Abstract
AIM
To review the clinical impact of machine perfusion (MP) of the liver on biliary complications post-transplantation, particularly ischaemic-type biliary lesions (ITBL).
METHODS
This systematic review was performed in accordance with the Preferred Reporting Systematic Reviews and Meta-Analysis (PRISMA) protocol. The following databases were searched: PubMed, MEDLINE and Scopus. The keyword “liver transplantation” was used in combination with the free term “machine perfusion”. Clinical studies reporting results of transplantation of donor human livers following ex situ or in situ MP were analysed. Details relating to donor characteristics, recipients, technique of MP performed and post-operative biliary complications (ITBL, bile leak and anastomotic strictures) were critically analysed.
RESULTS
Fifteen articles were considered to fit the criteria for this review. Ex situ normothermic MP was used in 6 studies, ex situ hypothermic MP in 5 studies and the other 4 studies investigated in situ normothermic regional perfusion (NRP) and controlled oxygenated rewarming. MP techniques which have per se the potential to alleviate ischaemia-reperfusion injury: Such as hypothermic MP and NRP, have also reported lower rates of ITBL. Other biliary complications, such as biliary leak and anastomotic biliary strictures, are reported with similar incidences with all MP techniques. There is currently less clinical evidence available to support normothermic MP as a mitigator of biliary complications following liver transplantation. On the other hand, restoration of organ to full metabolism during normothermic MP allows assessment of hepatobiliary function before transplantation, although universally accepted criteria have yet to be validated.
CONCLUSION
MP of the liver has the potential to have a positive impact on post-transplant biliary complications, specifically ITBL, and expand extended criteria donor livers utilisation.
Keywords: Liver transplantation, Ex situ machine perfusion of the liver, Donation after circulatory death, Non-anastomotic intra-hepatic stricture, Ischemic-type biliary lesions, Extended criteria donors
Core tip: Post-transplant biliary complications are one of the main culprits responsible for the high patient morbidity following extended criteria donor liver transplantation. In its most severe form, ischaemic-type biliary lesions, can lead to graft failure and re-transplantation. Machine perfusion (MP) of the liver is a promising approach in reconditioning high-risk organs. Clinical studies have, so far, focussed on the impact of MP on hepatocellular function recovery and assessment. In this review we present the clinical evidence of the effect of MP on post-transplant biliary complications and discuss how, in the future, this approach can reduce these complications further.
INTRODUCTION
Post-transplant biliary complications: The current scenario
Post-transplant biliary complications often require laborious and costly interventions, placing a heavy burden on health resources and adversely affecting patient outcomes[1,2]. The incidence of these complications is increasing as a result of the growing utilisation of extended criteria donor (ECD) organs, mainly from donation after circulatory death (DCD). Biliary complications such as biliary leak and anastomotic strictures are primarily related to surgical technicalities and are usually successfully managed with endoscopic procedures[3]. The most severe form of post-transplant biliary complication is non-anastomotic intrahepatic strictures (NAS). NAS is characterised by the occurrence of diffuse intra-hepatic strictures in the biliary tree and it was initially associated with hepatic artery thrombosis[4]. The ischaemic donor biliary tree was found to develop necrosis with fibrotic strictures, dilatations and potentially biliary casts[4]. Thereafter it was demonstrated that similar lesions occurred in the presence of a patent hepatic artery without evidence of recurrence of biliary disease. This entity was subsequently classified as ischaemic-type biliary lesion (ITBL)[5].
The reported incidence of ITBL is approximately 10%-30% for controlled DCD and 1%-3% for donation after brain death (DBD) organs[6-10]. Patients generally present with elevated liver function tests suggesting cholestasis (bilirubin, alkaline phosphatase and gamma-glutamyltransferase) within a few months of transplantation and may be asymptomatic initially. Initial work-up includes exclusion of hepatic artery thrombosis and anastomotic biliary strictures. Imaging investigations consist of non-invasive magnetic resonance cholangiopancreatography (MRCP) and computed tomographic cholangiography, or direct cholangiographic methods, such as endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography. Due to the high reliability of current non-invasive imaging techniques in diagnosing biliary strictures, invasive procedures are currently reserved for scenarios where an intervention is planned, such as stricture dilatation, stenting or stone extraction[11,12]. With ITBL, imaging confirms the presence of fibrotic strictures, in most cases located around the bifurcation of the common bile duct leading to dilatation of the intra-hepatic biliary system[1,8]. Figure 1 illustrates these typical imaging features of ITBL following liver transplantation. The obstructive strictures cause cholestasis with formation of sludge and casts that predispose to cholangitis, frequently requiring surgical or endoscopic intervention. Despite these measures, approximately 50% of patients with ITBL require re-transplantation or die[13].
Figure 1.
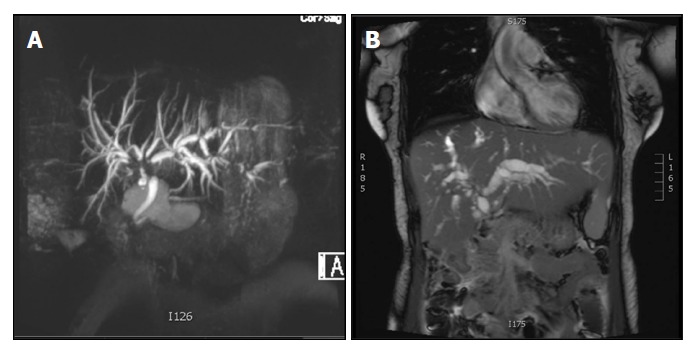
Magnetic resonance cholangiopancreatography images of ischemic-type biliary lesions following liver transplantation. The images show two recipients of livers from donation after circulatory death donors that developed ischemic-type biliary lesions within 60 d following transplantation. Hepatic artery thrombosis and anastomotic biliary strictures were ruled out. A: A typical lesion is seen affecting the bifurcation of the common hepatic bile duct with moderate dilatation of the intrahepatic biliary tree; B: The image shows strictures at the bifurcation of the common hepatic bile duct, diffuse intra-hepatic strictures and a severe dilatation of the intrahepatic biliary tree.
