (Multivariable model).
BDNF had a skewed distribution and therefore was log-transformed. Units for BDNF levels are log ng/ml.
denotes P values <0.1
denotes P values <0.05
The unadjusted longitudinal trajectories of any ASQ failure (in 5 domains) were similar between children with neonatal BDNF in the lowest tertile and the rest of children (in the primary cohort, term infants and preterm infants, Figure 1). In longitudinal analyses of neonatal BDNF and children’s development up to age 36 months, we found no association between neonatal BDNF and any ASQ failure or domain specific fail in the primary cohort (Table 3). When the alternative cut-off was used (1.5 SD of the scores in the study population), results remained essentially unchanged, except that there was a significant association between neonatal BDNF and failure in the domain of personal-social skills (Table A.2 shows the results in the primary cohort and with five ASQ domains). There was no interaction between plurality and BDNF in relation to ASQ failure; however, further testing suggested an interaction between preterm birth and BDNF (p for interaction: 0.06). When we stratified analyses for preterm birth, we found that among preterm infants, a higher neonatal BDNF was associated with lower odds of failing in any of the five ASQ domains (OR per log unit increase in BDNF: 0.29, 95%CI: 0.10–0.79). Post-hoc analyses on domain specific failure revealed that this association was significant for failure in communication (OR: 0.23, 95%CI: 0.07–0.76). Table A.3 presents the results in singletons and twins separately. Results of analyses with BDNF levels categorized in quartiles confirmed a linear association between BDNF levels and odds of any ASQ failure (data not shown).
Figure 1. Probabilities of failing Ages and Stages Questionnaire through age 36 months by neonatal BDNF levels (lowest tertile vs. highest two tertiles) and gestational age (term vs. preterm).
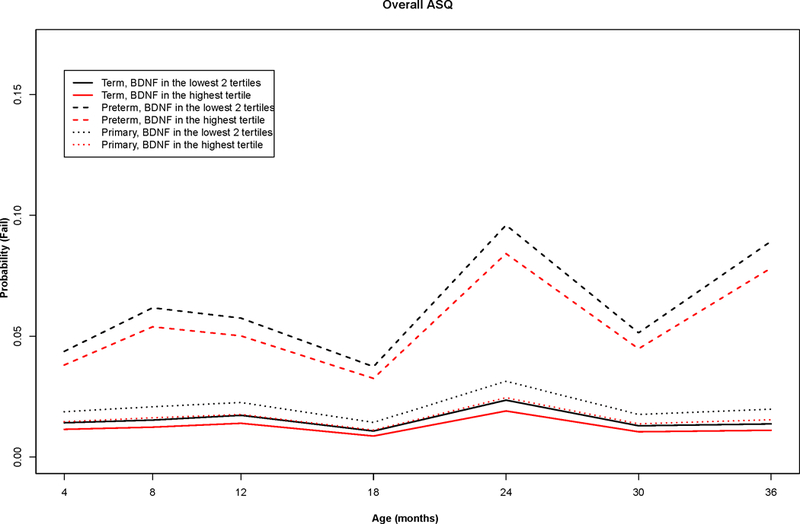
The longitudinal probabilities of failing ASQ by neonatal BDNF for children in the primary cohort (singletons and one randomly select of twin pairs) and non-stratified and stratified by gestational age (term vs. preterm) were estimated by generalized linear mixed-effects models using ASQ data from 4 to 36 months in the Upstate KIDS Study. Overall failure indicated failure in any one of the five domains that include fine motor, gross motor, communication, personal-social, and problem solving.
BDNF: Brain Derived Neurotrophic Factor; ASQ: Ages and Stages Questionnaire
Table 3.
