Abstract
Over the past 25 years, the immune system has appeared as a key regulator of adipose tissue biology and metabolic homeostasis. In lean animals, adipose-resident leukocytes maintain an anti-inflammatory environment that preserves the proper functioning of the tissue. Several adipose leukocyte classes have been described, each with unique contributions to the tissue microenvironment. Here, we describe two populations of innate T cells enriched in adipose tissue, iNKT and γδ T cells, and how they serve overlapping and non-redundant roles in controlling adipose tissue functions. Both cell types interact with and expand anti-inflammatory leukocytes, thereby driving a metabolically beneficial tissue milieu. Surprisingly, we have found that adipose iNKT and γδ T cells also promote weight loss and heat production in a process called “non-shivering thermogenesis.” The data surrounding these two cell types highlight their powerful ability to regulate not only other leukocytes, but also tissue-wide processes that affect an entire organism.
Introduction: Adipose-resident immune cells influence adipose tissue biology and systemic metabolism.
Adipose tissue contains a unique and highly developed immune system that maintains a non-inflammatory, Th2-biased state to coordinate metabolic responses. Alternatively-activated (M2) macrophages, type 2 innate lymphoid cells (ILC2), eosinophils, T regulatory cells (Tregs), and other leukocytes all cooperate to prevent inflammation (1). However, during chronic overfeeding and obesity, however, changes occur in the adipose tissue that promote inflammatory responses, including adipocyte hypertrophy and endoplasmic reticulum stress, and ultimately the release of tissue debris and toxic free fatty acids (FFAs) as a consequence of adipocyte bursting (2). This is accompanied by a shift in the adipose immune compartment towards more inflammatory cell populations. Monocyte-derived M1 macrophages are recruited to adipose tissue at the onset of obesity (3–5) and are followed by CD8+ T cells (6) and proinflammatory B cells (7) at later stages, promoting a positive feedback cycle of inflammation. Obesity-associated inflammation disrupts metabolic pathways in adipose tissue, resulting in systemic metabolic diseases such as cardiovascular disease and diabetes mellitus. TNF signaling profoundly alters adipocyte biology (8) and metabolic deficits have been described in adipocytes exposed to IFNγ (9) and IL-1β (10–12). In this way, the composite leukocyte composition and cytokine millieu of the adipose tissue determine its inflammatory state and metabolic functions. For additional information on the effects of inflammation on adipocyte biology, we refer the reader to an extensive review (13).
Unlike stereotypical lymphoid organs such as the spleen and lymph nodes, which primarily contain adaptive immune cells, adipose tissue is enriched with several types of innate leukocytes. Over 60% of the CD45+ cells in lean adipose tissue are macrophages, and this proportion increases to over 80% during obesity (14). Moreover, adipose tissue contains several subsets of innate lymphoid cells and innate lymphocytes. ILC2s were first identified in adipose tissue in 2013, and are a crucial source of the Th2 cytokines IL-5 and IL-13 (15). Recently, large populations of natural killer (NK) cells and type 1 innate lymphoid cells (ILC1) have been described in lean and obese adipose tissue, although whether these cells play a dominant role in limiting or promoting tissue inflammation remains a topic of active investigation (16–18). Additionally, mucosal-associated invariant T (MAIT) cells are highly expanded in obese human adipose tissue and exhibit a skewed Th-17 profile (19).
Recently several groups including ours have characterized two other classes of innate T lymphocytes in adipose tissue: invariant natural killer T (iNKT) and γδ T cells. While these cell types are relatively rare in most peripheral organs, they are highly enriched in human and murine adipose tissue where they have unique properties and functions. By distinct mechanisms, iNKT and γδ T cells maintain adipose tissue homeostasis by regulating the numbers and functions of other adipose-resident immune cells. Surprisingly, both iNKT cells and innate Vγ6 γδ T cells also regulate thermogenesis, which impacts total body metabolism, utilization of lipid substrates, and weight loss. In this review, we describe how iNKT cells and innate Vγ6+ γδ T cells play key, non-redundant roles in regulating adipose tissue inflammation and thermogenesis.
iNKT and γδ T cells are innate T lymphocytes that mediate host defense and regulate a variety of immune responses
iNKT cells, γδ T cells and MAIT cells are members of a family of T lymphocytes that are not restricted to major histocompatibility complex (MHC) molecules, but instead recognize CD1 and MR1 antigen presenting molecules and other types of cell surface molecules (20). These cells uniquely exist in the periphery in a poised state and are capable of rapid immune responses, much like NK cells and other innate leukocytes. However, they differ from other innate lymphocytes in using their T cell receptor (TCR) as a major sensor for specific recognition and activation (21). Here we briefly introduce iNKT and γδ T cells before focusing on their biology and function in adipose tissue.
In stark contrast to most MHC-restricted T cells with diverse TCRs, iNKT cells use canonical TCRα rearrangements paired with a restricted set of Vβ gene segments. In mice, the iNKT TCR is formed from pairing of the Vα14-Jα18 chain with Vβ8.2, Vβ7, or Vβ2, whereas in humans this TCR is generated from the Vα24-Jα18 chain paired almost exclusively with the Vβ11 chain (22). The iNKT TCR recognizes predominantly alpha-anomeric glycolipid antigens presented by CD1d molecules (22). The prototypical antigen for the iNKT TCR is alpha-galactosylceramide (αGalCer) (KRN7000), a synthetic glycolipid isolated from a marine sponge. Both murine and human iNKT cells produce copious amounts of IFNγ and IL-4 immediately upon stimulation with αGalCer bound to CD1d on antigen presenting cells (APCs) (23). Furthermore, they can also be activated by a combination of TCR stimulation plus signals from innate cytokines such as IL-12 or IL-18(24). Their “poised effector state” is driven by expression of the promyelocytic leukemia zinc finger protein (PLZF) transcription factor, which is originally expressed during positive selection in the thymus and whose expression is maintained in the periphery(25, 26). Within hours of pathogen challenge, iNKT cells are activated to produce massive amounts of proinflammatory cytokines and transactivate other immune cells to initiate immune responses. In fact, iNKT cells largely function by regulating the activities of other immune cells since iNKT cell activation results in transactivation of natural killer cells and macrophages, via secreted factors or cell-cell interactions (21). In this way, iNKT cells serve as cellular adjuvants that govern immune and inflammatory processes. Due to their ability to be activated by both TCR signals and cytokines, iNKT cells are protective against pathogens that contain iNKT lipid antigens such as S. pneumoniae, B. burgdorferi, and Sphingomonas, as well as those that do not contain lipid antigens such as influenza A virus or the fungus A. fumigatus (27). In the latter case, TCR signals come in the form of self lipids antigens which are upregulated in APCs in response to microbial danger signals (28–30).
