Abstract
The number of patients receiving hematopoietic stem cell transplantation (HSCT) is rapidly rising worldwide. Despite substantial improvements in peri-transplant care, pulmonary complications resulting in respiratory failure remain a major contributor to morbidity and mortality in the post-transplant period, and represent a major barrier to the overall success of HSCT. Infectious complications include pneumonia due to bacteria, viruses, and fungi, and most commonly occur during neutropenia in the early post-transplant period. Non-infectious complications include idiopathic pneumonia syndrome, peri-engraftment respiratory distress syndrome, diffuse alveolar hemorrhage, pulmonary veno-occlusive disease, delayed pulmonary toxicity syndrome, cryptogenic organizing pneumonia, bronchiolitis obliterans syndrome, and post-transplant lymphoproliferative disorder. These complications have distinct clinical features and risk factors, occur at differing times following transplant, and contribute to morbidity and mortality.
Keywords: Respiratory failure, Pulmonary complications, Hematopoietic stem cell transplantation, Stem cell transplant, Immunocompromised host
Core tip: Respiratory failure in the hematopoietic stem cell transplant recipient is common and is a major contributor of morbidity, mortality, and healthcare utilization. Etiology may be infectious or non-infectious in nature, and in some cases these may coexist. While identification remains challenging, infectious and non-infectious syndromes have distinct clinical features and risks.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is increasingly utilized worldwide for definitive treatment of hematologic malignancy and other conditions, with over 50000 transplants performed annually[1]. During HSCT, patients undergo high dose conditioning chemotherapy and/or radiation therapy with a view to eradicate their immune system along with any residual malignant cells. Stem cells are collected beforehand and are administered after conditioning is complete to reconstitute the immune system. HSCT may be autologous (where the donor stem cells are the patient’s own) or allogeneic (where the donor stem cells are from an appropriately matched donor).
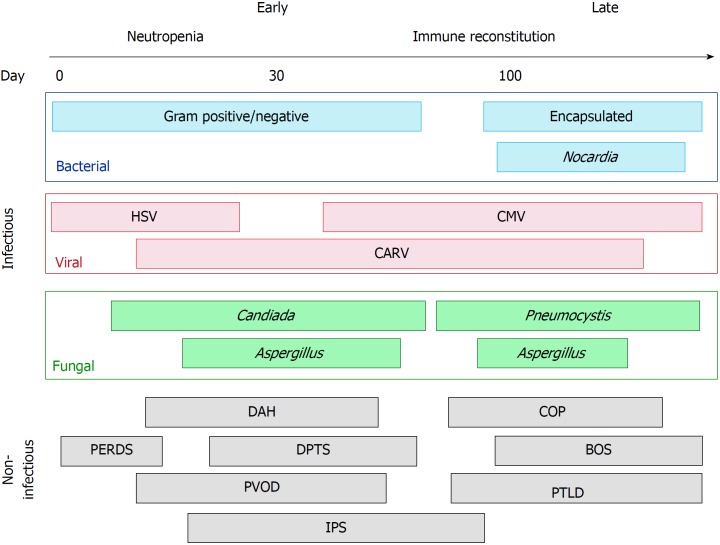
The post-transplantation period is temporally separated into three phases and represents a dynamic, individualized spectrum of risk (Figure 1). The first phase is the pancytopenic phase immediately following transplantation, typically lasting 10-21 d following HSCT. Autologous transplant recipients typically engraft before allogeneic, and several peri-transplant factors such as peripheral stem cell harvest and the use of granulocyte stimulating factors in the post-transplant period promote earlier marrow recovery. The second phase occurs after neutrophil engraftment, once the absolute neutrophil count consistently exceeds 500 cells per mm3. The second phase typically lasts for the first 100 or so days following transplantation. The third phase can be considered “late” complications of transplantation, occurring more often in allogeneic transplantation where graft-versus-host effects have pulmonary manifestations. Pulmonary complications and respiratory failure are common, occurring in up to two-thirds of HSCT recipients, and are associated with significant morbidity and mortality[2-4]. These pulmonary complications can be characterized by the phase of the post-transplant period when they are most likely to occur (Figure 1). The purpose of this mini-review is to highlight the infectious and non-infectious sources of respiratory failure in the HSCT recipient.
Figure 1.
Time-course of pulmonary complications following hematopoietic stem cell transplantation. BOS: Bronchiolitis obliterans syndrome; CARV: Community-acquired respiratory viruses; CMV: Cytomegalovirus; COP: Cryptogenic organizing pneumonia; DAH: Diffuse alveolar hemorrhage; DPTS: Delayed pulmonary toxicity syndrome; HSV: Herpes simplex virus; IPS: Idiopathic pneumonia syndrome; PERDS: Peri-engraftment respiratory distress syndrome; PTLD: Post-transplant lymphoproliferative disorder; PVOD: Pulmonary veno-occlusive disease.
INITIAL APPROACH IN THE ACUTELY ILL PATIENT
Respiratory failure following HSCT presents on a spectrum of severity. Several aspects of the clinical presentation provide clues about possible etiologies: acute versus subacute, early post-HSCT or late post-HSCT, diffuse versus focal. A substantial number of patients on the more severe end of this spectrum present with acute hypoxemic respiratory failure and diffuse pulmonary infiltrates, meeting criteria for the acute respiratory distress syndrome (ARDS)[2]. While the underlying etiology is often not known at the time of presentation, the principles of ARDS management and prevention are equally valid in this population. Specifically, this includes lung-protective mechanical ventilation with low tidal volume strategies, appropriate recruitment, and use of neuromuscular blockade where appropriate[5-7]. In addition, there should be a focus on preventing iatrogenic “second-hits” through judicious fluid and blood product administration, aspiration precautions, and early focus on mobilization and ventilator liberation[7-10]. These lung injury prevention guidelines have been conceptualized into the Checklist for Lung Injury Prevention, which was recently implemented as part of an ARDS prevention clinical trial[7,11]. Patients with pre-existing pulmonary disease are more susceptible to pulmonary complications, particularly those receiving high dose radiation to the lungs as part of their conditioning program[12,13]. Concurrently, patients should be evaluated for possible etiologies for their presentation. These can be divided broadly into infectious and non-infectious causes.