Brain Derived Neurotrophic Factor (BDNF) in infancy and children’s developmental delay.
| Child Development |
||||||
|---|---|---|---|---|---|---|
| Exposure: BDNF levels |
Any fail | Fine motor | Gross motor | Communication | Personal-social | Problem solving |
| OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
|
| Primary cohort (n=2637) | ||||||
| Unadjusted | 0.73 (0.47, 1.14) 4.40 (0.30) 5347.32 |
0.54 (0.29, 1.00)* 6.20 (0.58) 2287.48 |
0.58 (0.27, 1.26) 8.13 (0.93) 2020.1 |
0.71 (0.38, 1.31) 6.84 (0.58) 2763.6 |
0.76 (0.39, 1.48) 6.35 (0.52) 2494.27 |
0.66 (0.34, 1.31) 7.91 (0.90) 2119.05 |
| Adjusted | 0.83 (0.53, 1.30) 4.08 (0.29) 5281.44 |
0.65 (0.34, 1.22) 5.39 (0.52) 2300.29 |
0.61 (0.28, 1.33) 7.87 (0.90) 2024.44 |
0.89 (0.48, 1.63) 6.66 (0.59) 2750.65 |
0.91 (0.48, 1.75) 5.91 (0.52) 2495.81 |
0.87 (0.44, 1.70) 7.16 (0.83) 2172.47 |
| Term infants (n=2186) | ||||||
| Unadjusted | 0.99 (0.61, 1.59) 4.97 (0.35) 4625.42 |
0.57 (0.29, 1.13) 7.92 (0.98) 1889.86 |
0.90 (0.45, 1.78) 12.50 (2.51) 1653.51 |
1.03 (0.53, 2.00) 9.06 (1.10) 2312.58 |
1.18 (0.64, 2.17) 7.64 (0.78) 2146.78 |
0.87 (0.45, 1.69) 11.88 (2.17) 1710.93 |
| Adjusted | 0.98 (0.60, 1.59) 4.72 (0.35) 4593.30 |
0.69 (0.34, 1.40) 6.62 (0.74) 1916.84 |
0.84 (0.39, 1.77) 11.37 (2.13) 1680.32 |
1.26 (0.62, 2.57) 8.70 (0.99) 2323.55 |
1.35 (0.70, 2.59) 6.77 (0.67) 2164.52 |
0.99 (0.48, 2.03) 10.26 (1.69) 1782.53 |
| Preterm infants (n=451) | ||||||
| Unadjusted | 0.25 (0.09, 0.70)* 3.67 (0.65) 1612.23 |
0.71 (0.15, 3.43) 4.73 (0.74) 869.65 |
0.21 (0.03, 1.33) 6.65 (1.33) 736.24 |
0.26 (0.08, 0.86)* 4.48 (0.93) 958.37 |
0.21 (0.04, 1.13) 5.24 (0.99) 750.3 |
0.39 (0.09, 1.81) 4.98 (0.97) 852.87 |
| Adjusted | 0.29 (0.10, 0.85)* 3.10 (0.59) 1652.51 |
0.72 (0.15, 3.40) 3.62 (0.69) 915.63 |
0.23 (0.03, 1.52) 5.32 (1.22) 791.01 |
0.26 (0.07, 0.92)* 4.18 (0.82) 1006.62 |
0.22 (0.03, 1.55) 4.40 (1.16) 787.37 |
0.43 (0.07, 2.59) 4.61 (0.94) 918.76 |
BIC: Bayesian Information Criterion; OR: odds ratio; CI: confidence interval; SE: Standard Error
Primary cohort: singletons and one randomly selected twin of each pair
Developmental delay was assessed up to age three years using the Ages and Stages Questionnaires.
BDNF had a skewed distribution and therefore was log-transformed.
Models were adjusted for child gender, preterm birth (in not-stratified models), plurality, parity, infertility treatment, maternal age, race, education, history of smoking and drinking, pre-pregnancy body mass index, and having private insurance.
denotes P values <0.05
Table A.2.