iNKT cells are primarily tissue resident, and in C57BL/6 mice they comprise 1–2% of splenic T cells and 20–30% of all liver T cells (31, 32). Analogous to CD4+ T helper cell subsets, iNKT cell subsets exist, and these subset have tissue-specific distribution. The majority of iNKT cells in C57BL/6 mice are Th1-like (NKT1), reside in the liver and spleen, and produce IFNγ upon activation. Th2-like iNKT cells (NKT2) are major drivers of allergic diseases, and primarily produce IL-4 and IL-13 in the lungs. iNKT cells producing the Th-17 cytokines, IL-17, IL-21, and IL-22 (NKT17), are relatively rare in mice and humans, but significantly contribute to immune responses in the skin, lymph nodes, and lung(21). The relative contribution of thymic instruction versus local microenvironment to iNKT cell phenotype is a subject of active research, iNKT cells in the thymus producing discreet Th1, Th2, or Th17 cytokines suggest thymic instruction contributes to specialization of these subsets (33, 34).
While some subsets of γδ T cells exhibit TCR junctional diversity suggesting adaptive immune functions, several γδ T cell subsets have became associated with innate immunity based on their limited TCR diversity, anatomical location and rapid response kinetics (35). Unlike αβ T cells that require antigen priming and differentiation cues in secondary lymphoid organs, γδ T cells resemble iNKT cells and emerge from the thymus developmentally preprogrammed and have acquired innate-effector phenotypes that are important for tissue-specific functions. During development, γδ T cells emerge from the thymus and undergo TCRγ and TCRδ gene rearrangement in discrete waves. The earliest wave begins at embryonic day 13 where Vγ5+ fetal thymocytes become the first T lymphocytes to rearrange their TCRs. The population of Vγ5+ thymocytes are positively selected on Skint-1+ thymic stroma, before subsequently homing to the epidermis to mature into dendritic epidermal T cells (DETCs) where they limit inflammation, promote wound-healing responses, and increase barrier functions in response to cutaneous carcinogens (36) (Heilig and Tonegawa nomenclature) (37–40).
The next wave of Vγ6+ fetal thymocytes begin to rearrange at embryonic day 15.5 and seed the lung, dermis, tongue, epithelium of the uterus, and vaginal tract (41, 42). Vγ6+ cells secrete IL-17A and chemokines within 24 hours of bacterial or fungal encounter at mucosal sites and in the lung to rapidly mount a response (43–45). Owing to their early development and lack of deoxynucleotidyl transferase (TdT) expression (46) at the time of TCR rearrangement, these receptors have limited diversity at the variable (V), diversity (D), and junction (J) segments and therefore have invariant Vγ5+Vδ1+ and Vγ6+Vδ1+ TCRs (47). During the late stages of fetal and well into adult thymic development, however, Vγ7+, Vγ4+, and Vγ1+ cells develop with diverse TCRs owing to expression of TdT and seed the intestinal epithelium (Vγ7+ IELs), spleen (Vγ4+ and Vγ1+), liver (Vγ1+Vδ6.3+), and lymph nodes (Vγ4+ and Vγ1+)(48, 49). Vγ7+ IELs regulate enterocyte differentiation and turnover (50), and Vγ1+Vδ6.3+ T cells produce IFNγ and IL-4 within the first couple of hours after stimulation in vitro, and help to enhance IgE production by B cells through secretion of IL-4 in vivo(49, 51). Vγ4+ cells have been shown to infiltrate inflamed dermis during cutaneous infection and provide a continual source of IL-17 (43, 52). More recently, IL-17 producing Vγ4+ and Vγ6+ γδ T cells have been shown to either exacerbate or protect from autoimmunity or cancer and can modulate tissue pathology during chronic inflammation (53–56). Preferential tissue localization and function of Vγ2+ and Vγ3+ T cells is not yet known, but these cells exhibit extensive junctional diversity. Thus, γδ T cells are capable of engaging a wide variety of effector functions important to tissue immunity and homeostasis and their actions depend greatly on the location, extrinsic cues sees in situ, and their TCR rearrangement.
Regulating the Regulators in Adipose Tissue: iNKT and γδ T cells drive the expansion and function of adipose-resident Tregs and macrophages
Due to their tissue localization and rapid-response kinetics, innate T cells contribute to immune responses directly and by modulating the actions of other immune cells. In this section, we describe how iNKT and γδ T cells enhance the number and functions of two adipose leukocyte subsets: M2 macrophages and Tregs in the case of iNKT cells, and Tregs in the case of γδ T cells.