INFECTIOUS RESPIRATORY FAILURE
Infectious pulmonary complications are most common in the immediate post-transplant period during neutropenia. Recipients of allogeneic HSCT are typically more prone to infectious pulmonary complications due to a longer period of neutropenia and the need for immunosuppressant medication administration to prevent graft-versus-host disease[14]. Routine infectious prophylaxis during neutropenia has dramatically reduced the burden of infectious complications. However, breakthrough infections can occur from a variety of causative organisms and vary dependent on patient and transplant characteristics, and time elapsed following transplant (Figure 1)[3].
Bacterial
Bacterial pneumonias most commonly occur in the early transplant period[15]. Risk for bacterial pneumonias in allotransplants is greater if myeloablative (as opposed to non-myeloablative or reduced intensity) conditioning is used, the patient has graft-versus-host disease, there is delayed engraftment and a prolonged period of neutropenia, or if there are indwelling devices[16-18]. In the early post-transplant period, gram-negative organisms such as Pseudomonas aeruginosa and Klebsiella pneumoniae should be suspected, whereas encapsulated organisms are a concern late after HSCT[19]. When patients develop hypoxemic respiratory failure and new pulmonary infiltrates following HSCT, infection is typically presumed. This approach is reasonable given the substantial mortality associated with delayed antimicrobial therapy in immunocompromised patients. Ideally, microbiological sampling from bronchoalveolar lavage (BAL) is preferred, although the risk and benefits of invasive sampling need to be individually assessed. If patients are on antibacterial infectious prophylaxis when pneumonia is suspected, antibacterial agents should be broadened to cover nosocomial pathogens[20,21].
Certain infectious syndromes are worthy of additional discussion. Encapsulated bacteria, particularly Streptococcus pneumoniae, should be suspected later following HSCT, most commonly after 6 mo[22]. Invasive pneumococcal disease has been reported to be 30 times more prevalent in HSCT recipients compared to the general population[15], and up to 88% of cases have bacteremia[23]. Nocardia pneumonia can occur in the late post-transplant period, usually after 6 mo[24]. While nocardial infection is uncommon after HSCT, it should be suspected in non-responders to initial antimicrobial therapy. Sulfamethoxazole-trimethoprim is the treatment of choice and response to therapy is typically robust[24,25]. Routine use of sulfamethoxazole-trimethoprim for Pneumocystis prophylaxis does not adequately protect against nocardiosis. Mycobacterial pneumonia is rare, but can occur in the late post-transplant period, and typically presents one year after HSCT[26,27]. Incidence of Mycobacteria tuberculosis among HSCT recipients is higher in endemic areas and those receiving allogeneic grafts[27]. Presentation and management of these infections and non-tuberculous Mycobacteria are similar to that of the general population[27,28].
Viral
Herpes simplex virus (HSV) infection is relatively uncommon following HSCT due to routine infectious prophylaxis with acyclovir[29]. HSV pneumonia typically occurs in the early post-transplant period and is a result of latent reactivation (Figure 1). Allotransplants receiving grafts from seropositive donors and those with graft-versus-host disease are at increased risk of HSV[29,30]. Diagnosis of HSV pneumonia can be challenging since low-grade HSV reactivation and viral shedding is not uncommon in critical illness, and qualitative polymerase chain reaction (PCR) on BAL samples is exquisitely sensitive.
Cytomegalovirus (CMV) pneumonia occurs in up to 30% of allotransplants and typically presents after engraftment until around 4 mo (Figure 1)[31,32]. It occurs most commonly when a seropositive allograft recipient receives a seronegative transplant. Pulmonary imaging findings are nonspecific, typically bilateral and diffuse, with both alveolar and nodular opacities[33]. BAL fluid should be analyzed to confirm the presence of CMV by PCR (most common), shell assay, or viral culture. Again, low grade CMV shedding is not uncommon in critical illness and doesn’t necessarily indicate pneumonitis. Definitive diagnosis requires demonstration of tissue involvement on lung biopsy[34], but this is rarely performed. In the presence of CMV in BAL and a compatible clinical/radiographic picture, supportive evidence of widespread CMV reactivation is usually needed before initiation of treatment. Elevated and escalating quantitative serum PCR, or evidence of CMV involvement in other organs (e.g. gut, CNS) all support systemic CMV infection. Ganciclovir is the treatment of choice for invasive CMV disease, though treatment can be limited by leukopenia, particularly problematic among the HSCT population[35]. The epidemiology of post-HSCT CMV pneumonitis may change if novel CMV prophylactic agents are routinely administered[36].
The community-acquired respiratory viruses (CARV) including influenza virus, parainfluenza virus, respiratory syncytial virus (RSV), adenovirus, rhinovirus, enterovirus, and coronavirus, can occur during the entire post-transplant period (Figure 1)[37]. Diagnosis occurs most commonly by nasal PCR-amplification assays, or with BAL. RSV is the most commonly isolated CARV, and is estimated to be recovered in up to a third of patients undergoing HSCT in the first three years[37-39]. In addition to hypoxia, patients typically present with fever, productive cough, and dyspnea[37,40]. Chest imaging findings include diffuse patchy alveolar opacities[40]. RSV in the HSCT population is highly morbid and has mortality rates reported up to 80%. Beyond supportive care no specific therapy has shown consistent benefit. Given the high mortality rates in HSCT recipients, high RSV titer immune globulin or aerosolized ribavirin may be considered[41].
Fungal
Pulmonary aspergillosis effects up to two-thirds of HSCT recipients, although incidence is declining with routine anti-Aspergillus prophylaxis during neutropenia and more effective treatment of graft-versus-host disease[42-44]. Pulmonary aspergillosis has been reported in upwards of 30% of HSCT recipients[3,42]. Risk factors include allogeneic transplant, unrelated donors, prolonged neutropenia, immunosuppressant use for graft-versus-host disease, and CMV infection[45-47]. Most common findings radiologically include pulmonary nodules with or without halo sign, ground glass opacities, and an air crescent sign from necrotic tissue in advanced cases[47-49]. Hemoptysis can be present and is typically associated with poor prognosis[50-52]. Diagnosis is confirmed by Aspergillus-specific PCR or Aspergillus sp. antigen in BAL[53,54]. Monotherapy with isavuconazole or voriconazole is the preferred first-line treatment and therapeutic drug monitoring should be utilized to ensure adequacy of dosing[55]. Severe cases refractory to medical therapy or recurrent hemoptysis may be considered for surgical evaluation, though lung resection is highly morbid and associated with significant mortality in this population[56].