Brain Derived Neurotrophic Factor (BDNF) in infancy and children’s developmental delay in the primary cohort (alternative cut-off)
| Child Development |
|||||
|---|---|---|---|---|---|
| Exposure: BDNF levels |
Fine motor | Gross motor | Communication | Personal-social | Problem solving |
| OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
|
| Unadjusted | 0.58 (0.33, 1.02) 7.16 (0.41) 7407.02 |
0.50 (0.27, 0.94)* 7.16 (0.44) 5055.32 |
0.45 (0.25, 0.82)* 8.17 (0.49) 6392.33 |
0.42 (0.24, 0.74)* 6.69 (0.41) 6066.81 |
0.53 (0.30, 0.95)* 7.51 (0.46) 6550.98 |
| Adjusted | 0.68 (0.38, 1.22) 7.05 (0.43) 7328.95 |
0.61 (0.33, 1.14) 7.21 (0.48) 5004.21 |
0.63 (0.33, 1.20) 8.32 (0.56) 6273.5 |
0.57 (0.33, 0.98)* 6.26 (0.40) 5978.04 |
0.67 (0.36, 1.23) 7.48 (0.49) 6457.89 |
BIC: Bayesian Information Criterion; OR: Odds Ratio; CI: Confidence Interval; SE: Standard Error
Primary cohort: singletons and one randomly selected twin of each pair
Developmental delay was assessed up to age 3 years using the Ages and Stages Questionnaires.
BDNF had a skewed distribution and therefore was log-transformed.
Models were adjusted for child gender, preterm birth, parity, infertility treatment, maternal age, race, education, history of smoking and drinking, pre-pregnancy body mass index, and having private insurance.
denotes P values <0.05
Table A.3.
Brain Derived Neurotrophic Factor (BDNF) in infancy and children’s developmental delay in twins and singletons
| Child Development |
||||||
|---|---|---|---|---|---|---|
| Exposure: BDNF levels |
Any fail | Fine motor | Gross motor | Communication | Personal-social | Problem solving |
| OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
OR (95%CI) Random intercept variability (SE) BIC |
|
| Singletons (n=2095) | ||||||
| Unadjusted | 0.79 (0.47, 1.32) 5.34 (0.36) 5041.46 |
0.49 (0.24, 1.00)* 8.45 (1.09) 2129.39 |
0.66 (0.32, 1.39) 17.10 (4.44) 1808.42 |
0.74 (0.36, 1.51) 9.58 (1.21) 2554.60 |
0.82 (0.39, 1.70) 9.50 (1.28) 2302.58 |
0.67 (0.33, 1.37) 12.66 (2.83) 1965.28 |
| Adjusted | 0.85 (0.51, 1.42) 4.94 (0.36) 4998.53 |
0.58 (0.28, 1.20) 7.02 (0.76) 2155.09 |
0.60 (0.25, 1.43) 12.74 (2.53) 1838.23 |
0.94 (0.45, 1.99) 9.60 (1.19) 2560.35 |
0.97 (0.45, 2.10) 7.97 (0.84) 2325.34 |
0.80 (0.37, 1.71) 9.66 (1.43) 2024.62 |
| Twins (n=542) | ||||||
| Unadjusted | 0.43 (0.19, 0.98)* 3.44 (0.58) 1493.24 |
1.01 (0.29, 3.57) 4.51 (0.92) 738.22 |
0.64 (0.16, 2.52) 6.94 (1.22) 813.30 |
0.55 (0.22, 1.38) 3.48 (0.70) 926.39 |
0.70 (0.26, 1.89) 2.58 (0.58) 901.82 |
0.94 (0.27, 3.29) 4.11 (1.11) 694.32 |
| Adjusted | 0.49 (0.21, 1.17) 3.21 (0.57) 1502.87 |
1.28 (0.32, 5.1) 3.56 (0.85) 760.51 |
0.81 (0.19, 3.49) 6.77 (1.22) 855.86 |
0.82 (0.32, 2.06) 2.99 (0.75) 930.35 |
0.85 (0.32, 2.28) 2.14 (0.57) 905.13 |
1.40 (0.40, 4.93) 3.31 (0.94) 742.87 |
BIC: Bayesian Information Criterion; OR: Odds Ratio; CI: Confidence Interval; SE: Standard Error
Primary cohort: singletons and one randomly selected twin of each pair
Developmental delay was assessed up to age 3 years using the Ages and Stages Questionnaires.
BDNF had a skewed distribution and therefore was log-transformed.