Although the pathogenesis of ITBL is still not fully understood a growing body of evidence suggest that it is partially associated with ischaemia-reperfusion injury (IRI)[14,15]. Noack et al[16] in a well-designed in-vitro study using rat-derived bile duct cells showed that they were more resistant to anoxia than hepatocytes, however during reoxygenation they produced higher amounts of reactive oxygen species (ROS). This was associated with increased rates of bile duct cell death when compared to hepatocytes[16]. It has been shown that mitochondrial ischaemic induced injury leads to ROS production during reperfusion which in turn causes oxidative injury and activation of the inflammatory cascade[17,18]. Conversely, clinical series have reported severe injury to the biliary epithelium just after cold static storage[19,20]. Garcia-Valdecasas et al[15] using a porcine transplantation model suggested a direct relationship between prolonged ischaemic times and cell injury. Indeed, other clinical series have confirmed the association of longer cold ischaemic time (CIT) and higher rates of ITBL[21-24]. A similar relationship has been observed with warm ischemic time in DCD liver transplantation[15,25]. A large clinical series of donor bile duct biopsies before liver transplantation showed similar injury to the biliary epithelium after static cold storage (SCS), and that it was exacerbated after reperfusion; however, this did not correlate with the development of ITBL[26]. Nevertheless, the authors reported a strong association between ITBL and damage to the peribiliary vascular plexus and peribiliary glands. As progenitor biliary cells are known to reside in the peribiliary glands, the former finding suggests an association between ITBL and an attenuated regenerative capacity of the biliary epithelium[26,27]. Ischaemic injury is likely to play a major role in ITBL pathogenesis, although other factors have also been shown to be implicated. Immunological mediated injury to the biliary epithelium has been associated with ITBL[28]. It may be the result of direct immunological damage to the biliary epithelium via a rejection reaction[29]; or, indirect, secondary to the development of arteriopathy[29,30]. This cross reactivity is described in scenarios of cytomegalovirus infection[30], ABO incompatibility[31] and transplantation for primary sclerosing cholangitis[1]. Bile salt toxicity has also been investigated as a potential cause for ITBL by having a direct detergent effect on phospholipid cellular membranes of the biliary epithelium[28]. Flushing of the biliary tree during organ procurement is necessary in order to remove all bile salts that could damage cholangiocytes[5,28]. Furthermore, an imbalance in the post-transplant bile composition, with a higher bile salt/phospholipid ratio, due to inefficient ATP-dependent biliary transporters has been suggested as a predictive factor for ITBL[32]. While detail of the pathogenesis of ITBL is beyond the scope of this review, information on the implicated mechanisms can be found in a number of published reviews[9,28].
Machine perfusion of donor livers
The utilisation of DCD livers is increasing. In 2017, in the United Kingdom, they constituted 28% of the livers transplanted[33]. Furthermore, the rising prevalence of donor obesity (body mass index greater than 30 kg/m2) and an ageing population continue to compound the risks to those livers[33]. These high-risk ECD organs are associated not only with a higher risk of graft dysfunction post-transplantation but also increased rates of ITBL[34]. Despite these disadvantages, their utilisation is required to tackle the ever-growing discrepancy between organ donor supply and demand. Machine perfusion (MP) of the liver is being developed as a means of assessment and reconditioning of ECD donors, potentially allowing for safer transplantation of these high-risk livers[34,35]. Different techniques of MP have been developed; it can be performed in situ during organ procurement or ex situ after the procedure. With regards to livers, the only technique of in situ MP described so far is normothermic regional perfusion (NRP)[8]. Ex situ MP protocols vary in terms of oxygenation (active or pre-charged oxygenation), perfusate temperature (hypothermic, subnormothermic, gradual rewarming and normothermic), timing of perfusion (preservation or end-ischemic) and via of organ perfusion (portal vein alone or dual portal vein and hepatic artery perfusion)[34,36].
Hypothermic machine perfusion (HMP) has been performed around 10 °C in most studies[37,38]. At this temperature liver metabolism is reduced; and, passive oxygen delivery by diffusion in an oxygen carrier-free perfusate is enough to support the organ[39]. The first published clinical series employed pre-charged oxygen delivery to the organs[37], technique that was later followed by active oxygenation of the perfusate[40]. Hypothermic oxygenated MP can be performed via portal vein alone (HOPE) or via portal vein and hepatic artery (dual hypothermic oxygenated perfusion - D-HOPE)[41-43]. Both techniques have shown the capacity of improve mitochondrial oxidative function prior to rewarming, resulting in increased adenosine triphosphate (ATP) synthesis and a reduction in ROS production, oxidative tissue injury and activation of the inflammatory cascade[42,43].
Normothermic machine perfusion (NMP) maintains the organ at physiological temperatures (37 °C) and therefore restores full metabolic activity. This enables the possibility of functional or viability assessment prior to transplantation, a major advantage of NMP when compared to other perfusion techniques[44,45]. It also opens up a window of opportunity for ex situ therapeutic interventions[34]. Furthermore, previous studies have reported on the safety of extended normothermic perfusion of organs, which may facilitate transportation and logistical management of busy transplant units[46]. However, potential drawbacks of NMP are that it requires obligatorily the inclusion of an oxygen carrier in the perfusate, and NMP inevitably induces reperfusion injury to some extent.
Subnormothermic machine perfusion (SMP) has been performed at around 20 °C in most studies. It encompasses purely SMP and the controlled oxygenated rewarming (COR) from 10 °C to 20 °C[47,48]. The increase in temperature from HMP to SMP is suggested to be enough to increase liver metabolism to an extent that it would allow assessment of organ function without inducing the detrimental changes associated with organ reperfusion at normothermic temperatures[48]. Evidence for the clinical benefits is available for COR perfusions, it was associated with lower markers of hepatocellular injury after transplantation and enhanced graft function through the avoidance of subtle changes in organ temperature[47].
For DCD livers, there are encouraging reports of in situ oxygenated NRP. It has been successfully applied to controlled DCD donors (withdrawal of life support in patients with irreversible clinical conditions) and uncontrolled DCD (witnessed cardiac arrest without response to resuscitative measures)[8,49,50]. NRP limits ischaemia and prevents depletion of energy stores prior to SCS and this is suggested to be essential for uncontrolled DCD donors and beneficial for controlled DCD[8].
More recently, combinations of MP techniques have been shown to merge the advantages of individual protocols, enhancing the rescue of liver function what may potentially improve graft function after transplantation[51,52]. Despite differences between techniques, MP has the potential to limit ischaemic injury to the organ, thus offering a safer preservation environment and an opportunity for organ reconditioning which could mitigate IRI.
As discussed herein, the current evidence shows that cholangiocytes are more vulnerable to IRI than hepatocytes and that the pathogenesis for biliary injury goes beyond IRI. Therefore, investigation of the impact of MP on biliary function specifically, and not only on hepatocellular function, is fundamental. The aim of this review was to investigate the current clinical evidence available regarding the effect of MP on post-transplant biliary complications, focusing on ITBL.
MATERIALS AND METHODS
This systematic review was performed in accordance with the Preferred Reporting Systematic Reviews and Meta-Analysis (PRISMA) protocol[53].
The following databases were searched for the development of this review: PubMed, MEDLINE and Scopus. The keyword “liver transplantation” was used in combination with the free term “machine perfusion”. The literature review was performed until June 20, 2018 and there were no limits on the date for inclusion of publications. The literature search strategy used for one database is presented in the Supplementary Table S1.