Incidence of Pneumocystis jirovecii pneumonia (PCP) has marginally declined in recent years as the use of prophylaxis has increased[57,58]. However, there is limited guidance and no consensus on which patients outside of HIV-positive individuals should receive prophylaxis, and therefore PCP remains highly relevant in HSCT recipients. Our institution routinely implements prophylaxis from engraftment until the first 100 d (or longer if patients are immunosuppressed for graft-versus host disease). PCP occurs late after HSCT and presents with acute onset severe respiratory failure[58-60]. Diagnosis is confirmed by the identification of Pneumocystis organisms in respiratory samples by PCR or fungal smear[58,61]. Sulfamethoxazole-trimethoprim is the treatment of choice and is highly effective in killing Pneumocystis sp[58]. Patients with PCP typically die due to refractory hypoxemia from severe respiratory failure, and corticosteroids have failed to demonstrate benefit outside of the HIV population[62,63]. Nonetheless, adjunctive corticosteroids are typically administered in individuals with HSCT who develop PCP.
NON-INFECTIOUS RESPIRATORY FAILURE
Noninfectious respiratory failure syndromes are common throughout the entire post-HSCT period, and our understanding of them remains incomplete. The risks of these syndromes vary based on transplant type, and a variety of modifiable and non-modifiable transplant and patient characteristics. In addition to key distinguishing clinical criteria, non-infectious complications are categorized by when they occur temporally following HSCT (Figure 1). Often infection cannot be ruled out at the time of initial presentation and should be concurrently treated given the substantial mortality associated with delayed antimicrobial administration.
Peri-engraftment respiratory distress syndrome
The peri-engraftment respiratory distress syndrome (PERDS) is a pulmonary subset of the engraftment syndrome, a systemic capillary leak disorder that develops around the time of immune system reconstitution early after autologous HSCT (Figure 1)[64]. PERDS is defined as hypoxemic respiratory failure and bilateral pulmonary infiltrates that occur in the 5 d surrounding neutrophil engraftment, not fully explained by cardiac dysfunction or infection.
Focused studies of PERDS patients found an incidence of nearly 5% in autotransplants[65,66]. Case-fatality rates in excess of 20% nearly two decades ago have substantially reduced to 6% in the current era[65,66]. Risk factors include female gender, blood product administration, rapid engraftment, and HSCT for the POEMS syndrome. We recently found radiographic changes consistent with lung injury precede neutrophil engraftment and may aid in early identification of the syndrome[66]. Treatment consists of short courses of high dose corticosteroids, most commonly 1 to 2 mg/kg methylprednisolone twice daily for 3 d, followed by a rapid taper[65,67]. Response is typically prompt with improvements in oxygenation in most within 24 h of steroid initiation.
Diffuse alveolar hemorrhage
Diffuse alveolar hemorrhage (DAH) is a syndrome characterized by diffuse, bilateral pulmonary infiltrates, progressively bloody return during BAL, and presence of > 20% hemosiderin-laden macrophages in alveolar lavage fluid[64]. While hemoptysis can be seen, it is often absent[68]. DAH mainly occurs during the early post-transplant period (Figure 1).
DAH occurs in 5%-12% of HSCT recipients and is highly morbid with reported mortality rates as high as 60% to 100%[68-72]. Risk factors include age over 40 years, higher intensity conditioning therapies, total body irradiation, and HSCT for acute leukemia and myelodysplastic syndrome[69,70,73]. Our understanding of DAH following HSCT is limited. While some cases of alveolar hemorrhage occur during the thrombocytopenic period following transplant, many cases occur after platelet counts are adequate. Also, while DAH may occur in the setting of ARDS or pneumonia, some DAH cases occur in the absence of both.
Treatment of DAH consists of high-dose corticosteroids, most commonly 500 to 1000 mg methylprednisolone per day for 5 d[70,72,74-76]. While one study showed improved survival in 8 patients treated with anti-fibrinolytic aminocaproic acid[70], a subsequent larger study failed to show benefit[75]. Further, even in the presence of thrombocytopenia, platelet transfusion did not affect morbidity or mortality in DAH[68].
Idiopathic pneumonia syndrome
Idiopathic pneumonia syndrome (IPS) is an umbrella term for widespread alveolar injury occurring in the absence of cardiac or renal dysfunction, iatrogenic-induced circulatory overload, and infection[64]. Symptoms are consistent with ARDS and pulmonary imaging typically reveals diffuse, bilateral pulmonary infiltrates. There are many similarities and overlap in the clinical presentation of IPS and other non-infectious complications discussed in this review. Those conditions have key distinguishing features and are therefore discussed separately.
IPS effects up to 10% of HSCT recipients, more so allotransplants, and typically occurs during the early post-transplant period (Figure 1)[64]. Mortality is as high as 80% and even greater in those requiring respiratory support with the mechanical ventilator[45,64]. Risk factors include higher intensity conditioning therapies, radiation administration, allogeneic transplant, age, and the presence of graft-versus-host disease.
Treatment of IPS is controversial, and no therapy has shown favorable outcome. Corticosteroids may be administered, though while some studies have shown benefit[45,77], others have not[78,79]. When given, higher doses (4 mg/kg per day, prednisolone equivalent) have been shown to be no better than lower doses (2 mg/kg per day or less, prednisolone equivalent), but have the potential to carry greater risk of adverse effects[45]. There has been an ongoing interest in tumor necrosis factor (TNF)-α inhibition due to the observation that patients with IPS have cytokine-rich BAL fluid[64]. Preliminary retrospective studies have shown promise with increased response rates and improved overall survival when TNF-α inhibitor, etanercept, was added to corticosteroid therapy[80,81], though these findings were not replicated when a randomized controlled trial design as applied[82]. Further studies are needed to better phenotype what IPS truly represents, and whether any therapies can be effective.
Pulmonary veno-occlusive disease
Pulmonary veno-occlusive disease (PVOD) is a rare complication of HSCT with high associated mortality, typically occurring late after HSCT (Figure 1)[83-85]. PVOD should be suspected in those who are progressively dyspneic, have evidence of pulmonary hypertension in the absence of left heart failure, and imaging suggestive of pulmonary edema[64,83,85]. PVOD may occur in the absence of these and therefore, diagnosis must be confirmed by the presence of fibrous intimal proliferation of the pulmonary venules on open surgical lung biopsy[64,86].