Models were adjusted for child gender, preterm birth, parity, infertility treatment, maternal age, race, education, history of smoking and drinking, pre-pregnancy body mass index, and having private insurance.
denotes P values <0.05
Discussion
In our population-based cohort, we identified several determinants of BDNF among which three potentially modifiable factors (i.e., maternal cigarette smoking, history of drinking alcohol in pregnancy, and pre-pregnancy obesity) were associated with lower BDNF levels in neonates. No association was observed between neonatal BDNF and child development, except that when we used an alternative cut-off to define delay, higher neonatal BDNF was associated with lower odds of delay in personal-social skills. Nevertheless, when the sample was limited to preterm infants only, we observed that preterm infants with higher DBS BDNF levels were less likely to fail developmental screening up to age three years. This association was particularly present for failure in the communication domain.
Determinants of BDNF
Several studies have investigated the role of BDNF as a regulator of neuronal development and plasticity throughout the life span (Primiani et al., 2014). Due to high correlation between BDNF levels in the central nervous system and serum, neonatal BDNF has been used as a marker of plasticity to evaluate the effect of prenatal exposures on fetal brain development in both animal and human studies (Prickaerts, Gieling, Bruder, van der Staay, & Vanmierlo, 2014; Spulber et al., 2010; Uguz et al., 2013). In the present study, we examined a range of socioeconomic and lifestyle factors simultaneously and found that exposures during the prenatal period, including cigarette smoking in pregnancy and history of drinking alcohol, were associated with lower BDNF levels in neonates. Two other factors that were associated with lower neonatal BDNF were parity and maternal ethnicity/race. We found no association between maternal depressive symptoms and neonatal BDNF, likely due to a small number of women with depressive symptoms in this sample. The observation that neonates born to mothers who smoked during pregnancy averaged lower levels of BDNF than neonates of non-smoking mothers agrees with previous studies (Harrod et al., 2011; Spulber et al., 2010). Whether BDNF expression in the brain (and correlated peripheral levels) might be a possible neurobiological mechanism underlying the association of cigarette smoking during pregnancy and adverse neurodevelopment outcomes in the offspring, as investigated by others (Wiebe et al., 2015), requires further research.
Results on the association between prenatal alcohol exposure and neonatal BDNF levels are less conclusive. Animal studies show an overexpression of BDNF genes and upregulation of the BDNF protein in the hippocampus in rats exposed to third-trimester equivalent binge drinking and suggest a reactive BDNF response and neuroprotective mechanism in the central nervous system as a result of acute exposure to alcohol (Boschen, Criss, Palamarchouk, Roth, & Klintsova, 2015; Heaton, Mitchell, Paiva, & Walker, 2000). In this study, we did not use any measure of excessive alcohol exposure or binge drinking; rather, we defined any alcohol intake during pregnancy. Therefore, comparison with studies on excessive alcohol exposure or binge drinking in pregnancy should be done with caution.
We also observed that children born to women with pre-pregnancy obesity had lower BDNF levels shortly after birth. Due to the increase in the number of obese women in reproductive age, concerns regarding the impact of obesity in children of these women have increased considerably. In a study with mice, Tozuka et al. showed that maternal obesity resulted in a significant decrease in the hippocampal BDNF levels during the early postnatal period of the offspring (Tozuka et al., 2010). Also, offspring of obese mice had fewer neuronal branches in the hippocampus and subsequently had impaired hippocampus-dependent cognitive function, i.e. spatial learning. The hippocampus is one of the brain regions with high expression of BDNF (Ward & Hagg, 2000), and factors affecting BDNF expression in the hippocampus may influence not only learning and memory but also cognitive information necessary to control food intake (Kanoski & Grill, 2015). Our findings of an association between maternal obesity and lower DBS BDNF, and separately of maternal obesity with childhood development (Yeung, E., Sundaram, R., Ghassabian, A., Xie, Y., & Buck Louis, G. M. 2016a) seem to follow the above findings from experimental studies. However, it remains premature evidence, as we admittedly also did not find an association (except among preterm infants) between BDNF and development, suggesting other explanatory mechanisms may be at play. Future studies are suggested to examine whether the effect of maternal socioeconomic and lifestyle characteristics on BDNF expression is through methylation processes on the BDNF polymorphism or otherwise (Braithwaite, Kundakovic, Ramchandani, Murphy, & Champagne, 2015; Roth, Matt, Chen, & Blaze, 2014).