The screening and selection of articles were independently performed by two authors (Yuri L Boteon and Amanda PCS Boteon). There was no disagreement in study selection between authors. Manuscript titles that were not related to the main scope of the review were excluded. Full abstracts were then read and excluded if found not to be relevant to the review. Finally, full papers were assessed for eligibility and included in this review. The flow diagram for the literature selection process is shown in Figure 2.
Figure 2.
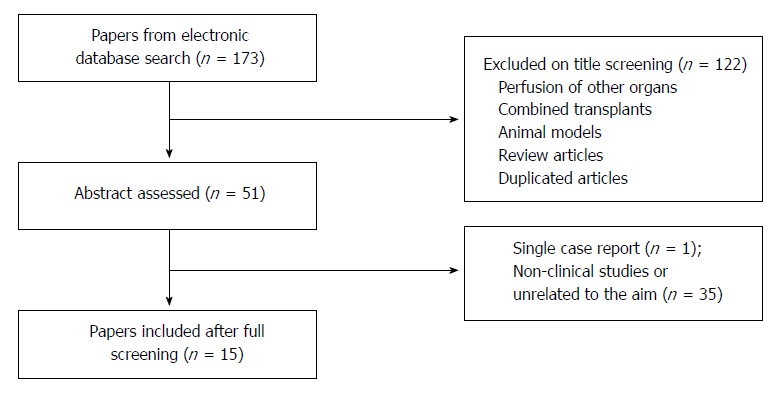
Study flow diagram for systematic review of the literature on the impact of machine perfusion of the liver and post-transplant biliary complications. Following literature search duplicate articles were excluded and the titles screened. The selected abstracts were then read and non-clinical studies or reports unrelated to the aim of the review were excluded.
Inclusion criteria were: (1) clinical studies reporting results of transplantation of donor human livers following ex situ or in situ MP; and (2) articles written in English and published. Exclusion criteria were: (1) absence of transplantation following MP; (2) exclusively animal models; (3) single case report; (4) review articles; and (5) articles not written in English.
Details relating to donor characteristics [type, age, donor risk index (DRI), warm ischaemic time (WIT), CIT], recipients [age, model for end-stage liver disease (MELD)], perfusion (type of perfusion, oxygenation, timings) and post-operative biliary complications (ITBL, leak and anastomotic strictures) were retrieved from each manuscript and critically analysed. Studies were assessed in terms of study design, methods and outcomes. No review protocol was registered before this review was started. No simplifications or assumptions were made, and any identified risk of bias is discussed throughout the review.
RESULTS
Fifteen articles were considered to fit the criteria for this review. A diagrammatic summary of the screening process is provided in Figure 2.
MP and ischemic type biliary lesions (ITBL)
Eight out of fifteen clinical studies utilised an end-ischemic model of MP (MP commenced after a variable period of SCS), 4 studies utilised preservation MP (MP from organ procurement up to transplantation) and 3 employed NRP. NMP was used in 6 studies, HMP in 5 studies and the other 4 studies investigated NRP and COR. HMP with active perfusate oxygenation (HOPE and D-HOPE) studies were seen to be currently focused on DCD organs and HMP with pre-charged oxygenation on DBD organs. NMP studies used both donor types, however preservation studies explored a higher proportion of DBD compared to DCD organs. The contrary was seen for end-ischemic NMP.
Donor and recipients characteristics, of the cases included in individual studies, are presented in Table 1. It also reports the rates of ITBL. Table 2 describes the incidence of bile leak and anastomotic biliary stricture within the different studies. Studies characteristics were described therein, as it was their design.
Table 1.
Comparison between donor, recipient, perfusion characteristics and the reported rates of ischemic-type biliary lesions
| Ref. | Yr | Perfusion type | Timing MP | n | Donor age | Donor risk index | Recipient age | Recipient MELD | DBD (n) | DCD (n) | DBD ITBL (%) | DCD ITBL (%) | CIT (min) | Func. WIT (min) | Re-Tx (n) |
| Ex situ normothermic machine perfusion | |||||||||||||||
| Nasralla et al[46] | 2018 | NMP | Preserv | 121 | 56 (16-84) | 1.73 | 55 | 13 (6-35) | 87 | 34 | 7.4 | 11.1 | 126 | 21 | 3 |
| Selzner et al[54] | 2016 | NMP | Preserv | 10 | 48 (17-75) | 1.9 | 57 | 21 (8-40) | 8 | 2 | 0 | 0 | 103 | NA | 0 |
| Bral et al[56] | 2017 | NMP | Preserv | 9 | 56 (14-71) | 1.6 (0.9-2.7) | 53 (28-67) | 13 (9-32) | 6 | 3 | 0 | 0 | 167 (95-293) | 22 | 0 |
| Ravikumar et al[55] | 2016 | NMP | Preserv | 20 | 58 (21-85) | NA | NA | 12 (7-27) | 16 | 4 | 0 | 0 | NA | 21 | 0 |
| Watson et al[58] | 2018 | NMP | End-Isc | 22 | 57 | 2.3 | NA | NA | 6 | 16 | 0 | 25 | 386 | 12 | 3 |
| Mergental et al[57] | 2016 | NMP | End-Isc | 5 | 49 (29-54) | 2.3 | 56 (47-66) | 8 (8-13) | 1 | 4 | 0 | 0 | 422 | 28 | 0 |
| Ex situ hypothermic non-oxygenated machine perfusion | |||||||||||||||
| Guarrera et al[59] | 2015 | HMP | End-Isc | 31 | 57 (± 18)1 | 1.9 (± 0.5)1 | 57 (± 8.0)1 | 19 (± 5.9)1 | 31 | 0 | 9.7 | NA | 558 | NA | 0 |
| Guarrera et al[37] | 2010 | HMP | End-Isc | 20 | 39 (± 2.5)1 | NA | 55 (± 6.2)1 | 17 (± 7.4)1 | 20 | 0 | 5 | NA | 306 | 26 | 0 |
| Ex situ hypothermic oxygenated machine perfusion | |||||||||||||||
| van Rijn et al[43] | 2017 | DHOPE | End-Isc | 10 | 53 (47-57) | 1.9 (1.5-2.2) | 57 (54-62) | 16 (15-22) | 0 | 10 | NA | 10 | 331 | 15 | 0 |
| Dutkowski et al[38] | 2015 | HOPE | End-Isc | 25 | 54 (36-63) | NA | 60 (57-64) | 13 (9-15) | 0 | 25 | NA | 0 | 188 (141-264) | 31 (26-36) | 0 |
| Dutkowski et al[40] | 2014 | HOPE | End-Isc | 8 | 54 (NA) | 2.2 (NA) | 60 (NA) | 12 (NA) | 0 | 8 | NA | 0 | 141 (NA) | 31 (22-41) | 0 |
| In situ normothermic regional perfusion | |||||||||||||||
| De Carlis et al[60]2 | 2017 | NRP | NRP | 7 | 481 | NA | 541 | 10.61 | 0 | 7 | NA | 0 | 4141 | 33 | 0 |
| Oniscu et al[49] | 2014 | NRP | NRP | 11 | 46 (16-74) | NA | 68 (43-74) | NA | 0 | 11 | NA | 0 | 389 (169-450) | 26 (13-48) | 0 |
| Minambres et al[50] | 2017 | NRP | NRP | 11 | 58 (50-67) | NA | 55 (± 13)1 | NA | 0 | 11 | NA | 0 | 266 (± 82.7)1 | 12 (11-16) | 0 |
| Controlled oxygenated rewarming | |||||||||||||||
| Hoyer et al[47] | 2016 | COR | End-Isc | 6 | 58 (51-71) | 1.9 (1.5-2.5) | 52 (43-65) | 18 (11-23) | 6 | 0 | 0 | NA | 508 (369-870) | NA | 0 |
Data presented as median or median (± SD), if available. Otherwise, all data presented as median (Interquartile range); 2Combined hypothermic oxygenated machine perfusion after normothermic regional perfusion. Six uncontrolled DCD were included in this study;
Eurotransplant DRI. MP: Machine perfusion; MELD: Model for end stage liver disease; DBD: Donation after brain death; DCD: Donation after circulatory death; ITBL: Ischemic-type biliary lesions; CIT: Cold ischemic time; Func; WIT: Functional warm ischemic time; Re-Tx: Re-transplantation; NA: Not applicable or not available; Preserv: Preservation; End-Isc: End ischemic; NMP: Normothermic machine perfusion; HMP: Hypothermic machine perfusion; DHOPE: Dual vessel hypothermic oxygenated machine perfusion; HOPE: Hypothermic oxygenated machine perfusion; NRP: Normothermic regional perfusion; COR: Controlled oxygenated rewarming.