Due to the low incidence of PVOD following HSCT and inability to study large numbers of cases, risk factors are extrapolated from the non-HSCT population. These include viral infections, genetic predisposition, autoimmune disorders, and toxic insult to endothelia[86]. In the context of HSCT, these insults include conditioning chemotherapies bleomycin, mitomycin, and carmustine, and irradiation[86-89]. Despite their use in primary pulmonary hypertension, pulmonary vasodilators may be detrimental in PVOD and should be avoided. Dilating the pulmonary arterial vasculature in the setting of fixed venous resistance may precipitate pulmonary edema and worsen respiratory status[86]. Corticosteroids may be administered, though data is sparse[83,86]. Overall, prognosis is poor and patients may consider evaluation for lung transplantation if eligible.
Delayed pulmonary toxicity syndrome
The delayed pulmonary toxicity syndrome (DPTS) is a constellation of interstitial pneumonitis and fibrosis occurring in the late transplant period, and can present years after HSCT[64]. Characteristically, DPTS appears to be confined to patients receiving high-dose chemotherapy followed by autologous stem cell rescue for breast cancer[90-93]. Accordingly, the incidence of DPTS in this specific population is reported to be as high as 72%[91]. Symptoms are non-specific and include dyspnea, fevers, and nonproductive cough[64]. Similarly, chest imaging reveals bilateral interstitial infiltrates and ground glass opacities. DPTS occurs late following HSCT and can present several years following transplant (Figure 1)[90-93]. The syndrome is highly responsive to corticosteroids and typically associated with favorable outcomes[91,92].
Cryptogenic organizing pneumonia
Cryptogenic organizing pneumonia (COP) is an interstitial and airspace disease with symptoms mimicking classic pneumonia. Imaging findings include nodular lesions, ground glass attenuation, and patchy peribronchovascular, peripheral, band-like consolidative distributions[64,94]. Biopsy reveals chronic alveolar inflammation and extensive granulation of the alveolar ducts and small airways[94]. Bronchoscopy is useful to distinguish COP from infectious pneumonia, and analysis of lavage fluid reveals a predominant lymphocytosis[95]. Previously referred to as bronchiolitis obliterans-organizing pneumonia, COP is a distinct entity from the bronchiolitis obliterans syndrome (BOS), which is discussed separately and should not be confused.
COP occurs in up to 10% of HSCT recipients and typically presents late following transplant (Figure 1)[94,96]. Risk factors include cyclophosphamide conditioning, total body irradiation, male allotransplants with a female cell donor, presence of graft-versus-host disease, and HSCT for leukemia[94,95,97]. Generally, COP is responsive to corticosteroid therapy and typical regimens include 1 mg/kg prednisone daily with an extended taper up to 6 mo[94]. Case fatality rates are reported up to 20%, and are usually due to respiratory failure in the setting of relapsed, steroid-refractory disease[97,98].
BOS
BOS is a slow progression of small airway obstruction believed to be a consequence of graft-versus-host disease[99]. While BOS classically manifests over months to years, abrupt decompensation and severe respiratory failure is not uncommon[100-102]. Histology will reveal intraluminal fibrosis, however yield on transbronchial biopsy is highly dependent on disease presence in the area sampled and open surgical biopsy is very high risk in this population[64,103]. Therefore in the acute setting, diagnosis is established on the basis of reduced expiratory flow with obstructive airflow and radiologic findings include hyperinflation, air trapping, and a mosaic pattern of attenuation[64,95,103].
The incidence of BOS is estimated to be up to 20% and more likely associated with the presence of chronic graft-versus-host disease[99,104,105]. Other risk factors include elder age, reduced expiratory capacity pre-transplantation, unrelated graft donor, irradiation, and viral infection post-HSCT[99,105,106]. High-dose corticosteroids administered for weeks to months are the mainstay of treatment, though response rates are poor as BOS is irreversible, and mortality rates can be as high as 40%[4,95,99,103]. Despite extensive extrapolated use from solid organ transplant patients, macrolides have shown to worsen airflow decline-free survival in HSCT recipients[107]. Other therapies with inconclusive utility include inhaled corticosteroids, intravenous immune globulin, TNF-α inhibitors, cyclosporine, and tacrolimus[4]. Extracorporeal photophoresis is a promising therapy with increasing evidence suggesting its potential benefit[108,109]. Lung transplantation for advanced BOS has been reported[110-113].
Post-transplant lymphoproliferative disorder
Post-transplant lymphoproliferative disorder (PTLD) is a rare form of malignancy secondary to Epstein Barr virus (EBV)-infected B lymphocytes occurring in the first six months following allotransplant (Figure 1)[64,114,115]. Risk factors include T-cell depleted donors, HLA donor mismatch, T-cell depleting therapies including antithymocyte globulin and anti-CD3 antibodies, and CMV antigens[114,115]. In addition to hypoxia, symptoms are consistent with viral illness, and chest imaging reveals diffuse basal and subpleural infiltrates[64,114]. Definitive diagnosis is established when EBV-associated lymphoid proliferation is demonstrated on biopsy[64,116]. Treatment includes modulation of T-cell depleting immunosuppression and administration of rituximab, an anti-B cell antibody[117,118]. Preliminary reports demonstrate promise of infusion of EBV-specific T-cells as a therapeutic for PTLD, though others have demonstrated resistance to such therapy[119].
CONCLUSION
Respiratory failure due to infectious and non-infectious complications is common following HSCT and is associated with significant mortality, especially in those necessitating mechanical ventilation. Pulmonary complications are differentiated by key distinguishing features and their time-course following transplantation. In acutely ill patients meeting ARDS criteria, routine use of best-practice lung-protective strategies is recommended even once the underlying explanation for the respiratory failure is identified.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Peer-review started: August 3, 2018
First decision: August 24, 2018
Article in press: October 10, 2018
P- Reviewer: Cascella M, Grawish ME, Lin JA, Zhao L S- Editor: Ji FF L- Editor: A E- Editor: Yin SY
Contributor Information
Patrick M Wieruszewski, Department of Pharmacy, Mayo Clinic, Rochester, MN 55905, United States; Multidisciplinary Epidemiology and Translational Research in Intensive Care Group, Mayo Clinic, Rochester, MN 55905, United States.
Svetlana Herasevich, Multidisciplinary Epidemiology and Translational Research in Intensive Care Group, Mayo Clinic, Rochester, MN 55905, United States; Department of Anesthesiology, Mayo Clinic, Rochester, MN 55905, United States.
Ognjen Gajic, Multidisciplinary Epidemiology and Translational Research in Intensive Care Group, Mayo Clinic, Rochester, MN 55905, United States; Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN 55905, United States.
Hemang Yadav, Multidisciplinary Epidemiology and Translational Research in Intensive Care Group, Mayo Clinic, Rochester, MN 55905, United States; Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN 55905, United States. yadav.hemang@mayo.edu.