Adipose iNKT cells regulate Tregs and macrophages through production of IL-2 and IL-10, respectively
iNKT cells were first described in lean human omental adipose tissue in 2009, where they comprise 10–50% of the T cells making it the most iNKT cell-rich organ in the human body (57). Adipose iNKT cell numbers were found to be significantly reduced in obese humans (57), and this finding was later echoed in several reports in mice (14, 58). While the preponderance of iNKT cells in C57BL/6 mice are Th1 skewed and produce high levels of proinflammatory cytokines, we and others have found that adipose iNKT cells produce little IFNγ and TNFα after in vivo stimulation with αGalCer (58, 59). Instead, we found that adipose iNKT cells produce large amounts of IL-2 and IL-10, regulatory cytokines important for adipose tissue homeostasis (59). Other investigators have reported that adipose iNKT cells produce high levels of the Th2 cytokines IL-4 and IL-13, both at steady state and after activation (14, 58). These data suggest that iNKT cells positively contribute to the anti-inflammatory environment required for proper adipose tissue function.
Murine iNKT cells have a characteristic transcriptional profile that distinguishes them from other T cells (60–62). To determine where adipose iNKT cells fit within the iNKT cell compendium, we performed transcriptional comparisons of adipose iNKT cells with splenic iNKT cells, a typical proinflammatory iNKT cell subset. We found that iNKT cells in adipose tissue differed greatly from splenic iNKT cells. Importantly, adipose iNKT cells lack expression of PLZF, the transcription factor previously thought to be characteristic of all iNKT cells. Instead of PLZF, adipose iNKT cells express a basic leucine zipper transcription factor, E4BP4, that drives their production of IL-10 (59). Additionally, adipose iNKT cells appear to be persistently stimulated through their TCRs at steady state as evidenced by their high expression of Nr4a1, which encodes the early TCR activation protein Nur77. This is further supported by their increased expression of CD69 and their high proliferation rate, as measured by intracellular staining for Ki67 (LaMarche and Brenner, unpublished observations). Whether this persistent stimulation is critical for adipose iNKT cell biology and function remains to be determined.
Because of their relatively low numbers in most organs, iNKT cells typically mediate their powerful immunological effects by regulating the activities and numbers of other immune cells such as macrophages, dendritic cells, and natural killer cells (21, 27). To determine if adipose iNKT cells interact with other immune cells in adipose tissue at steady state, we performed whole-mount microscopy of adipose tissue using fluorescently-labeled CD1d:αGalCer tetramers. We found that iNKT cells colocalized with macrophages (as measured by CD68) and Tregs (as measured by FoxP3), two tissue-resident cells known to maintain the anti-inflammatory adipose microenvironment (3–5). Further coculture and antibody blocking experiments revealed that adipose iNKT cells, but not splenic iNKT cells, induced expansion of M2 macrophages and Tregs, and that this expansion was dependent on IL-10 and IL-2, respectively (59).
Importantly, in the absence of iNKT cells adipose Treg numbers are markedly reduced, and their functions are significantly impaired. Tregs in iNKT-deficient mice express lower levels of KLRG1, a marker of enhanced suppressive function, than their WT counterparts, and they produce less IL-10 at steady-state. (59). Similarly, adipose macrophages in Ja18−/− (iNKT deficient) mice express higher levels of iNOS and CD11c and lower levels of Arginase, CD206, and CD301. The percentage of iNKT cells colocalizing with macrophages increased significantly when mice were injected with αGalCer (59). This colocalization was blunted in mice with macrophage-specific deletion of CD1d, suggesting that iNKT-macrophage interactions in adipose tissue rely on antigen presentation (63). Together, these results indicate that the important functions of Tregs and M2 macrophages in adipose tissue homeostasis are critically dependent on iNKT cell instruction.
Adipose γδ T cells: Innate IL-17A producing cells are required for age-related Treg expansion in adipose tissue
γδ T cells play important roles in providing early protection against pathogens by rapidly producing inflammatory cytokines such as TNF, IFNγ and IL-17A, and chemokines to mediate the recruitment of phagocytes like monocytes and neutrophils to the infected tissue. Although their function at barrier sites is well appreciated, the role of γδ T cells in adipose tissue is only now unfolding. We and others have recently reported an enriched population of IL-17A producing γδ T cells in visceral adipose tissue (64–67). Parabiosis experiments revealed that γδ T cells are resident in adipose tissue and phenotypic analysis has uncovered the presence of two discrete populations of γδ T cells based on CD27 and CD3 expression (42, 67, 68). CD3hiCD27neg γδ T cells are more abundant in adipose tissue than their CD3loCD27pos counterparts. Furthermore, transcriptional profiling of CD3hiCD27neg γδ T cells show that this population expresses PLZF, Sox13, and Rorc, and surface markers such as Il1r1, Il23r, Cd44, and Il7r (Cd127), classifying them as innate-IL-17 producing cells. Conversely, CD3loCD27pos γδ T cells transcriptionally appear to look more like cytotoxic, NK cells. Interestingly, this enriched population of PLZF+ γδ T cells expresses the canonical Vγ6Vδ1 T-cell receptor and greatly expands in visceral adipose tissue with age. In adipose tissue, there is a large population of innate-IL17A producing γδ T cells that are tissue-resident, express PLZF, and become a substantial proportion of innate lymphocytes with age (67).
Our group has recently uncovered a role for γδ T cells in the age-related expansion of adipose tissue Tregs. Adipose Tregs arrive from the thymus and seed the tissue early in life (69). With age, they expand (70), comprising 40–80% of CD4+ T cells in the visceral adipose tissue (69, 71) Unlike Tregs elsewhere, adipose Tregs express high levels of CD25, ST2, KLRG1, and IL-10 (72). Our group has recently found that PLZF+ γδ T cells are important for age-dependent Treg accumulation in adipose tissue. Similar to accumulation kinetics of adipose Tregs, timecourse analysis of adipose tissue showed an increase in the numbers of PLZF+ innate IL-17A producing γδ T cells and increase in cytokine levels of IL-17A with age. Remarkably, the numbers of adipose Tregs in mice lacking γδ T cells or IL-17A were also significantly diminished. Moreover, many of the suppressive features of adipose Tregs including high expression of IL-10, ST2, and KLRG1 were decreased in older mice that lack γδ T cells compared to WT mice. The expansion of γδ T cells and Tregs in adipose tissue, inversely correlated with the declining numbers of iNKT cells and ILC2 numbers, two populations that were previously been shown to also regulate Treg numbers in adipose tissue (67).