Table 2.
Prevalence of bile leak and anastomotic biliary strictures between clinical studies using different techniques of machine perfusion of donor livers
| Ref. | Yr | Study design | Perfusion type | Timing machine perfusion | n | DBD (n) | DCD (n) | Bile leak (n) | Anastomotic stricture (n) |
| Ex situ normothermic machine perfusion | |||||||||
| Nasralla et al[46] | 2018 | RCT | NMP | Preservation | 121 | 87 | 34 | 0 | 0 |
| Selzner et al[54] | 2016 | PS | NMP | Preservation | 10 | 8 | 2 | 0 | 0 |
| Bral et al[56] | 2017 | PS | NMP | Preservation | 9 | 6 | 3 | 0 | 0 |
| Ravikumar et al[55] | 2016 | PS | NMP | Preservation | 20 | 16 | 4 | 0 | 4 (DBD) |
| Watson et al[58] | 2018 | DS | NMP | End-Ischaemic | 22 | 6 | 16 | 0 | 0 |
| Mergental et al[57] | 2016 | DS | NMP | End-Ischaemic | 5 | 1 | 4 | 0 | 0 |
| Ex situ hypothermic non-oxygenated machine perfusion | |||||||||
| Guarrera et al[59] | 2015 | PS | HMP | End-Ischaemic | 31 | 31 | 0 | 1 | 0 |
| Guarrera et al[37] | 2010 | NCS | HMP | End-Ischaemic | 20 | 20 | 0 | 1 | 1 |
| Ex situ hypothermic oxygenated machine perfusion | |||||||||
| van Rijn et al[43] | 2017 | PS | DHOPE | End-Ischaemic | 10 | 0 | 10 | 0 | 2 |
| Dutkowski et al[38] | 2015 | PS | HOPE | End-Ischaemic | 25 | 0 | 25 | 5 (in total) | |
| Dutkowski et al[40] | 2014 | PS | HOPE | End-Ischaemic | 8 | 0 | 8 | 1 | 1 |
| In situ normothermic regional perfusion | |||||||||
| De Carlis et al[60]1 | 2017 | DS | NRP | NRP | 7 | 0 | 7* | 0 | 1 |
| Oniscu et al[49] | 2014 | DS | NRP | NRP | 11 | 0 | 11 | 1 | 1 |
| Minambres et al[50] | 2017 | DS | NRP | NRP | 11 | 0 | 11 | NA | NA |
| Controlled Oxygenated Rewarming | |||||||||
| Hoyer et al[47] | 2016 | PS | COR | End-Ischaemic | 6 | 6 | 0 | NA | NA |
1Combined hypothermic oxygenated machine perfusion after normothermic regional perfusion. Six uncontrolled DCD were included in this study. RCT: Randomised controlled trial; PS: Single-arm non-randomised pilot study; DS: Descriptive study; NCS: Non-randomised cohort studies; DBD: Donation after brain death; DCD: Donation after circulatory death; NA: Not applicable or not available; NMP: Normothermic machine perfusion; HMP: Hypothermic machine perfusion; DHOPE: Dual vessel hypothermic oxygenated machine perfusion; HOPE: Hypothermic oxygenated machine perfusion; NRP: Normothermic regional perfusion; COR: Controlled oxygenated rewarming.
NMP and post-transplant biliary complications
The largest clinical trial involving NMP as a preservation strategy was recently published by Nasralla et al[46]. Following procurement, transplantable livers were randomised and allocated to the intervention group that had NMP up to the point of transplantation or a control group that had conventional SCS. From the 121 livers perfused, 87 were from DBD donors and 34 from DCD donors. Results did not shown differences in bile duct complications between groups, with one patient in each arm developing ITBL within the first year, both requiring re-transplantation. On MRCP, the rates of NAS were similar between groups for DBD (NMP 7.4% vs SCS 5.4%; P = 0.678) and DCD (NMP 11.1% vs SCS 26.3%; P = 0.180). The incidence of anastomotic strictures was also similar for DBD or DCD organs (NMP 40.7% vs SCS 41.8%; P = 0.909; and, NMP 48.1% vs SCS 57.9%; P = 0.515, respectively)[46].
Other clinical studies investigating NMP using a preservation approach[54-56] involved smaller patient numbers, the majority of which were from DBD donors, and did not specifically report the incidence of ITBL (Table 1). Ravikumar et al[55] published the first phase 1 clinical trial demonstrating the safety and feasibility of NMP in a preservation approach, as an alternative to SCS. In all, 20 donor livers (16 DBD and 4 DCD) were transplanted following NMP. The 30-day graft survival was similar to static cold stored livers and the median peak aspartate aminotransferase within the first 7 post-operative days was lower. In terms of biliary complications, the authors reported the occurrence of 4 cases of anastomotic biliary strictures in the NMP group[55].
