Introduction
A growing body of literature shows that visceral fat, but not subcutaneous fat, is associated with development of cardiovascular disease (CVD),1, 2, 3, 4 and there are disparate functions and implications of fat stored in these 2 different depots.5 Although the overall relationship between visceral fat and CVD is rarely contested, the relationship in older adults has been heterogeneous. For example, pooled analyses from large, well‐characterized, epidemiologic cohorts have shown that the magnitude of association between obesity (measured by body mass index) and atherosclerosis does not differ across the age spectrum.6 This is in contrast to a body of literature showing that the relationship between obesity and CVD is weaker with increasing age.7 As such, questions remain about the role of central obesity in predicting atherosclerotic CVD (ASCVD) in older adults. This is an important issue given the global obesity epidemic and an increasingly aging population.
In this issue of the Journal of the American Heart Association (JAHA), Schousboe et al examine associations of central adiposity and incident ASCVD in the study of MrOS Sleep (Outcomes of Sleep Disorders in Older Men), an ancillary to the study of MrOS (Osteoporotic Fractures in Men).8 MrOS Sleep is a large, epidemiologic cohort of older white men with comprehensively measured and adjudicated ASCVD outcomes. The ability to comprehensively assess and evaluate central obesity as a predictor of ASCVD outcomes in a large study of older adults is uncommon, and to our knowledge, this is only the second study to do so. Schousboe et al8 found no significant associations between visceral adipose tissue or android‐gynoid fat mass ratio and ASCVD; and this finding persisted in the subset of their cohort who did not have comorbid conditions and the subset who were overweight or normal weight.
The null and paradoxical findings of this study leave us grappling with the clinical and public health implications of developing and/or maintaining higher levels of central adiposity over the life course. Although the MrOS Sleep study data set is a rich resource within which to examine the relationship between central obesity and incident ASCVD, as with all epidemiologic cohorts with data for older adults, there are complexities and potential biases that may be exacerbated in this context. Schousboe et al acknowledged concerns, such as their inability to use computed tomography or magnetic resonance imaging to measure visceral adipose tissue and the unavailability of medical records to confirm the self‐reported health conditions of participants at study baseline. Additional biases that influence these types of analyses include measurement error, collider stratification bias (the “obesity paradox”), and reverse causality.
With regard to measurement error, the increasing use of direct measurements of central obesity, as done by Schousboe et al8 with dual‐energy x‐ray absorptiometry for visceral adipose tissue, helps to minimize the concerns that emerge when proxy measures, such as body mass index, are used in obesity research (Figure). A contemporary approach is the concordant measurement and analysis of subcutaneous fat and lean mass, which allows for better understanding of the underlying cause of CVD in older adults. Widespread utility of concordant measures in observational epidemiologic studies is limited by cost and the participant burden associated with multiple in‐clinic visits and imaging.
Figure 1.
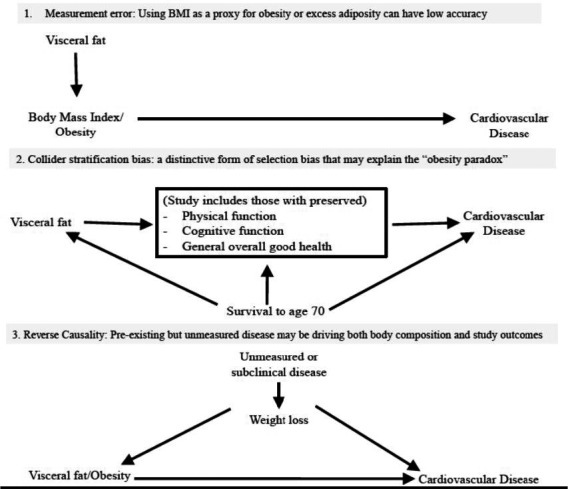
Potential biases in observational epidemiologic studies of obesity. BMI indicates body mass index.
Collider stratification bias (Figure) is a distinctive form of selection bias that may account for the paradoxical findings that were observed.9 This bias emerges from the fact that to participate in a study of central adiposity at ≥70 years, it is necessary to have survived to older age and to have preserved physical and cognitive function. Few Americans are truly free of cardiometabolic disease at the age of 70 years, and this population has already experienced multiple selective survival pressures that can result in biased estimates. Because of this, some have suggested that the obesity paradox does not exist and the relationship of adiposity to CVD should be addressed entirely in the context of unmitigated bias.10 It has even been argued that journals should reject all “future studies on the obesity paradox that fall into the collider bias trap.”11
In all studies of individuals above a certain age or where mortality is the study outcome, reverse causality is a serious concern (Figure) because unmeasured or subclinical disease can result in weight loss or decreased central obesity. This subclinical disease affects the likelihood of the outcome being studied and can bias study estimates of associations. There is increasing evidence of a relationship between adiposity and noncardiometabolic outcomes, such as some cancers, cognitive function, and physical disability. This increases the possibility of conflating intentional weight loss (or reduction in adiposity) with the consequences of unintentional weight loss (or reduction in adiposity). Solutions include beginning follow‐up at younger ages and excluding the 5 years of follow‐up that are adjacent to the time that measurements of adiposity were taken. Doing so would change the focus of this field away from evaluating whether central adiposity after the age of 70 years results in ASCVD in the next 5 to 10 years, toward how to best intervene on excess adiposity over the life course to prevent ASCVD in this age group. The process and consequences of developing obesity as an older adult are likely to differ from those of developing obesity earlier in life. Although the MrOS Sleep study is a longitudinal cohort, it can only provide a snapshot of exposure to central obesity and resulting outcomes, so the challenge to understand the cardiovascular consequences of cumulative exposure to central obesity remains.