IL-33 is a critical survival factor for Tregs in adipose tissue owing to their expression of ST2, the IL-33 receptor (69, 73, 74). Mice lacking ST2 have severe impairments in adipose Treg numbers (69). γδ- and IL17A-deficient mice have decreased protein levels of IL-33 and administration of recombinant IL-33 rescued the low Treg numbers in situ, suggesting a role for both γδ T cells and IL-17A in IL-33 regulation. The source of IL-33 in adipose tissue has been characterized by our group and others (69, 75–77). IL-33 expressing mesenchymal cells include cadherin-11+ (Cdh11) cells, podoplanin+ fibroblasts, PDGFRα+ preadipocytes, and CD31+ endothelial cells (69, 73, 76, 78). Recently, we defined a specific population of Pdpn+PDGFRα-Cdh11+ stromal cells that highly expressed IL-33 in murine adipose tissue at steady state (67). Interestingly in vivo, TNF and IL-17A cytokine administration acted to expand the numbers of Pdpn+Pdgfrα-Cdh11+ stromal cells in adipose tissue, and synergistically induced expression level of IL-33 in PdpnloPDGFRα+ preadipocytes. Together, both the increased numbers of IL-33 expressing stromal cells and increased transcription of IL-33 by PdpnloPDGFRα+ preadipocytes, work in concert to increase IL-33 protein levels in visceral adipose tissue. Together, IL-17A producing γδ T cells promote production of IL-33 by adipose stromal cells and appear to be important for the age-dependent accumulation of Tregs in adipose tissue (59, 73).
The effects mediated by γδ T cells are intriguing given the defined roles for iNKT cells that also regulate Treg numbers and function (59). While iNKT cells regulate Treg homeostasis in young mice and via production of IL-2, PLZF+ γδ T cells become a dominant player in adult mice when iNKT cell numbers decline and do so via a stromal cell-IL-33 axis. As regulators of type 2 immunity, ILC2s and iNKT cells, decline with age, a new wave of immune cells composed of γδ T cells and Tregs expand to fill a regulatory niche (Figure 1). This temporal regulation of adipose lymphocytes may ensure redundancies in the molecular pathways that maintain healthy adipose tissue, which is critical for local and systemic metabolic homeostasis at steady state.
Figure 1. Cellular changes in adipose tissue in young and aged mice.
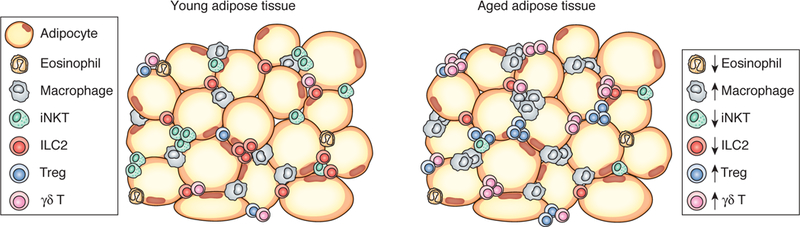
Young adipose tissue is highly enriched with iNKT cells, which support M2 macrophage and Treg expansion by production of IL-10 and IL-2, respectively. As mice age, iNKT cells are gradually replaced by a population of IL-17A-secreting γδ T cells which support Tregs by a stromal cell-IL-33 axis.
Innate Rheostats: iNKT and γδ T cells drive adaptive thermogenesis via non-redundant pathways
Innate immunity and adaptive thermogenesis in brown and beige adipose tissue
Non-shivering thermogenesis is a biological process that enables mammals to adapt to environmental cold by producing heat in beige and brown adipose tissues (79). This process is largely controlled by uncoupling proteins (UCPs) that are induced on the inner mitochondrial membrane and facilitate ‘uncoupled respiration’ – proton leak without ATP synthesis leading to increases in body temperature (79). UCP1 was the first uncoupling protein to be identified and is potently induced upon cold exposure as a strategy to generate heat and maintain core body temperature (80). In addition to UCP1, there is emerging evidence of other pathways the body utilizes to activate non-shivering thermogenesis (81, 82).
In recent years, the innate immune system has emerged as an active participant in body temperature control in response to cold and β-adrenergic stimulation. ILC2s resident in subcutaneous adipose tissue secrete methionine-enkephalin (MetEnk) peptides to boost local UCP1 expression and induce beiging (83). In response to cold stimuli, production of a skeletal hormone, meteorin-like, and the chemokine, CCL11, recruits and induces, respectively, eosinophil production of IL-4 and IL-13 (84, 85). Together, this type 2 immune response creates a milieu that is favorable for thermogenesis (85–87). Lastly, brown adipose tissue macrophages expressing CX3CR1+ was shown to regulate sympathetic nerve innervation (88). Genetic ablation of CX3CR1+ macrophages decreased local norepinephrine levels and consequently affected brown adipocyte function at thermoneutrality (88). Here we describe recent insights into the ways in which iNKT cells and γδ T cells modulate non-shivering thermogenesis, the metabolic adaptation to cold.
iNKT-FGF21 Axis for Thermogenic Responses
Our group has described a mechanism by which activation of adipose iNKT cells induces weight loss though non-shivering thermogenesis. In 2012, Lynch and colleagues showed that injection of obese mice with αGalCer induced potent weight loss due to a reduction in fat mass, not lean mass, in an iNKT cell-dependent manner (14). After injection with αGalCer obese mice lost roughly 10% of their body weight in 4 days and increased their body temperature by 1° Celsius. Further analysis revealed that these changes were due to increased energy expenditure associated with browning of white fat with upregulation of UCP1 in visceral and subcutaneous fat depots. The effects of αGalCer occurred in the absence of changes in activity level or food consumption (89).
