Abstract
Background
Osteoprotegerin is a cytokine involved in bone metabolism as well as vascular calcification and atherogenesis. Although circulating osteoprotegerin levels are robustly associated with incident cardiovascular disease (CVD) in the general population, its relevance as a biomarker among populations at high CVD risk is less clear.
Methods and Results
Three independent reviewers systematically searched PubMed, EMBASE, and Web of Science to identify prospective studies that had recruited participants on the basis of having conditions related to high CVD risk. A total of 19 studies were eligible for inclusion, reporting on 27 450 patients with diabetes mellitus (2 studies), kidney disease (7 studies), preexisting heart disease (5 studies), or recent acute coronary syndromes (5 studies) at baseline. Over a mean follow‐up of 4.2 years, 4066 CVD events were recorded. In a random‐effects meta‐analysis, the pooled risk ratio for CVD events comparing people in the top versus the bottom tertile of osteoprotegerin concentration was 1.30 (95% confidence interval, 1.12–1.50; P<0.001; I2=68.3%). There was evidence for presence of publication bias (P value from Egger's test=0.013). Correction for publication bias using the trim‐and‐fill method reduced the risk ratio to 1.21 (95% confidence interval, 1.03–1.42; P<0.001). The risk ratios did not vary significantly by population type, geographical region, statistical adjustment, sample or assay type, age, sex, or length of follow‐up.
Conclusions
In populations at high CVD risk, elevated circulating osteoprotegerin levels are associated with a higher risk for future CVD events. The magnitude of association appears weaker than in the general population.
Keywords: cardiovascular disease, high‐risk population, meta‐analysis, osteoprotegerin, prospective cohorts
Subject Categories: Cardiovascular Disease, Biomarkers, Meta Analysis, Mortality/Survival
Clinical Perspective
What Is New?
In the present report, we systematically reviewed and combined the published evidence on the relevance of circulating osteoprotegerin concentration to cardiovascular events in high‐risk populations.
Our meta‐analysis demonstrated a significant positive association between osteoprotegerin concentration and cardiovascular disease risk.
The magnitude of association was similar in various clinically significant subgroups, including those defined by age and sex.
What Are the Clinical Implications?
This work highlights the potential of osteoprotegerin as a biomarker for cardiovascular disease risk.
Introduction
Osteoprotegerin is a member of the tumor necrosis factor (TNF) receptor superfamily and is involved in bone homeostasis.1 It inhibits osteoclastogenesis by binding to the receptor activator of nuclear factor‐κB ligand (RANKL), which prevents RANKL from binding to the receptor activator of nuclear factor‐κB (RANK).2 Inhibition of the RANK/RANKL pathway results in less osteoclast differentiation as well as reduced activation and survival of mature osteoclasts.2, 3 TNF‐related apoptosis‐inducing ligand, a protein that belongs to the TNF superfamily as well, also serves as an osteoprotegerin ligand.4 Osteoprotegerin thereby contributes to maintaining the balance between bone resorption and bone formation.2, 5
In addition to its role in bone homeostasis, osteoprotegerin has been implicated in the development of cardiovascular diseases (CVDs).6 It is found in atherosclerotic plaques,7, 8 may regulate vascular calcification,9, 10 and may thereby influence cardiovascular risk. Furthermore, genetic studies have demonstrated associations of osteoprotegerin gene polymorphisms with CVD.11, 12, 13, 14, 15 In a literature‐based meta‐analysis of 9 general population studies, we recently demonstrated robust positive associations between osteoprotegerin concentration and incident CVD.16 However, it is unclear whether these associations equally apply to high‐risk populations. Although several individual studies have investigated the predictive significance of osteoprotegerin in these settings,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 the interpretation of their findings has been complicated by differing in scales of association, levels of adjustment, and outcome definitions.
The principal aim of this report is to review comprehensively the available literature and to perform a meta‐analysis of reported associations between osteoprotegerin and risk for future CVD in high‐risk populations (ie, in studies that have recruited participants on the basis of having conditions related to high CVD risk). Secondary analyses will assess associations with coronary heart disease (CHD) events and stroke separately and will clarify whether the magnitude of association differs according to study‐level characteristics.