A possible misinterpretation of the findings from the study by Schousboe et al8 is that central adiposity does not confer cardiovascular risk and that peripheral adiposity may even be beneficial. The investigators report a significant inverse association between ASCVD and gynoid fat mass (presumably containing a preponderance of subcutaneous fat). Although consistent with studies examining hip circumference and other proxy measures of fat mass traditionally differentiated by sex, the findings from the analyses of the MrOS Sleep study could generate 2 opposing hypotheses: first, subcutaneous fat, itself, is protective against cardiometabolic dysfunction12; and second, the ability to store excess fat in the subcutaneous compartment instead of in the body cavity and in and around the organs mitigates the pathological condition of excess adiposity.13 This second hypothesis suggests that it is not total subcutaneous fat volume, but how much capacity there is to store fat in the subcutaneous depot, that explains both differences in the relationship between visceral and subcutaneous fat with CVD, and potentially the sex differences in CVD risk. To date, subcutaneous fat has not been found to be associated with incident CVD after the impact of visceral fat has been accounted for.1, 2, 3, 4 New research findings in both the clinical and basic science realms present growing support for the importance of subcutaneous capacity,14, 15 and offer additional reminders to exercise caution when ascribing “cause or consequence.” This study in older white men may not be generalizable to other populations (eg, some Asians), who at apparently normal weight, as determined by their body mass index, have excessive visceral fat and higher risks for cardiometabolic diseases.16 Efforts to prevent central obesity through healthy lifestyles seem prudent for all.
No one study can address all methodologic issues, and despite the complexities inherent to this licne of research, the analyses conducted by Schousboe et al8 extend our thinking about this important health issue. Their findings beg the question as to what should be the health priority for adults older than 70 years? Is the priority to extend life by preventing the cardiovascular consequences of central adiposity? Or is the priority to prolong quality of life? And, are there competing conditions that are more likely to affect life expectancy and quality of life, making the focus on primary prevention of CVD less important? It is still unknown whether the ability to retain or accumulate fat mass in older adulthood is a marker of resilience in aging and the absence of disease. It is exceptionally challenging to disentangle the multiple potential sources of potential bias in observational epidemiologic studies of central obesity and ASCVD in older adults.
Disclosures
None.
J Am Heart Assoc. 2018;7:e010119 DOI: 10.1161/JAHA.118.010119.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all‐cause mortality. J Am Coll Cardiol. 2013;62:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Figueroa AL, Takx RA, MacNabb MH, Abdelbaky A, Lavendar ZR, Kaplan RS, Truon QA, Lo J, Ghoshhajra BB, Grinspoon SK, Hoffman U, Tawakol A. Relationship between measures of adiposity, arterial inflammation, and subsequent cardiovascular events. Circ Cardiovasc Imaging. 2016;9:e004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–749. [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell‐Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese‐American men: the 10‐year follow‐up results of the Seattle Japanese‐American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. [DOI] [PubMed] [Google Scholar]
- 5. Mohsen IM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 6. Howard G, Manolio TA, Burke GL, Wolfson SK, O'Leary DH; The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) Investigators. Does the association of risk factors and atherosclerosis change with age? An analysis of the combined ARIC and CHS cohorts. Stroke. 1997;28:1693–1701. [DOI] [PubMed] [Google Scholar]
- 7. Global BMI Mortality Collaboration . Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schousboe JT, Kats AM, Langsetmo L, Vo TN, Taylor BC, Schwartz AV, Cawthon PM, Lewis CE, Barrett‐Connor E, Hoffman AR, Orwoll E, Ensrud KE. Central obesity and visceral adipose tissue are not associated with incident atherosclerotic cardiovascular disease events in older men. J Am Heart Assoc. 2018;7:e009172 DOI: 10.1161/JAHA.118.009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. [DOI] [PubMed] [Google Scholar]
- 10. Banack HR, Stokes A. The “obesity paradox” may not be a paradox at all. Int J Obes. 2017;41:1162. [DOI] [PubMed] [Google Scholar]
- 11. Peeters A. Journals should no longer accept “obesity paradox” articles. Int J Obes (Lond). 2018;42:584. [DOI] [PubMed] [Google Scholar]
- 12. Porter S, Massaro J, Hoffmann U, Vasan R, O'Donnel C, Fox C. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lemieux I. Energy partitioning in gluteal‐femoral fat: does the metabolic fate of triglycerides affect coronary heart disease risk? Arterioscler Thromb Vasc Biol. 2004;24:795–797. [DOI] [PubMed] [Google Scholar]
- 14. Lessard J, Laforest S, Pelletier M, Leboeuf M, Blackburn L, Tchernof A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte. 2014;3:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gujral UP, Vittinghoff E, Mongraw‐Chaffin M, Vaidya D, Kandula NR, Allison M, Carr J, Liu K, Narayan V, Kanaya A. Cardiometabolic abnormalities among normal‐weight persons from five racial/ethnic groups in the United States: a cross‐sectional analysis of two cohort studies. Ann Intern Med. 2017;166:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]