Fibroblast growth factor 21 (FGF21) is an important insulin-sensitizing hormone that acts directly and indirectly to regulate thermogenesis. FGF21 can directly influence the central nervous system to induce its thermogenic effects (90). Additionally, FGF21 can have indirect effects by upregulating CCL11 in white adipose tissue to recruit eosinophils that initiate a beiging program in inguinal depots (85). We determined that weight loss after αGalCer administration in mice was largely dependent on FGF21 likely produced by adipocytes themselves downstream of iNKT cell activation. In separate experiments, we found that adipose iNKT cells were activated by liraglutide, a glucagon-like peptide 1 receptor agonist used to treat metabolic disease in obese humans. Liraglutide induced weight loss and browning of white fat in an iNKT and FGF21-dependent manner such that the ability of liraglutide to induce weight loss was reduced in iNKT deficient mice (89). While the exact mechanism of the iNKT-FGF21 axis is still a topic of active investigation, our work suggests that specific activation of adipose iNKT cells could serve as an immune-based treatment for weight loss. Furthermore, these findings have important implications for the treatment of human obesity and diabetes: complementing our mouse models, we found that obese patients treated with liraglutide expressed increased serum FGF21 in direct proportion to weight loss. Additionally, the blood of obese patients treated with liraglutide contained higher levels of iNKT cells than that of control obese patients (89).
γδ-IL17 Axis is Important for Body Temperature Regulation
Similar to γδ T cells in visceral adipose tissue, γδ T cells are also found in subcutaneous and brown adipose tissues and robustly produce IL-17A in response to stimulation. Recently, we uncovered surprising roles for γδ T cells and IL-17A in body temperature control at thermoneutrality and after cold stimulation (67). At steady state, mice that lacked γδ T cells and IL-17A showed decreased body temperature and delayed circadian temperature control, respectively, compared to WT mice. After cold challenge, γδ-deficient mice dropped body temperature more rapidly and correspondingly could not increase energy expenditure compared to WT controls. Similarly, at a critical time when mice increase body temperature to maintain body temperature in the cold, IL-17A-deficient mice were unable to expend energy and had to be rescued within the first 10 hours. In support of both observations, mice without γδ T cells or IL-17A were unable to induce a thermogenic program at the molecular level where genes such as Ucp1, Ppargc1a, Dio2, and Cox7a1 were decreased compared to WT mice. Histologically, there are increased lipid stores in brown and subcutaneous adipose tissues of γδ- and IL-17A-deficient mice and expression of lipolysis enzymes are correspondingly impaired (67). Interestingly, of the immune cells present in brown and subcutaneous adipose tissue, γδ T cells are the dominant source of IL-17A, further bolstering their contribution during thermogenic responses. Together, the data suggest that γδ T cells and IL-17A play important regulatory roles at thermoneutrality and are key for mobilizing lipid stores for substrate utilization and thermogenic induction after cold.
IL-33 is critical for maintaining body temperature during cold challenge. Mechanistically, IL-33 signals for proper UCP1 splicing and mitochondrial function in newborns and adults, respectively (91). In addition to regulating IL-33 levels in visceral adipose tissue, γδ T cells were shown to affect IL-33 levels in subcutaneous and brown adipose depots (67). Mice lacking γδ T cells and IL-17A exhibit decreased IL-33 levels at thermoneutrality and after cold challenge in adipose sites important for heat generation. TNF and IL-17A appear to be sufficient to upregulate thermogenic genes and IL-33 levels in subcutaneous adipose tissue stromal cells, highlighting another γδ-IL17-stromal axis to regulate adipose tissue function, this time for body temperature regulation. There are many outstanding questions that remain regarding the nature by which γδ T cells sense or get activated in response to temperature fluctuations. These questions include the identity of the natural ligands that stimulate γδ T cells after cold exposure, and if those ligands activate γδ T cells in a TCR-dependent manner. It is intriguing to consider if γδ T cells evolved as an innate cell type to help regulate body temperature during cold and induce febrile responses as a protective mechanism against pathogens. Moreover, a more detailed understanding of how IL-17A acts on local stromal cells and adipocytes in subcutaneous and brown adipose depots to boost adaptive thermogenesis is warranted. It is not known if adipocytes or stromal cells in brown and subcutaneous depots express IL-17R, or if brown and beige adipocytes are affected in IL-17A-deficient mice at steady state of after cold exposure. Nor is the critical IL-33-expressing cell type in brown and subcutaneous adipose tissue that is important for proper UCP1 expression and function known. Despite these mysteries, initial characterizations of IL-17A producing γδ T cells pave the way for future work investigating innate T cells in body temperature regulation.
Conclusions: Mechanistic roles of iNKT cells and γδ T cells in adipose tissue homeostasis
Far from being only a site for fat storage, adipose tissue acts as a dynamic endocrine and immune organ, where visceral, subcutaneous, and brown adipose depots are spatially and differentially regulated. We now appreciate that each site harbors a unique immune system that carries out a wide variety of functions to promote metabolic fitness and tissue homeostasis. iNKT cells and γδ cells, two innate T lymphocytes, have emerged as key regulators of immune homeostasis in visceral adipose tissue, and rheostats controlling body temperature in response to environmental fluctuations in subcutaneous and brown adipose depots (Figure 2). iNKT cells control adipose Tregs and macrophage frequencies through IL-2 and IL-10 production, respectively, while γδ T cells influence stromal cell expression of IL-33 through IL-17A production, and this in turn affects ST2+ Treg numbers in visceral adipose tissue. By producing different sets of cytokines, iNKT and γδ T cells are both able to support adipose Tregs at adolescence and with age, respectively. It should be noted that some studies found that adipose iNKT cells drive proinflammatory, pathogenic immune responses during obesity (92–94). These differences have not yet been reconciled, but identifying the mechanisms behind them may reveal further insights into iNKT cell biology and how tissue-specific cues can drive different cell phenotypes.