References
- 1.Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, Bouzas LF, Confer D, Greinix H, Horowitz M, et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016;51:778–785. doi: 10.1038/bmt.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav H, Nolan ME, Bohman JK, Cartin-Ceba R, Peters SG, Hogan WJ, Gajic O, Kor DJ. Epidemiology of Acute Respiratory Distress Syndrome Following Hematopoietic Stem Cell Transplantation. Crit Care Med. 2016;44:1082–1090. doi: 10.1097/CCM.0000000000001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest. 2013;144:1913–1922. doi: 10.1378/chest.12-1708. [DOI] [PubMed] [Google Scholar]
- 4.Soubani AO, Pandya CM. The spectrum of noninfectious pulmonary complications following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2010;3:143–157. doi: 10.1016/s1658-3876(10)50025-6. [DOI] [PubMed] [Google Scholar]
- 5.Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Gergshengorn HB, Dinkels M, Hou P, Talmor DS, Gajic O, Gong MN, Group LIPS. Checklist for lung injury prevention (CLIP): A pilot study on implementation across multiple hospitals and multiple clinical areas. Am J Respir Crit Care Med. 2012;185:A6567. [Google Scholar]
- 8.Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med. 2017;195:725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- 9.Gong MN, Schenk L, Gajic O, Mirhaji P, Sloan J, Dong Y, Festic E, Herasevich V. Early intervention of patients at risk for acute respiratory failure and prolonged mechanical ventilation with a checklist aimed at the prevention of organ failure: protocol for a pragmatic stepped-wedged cluster trial of PROOFCheck. BMJ Open. 2016;6:e011347. doi: 10.1136/bmjopen-2016-011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 11.Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN; US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A) Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AK, Karimpour SE, Savani BN, Guion P, Hope AJ, Mansueti JR, Ning H, Altemus RM, Wu CO, Barrett AJ. Pretransplant pulmonary function tests predict risk of mortality following fractionated total body irradiation and allogeneic peripheral blood stem cell transplant. Int J Radiat Oncol Biol Phys. 2006;66:520–527. doi: 10.1016/j.ijrobp.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172:384–390. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coomes SM, Hubbard LL, Moore BB. Impaired pulmonary immunity post-bone marrow transplant. Immunol Res. 2011;50:78–86. doi: 10.1007/s12026-010-8200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar D, Humar A, Plevneshi A, Siegal D, Franke N, Green K, McGeer A; Toronto Invasive Bacterial Diseases Network. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant. 2008;41:743–747. doi: 10.1038/sj.bmt.1705964. [DOI] [PubMed] [Google Scholar]
- 16.Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996;109:1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- 17.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 18.Sirithanakul K, Salloum A, Klein JL, Soubani AO. Pulmonary complications following hematopoietic stem cell transplantation: diagnostic approaches. Am J Hematol. 2005;80:137–146. doi: 10.1002/ajh.20437. [DOI] [PubMed] [Google Scholar]
- 19.Lossos IS, Breuer R, Or R, Strauss N, Elishoov H, Naparstek E, Aker M, Nagler A, Moses AE, Shapiro M. Bacterial pneumonia in recipients of bone marrow transplantation. A five-year prospective study. Transplantation. 1995;60:672–678. doi: 10.1097/00007890-199510150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR; Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 21.Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, Herrstedt J; ESMO Guidelines Committee. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27:v111–v118. doi: 10.1093/annonc/mdw325. [DOI] [PubMed] [Google Scholar]
- 22.Olarte L, Lin PL, Barson WJ, Romero JR, Tan TQ, Givner LB, Hoffman JA, Bradley JS, Hultén KG, Mason EO, et al. Invasive pneumococcal infections in children following transplantation in the pneumococcal conjugate vaccine era. Transpl Infect Dis. 2017;19:e12630. doi: 10.1111/tid.12630. [DOI] [PubMed] [Google Scholar]
- 23.Torda A, Chong Q, Lee A, Chen S, Dodds A, Greenwood M, Larsen S, Gilroy N. Invasive pneumococcal disease following adult allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2014;16:751–759. doi: 10.1111/tid.12268. [DOI] [PubMed] [Google Scholar]
- 24.van Burik JA, Hackman RC, Nadeem SQ, Hiemenz JW, White MH, Flowers ME, Bowden RA. Nocardiosis after bone marrow transplantation: a retrospective study. Clin Infect Dis. 1997;24:1154–1160. doi: 10.1086/513654. [DOI] [PubMed] [Google Scholar]
- 25.Daly AS, McGeer A, Lipton JH. Systemic nocardiosis following allogeneic bone marrow transplantation. Transpl Infect Dis. 2003;5:16–20. doi: 10.1034/j.1399-3062.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Akan H, Arslan O, Akan OA. Tuberculosis in stem cell transplant patients. J Hosp Infect. 2006;62:421–426. doi: 10.1016/j.jhin.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Russo RL, Dulley FL, Suganuma L, França IL, Yasuda MA, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis. 2010;14 Suppl 3:e187–e191. doi: 10.1016/j.ijid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Al-Anazi KA, Al-Jasser AM, Al-Anazi WK. Infections caused by non-tuberculous mycobacteria in recipients of hematopoietic stem cell transplantation. Front Oncol. 2014;4:311. doi: 10.3389/fonc.2014.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, Ljungman P, Engelhard D; Second European Conference on Infections in Leukemia. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–770. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- 30.Przybylski M, Majewska A, Dzieciatkowski T, Rusicka P, Basak GW, Nasilowska-Adamska B, Bilinski J, Jedrzejczak WW, Wroblewska M, Halaburda K, et al. Infections due to alphaherpesviruses in early post-transplant period after allogeneic haematopoietic stem cell transplantation: Results of a 5-year survey. J Clin Virol. 2017;87:67–72. doi: 10.1016/j.jcv.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Pergam SA, Xie H, Sandhu R, Pollack M, Smith J, Stevens-Ayers T, Ilieva V, Kimball LE, Huang ML, Hayes TS, et al. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biol Blood Marrow Transplant. 2012;18:1391–1400. doi: 10.1016/j.bbmt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konoplev S, Champlin RE, Giralt S, Ueno NT, Khouri I, Raad I, Rolston K, Jacobson K, Tarrand J, Luna M, et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplant. 2001;27:877–881. doi: 10.1038/sj.bmt.1702877. [DOI] [PubMed] [Google Scholar]