Longitudinal association of BDNF and neurodevelopment
BDNF is implicated in several psychiatric disorders (Lee & Kim, 2010; Mueller et al., 2013; Wang et al., 2015) and epilepsy (Connolly et al., 2006), has a role in the pathogenesis of obesity (Mou et al., 2015), and is involved in immunological reaction and asthma (Lommatzsch et al., 2005). BDNF plays a crucial role in antenatal and postnatal brain developmental processes including neurogenesis, neural migration, and synapse formation. Neurotrophins such as BDNF may contribute to the pathogenesis of the neurodevelopmental delays as suggested by animal models (Janke, Cominski, Kuzhikandathil, Servatius, & Pang, 2015) or postmortem studies (Weickert et al., 2003), or may be an early biomarker in children who later develop brain abnormalities. For this reason, several studies to date examined the prospective association of neonatal BDNF and children’s neurodevelopment (Abdallah et al., 2013; P. G. Nelson et al., 2006) but have yielded inconsistent results. In this study, there were no associations between neonatal BDNF levels and failure of developmental screening in children. This finding is consistent with a study by Nelson et al. that showed BDNF did not differ in children with developmental disabilities such as autism or Down syndrome and controls (P. G. Nelson et al., 2006). We observed an effect modification by preterm birth such that in children born before week 37, BDNF levels shortly after birth were associated with failing the developmental screening and, in particular, communication skills. Neonatal BDNF may reflect the degree of neuronal maturity in preterm infants, as BDNF is shown to play a significant role in cortical maturation and synaptic plasticity in perinatal and early postnatal period (Ward & Hagg, 2000). During neuronal migration in human cortex between weeks 12 and 24, the “inside-out” pattern of neuronal layering happens under the influence of reelin, an extracellular matrix protein secreted by Cajal-Retzius cells. In late prenatal and early postnatal period, reelin expression is downregulated by the BDNF to initiate developmental maturation and synaptogenesis in human brain (Ringstedt et al., 1998). As BDNF increases with gestational age, the levels are lower in preterm infants (Chouthai, Sampers, Desai, & Smith, 2003), and therefore, the regulatory effect on developmental processes in the cortex of the preterm infants might be limited. This may explain the association observed between neonatal BDNF levels in preterm infants and not the children born at term. Furthermore, disruption in the reelin signaling system is suggested as the underlying pathophysiology in autism (Reiner, Karzburn, Kshirsagar, & Kaibuchi, 2016) and may explain the possible link between neonatal BDNF and communication problems or ASD diagnosis.
The strengths of this study include having a large sample of singletons and twins that allowed us to test interactions and longitudinal and repeated assessment of children’s development up to age 36 months. We considered many socioeconomic and lifestyle factors as determinants of BDNF and potential confounders in the analyses. Previous studies suggested that the length of sample storage affects BDNF measurement (Bus et al., 2011); in this prospective study, BDNF was assessed in samples stored for less than a year. However, we faced limitations. First, not all Upstate KIDS participants gave consent for the use of DBS for BDNF assessment. Although there were small absolute differences in sociodemographic characteristics of parents who gave consent for DBS use, these differences should be random with respect to BDNF levels and were not related to ASQ fails. Such random misclassification might have reduced our ability to detect small associations but should not introduce any bias to our results. Also, the children with BDNF data did not differ in ASQ failure compared to children without BDNF data. Second, similar to other follow-up studies of children’s development, there was attrition over time in response to ASQ screening. Nevertheless, we used generalized linear mixed effect models that are robust to loss to follow-up under the missing at random assumption (Fitzmaurice, Laird, & Ware; Molenberghs & Verbeke, 2006). Third, even though a large number of factors were considered as determinants of BDNF, information on important determinants of BDNF levels such as physical activity during pregnancy (Vega et al., 2011) was not available.