The two studies of NMP after a period of SCS (end-ischaemic model) involved organs that were deemed too high risk for transplantation[57,58]. These studies predominantly used DCD livers and applied predefined viability criteria prior to transplantation. Mergental et al[57] did not observe any biliary complications at 7 mo of follow up post-transplantation. Watson et al[58] reported the occurrence of 4 cases of ITBL in 16 DCD liver transplants, of which 3 needed re-transplantation. The authors of the latter study concluded that that NMP per se does not prevent ITBL but may provide biomarkers to identify livers that are high risk, such as maximum bile pH > 7.5 and bile glucose ≤ 3 mmol/L or ≥ 10 mmol less than perfusate glucose[58].
HMP and post-transplant biliary complications
The first clinical study using HMP prior to transplantation was performed by Guarrera et al[37] Twenty DBD livers were perfused after a period of SCS in a non-actively oxygenated model of HMP. ITBL rate was reported as 5%, half of the incidence of the control matched cohort that was subjected to SCS. Additionally, there was one case of bile leak and 1 report of anastomotic biliary stricture[37]. The same approach was repeated later in a study of DBD livers declined by the United Network for Organ Sharing region for transplantation[59]. The authors found a significant decrease in the rate of biliary stricture in comparison with SCS (10% vs 33%, P = 0.031). One report of bile leak was noted in the HMP group and 3 in SCS respectively (Table 2).
Following these initial studies, the Zurich group developed the concept of HOPE, with active oxygenation of the perfusate, and applied this MP strategy to DCD donors[38,40]. Their first clinical trial was published in 2015, reporting the results of transplantation of 25 DCD livers[38]. The authors reported no cases of ITBL at one year follow-up of patients who received perfused DCD livers, whereas control livers subjected to SCS developed a significantly higher rate of ITBL (0/25 vs 11/50, P = 0.013). The same benefit of HOPE was not seen for extra-hepatic biliary complications, as the reported rates of leaks and anastomotic strictures were similar (HOPE 5/25 vs Control 12/50)[38].
The Groningen group published the first clinical series using D-HOPE in 2017[43]. Ten DCD livers were transplanted following two hours of D-HOPE, one patient in the perfusion group developed ITBL compared to 7 out of 20 in the control group. The case in the D-HOPE group was described as NAS in segments II and III of the liver and was managed with endoscopic stenting. Three control livers which developed ITBL required re-transplantation. The rate of anastomotic biliary strictures was comparable between groups (D-HOPE 2 vs Control 3, P = 1.000) as was the reported rate of biliary cast formation (D-HOPE 3 vs Control 3, P = 0.372)[43].
Normothermic reginal perfusion and post-transplant biliary complications
The first series reporting the results for transplantation of livers following NRP was published in 2014 by Oniscu et al[49] The authors reported the results of transplantation of 11 controlled DCD livers, with a minimum follow-up of 3 mo, with no clinical or radiological evidence of ITBL. One patient developed an anastomotic stricture, treated endoscopically by cholangio-pancreatography (exact intervention performed is not described), and one patient had a bile leak[49]. Minambres et al[50] 2017, studying controlled DCD transplantation after NRP, reported no cases of ITBL after 1-year follow-up. De Carlis et al[60] 2017 performed NRP on 1 controlled DCD liver and 6 uncontrolled DCD. On arrival at the transplant centre, the livers were subjected to D-HOPE until transplantation. No cases of ITBL were observed and one patient had an anastomotic biliary stricture 45 d after transplantation, which was successfully treated with endoscopic stenting[60]. In terms of SMP, Hoyer et al[47] reported transplantation of 6 DBD livers following COR perfusion. No biliary complications were reported within a follow-up period of six months.
DISCUSSION
Post-transplant biliary complications are associated with high rates of morbidity and re-transplantation and are a major obstacle to the wider clinical utilisation of ECD livers. There is a growing body of evidence suggesting that MP can offer safer organ preservation when compared to SCS, and also offer an opportunity for organ assessment and/or reconditioning prior to transplantation[38,43,46,49,58]. In this review we have assessed the available literature investigating the impact of MP on post-transplant biliary complications, with special reference to ITBL. MP techniques which have per se the potential to alleviate IRI, such as HMP and NRP, have also reported lower rates of ITBL. Other biliary complications, such as biliary leak and anastomotic biliary strictures, are reported with similar incidences with all MP techniques.
Liver IRI is thought to be a major driver of biliary injury and, therefore, it is associated with complications following transplantation. More specifically, during ischemia, without oxygen as a terminal acceptor of electrons in the electron transport chain, succinate accumulates and acts as a store for electrons. Succinate oxidation during the early stage of reperfusion, blocks mitochondrial complex II of the electron transport chain resulting in a reverse flow of electrons towards mitochondrial complex I leading to accentuated leakage of electrons, and generation of ROS[61]. Various experimental findings using the HOPE technique have shown that oxygen at hypothermic temperatures is able to promote mitochondrial metabolism of succinate prior to reperfusion[36,42,62]. By re-establishing adequate mitochondrial oxidative function, HOPE is able to recover ATP stores, since during hypothermia mitochondria have lower energy requirements due to a minimum activation of the organ metabolism. Therefore, mechanistically, HOPE can in theory prevent the reverse flow of electrons during reperfusion, ROS generation and activation of the inflammatory cascade[36]. These factors may mitigate IRI, which would be beneficial not only for hepatocellular function but also for the prevention of further biliary injury.
Extensive research focussing on the effect of oxygenated HMP on post-transplant biliary complications has been performed by the Groningen group. In a recent publication exploring the effects of D-HOPE on bile duct biopsies from a previous published series of cases, they showed less injury to deep and periluminal peribiliary glands after reperfusion during transplantation in the perfused group in comparison with SCS control livers[43,63]. Peribiliary glands have been described as stores for biliary progenitor cells, therefore injury to them would potentially decrease the regenerative capacity of the biliary system[64,65]. The authors acknowledge that definitive evidence to support this would require a clinical randomized trial that has since been initiated at their centre[63].
There is currently less clinical evidence available to support NMP as a mitigator of biliary complications following liver transplantation[58]. Preservation NMP shortens the ischaemic injury and offers a more physiological environment for the organ before transplantation. Nevertheless, as previously discussed, the injury to biliary cells would not be restricted to an ischaemic mechanism but may also be worsened during reperfusion. This observation could imply that NMP is of limited benefit in terms of biliary complications, since biliary injury may worsen during organ reperfusion on the machine and is not prevented or mitigated beforehand. NMP restores the full metabolism of the organ, resulting inevitably in the production and circulation of ROS and potential activation of the inflammatory response leading to tissue injury[66]. On the other hand, restoration of organ to full metabolism allows assessment of hepatobiliary function before transplantation, although universally accepted criteria have yet to be validated[35]. Watson et al[58] suggested bile pH and glucose content as markers of bile duct injury and associated those with the development of ITBL, however the authors recognise that NMP was not able to prevent biliary damage.