- 33.Leung AN, Gosselin MV, Napper CH, Braun SG, Hu WW, Wong RM, Gasman J. Pulmonary infections after bone marrow transplantation: clinical and radiographic findings. Radiology. 1999;210:699–710. doi: 10.1148/radiology.210.3.r99mr39699. [DOI] [PubMed] [Google Scholar]
- 34.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa T. Betaherpesvirus Complications and Management During Hematopoietic Stem Cell Transplantation. Adv Exp Med Biol. 2018;1045:251–270. doi: 10.1007/978-981-10-7230-7_12. [DOI] [PubMed] [Google Scholar]
- 36.Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhäuser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370:1781–1789. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 37.Community-acquired respiratory viruses. Am J Transplant. 2004;4 Suppl 10:105–109. doi: 10.1111/j.1600-6135.2004.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavergne V, Ghannoum M, Weiss K, Roy J, Béliveau C. Successful prevention of respiratory syncytial virus nosocomial transmission following an enhanced seasonal infection control program. Bone Marrow Transplant. 2011;46:137–142. doi: 10.1038/bmt.2010.67. [DOI] [PubMed] [Google Scholar]
- 39.Chatzis O, Darbre S, Pasquier J, Meylan P, Manuel O, Aubert JD, Beck-Popovic M, Masouridi-Levrat S, Ansari M, Kaiser L, et al. Burden of severe RSV disease among immunocompromised children and adults: a 10 year retrospective study. BMC Infect Dis. 2018;18:111. doi: 10.1186/s12879-018-3002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebbert JO, Limper AH. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration. 2005;72:263–269. doi: 10.1159/000085367. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, Champlin RE, Englund J, Giralt SA, Rolston K, Raad I, Jacobson K, Neumann J, Ippoliti C, Mallik S, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25:751–755. doi: 10.1038/sj.bmt.1702228. [DOI] [PubMed] [Google Scholar]
- 42.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 43.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, Maloney DG, Deeg HJ, Martin PJ, Storb RF, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102:2777–2785. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 46.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 47.Salman N, Törün SH, Budan B, Somer A. Invasive aspergillosis in hematopoietic stem cell and solid organ transplantation. Expert Rev Anti Infect Ther. 2011;9:307–315. doi: 10.1586/eri.11.13. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho-Dias VM, Sola CB, Cunha CA, Shimakura SE, Pasquini R, Queiroz-Telles Fd. Invasive aspergillosis in hematopoietic stem cell transplant recipients: a retrospective analysis. Braz J Infect Dis. 2008;12:385–389. doi: 10.1590/s1413-86702008000500008. [DOI] [PubMed] [Google Scholar]
- 49.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Shao C, Song Y. Analysis of the clinical features of invasive bronchopulmonary aspergillosis. Clin Respir J. 2018;12:1635–1643. doi: 10.1111/crj.12722. [DOI] [PubMed] [Google Scholar]
- 51.Herbrecht R, Natarajan-Amé S, Letscher-Bru V, Canuet M. Invasive pulmonary aspergillosis. Semin Respir Crit Care Med. 2004;25:191–202. doi: 10.1055/s-2004-824903. [DOI] [PubMed] [Google Scholar]
- 52.Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983;38:572–578. doi: 10.1136/thx.38.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehrnbecher T, Robinson PD, Fisher BT, Castagnola E, Groll AH, Steinbach WJ, Zaoutis TE, Negeri ZF, Beyene J, Phillips B, et al. Galactomannan, β-D-Glucan, and Polymerase Chain Reaction-Based Assays for the Diagnosis of Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016;63:1340–1348. doi: 10.1093/cid/ciw592. [DOI] [PubMed] [Google Scholar]
- 54.Sakata KK, Klassen CL, Bollin KB, Grys TE, Slack JL, Wesselius LJ, Vikram HR. Microbiologic yield of bronchoalveolar lavage specimens from stem cell transplant recipients. Transpl Infect Dis. 2017;19:e12684. doi: 10.1111/tid.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24 Suppl 1:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Wu GX, Khojabekyan M, Wang J, Tegtmeier BR, O’Donnell MR, Kim JY, Grannis FW, Raz DJ. Survival following lung resection in immunocompromised patients with pulmonary invasive fungal infection. Eur J Cardiothorac Surg. 2016;49:314–320. doi: 10.1093/ejcts/ezv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128:573–579. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 58.Carmona EM, Limper AH. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther Adv Respir Dis. 2011;5:41–59. doi: 10.1177/1753465810380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 60.De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, Gluckman E, Socié G, Molina JM. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant. 2005;36:879–883. doi: 10.1038/sj.bmt.1705149. [DOI] [PubMed] [Google Scholar]
- 61.Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298–308. doi: 10.1038/nrmicro1621. [DOI] [PubMed] [Google Scholar]
- 62.Injean P, Eells SJ, Wu H, McElroy I, Gregson AL, McKinnell JA. A Systematic Review and Meta-Analysis of the Data Behind Current Recommendations for Corticosteroids in Non-HIV-Related PCP: Knowing When You Are on Shaky Foundations. Transplant Direct. 2017;3:e137. doi: 10.1097/TXD.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wieruszewski PM, Barreto JN, Frazee E, Daniels CE, Tosh PK, Dierkhising RA, Mara KC, Limper AH. Early Corticosteroids for Pneumocystis Pneumonia in Adults Without HIV Are Not Associated With Better Outcome. Chest. 2018;pii:S0012–3692(18)30648-2. doi: 10.1016/j.chest.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 64.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR; American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capizzi SA, Kumar S, Huneke NE, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Gastineau DA, Prakash UB, Tefferi A. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:1299–1303. doi: 10.1038/sj.bmt.1703075. [DOI] [PubMed] [Google Scholar]