Methods
Research Data Availability
The database of published results from studies included in the meta‐analysis is made available to other researchers for purposes of reproducing the results or replicating the procedure.36
Literature Search, Study Selection, and Data Extraction
We systematically sought PubMed, Web of Science, and EMBASE for prospective studies published between January 1970 and April 2017 that reported on associations of osteoprotegerin concentration with CVD outcomes (defined as nonfatal CHD [ie, myocardial infarction, unstable or stable angina, or coronary revascularization procedures], nonfatal stroke, or cardiovascular death). We also scanned reference lists of articles (including reviews) and corresponded with several study investigators. Table S1 provides a detailed description of search terms used in the literature search. Studies were eligible for inclusion if they (1) had a prospective design; (2) had recruited study participants on the basis of having preexisting conditions favoring risk of future CVD; and (3) had recorded incident CVD outcomes over a period of >1 month. Studies that did not report on the predefined outcome definition (including those reporting on the combination of CVD events and all‐cause mortality) were excluded from the analysis.
For each eligible study, 3 reviewers (L.T., G.K., P.W.) independently extracted the following pieces of information: type of baseline disease, study location, year of baseline, duration of follow‐up, mean or median age at baseline, proportion of male participants, osteoprotegerin assay type (ELISAs versus immunofluorometric assays), osteoprotegerin assay manufacturers, and sample types (plasma versus serum). In addition, we extracted information on the statistical adjustment used, categorizing adjustments as “unadjusted” if no adjustment was employed; “+” for adjustment for age and sex; “++” for adjustment for age, sex, and non–blood‐based risk factors, such as smoking, blood pressure, and diabetes mellitus; and “+++” for further adjustments for blood‐based risk factors (eg, cholesterol and C‐reactive protein). If a study reported different adjustment models, the most adjusted model was used to minimize the scope for confounding. If information about the same study was published twice or more often, we used the most recent publication. Study quality was evaluated using the Newcastle‐Ottawa scale for cohort studies.37 The meta‐analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.38
Statistical Analyses
We conducted analyses according to a predefined statistical analysis plan. The primary outcome was CVD events (as defined above); secondary outcomes were CHD events and stroke events. Because studies reported effect estimates on different scales (eg, per standard deviation or across quartiles), we converted risk ratios and 95% confidence intervals (CIs) to reflect a comparison of the top versus bottom tertiles of baseline osteoprotegerin distribution using methods described elsewhere.39 One study29 did not provide sufficient information on the osteoprotegerin distribution—a prerequisite for converting its risk ratio—and we therefore estimated its distribution on the basis of comparable study populations.26, 28, 30 We pooled study‐specific risk ratios using random‐effects meta‐analysis (sensitivity analyses used fixed‐effect meta‐analysis). The I2 statistic was used to assess heterogeneity across studies.40 Subgroup analyses were conducted using meta‐regression across prespecified study‐level characteristics.40 We evaluated whether publication bias was present by visually inspecting a funnel plot and applying Egger's asymmetry test.41 We estimated a risk ratio corrected for publication bias using the trim‐and‐fill method, which imputes artificial studies to achieve symmetry of the funnel plot.42 In addition, to evaluate the influence of single studies on the overall result, we performed a leave‐one‐out cross‐validation, which reestimates the pooled risk ratio while omitting each study in turn. All statistical tests were 2‐sided; P<0.05 was deemed as statistically significant. Data were analyzed using the statistical software Stata, version 14.1 (StataCorp). Because our analysis relied entirely on data available in the published literature, approval by the institutional review board of the project was not required.