The results of this large population-based study support the evidence from animal models that have shown BDNF levels in offspring are influenced by various prenatal factors. Therefore, neonatal BDNF might be used as a marker of plasticity to evaluate the effect of prenatal exposures on fetal brain development. Furthermore, in line with studies in children with neuropsychiatric disorders, we did not find a longitudinal association between neonatal BDNF and developmental delays in children born at term. Our findings cautiously suggest that lower neonatal BDNF levels might be an early marker of aberrant neurodevelopment in preterm infants. Identification of a biomarker that is present prior to abnormal behavior may allow for earlier recognition of children at risk and earlier interventions. We used a double-antibody immunoaffinity assay (Luminex) to measure BDNF in the residual DBSs. Others have discussed the advantage of this method over immunoaffinity chromatography (RIC) for measurement of BDNF (P. G. Nelson et al., 2006). Evidence suggests that methods of assessment and biological sample could be the source of heterogeneity in existing studies on neonatal BDNF and child development (P. G. Nelson et al., 2006; Qin et al., 2016). Future studies are suggested to consider this methodological issue in assessment of BDNF in dried blood spots, plasma or serum.
Acknowledgments
This research was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; contracts #HHSN275201200005C, #HHSN267200700019C). The authors thank all the Upstate KIDS families and staff for their important contributions.
References
- Abdallah MW, Mortensen EL, Greaves-Lord K, Larsen N, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, … Grove J (2013). Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatrica Scandinavica, 128, 61–69. [DOI] [PubMed] [Google Scholar]
- Barde YA (1990). The nerve growth factor family. Progress in Growth Factor Research, 2, 237–248. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Criss KJ, Palamarchouk V, Roth TL, & Klintsova AY (2015). Effects of developmental alcohol exposure vs. intubation stress on BDNF and TrkB expression in the hippocampus and frontal cortex of neonatal rats. International Journal of Developmental Neuroscience, 43, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, & Champagne FA (2015). Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics, 10, 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Bell EM, Kus CA, Sundaram R, McLain AC, … Druschel CM (2014). Methodology for establishing a population-based birth cohort focusing on couple fertility and children’s development, the Upstate KIDS Study. Paediatric and Perinatal Epidemiology, 28, 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, … Voshaar RC (2011). Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology, 36, 228–239. [DOI] [PubMed] [Google Scholar]
- Chouthai NS, Sampers J, Desai N, & Smith GM (2003). Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatric Research, 53, 965–969. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, … Deuel RM (2006). Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biological Psychiatry, 59, 354–363. [DOI] [PubMed] [Google Scholar]
- Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, … Van de Water J (2008). Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Research, 1, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, & Ware J (2004) Applied Longitudinal Analysis Hoboken: John Wiley & Sons, Inc. [Google Scholar]
- Flock A, Weber SK, Ferrari N, Fietz C, Graf C, Fimmers R, … Merz WM (2015). Determinants of brain-derived neurotrophic factor (BDNF) in umbilical cord and maternal serum. Psychoneuroendocrinology, 63, 191–197. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, Lynch CD, Jackson LW, McGuinness BM, & Msall ME (2010). Concurrent validity of the parent-completed Ages and Stages Questionnaires, 2nd Ed. with the Bayley Scales of Infant Development II in a low-risk sample. Child: Care, Health and Development, 36, 485–490. [DOI] [PubMed] [Google Scholar]
- Guevara JP, Gerdes M, Localio R, Huang YV, Pinto-Martin J, Minkovitz CS, … Pati S (2013). Effectiveness of developmental screening in an urban setting. Pediatrics, 131, 30–37. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Zhu J, Hughes BA, Perna MK, & Brown RW (2011). Gestational IV nicotine produces elevated brain-derived neurotrophic factor in the mesocorticolimbic dopamine system of adolescent rat offspring. Synapse, 65, 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M, & Walker DW (2000). Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Brain Research. Developmental Brain Research, 121, 97–107. [DOI] [PubMed] [Google Scholar]