Figure 2. Non-redundant actions of iNKT and γδ T cells promote adipose tissue homeostasis.
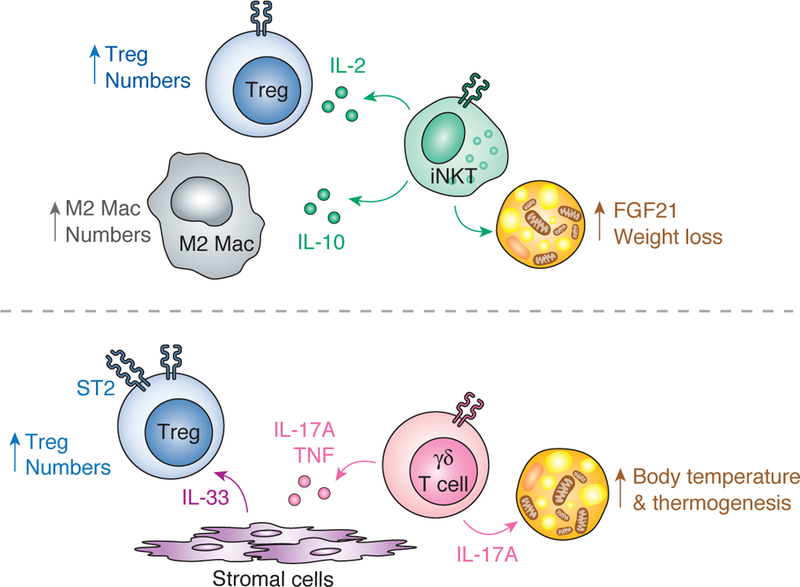
(Top) Adipose iNKT cells expands Tregs via production of IL-2 and M2 macrophages via production of IL-10. Furthermore, activation of adipose iNKT cells induces weight loss and thermogenesis through an FGF21-dependent mechanism. (Bottom) IL-17A-producing γδ T cells in adipose tissue drive Treg expansion by inducing stromal cells to produce IL-33. Additionally, a γδ – IL-17A axis in adipose tissue is critical for maintenance of body temperature at thermoneutrality and upon cold challenge.
More remarkably, recent data highlights the surprising role of iNKT and γδ T cells in non-shivering thermogenesis. iNKT cells when activated with αGalCer induce FGF21 production by adipocytes and promote UCP1-driven increases in body temperature. Conversely, γδ T cells that are enriched in subcutaneous and brown adipose tissue make IL-17A and are important for maintaining proper body temperature control at thermoneutrality and after cold challenge. Together, these studies draw attention to the enriched localization and non-redundant roles of both resident innate T cell populations in regulating adipose tissue biology, as well as their dynamic and complementary function. Future work investigating non-infectious, tissue-specific roles of iNKT and γδ T cells (and possibly MAIT cells) is promising for understanding basic adipose tissue biology and function and systemic metabolism.
Acknowledgments
Funding: M.B.B. is supported by US National Institutes of Health grant# R01 AI113046 and R01 AI063428. N.M.L. is supported by US National Institutes of Health grant# 1F31 AI138353-01 and A.C.K. is supported by T32 AR007530.
References
- 1.Mathis D 2013. Immunological Goings-on in Visceral Adipose Tissue. Cell Metab. 17: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kammoun HL, Kraakman MJ, and Febbraio MA. 2013. Adipose tissue inflammation in glucose metabolism. Rev. Endocr. Metab. Disord. 15: 31–44. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, Bodzin JL, and Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, DelProposto JB, Westcott DJ, and Saltiel AR. 2008. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57: 3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M. Leibel RL, and Ferrante AW Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, and Nagai R. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15: 914–920. [DOI] [PubMed] [Google Scholar]
- 7.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, and Engleman EG. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthorn WP and Sethi JK. 2008. TNF‐α and adipocyte biology. FEBS Letters 582: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, and Reilly MP. 2009. Interferon γ attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J. Biol. Chem. 284: 31936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, Hunter L, and Bing C. 2014. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 307: E289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Müller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack C, and Netea MG. 2010. The Inflammasome-Mediated Caspase-1 Activation Controls Adipocyte Differentiation and Insulin Sensitivity. Cell Metab. 12: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, and Dixit VD. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregor MF and Hotamisligil GS. 2011. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29: 415–45. [DOI] [PubMed] [Google Scholar]
- 14.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, and Exley MA. 2012. Adipose Tissue Invariant NKT Cells Protect against Diet-Induced Obesity and Metabolic Disorder through Regulatory Cytokine Production. Immunity 37: 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molofsky AB, Nussbaum JC, Liang HE, Dyken SJ, Cheng LE, Mohapatra A, Chawla A, and Locksley RM. 2013. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp Med. 210: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan A, Dold C, O’Connor D, Stutte S, Tavakkoli A, Winters D, Exley MA, O’Shea D, Brenner MB, von Andrian U, and Lynch L. 2017. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity 46: 273–286. [DOI] [PubMed] [Google Scholar]
- 17.Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé D, Shimada T, Kamei N, Park H-S, Sasorith S, Woo J, You J, Mosher W, Brady HJ, Shoelson SE, and Lee J. 2016. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 23: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, and Sun JC. 2016. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity 45: 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Silva J, Allatif O, Rossjohn J, Kjer-Nielsen L, McCluskey J, Ledoux S, Genser L, Torcivia A, Soudais C, Lantz O, Boitard C, Aron-Wisnewsky J, Larger E, Clément K, and Lehuen A. 2015. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J. Clin. Invest. 125: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori L, Lepore M, and De Libero G. 2016. The Immunology of CD1- and MR1-Restricted T Cells. Annu. Rev. Immunol. 34: 479–510. [DOI] [PubMed] [Google Scholar]
- 21.Brennan PJ, Brigl M, and Brenner MB. 2013. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13: 101–117. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, and Van Kaer L. 2004. NKT cells: what’s in a name? Nat. Rev. Immunol. 4: 231–237. [DOI] [PubMed] [Google Scholar]
- 23.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, and Taniguchi M. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278: 1626–1629. [DOI] [PubMed] [Google Scholar]