- 66.Wieruszewski PM, Personett HA, Peters SG, Gajic O, Hogan WJ, Dierkhising RA, Alkhateeb H, Yadav H. The Peri-Engraftment Respiratory Distress Syndrome Following Autologous Hematopoietic Cell Transplant. Am J Respir Crit Care Med. 2018;197:A5161. [Google Scholar]
- 67.Carreras E, Fernández-Avilés F, Silva L, Guerrero M, Fernández de Larrea C, Martínez C, Rosiñol L, Lozano M, Marín P, Rovira M. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant. 2010;45:1417–1422. doi: 10.1038/bmt.2009.363. [DOI] [PubMed] [Google Scholar]
- 68.Robbins RA, Linder J, Stahl MG, Thompson AB 3rd, Haire W, Kessinger A, Armitage JO, Arneson M, Woods G, Vaughan WP. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. 1989;87:511–518. doi: 10.1016/s0002-9343(89)80606-0. [DOI] [PubMed] [Google Scholar]
- 69.Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26:539–543. doi: 10.1038/sj.bmt.1702546. [DOI] [PubMed] [Google Scholar]
- 70.Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant. 2006;12:949–953. doi: 10.1016/j.bbmt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12:1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166:1364–1368. doi: 10.1164/rccm.200208-792OC. [DOI] [PubMed] [Google Scholar]
- 73.Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166:641–645. doi: 10.1164/rccm.200112-141cc. [DOI] [PubMed] [Google Scholar]
- 74.Metcalf JP, Rennard SI, Reed EC, Haire WD, Sisson JH, Walter T, Robbins RA. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med. 1994;96:327–334. doi: 10.1016/0002-9343(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 75.Rathi NK, Tanner AR, Dinh A, Dong W, Feng L, Ensor J, Wallace SK, Haque SA, Rondon G, Price KJ, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant. 2015;50:420–426. doi: 10.1038/bmt.2014.287. [DOI] [PubMed] [Google Scholar]
- 76.Raptis A, Mavroudis D, Suffredini A, Molldrem J, Rhee FV, Childs R, Phang S, Barrett A. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999;24:879–883. doi: 10.1038/sj.bmt.1701995. [DOI] [PubMed] [Google Scholar]
- 77.Griese M, Rampf U, Hofmann D, Führer M, Reinhardt D, Bender-Götze C. Pulmonary complications after bone marrow transplantation in children: twenty-four years of experience in a single pediatric center. Pediatr Pulmonol. 2000;30:393–401. doi: 10.1002/1099-0496(200011)30:5<393::aid-ppul5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 78.Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1393–1400. doi: 10.1164/ajrccm/147.6_Pt_1.1393. [DOI] [PubMed] [Google Scholar]
- 79.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation. 1997;63:1079–1086. doi: 10.1097/00007890-199704270-00006. [DOI] [PubMed] [Google Scholar]
- 80.Tizon R, Frey N, Heitjan DF, Tan KS, Goldstein SC, Hexner EO, Loren A, Luger SM, Reshef R, Tsai D, et al. High-dose corticosteroids with or without etanercept for the treatment of idiopathic pneumonia syndrome after allo-SCT. Bone Marrow Transplant. 2012;47:1332–1337. doi: 10.1038/bmt.2011.260. [DOI] [PubMed] [Google Scholar]
- 81.Thompson J, Yin Z, D’Souza A, Fenske T, Hamadani M, Hari P, Rizzo JD, Pasquini M, Saber W, Shah N, et al. Etanercept and Corticosteroid Therapy for the Treatment of Late-Onset Idiopathic Pneumonia Syndrome. Biol Blood Marrow Transplant. 2017;23:1955–1960. doi: 10.1016/j.bbmt.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Yanik GA, Horowitz MM, Weisdorf DJ, Logan BR, Ho VT, Soiffer RJ, Carter SL, Wu J, Wingard JR, Difronzo NL, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20:858–864. doi: 10.1016/j.bbmt.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hackman RC, Madtes DK, Petersen FB, Clark JG. Pulmonary venoocclusive disease following bone marrow transplantation. Transplantation. 1989;47:989–992. doi: 10.1097/00007890-198906000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Williams LM, Fussell S, Veith RW, Nelson S, Mason CM. Pulmonary veno-occlusive disease in an adult following bone marrow transplantation. Case report and review of the literature. Chest. 1996;109:1388–1391. doi: 10.1378/chest.109.5.1388. [DOI] [PubMed] [Google Scholar]
- 85.Troussard X, Bernaudin JF, Cordonnier C, Fleury J, Payen D, Briere J, Vernant JP. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax. 1984;39:956–957. doi: 10.1136/thx.39.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandel J, Mark EJ, Hales CA. Pulmonary veno-occlusive disease. Am J Respir Crit Care Med. 2000;162:1964–1973. doi: 10.1164/ajrccm.162.5.9912045. [DOI] [PubMed] [Google Scholar]
- 87.Knight BK, Rose AG. Pulmonary veno-occlusive disease after chemotherapy. Thorax. 1985;40:874–875. doi: 10.1136/thx.40.11.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joselson R, Warnock M. Pulmonary veno-occlusive disease after chemotherapy. Hum Pathol. 1983;14:88–91. doi: 10.1016/s0046-8177(83)80052-5. [DOI] [PubMed] [Google Scholar]
- 89.Doll DC, Yarbro JW. Vascular toxicity associated with antineoplastic agents. Semin Oncol. 1992;19:580–596. [PubMed] [Google Scholar]
- 90.Cao TM, Negrin RS, Stockerl-Goldstein KE, Johnston LJ, Shizuru JA, Taylor TL, Rizk NW, Wong RM, Blume KG, Hu WW. Pulmonary toxicity syndrome in breast cancer patients undergoing BCNU-containing high-dose chemotherapy and autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2000;6:387–394. doi: 10.1016/s1083-8791(00)70015-2. [DOI] [PubMed] [Google Scholar]
- 91.Bhalla KS, Wilczynski SW, Abushamaa AM, Petros WP, McDonald CS, Loftis JS, Chao NJ, Vredenburgh JJ, Folz RJ. Pulmonary toxicity of induction chemotherapy prior to standard or high-dose chemotherapy with autologous hematopoietic support. Am J Respir Crit Care Med. 2000;161:17–25. doi: 10.1164/ajrccm.161.1.9903059. [DOI] [PubMed] [Google Scholar]
- 92.Wilczynski SW, Erasmus JJ, Petros WP, Vredenburgh JJ, Folz RJ. Delayed pulmonary toxicity syndrome following high-dose chemotherapy and bone marrow transplantation for breast cancer. Am J Respir Crit Care Med. 1998;157:565–573. doi: 10.1164/ajrccm.157.2.9705072. [DOI] [PubMed] [Google Scholar]
- 93.Todd NW, Peters WP, Ost AH, Roggli VL, Piantadosi CA. Pulmonary drug toxicity in patients with primary breast cancer treated with high-dose combination chemotherapy and autologous bone marrow transplantation. Am Rev Respir Dis. 1993;147:1264–1270. doi: 10.1164/ajrccm/147.5.1264. [DOI] [PubMed] [Google Scholar]