Promisingly, in situ NRP has shown excellent biliary outcomes after transplantation of DCD livers[49,50,60]. NRP may potentially prevent ischaemic injury and deterioration of ATP stores during organ procurement. Additionally, NRP allows assessment of the liver metabolism even before SCS[8]. Despite these points, there is no mechanistic evidence available to demonstrate any alleviation in IRI after reperfusion. It is also difficult to rule out the possibility that this beneficial effect was as a result of a potential selection bias when recruiting organs for transplantation during the procedure.
The present body of work has several limitations. First and foremost, donor livers and recipient characteristics as well as MP technique protocols exhibit a high degree of variability between studies. So far, there has been no standardisation in terms of methodology and reporting of results. Furthermore, some studies neglect to report important data variables, such as DRI, recipient age, recipient MELD and CIT. All these features are presented in Table 1 to allow an unbiased assessment of the retrieved information by the readers. Additionally, few clinical studies from each MP technique are available and most of them are originated from small pilot studies, which limit definitive interpretation of the data. MRCP was performed in some of the studies at different post-operative periods, but the significance of findings without clinical correlation is not clear. In addition, they have focussed mainly on evaluation of hepatocellular function rather than biliary function and injury. Despite the subject of this review being a relevant topic with important clinical implications, the direct effects of MP on biliary tree integrity are still relatively under-researched. More clinical randomized trials will be reported in the field in the next few years.
Higher rates of ITBL following transplantation of ECD livers, mainly DCD, place a major restraint on the wider use of these marginal livers. Each technique of MP offers different advantages and they all have the potential to tackle this problem. A feasibility study has shown that a combination of HOPE and NMP increased the rescue of metabolic parameters of high-risk ECD organs[52]. This approach may derive benefits from the individual methods, thus optimising gains also in terms of biliary function. Pharmacological interventions during NMP may potentially alleviate IRI, positively affecting biliary cells[67], and may have a direct effect on post-transplant biliary complications. Supplementation of the perfusate with substances that may induce proliferation and maturation of progenitor cells from peribiliary glands may be a feasible option to be considered[9]. We hypothesize that therapies promoting increase in secretion of phospholipids and cholesterol in the bile would equilibrate the phospholipids/bile salts balance mitigating further injury to the biliary tree. Although promising, these are options that still need to be explored in future studies. A diagrammatic summary of the current and future impact of MP on ITBL is presented in Figure 3.
Figure 3.
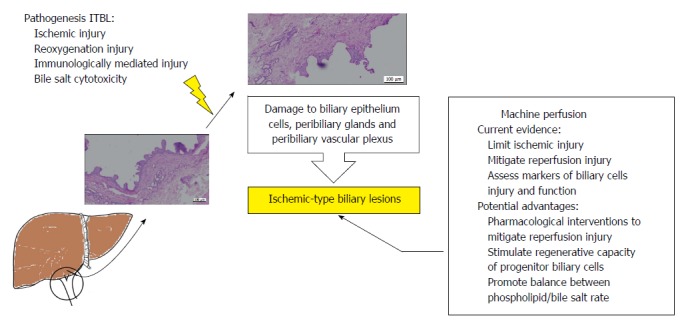
Diagrammatic summary of the current evidence for the impact of machine perfusion of the liver on post-transplant ischemic-type biliary lesions and future perspectives. The current evidence suggests that ischaemic-type biliary lesions (ITBL) have a multifactorial pathogenesis. These diverse factors lead to injury to the biliary epithelium, peribiliary glands and peribiliary vascular plexus. Currently, there is evidence for the potential benefits of machine perfusion on post-transplant ITBL. The figure summarises those and possible future interventions that could enhance increase these benefits further.
The high incidence of post-transplant biliary complications, specifically ITBL, is a major constraint to wider utilisation of ECD livers. MP is currently considered a promising tool to increase ECD utilisation. However, the focus of most of the studies up to date has been the effect of MP on hepatocellular function. In this review we explored the clinical evidence currently available for the impact of MP on post-transplant biliary complications. From those studies that have looked at the effects of MP on biliary integrity, oxygenated HMP and NRP studies have been shown to exhibit better postoperative biliary outcomes in comparison with NMP and non-oxygenated HMP. However, larger clinical studies and randomised clinical trials powered for the occurrence of biliary complications as a primary endpoint are needed to confirm this data.
ARTICLE HIGHLIGHTS
Research background
The ever-growing discrepancy between donor organ availability and patients on the transplant waiting list has led to increased acceptance of extended criteria donors (ECD). However, ECD liver transplantation, mainly donation after circulatory death, is associated with poor patient and graft outcome. A major factor is the increased risk of biliary complications, in particular ischaemic type biliary lesions (ITBL). Machine perfusion (MP) of the liveris a promising tool to recondition ECD organs prior to transplantation. Therefore investigation of the impact of MP on post-transplant biliary complications is a highly relevant topic.
Research motivation
Understanding the current evidence available for the effect of MP on post-transplant biliary complications, in particular ITBL, may guide further studies in this field.
Research objectives
Revise the current clinical evidence available regarding the effect of MP on post-transplant biliary complications, focusing on ITBL.
Research methods
A systematic review was carried out with literature searches in PubMed, MEDLINE and Scopus databases. The keyword “liver transplantation” was used in combination with the free term “machine perfusion”. Only clinical studies reporting results of transplantation of donor human livers following ex situ or in situ MP were included.
Research results
MP techniques which have demonstrated the potential to mitigate ischaemia reperfusion injury, such as ex situ oxygenated hypothermic MP and in situ normothermic regional perfusion, have also reported lower rates of ITBL. Other biliary complications, such as biliary leak and anastomotic biliary strictures, are reported with similar incidences with all MP techniques. Clinical studies have focused on evaluation of hepatocellular function rather than biliary function and injury so far. The direct effects of MP on biliary tree integrity are still relatively under-researched and further studies are needed.
Research conclusions
Post-transplant biliary complications are a major obstacle to the wider utilisation of ECD livers. MP has the potential to have a positive impact on this issue, specifically ITBL, and expand ECD livers utilisation. Mechanistically, mitigation of ischaemia-reperfusion injury appears to be the key mechanism involved.
Research perspectives
Supplementation of the perfusion fluid during ex situ MP with drugs can stimulate protective/regenerative mechanisms of the biliary tree. Pharmacological strategies may potentially modulate progenitor cells proliferation and equilibrate the phospholipid/bile salts balance in the bile.