- 24.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, and Brenner MB. 2011. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant’Angelo DB. 2008. The BTB–zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage AK , Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, and Bendelac A. 2008. The Transcription Factor PLZF Directs the Effector Program of the NKT Cell Lineage. Immunity 29: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlgruber AC, Donado CA, LaMarche NM, Brenner MB, and Brennan PJ. 2016. Activation strategies for invariant natural killer T cells. Immunogenetics 68: 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, Zhao M, Self K, Teyton A, Everett C, Kronenberg M, Zajonc DM, Bendelac A, Savage PB, and Teyton L. 2014. The Identification of the Endogenous Ligands of Natural Killer T Cells Reveals the Presence of Mammalian α-Linked Glycosylceramides. Immunity 41: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan PJ, Tatituri RV, Heiss C, Watts GF, Hsu FF, Veerapen N, Cox LR, Azadi P, Besra GS, and Brenner MB. 2014. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc. Natl. Acad. Sci. USA 111: 13433–13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, and Brenner MB. 2011. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12: 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benlagha K, Weiss A, Beavis A, Teyton L, and Bendelac A. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191: 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, and Kronenberg M. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192: 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, and Hogquist KA. 2015. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity 43: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, and Hogquist KA. 2013. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vantourout P, and Hayday A. 2013. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison JP and Havran WL. 1991. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu. Rev. Immunol 9: 679–705. [DOI] [PubMed] [Google Scholar]
- 37.Turchinovich G, and Hayday AC. 2011. Skint-1 Identifies a Common Molecular Mechanism for the Development of Interferon-γ-Secreting versus Interleukin-17-Secreting γδ T Cells. Immunity 35: 59–68. [DOI] [PubMed] [Google Scholar]
- 38.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, and Lifton RP. 2008. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis J, Girardi M, Roberts SJ, Barbee SD, Hayday AC, and Tigelaar RE. 2006. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7: 843–850. [DOI] [PubMed] [Google Scholar]
- 40.Heilig JS and Tonegawa S. 1986. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322: 836–840. [DOI] [PubMed] [Google Scholar]
- 41.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, and Tonegawa S. 1990. Homing of a γδ thymocyte subset with homogeneous T-cellreceptors to mucosal epithelia. Nature 343: 754–757. [DOI] [PubMed] [Google Scholar]
- 42.Paget C, Chow MT, Gherardin NA, Beavis PA, Uldrich AP, Duret H, Hassane M, Souza-Fonseca-Guimaraes F, Mogilenko DA, Staumont-Sallé D, Escalante NK, Hill GR, Neeson P, Ritchie DS, Dombrowicz D, Mallevaey T, Trottein F, Belz GT, Godfrey DI, and Smyth MJ. 2014. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol. Cell Biol. 93: 198–212. [DOI] [PubMed] [Google Scholar]
- 43.Chien YH, Zeng X, and Prinz I. 2013. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. 34: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roark CL, Simonian PL, Fontenot AP, Born WK, and O’Brien RL. 2008. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 20: 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O’Brien RL, and Fontenot AP. 2009. IL-17A-Expressing T Cells Are Essential for Bacterial Clearance in a Murine Model of Hypersensitivity Pneumonitis. J. Immunol. 182: 6540–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aono A, Enomoto H, Yoshida N, Yoshizaki K, Kishimoto T, and Komori T. 2000. Forced expression of terminal deoxynucleotidyl transferase in fetal thymus resulted in a decrease in γδ T cells and random dissemination of Vγ3Vδ1 T cells in skin of newborn but not adult mice. Immunology 99: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, and Tonegawa S. 1989. Junctional sequences of T cell receptor γδ genes: Implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell 59: 859–870. [DOI] [PubMed] [Google Scholar]
- 48.Xiong N and Raulet DH. 2007. Development and selection of γδ T cells. Immunol Rev 215: 15–31. [DOI] [PubMed] [Google Scholar]
- 49.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, and von Boehmer H. 2009. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of γδ T cells with restricted TCR diversity. Proc. Natl. Acad. Sci. USA 106: 12453–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Mombaerts P, Tonegawa S, Yamamoto H, and Itohara S. 1995. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 92: 6147–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felices M, Yin CC, Kosaka Y, Kang J, and Berg LJ. 2009. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proc. Natl. Acad. Sci. USA 106: 8308–8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramírez-Valle F, Gray EE, and Cyster JG. 2015. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17–driven responses. Proc. Natl. Acad. Sci. USA 112: 8046–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akitsu A, Ishigame H, Kakuta S, Chung SH, Ikeda S, Shimizu K, Kubo S, Liu Y, Umemura M, Matsuzaki G, Yoshikai Y, Saijo S, and Iwakura Y. 2015. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2+Vγ6+γδ T cells. Nat Commun 6: 7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, and Silva-Santos B. 2014. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl. Acad. Sci. USA 111: E3562–E3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, and Mills KH. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341. [DOI] [PubMed] [Google Scholar]
- 56.Leger A, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, Raychaudhuri K, Gadjeva M, Iwakura Y, Lionakis MS, and Caspi RR. 2017. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells. Immunity 47: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, and O’Farrelly C. 2009. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 39: 1893–1901. [DOI] [PubMed] [Google Scholar]