- 94.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–3828. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]
- 95.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Patriarca F, Skert C, Sperotto A, Damiani D, Cerno M, Geromin A, Zaja F, Stocchi R, Prosdocimo S, Fili’ C, et al. Incidence, outcome, and risk factors of late-onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplant. 2004;33:751–758. doi: 10.1038/sj.bmt.1704426. [DOI] [PubMed] [Google Scholar]
- 97.Nakasone H, Onizuka M, Suzuki N, Fujii N, Taniguchi S, Kakihana K, Ogawa H, Miyamura K, Eto T, Sakamaki H, et al. Pre-transplant risk factors for cryptogenic organizing pneumonia/bronchiolitis obliterans organizing pneumonia after hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:1317–1323. doi: 10.1038/bmt.2013.116. [DOI] [PubMed] [Google Scholar]
- 98.Afessa B, Peters SG. Chronic lung disease after hematopoietic stem cell transplantation. Clin Chest Med. 2005;26:571–586, vi. doi: 10.1016/j.ccm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 99.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan CK, Hyland RH, Hutcheon MA, Minden MD, Alexander MA, Kossakowska AE, Urbanski SJ, Fyles GM, Fraser IM, Curtis JE. Small-airways disease in recipients of allogeneic bone marrow transplants. An analysis of 11 cases and a review of the literature. Medicine (Baltimore) 1987;66:327–340. doi: 10.1097/00005792-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Palmas A, Tefferi A, Myers JL, Scott JP, Swensen SJ, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol. 1998;100:680–687. doi: 10.1046/j.1365-2141.1998.00617.x. [DOI] [PubMed] [Google Scholar]
- 102.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 103.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 104.Curtis DJ, Smale A, Thien F, Schwarer AP, Szer J. Chronic airflow obstruction in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;16:169–173. [PubMed] [Google Scholar]
- 105.Yoshihara S, Tateishi U, Ando T, Kunitoh H, Suyama H, Onishi Y, Tanosaki R, Mineishi S. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant. 2005;35:1195–1200. doi: 10.1038/sj.bmt.1704985. [DOI] [PubMed] [Google Scholar]
- 106.Chien JW, Martin PJ, Flowers ME, Nichols WG, Clark JG. Implications of early airflow decline after myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33:759–764. doi: 10.1038/sj.bmt.1704422. [DOI] [PubMed] [Google Scholar]
- 107.Bergeron A, Chevret S, Granata A, Chevallier P, Vincent L, Huynh A, Tabrizi R, Labussiere-Wallet H, Bernard M, Chantepie S, et al. Effect of Azithromycin on Airflow Decline-Free Survival After Allogeneic Hematopoietic Stem Cell Transplant: The ALLOZITHRO Randomized Clinical Trial. JAMA. 2017;318:557–566. doi: 10.1001/jama.2017.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Del Fante C, Galasso T, Bernasconi P, Scudeller L, Ripamonti F, Perotti C, Meloni F. Extracorporeal photopheresis as a new supportive therapy for bronchiolitis obliterans syndrome after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:728–731. doi: 10.1038/bmt.2015.324. [DOI] [PubMed] [Google Scholar]
- 109.Hefazi M, Langer KJ, Khera N, Adamski J, Roy V, Winters JL, Gastineau DA, Jacob EK, Kreuter JD, Gandhi MJ, et al. Extracorporeal Photopheresis Improves Survival in Hematopoietic Cell Transplant Patients with Bronchiolitis Obliterans Syndrome without Significantly Impacting Measured Pulmonary Functions. Biol Blood Marrow Transplant. 2018;pii:S1083–8791(18)30193-9. doi: 10.1016/j.bbmt.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Redel-Montero J, Bujalance-Cabrera C, Vaquero-Barrios JM, Santos-Luna F, Arenas-De Larriva M, Moreno-Casado P, Espinosa-Jiménez D. Lung transplantation for bronchiolitis obliterans after allogenic bone marrow transplantation. Transplant Proc. 2010;42:3023–3025. doi: 10.1016/j.transproceed.2010.07.086. [DOI] [PubMed] [Google Scholar]
- 111.Cheng GS, Edelman JD, Madtes DK, Martin PJ, Flowers ME. Outcomes of lung transplantation after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1169–1175. doi: 10.1016/j.bbmt.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 112.Soubani AO, Kingah P, Alshabani K, Muma G, Haq A. Lung transplantation following hematopoietic stem cell transplantation: report of two cases and systematic review of literature. Clin Transplant. 2014;28:776–782. doi: 10.1111/ctr.12378. [DOI] [PubMed] [Google Scholar]
- 113.Rabitsch W, Deviatko E, Keil F, Herold C, Dekan G, Greinix HT, Lechner K, Klepetko W, Kalhs P. Successful lung transplantation for bronchiolitis obliterans after allogeneic marrow transplantation. Transplantation. 2001;71:1341–1343. doi: 10.1097/00007890-200105150-00028. [DOI] [PubMed] [Google Scholar]
- 114.Hou HA, Yao M, Tang JL, Chen YK, Ko BS, Huang SY, Tien HF, Chang HH, Lu MY, Lin TT, et al. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years’ experience in a single institute. Bone Marrow Transplant. 2009;43:315–321. doi: 10.1038/bmt.2008.325. [DOI] [PubMed] [Google Scholar]
- 115.Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon RP, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 116.Stevens SJ, Verschuuren EA, Verkuujlen SA, Van Den Brule AJ, Meijer CJ, Middeldorp JM. Role of Epstein-Barr virus DNA load monitoring in prevention and early detection of post-transplant lymphoproliferative disease. Leuk Lymphoma. 2002;43:831–840. doi: 10.1080/10428190290016971. [DOI] [PubMed] [Google Scholar]
- 117.Kunitomi A, Arima N, Ishikawa T. Epstein-Barr virus-associated post-transplant lymphoproliferative disorders presented as interstitial pneumonia; successful recovery with rituximab. Haematologica. 2007;92:e49–e52. doi: 10.3324/haematol.11142. [DOI] [PubMed] [Google Scholar]
- 118.Benkerrou M, Jais JP, Leblond V, Durandy A, Sutton L, Bordigoni P, Garnier JL, Le Bidois J, Le Deist F, Blanche S, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood. 1998;92:3137–3147. [PubMed] [Google Scholar]
- 119.McLaughlin LP, Bollard CM, Keller MD. Adoptive T Cell Therapy for Epstein-Barr Virus Complications in Patients With Primary Immunodeficiency Disorders. Front Immunol. 2018;9:556. doi: 10.3389/fimmu.2018.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]