- Janke KL, Cominski TP, Kuzhikandathil EV, Servatius RJ, & Pang KC (2015). Investigating the role of hippocampal BDNF in anxiety vulnerability using classical eyeblink conditioning. Frontiers in Psychiatry, 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, & Lind JN (2015). Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics, 135, e1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, & Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, & Grill HJ (2015). Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biological Psychiatry doi: 10.1016/j.biopsych.2015.09.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Schwald M, & Cisse M (2002). Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neuroscience Letters, 328, 261–264. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Stuart EA, Gross AL, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: The First 3 Years. Child Development, 84(2), 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-H, & Kim Y-K (2010). The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investigation, 7, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Jaffe AE, & Storey JD (2012). The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics, 28, 882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, … Virchow JC (2005). Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. American Journal of Respiratory and Critical Care Medicine, 171, 115–120. [DOI] [PubMed] [Google Scholar]
- McDade T, Williams S, & Snodgrass JJ (2007). What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography, 44, 899–925. [DOI] [PubMed] [Google Scholar]
- Molenberghs G, & Verbeke G (2006). Models for Discrete Longitudinal Data New York: Springer Science & Business Media, Inc. [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, & Elzinga BM (2014). Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular Psychiatry, 19, 791–800. [DOI] [PubMed] [Google Scholar]
- Mou Z, Hyde TM, Lipska BK, Martinowich K, Wei P, Ong CJ, … Han JC (2015). Human obesity associated with an intronic SNP in the Brain-Derived Neurotrophic Factor locus. Cell Reports, 13, 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, & Ernst M (2013). Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism? Journal of American Academy of Child & Adolescent Psychiatry, 52, 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Jelliffe LL, … Phillips TM (2001). Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Annals of Neurology, 49, 597–606. [PubMed] [Google Scholar]
- Nelson PG, Kuddo T, Song EY, Dambrosia JM, Kohler S, Satyanarayana G, … Nelson KB (2006). Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. International Journal of Developmental Neuroscience, 24, 73–80. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, & Michel TM (2011). The role of neurotrophic factors in autism. Molecular Psychiatry, 16, 478–490. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Gieling ET, Bruder AK, van der Staay FJ, & Vanmierlo T (2014). Long-term effects of prenatal allopurinol treatment on brain plasticity markers in low and normal birth weight piglets. International Journal of Developmental Neuroscience, 33, 29–32. [DOI] [PubMed] [Google Scholar]
- Primiani CT, Ryan VH, Rao JS, Cam MC, Ahn K, Modi HR, & Rapoport SI (2014). Coordinated Gene Expression of Neuroinflammatory and Cell Signaling Markers in Dorsolateral Prefrontal Cortex during Human Brain Development and Aging. PLoS ONE, 9, e110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Feng JC, Cao C, Wu HT, Loh YP, & Cheng Y (2016). Association of Peripheral Blood Levels of Brain-Derived Neurotrophic Factor With Autism Spectrum Disorder in Children: A Systematic Review and Meta-analysis. JAMA Pediatrics, 170, 1079–1086. [DOI] [PubMed] [Google Scholar]
- Reiner O, Karzburn E, Kshirsagar A, & Kaibuchi K (2016). Regulation of neuronal migration, an emerging topic in autism spectrum disorders (ASD). Journal of Neurochemistry, 136: 440–56 [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, … Ibanez CF (1998). BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron, 21, 305–315. [DOI] [PubMed] [Google Scholar]
- Roth TL, Matt S, Chen K, & Blaze J (2014). Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Developmental Psychobiology, 56, 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, & Lash TL (2008). Modern Epidemiology, Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, & Liu A (2006). The limitations due to exposure detection limits for regression models. American Journal of Epidemiology, 163, 374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhaut L, Armijo I, Schonstedt M, Alvarez J, & Cordero M (2013). Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics, 131, e1468–1474. [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Nørgaard-Pedersen B, & Hougaard DM (2008). Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. Journal of Immunological Methods, 336, 78–84. [DOI] [PubMed] [Google Scholar]