- 58.Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, Boon L, Nieuwenhuis EE, Elewaut D, Prakken B, Kersten S, Boes M, and Kalkhoven E. 2012. Natural killer T cells in adipose tissue prevent insulin resistance. J. Clin. Invest. 122: 3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch L, Michelet X, Zhang S, Brennan P, Moseman A, Lester C, Besra G, Vomhof-Dekrey E, Tighe M, Koay H-F, Godfrey D, Leadbetter E, Sant’Angelo D, von Andrian U, and Brenner M. 2015. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol.16: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, Brenner MB, Monach P, Shinton SA, Hardy RR, Jianu R, Koller D, Collins J, Gazit R, Garrison BS, Rossi DJ, Narayan K, Sylvia K, Kang J, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Best AJ, Knell J, Goldrath A, Jojic V, Koller D, Shay T, Regev A, Cohen N, Brennan P, Brenner M, Kreslavsky T, Bezman NA, Sun J, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Kim F, Rao TN, Wagers A, Heng T, Painter M, Ericson J, Davis S, Ergun A, Mingueneau M, Mathis D, and Benoist C. 2012. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat. Immunol. 14: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, Mock D, Vijayanand P, and Kronenberg M. 2016. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat. Immunol. 17: 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim EY, Lynch L, Brennan PJ, Cohen NR, and Brenner MB. 2015. The transcriptional programs of iNKT cells. Semin. Immunol. 27: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Xue R, Zhu S, Fu S, Chen Z, Zhou R, Tian Z, and Bai L. 2017. M2-specific reduction of CD1d switches NKT cell-mediated immune responses and triggers metaflammation in adipose tissue. Cell Mol Immunol. doi: 10.1038/cmi.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, and Butcher EC. 2010. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol. 185: 6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta P, Nuotio‐Antar AM, and Smith CW. 2015. γδ T cells promote inflammation and insulin resistance during high fat diet‐induced obesity in mice. J. Leuk. Biol. 97: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei YL, Han A, Glanville J, Fang F, Zuniga LA, Lee JS, Cua DJ, and Chien YH. 2015. A Highly Focused Antigen Receptor Repertoire Characterizes γδ T Cells That are Poised to Make IL-17 Rapidly in Naive Animals. Front. Immunol. 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, Banks A, Shay T, Brenner MB, and Lynch L. 2018. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19: 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ribot JC , deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, and Silva-Santos B. 2009. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17–producing γδ T cell subsets. Nat. Immunol. 10: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, and Mathis D. 2015. Antigen- and Cytokine-Driven Accumulation of Regulatory T Cells in Visceral Adipose Tissue of Lean Mice. Cell Metab. 21: 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, and Mathis D. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, and Mathis D. 2012. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panduro M, Benoist C, and Mathis D. 2016. Tissue Tregs. Annu. Rev. Immunol. 34: 609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, and Locksley RM. 2015. Interleukin-33 and Interferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 43: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, and Kallies A. 2015. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16: 276–285. [DOI] [PubMed] [Google Scholar]
- 75.Molofsky AB, Savage AK, and Locksley RM. 2015. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 42: 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson-Jones LH , Duncan SM, Magalhaes MS, Campbell SM, Maizels RM, McSorley HJ, Allen JE, and Bénézech C. 2016. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat. Commun. 7: 12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang SK, Kohlgruber AC, Mizoguchi F, Michelet X, Wolf BJ, Wei K, Lee PY, Lynch L, Duquette D, Ceperuelo-Mallafré V, Banks AS, and Brenner MB. 2017. Stromal cell cadherin-11 regulates adipose tissue inflammation and diabetes. J. Clin. Invest. 127: 3300–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, and Girard JP. 2012. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33–LacZ Gene Trap Reporter Strain. J. Immunol. 188: 3488–3495. [DOI] [PubMed] [Google Scholar]
- 79.Cannon B, and Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359. [DOI] [PubMed] [Google Scholar]
- 80.Nicholls DG . 2001. A history of UCP1. Biochem. Soc. Trans. 29: 751–755. [DOI] [PubMed] [Google Scholar]
- 81.Bertholet AM, Kazak L, Chouchani ET, Bogaczyńska MG, Paranjpe I, Wainwright GL, Bétourné A, Kajimura S, Spiegelman BM, and Kirichok Y. 2017. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 25: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu G, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi S, and Spiegelman BM. 2015. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 163: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, and Artis D. 2015. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, and Spiegelman BM. 2014. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 157: 1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L, Wang Y, Wong CM, and Xu A. 2017. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 26: 493–508. [DOI] [PubMed] [Google Scholar]
- 86.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley R, and Chawla A. 2015. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, and Chawla A. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Shalom H, Kuperman Y, Kalchenko V, Brandis A, David E, Segal-Hayoun Y, Chappell-Maor L, Yaron A, and Jung S. 2017. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol.18: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch L, Hogan AE, Duquette D, Lester C, Banks A, LeClair K, Cohen DE, Ghosh A, Lu B, Corrigan M, Stevanovic D, Maratos-Flier E, Drucker DJ, O’Shea D, and Brenner M. 2016. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab. 24: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, Holland WL, Kliewer SA, and Mangelsdorf DJ. 2017. FGF19, FGF21, and an FGFR1/β-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 26: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Odegaard JI, Lee MW, Sogawa Y, Bertholet AM, Locksley RM, Weinberg DE, Kirichok Y, Deo RC, and Chawla A. 2016. Perinatal Licensing of Thermogenesis by IL-33 and ST2. Cell 166: 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoé K, and Tsutsui H. 2010. Natural Killer T Cells Are Involved in Adipose Tissues Inflammation and Glucose Intolerance in Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 30: 193–199. [DOI] [PubMed] [Google Scholar]
- 93.Satoh M, Hoshino M, Fujita K, Iizuka M, Fujii S, Clingan CS, Kaer L, and Iwabuchi K. 2016. Adipocyte-specific CD1d-deficiency mitigates diet-induced obesity and insulin resistance in mice. Sci. Rep. 6: 28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH, and Kaer L. 2012. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. USA 109: E1143–E1152. [DOI] [PMC free article] [PubMed] [Google Scholar]