Results
General Characteristics of Included Studies
Of 2602 records retrieved from PubMed, Web of Science, and EMBASE, we excluded 1001 duplicates and 1318 records after review of titles and abstracts (Figure 1). When reviewing the full text of the remaining 283 articles, we excluded a further 264 additional articles, leaving 19 prospective studies17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 eligible for inclusion in the meta‐analysis. Patients were recruited on the basis of having diabetes mellitus in 2 studies, kidney disease in 7 studies, preexisting heart disease in 5 studies, and recent acute coronary syndromes in 5 studies. Details on the definitions of baseline conditions are provided in Table S2. Twelve studies were based in Europe, 4 were based in Asia, and 3 were located in multiple continents (Table 1). Of the 19 prospective studies, 7 were nested in a trial. The weighted mean age was 60.9 years; 68.3% of patients were men. The average quality of the studies assessed by the Newcastle‐Ottawa scale for cohort studies was 7.6. For measuring osteoprotegerin concentrations, 15 studies used ELISAs and 4 studies used immunofluorometric assays. Ten studies had measured osteoprotegerin concentration in plasma, and 9 had measured osteoprotegerin concentration in serum. In total, the studies involved 27 450 participants and reported on 4066 CVD outcomes recorded over a weighted mean follow‐up duration of 4.2 years (Table 2). One study reported unadjusted effect estimates; another 3 studies reported effect estimates adjusted for age, sex, and non–blood‐based markers; 14 studies reported multivariable adjusted effect estimates (including blood‐based markers); and 1 study reported unadjusted risk ratios for CVD events and multivariable adjusted risk ratios for stroke.
Figure 1.
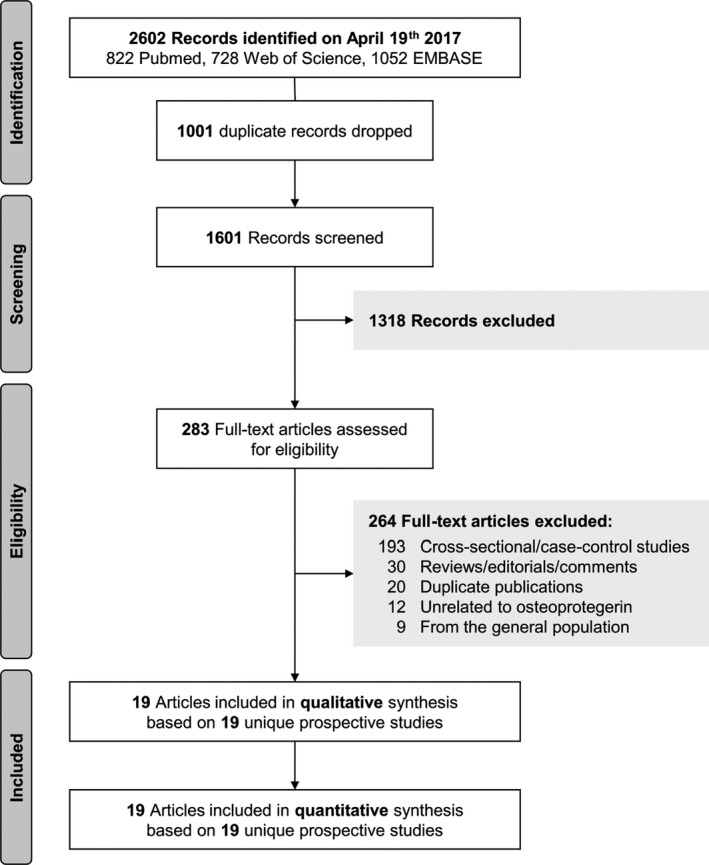
Study flow diagram.
Table 1.