- Skovgaard AM, Olsen EM, Christiansen E, Houmann T, Landorph SL, & Jorgensen T (2008). Predictors (0–10 months) of psychopathology at age 11/2 years -a general population study in The Copenhagen Child Cohort CCC 2000. Journal of Child Psychology and Psychiatry and Allied Disciplines, 49, 553–562. [DOI] [PubMed] [Google Scholar]
- Spulber S, Rantamaki T, Nikkila O, Castren E, Weihe P, Grandjean P, & Ceccatelli S (2010). Effects of maternal smoking and exposure to methylmercury on brain-derived neurotrophic factor concentrations in umbilical cord serum. Toxicological Sciences, 117, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J, & Bricker D (2009). Ages & Stages Questionnaires [R], (ASQ-3 [TM]): A Parent-Completed Child-Monitoring System Baltimore: Brookes Publishing Co. [Google Scholar]
- Squires J, Potter L, & Bricker D (1999). The ASQ user’s guide for the Ages & Stages Questionnaires: a parent-completed, child-monitoring system Baltimore: Brookes Publishing Co. [Google Scholar]
- Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, & Wada K (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochemistry International, 57, 235–247. [DOI] [PubMed] [Google Scholar]
- Uguz F, Sonmez EO, Sahingoz M, Gokmen Z, Basaran M, Gezginc K, … Tasyurek E (2013). Maternal generalized anxiety disorder during pregnancy and fetal brain development: a comparative study on cord blood brain-derived neurotrophic factor levels. Journal of Psychosomatic Research, 75, 346–350. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, … Blanco CE (2006). Prenatal stress and neonatal rat brain development. Neuroscience, 137, 145–155. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ (2013). Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutrition Reviews, 71 Suppl 1, S95–101. [DOI] [PubMed] [Google Scholar]
- Vega SR, Kleinert J, Sulprizio M, Hollmann W, Bloch W, & Struder HK (2011). Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology, 36, 220–227. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen H, Yu T, Cui G, Jiao A, & Liang H (2015). Increased serum levels of brain-derived neurotrophic factor in autism spectrum disorder. Neuroreport, 26, 638–641. [DOI] [PubMed] [Google Scholar]
- Ward NL, & Hagg T (2000). BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Experimental Neurology, 162, 297–310. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, & Kleinman JE (2003). Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Molecular Psychiatry, 8, 592–610. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Clark CA, De Jong DM, Chevalier N, Espy KA, & Wakschlag L (2015). Prenatal tobacco exposure and self-regulation in early childhood: Implications for developmental psychopathology. Development and Psychopathology, 27, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E, Sundaram R, Ghassabian A, Xie Y, & Buck Louis GM (2016a). Parental obesity and early childhood development. Pediatrics, in press. [DOI] [PMC free article] [PubMed]
- Yeung E, Buck Louis GM, Lawrence D, Kannan K, McLain AC, Caggana M, … Bell E. (2016b). Eliciting parental support for the use of newborn blood spots for pediatric research. BMC Medical Research Methodology, 16, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E, Sundaram R, Bell EM, Druschel C, Kus C, Ghassabian A, … Buck Louis GM (2016c). Examining Infertility Treatment and Early Childhood Development in the Upstate KIDS Study. JAMA Pediatrics, 170, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, & Charman T (2010). The prodrome of autism: early behavioral and biological signs, regression, peri-and post-natal development and genetics. Journal of Child Psychology and Psychiatry and Allied Disciplines, 51, 432–458. [DOI] [PubMed] [Google Scholar]
- Yochum C, Doherty-Lyon S, Hoffman C, Hossain MM, Zelikoff JT, & Richardson JR (2014). Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: role of altered catecholamines and BDNF. Experimental Neurology, 254, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chen L, Wang C, Yang X, Gao Y, & Tian Y (2016). The role of cord blood BDNF in infant cognitive impairment induced by low-level prenatal manganese exposure: LW birth cohort, China. Chemosphere, 163, 446–451. [DOI] [PubMed] [Google Scholar]