ACKNOWLEDGMENTS
This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We are extremely grateful to the Research Staff from the Centre for Liver and Gastrointestinal Research, whose continued support provides resources and intellectual input that is shaping our thoughts and future strategies for the continuing development of our research. We are also extremely grateful to all members of the Queen Elizabeth University Hospital Liver Transplant and Hepatobiliary Surgical Unit who are actively involved in the Birmingham MP projects, trials and organ procurement. YLB is funded by the Welcome Trust. We would like to thank the Liver Charities - University Hospitals Birmingham, Queen Elizabeth Hospital for their support to many projects involving machine perfusion.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
PRISMA 2009 Checklist statement: This systematic review was performed in accordance with the Preferred Reporting Systematic Reviews and Meta-Analysis (PRISMA) 2009 protocol.
Peer-review started: August 1, 2018
First decision: August 20, 2018
Article in press: October 9, 2018
P- Reviewer: Gerber DA, Gonzalez F, Hibberd AD, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Huang Y
Contributor Information
Yuri L Boteon, Liver Unit, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2WB, United Kingdom; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2 TT, United Kingdom; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom.
Amanda PCS Boteon, Liver Unit, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2WB, United Kingdom.
Joseph Attard, Liver Unit, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2WB, United Kingdom; Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2 TT, United Kingdom; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom.
Lorraine Wallace, Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2 TT, United Kingdom; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom.
Ricky H Bhogal, Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2 TT, United Kingdom; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom.
Simon C Afford, Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2 TT, United Kingdom; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom. s.c.afford@bham.ac.uk.
References
- 1.Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245–257. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 3.DeOliveira ML, Jassem W, Valente R, Khorsandi SE, Santori G, Prachalias A, Srinivasan P, Rela M, Heaton N. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from a matched control study in a single large volume center. Ann Surg. 2011;254:716–722; discussion 722-723. doi: 10.1097/SLA.0b013e318235c572. [DOI] [PubMed] [Google Scholar]
- 4.Zajko AB, Campbell WL, Logsdon GA, Bron KM, Tzakis A, Esquivel CO, Starzl TE. Cholangiographic findings in hepatic artery occlusion after liver transplantation. AJR Am J Roentgenol. 1987;149:485–489. doi: 10.2214/ajr.149.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 6.Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 7.Laing RW, Scalera I, Isaac J, Mergental H, Mirza DF, Hodson J, Wilkin RJ, Perera MT, Muiesan P. Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant. 2016;16:1795–1804. doi: 10.1111/ajt.13699. [DOI] [PubMed] [Google Scholar]
- 8.Hessheimer AJ, Cárdenas A, García-Valdecasas JC, Fondevila C. Can we prevent ischemic-type biliary lesions in donation after circulatory determination of death liver transplantation? Liver Transpl. 2016;22:1025–1033. doi: 10.1002/lt.24460. [DOI] [PubMed] [Google Scholar]
- 9.de Vries Y, von Meijenfeldt FA, Porte RJ. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta. 2018;1864:1507–1515. doi: 10.1016/j.bbadis.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259–264. doi: 10.1097/SLA.0b013e318204e658. [DOI] [PubMed] [Google Scholar]
- 11.Akbar A, Tran QT, Nair SP, Parikh S, Bilal M, Ismail M, Vanatta JM, Eason JD, Satapathy SK. Role of MRCP in Diagnosing Biliary Anastomotic Strictures After Liver Transplantation: A Single Tertiary Care Center Experience. Transplant Direct. 2018;4:e347. doi: 10.1097/TXD.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73:955–962. doi: 10.1016/j.gie.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517–524. doi: 10.1007/s00534-005-1080-2. [DOI] [PubMed] [Google Scholar]
- 14.Cutrin JC, Cantino D, Biasi F, Chiarpotto E, Salizzoni M, Andorno E, Massano G, Lanfranco G, Rizzetto M, Boveris A, et al. Reperfusion damage to the bile canaliculi in transplanted human liver. Hepatology. 1996;24:1053–1057. doi: 10.1002/hep.510240512. [DOI] [PubMed] [Google Scholar]
- 15.García-Valdecasas JC, Tabet J, Valero R, Deulofeu R, Taurá P, Rull R, Capdevila L, Cifuentes A, González FX, Net M, et al. Evaluation of ischemic injury during liver procurement from non-heart-beating donors. Eur Surg Res. 1999;31:447–456. doi: 10.1159/000008724. [DOI] [PubMed] [Google Scholar]
- 16.Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495–500. doi: 10.1097/00007890-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunner SM, Junger H, Ruemmele P, Schnitzbauer AA, Doenecke A, Kirchner GI, Farkas SA, Loss M, Scherer MN, Schlitt HJ, et al. Bile duct damage after cold storage of deceased donor livers predicts biliary complications after liver transplantation. J Hepatol. 2013;58:1133–1139. doi: 10.1016/j.jhep.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Hansen T, Hollemann D, Pitton MB, Heise M, Hoppe-Lotichius M, Schuchmann M, Kirkpatrick CJ, Otto G. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation--a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012;461:41–48. doi: 10.1007/s00428-012-1245-8. [DOI] [PubMed] [Google Scholar]
- 21.Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–890. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A, Miller CH. Ischemic-type biliary strictures in liver allografts: the Achilles heel revisited? Hepatology. 1995;21:589–591. [PubMed] [Google Scholar]
- 23.Theilmann L, Küppers B, Kadmon M, Roeren T, Notheisen H, Stiehl A, Otto G. Biliary tract strictures after orthotopic liver transplantation: diagnosis and management. Endoscopy. 1994;26:517–522. doi: 10.1055/s-2007-1009026. [DOI] [PubMed] [Google Scholar]
- 24.Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14–22. doi: 10.1111/j.1432-2277.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 25.Taner CB, Bulatao IG, Perry DK, Sibulesky L, Willingham DL, Kramer DJ, Nguyen JH. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012;25:838–846. doi: 10.1111/j.1432-2277.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 26.op den Dries S, Westerkamp AC, Karimian N, Gouw AS, Bruinsma BG, Markmann JF, Lisman T, Yeh H, Uygun K, Martins PN, et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60:1172–1179. doi: 10.1016/j.jhep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Sutton ME, op den Dries S, Koster MH, Lisman T, Gouw AS, Porte RJ. Regeneration of human extrahepatic biliary epithelium: the peribiliary glands as progenitor cell compartment. Liver Int. 2012;32:554–559. doi: 10.1111/j.1478-3231.2011.02721.x. [DOI] [PubMed] [Google Scholar]
- 28.Op den Dries S, Sutton ME, Lisman T, Porte RJ. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373–379. doi: 10.1097/TP.0b013e318223a384. [DOI] [PubMed] [Google Scholar]
- 29.Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9:204–209. doi: 10.1002/hep.1840090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martelius T, Krogerus L, Höckerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27:996–1002. doi: 10.1002/hep.510270415. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Ye S, Xu X, Xie H, Zhou L, Zheng S. Recipient outcomes after ABO-incompatible liver transplantation: a systematic review and meta-analysis. PLoS One. 2011;6:e16521. doi: 10.1371/journal.pone.0016521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, Porte RJ. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009;50:69–79. doi: 10.1016/j.jhep.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 33.NHS Blood and Transplant. 2017. Interim report on liver transplantation. REPORT FOR 2016/ pp. (1 October 2015–30 September 2016). Published April 2017. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/5947/nhsbt-interim-liver-report-1617.pdf. [Google Scholar]
- 34.Boteon YL, Afford SC, Mergental H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr Transplant Rep. 2018;5:113–120. doi: 10.1007/s40472-018-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson CJE, Jochmans I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr Transplant Rep. 2018;5:72–81. doi: 10.1007/s40472-018-0178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlegel A, Muller X, Dutkowski P. Hypothermic Machine Preservation of the Liver: State of the Art. Curr Transplant Rep. 2018;5:93–102. doi: 10.1007/s40472-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS Jr, Emond JC. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372–381. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 38.Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, DeOliveira ML, Kron P, Clavien PA. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann Surg. 2015;262:764–70; discussion 770-1. doi: 10.1097/SLA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 39.Dutkowski P, de Rougemont O, Clavien PA. Machine perfusion for ‘marginal’ liver grafts. Am J Transplant. 2008;8:917–924. doi: 10.1111/j.1600-6143.2008.02165.x. [DOI] [PubMed] [Google Scholar]
- 40.Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765–772. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Schlegel A, Kron P, De Oliveira ML, Clavien PA, Dutkowski P. Is single portal vein approach sufficient for hypothermic machine perfusion of DCD liver grafts? J Hepatol. 2016;64:239–241. doi: 10.1016/j.jhep.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014;260:931–7; discussion 937-8. doi: 10.1097/SLA.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 43.van Rijn R, Karimian N, Matton APM, Burlage LC, Westerkamp AC, van den Berg AP, de Kleine RHJ, de Boer MT, Lisman T, Porte RJ. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907–917. doi: 10.1002/bjs.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laing RW, Mergental H, Yap C, Kirkham A, Whilku M, Barton D, Curbishley S, Boteon YL, Neil DA, Hübscher SG, et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open. 2017;7:e017733. doi: 10.1136/bmjopen-2017-017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, Wiersema-Buist J, Lisman T, Leuvenink HG, Porte RJ. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 46.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 47.Hoyer DP, Mathé Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, Paul A, Minor T. Controlled Oxygenated Rewarming of Cold Stored Livers Prior to Transplantation: First Clinical Application of a New Concept. Transplantation. 2016;100:147–152. doi: 10.1097/TP.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 48.Bruinsma BG, Yeh H, Ozer S, Martins PN, Farmer A, Wu W, Saeidi N, Op den Dries S, Berendsen TA, Smith RN, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14:1400–1409. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oniscu GC, Randle LV, Muiesan P, Butler AJ, Currie IS, Perera MT, Forsythe JL, Watson CJ. In situ normothermic regional perfusion for controlled donation after circulatory death--the United Kingdom experience. Am J Transplant. 2014;14:2846–2854. doi: 10.1111/ajt.12927. [DOI] [PubMed] [Google Scholar]
- 50.Miñambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodríguez-San Juan JC, Ballesteros MA. Improving the Outcomes of Organs Obtained From Controlled Donation After Circulatory Death Donors Using Abdominal Normothermic Regional Perfusion. Am J Transplant. 2017;17:2165–2172. doi: 10.1111/ajt.14214. [DOI] [PubMed] [Google Scholar]
- 51.De Carlis L, De Carlis R, Lauterio A, Di Sandro S, Ferla F, Zanierato M. Sequential Use of Normothermic Regional Perfusion and Hypothermic Machine Perfusion in Donation After Cardiac Death Liver Transplantation With Extended Warm Ischemia Time. Transplantation. 2016;100:e101–e102. doi: 10.1097/TP.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 52.Boteon YL, Laing RW, Schlegel A, Wallace L, Smith A, Attard J, Bhogal RH, Neil DA, Hübscher S, Perera MTP, et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018 doi: 10.1002/lt.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Selzner M, Goldaracena N, Echeverri J, Kaths JM, Linares I, Selzner N, Serrick C, Marquez M, Sapisochin G, Renner EL, et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: First North American results. Liver Transpl. 2016;22:1501–1508. doi: 10.1002/lt.24499. [DOI] [PubMed] [Google Scholar]
- 55.Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC, et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779–1787. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 56.Bral M, Gala-Lopez B, Bigam D, Kneteman N, Malcolm A, Livingstone S, Andres A, Emamaullee J, Russell L, Coussios C, et al. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am J Transplant. 2017;17:1071–1080. doi: 10.1111/ajt.14049. [DOI] [PubMed] [Google Scholar]
- 57.Mergental H, Perera MT, Laing RW, Muiesan P, Isaac JR, Smith A, Stephenson BT, Cilliers H, Neil DA, Hübscher SG, et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am J Transplant. 2016;16:3235–3245. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 58.Watson CJE, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K, Gimson AE, Allison M, Upponi S, Brais R, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005–2020. doi: 10.1111/ajt.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, Ratner LE, Brown RS Jr, Kato T, Emond JC. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–169. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 60.De Carlis R, Di Sandro S, Lauterio A, Ferla F, Dell’Acqua A, Zanierato M, De Carlis L. Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl. 2017;23:166–173. doi: 10.1002/lt.24666. [DOI] [PubMed] [Google Scholar]
- 61.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant. 2016;21:308–314. doi: 10.1097/MOT.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 63.van Rijn R, van Leeuwen OB, Matton APM, Burlage LC, Wiersema-Buist J, van den Heuvel MC, de Kleine RHJ, de Boer MT, Gouw ASH, Porte RJ. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018;24:655–664. doi: 10.1002/lt.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiPaola F, Shivakumar P, Pfister J, Walters S, Sabla G, Bezerra JA. Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology. 2013;58:1486–1496. doi: 10.1002/hep.26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irie T, Asahina K, Shimizu-Saito K, Teramoto K, Arii S, Teraoka H. Hepatic progenitor cells in the mouse extrahepatic bile duct after a bile duct ligation. Stem Cells Dev. 2007;16:979–987. doi: 10.1089/scd.2007.0037. [DOI] [PubMed] [Google Scholar]
- 66.Selten J, Schlegel A, de Jonge J, Dutkowski P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract Res Clin Gastroenterol. 2017;31:171–179. doi: 10.1016/j.bpg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Boteon YL, Boteon APCS, Attard J, Mergental H, Mirza DF, Bhogal RH, Afford SC. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant. 2018;18:2384–2399. doi: 10.1111/ajt.14992. [DOI] [PubMed] [Google Scholar]