Design Features of Contributing Studies
| Study Acronym or First Author | Location | Year of Baseline, Range | Study Quality, NOS | Mean Age, y | Male Sex, % | Osteoprotegerin Assay Type (Manufacturer) | Sample Type |
|---|---|---|---|---|---|---|---|
| Populations with diabetes mellitus at baseline | |||||||
| Anand17 | United Kingdom | NR | 7 | 52.7 | 60.6 | ELISA (Biomedica) | Plasma |
| FINNDIANE18 | Finland | 1997–2004 | 8 | 36.9 | 49.8 | IFMA (R&D Systems) | Serum |
| Populations with kidney disease at baseline | |||||||
| ALERT19, a | Multicenter | 1997 | 7 | 49.6 | 65.8 | ELISA (Biomedica) | Serum |
| CRISIS20 | United Kingdom | 2002–2010 | 6 | 63.8 | 61.8 | ELISA (BioVendor) | Plasma |
| Kuzniewski21 | Poland | 2004 | 8 | 60.0 | 56.5 | ELISA (BioVendor) | Plasma |
| Nakashima22 | Japan | 2003 | 7 | 62.1 | 56.3 | ELISA (Immundiagnostik) | Plasma |
| Nishiura23 | Japan | 2000–2006 | 7 | 58.9 | 65.7 | ELISA (Immundiagnostik) | Serum |
| Speer24 | Hungary | 2004–2007 | 7 | 63.4 | 61.2 | ELISA (Immundiagnostik) | Serum |
| Yilmaz25 | Turkey | 2009–2013 | 7 | 48.9 | 51.9 | ELISA (RayBiotech) | Serum |
| Populations with preexisting heart disease at baseline | |||||||
| CLARICOR26, a | Denmark | 1999–2000 | 9 | 65.4 | 69.4 | IFMA (R&D Systems) | Serum |
| CORONA27, a | The Netherlands | 2003–2005 | 8 | 72.0 | 76.7 | ELISA (R&D Systems) | Plasma |
| Jono28 | Japan | 1999–2000 | 8 | 63.1 | 82.7 | ELISA (Cosmo Bio) | Serum |
| PEACE29, a | Multicenter | 1996–2000 | 6 | 63.7 | 81.0 | ELISA (R&D Systems) | Plasma |
| Pedersen (1)30 | Norway | 2000–2001 | 9 | 62.0b | 71.9 | ELISA (R&D Systems) | Serum |
| Populations with recent acute coronary syndromes at baseline | |||||||
| MERLIN‐TIMI3631, a | Italy | 2004–2006 | 8 | 64.0 | 64.9 | IFMA (R&D Systems) | Plasma |
| OPTIMAAL32 | Multicenter | 1998–1999 | 9 | 67.8 | 70.3 | ELISA (R&D Systems) | Plasma |
| PLATO33, a | Multicenter | 2006–2008 | 6 | 62.0 | 71.6 | ELISA (NR) | Plasma |
| PRACSIS34 | Sweden | 1996–2001 | 9 | 65.0 | 70.7 | ELISA (R&D Systems) | Serum |
| Pedersen (2)35 | Denmark | 2006–2008 | 9 | 63.5 | 41.3 | IFMA (R&D Systems) | Plasma |
| Total | 1996–2013 | 7.6 | 60.9 | 68.3 | |||
Summary statistics are ranges, weighted means, or sums, as appropriate. ALERT indicates Assessment of Lescol in Renal Transplantation Study; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CORONA, Controlled Rosuvastatin Multinational Trial; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; FINNDIANE, Finnish Diabetic Nephropathy Study; IFMA, immunofluorometric assay; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; NOS, Newcastle‐Ottawa scale; NR, not reported; OPTIMAAL, Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PLATO, Platelet Inhibition and Patient Outcomes Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
Nested in clinical trial.
Median.
Table 2.
Follow‐Up Data in the Contributing Studies
| Study Acronym or First Author | Maximum Follow‐Up, y | No. of Participants | No. of Events | Adjustment of Reported Risk Ratio | ||
|---|---|---|---|---|---|---|
| CVD | CHD | Stroke | ||||
| Populations with diabetes mellitus at baseline | ||||||
| Anand17 | 1.5a | 510 | 16 | ··· | ··· | Unadjusted |
| FINNDIANE18 | 10.5a | 1903 | 190 | 152 | 71 | ++ |
| Populations with kidney disease at baseline | ||||||
| ALERT19, b | 6.7a | 1889 | ··· | 285 | ··· | +++ |
| CRISIS20 | 3.8a | 463 | 108 | ··· | ··· | +++ |
| Kuzniewski21 | 7.0 | 69 | 31 | ··· | ··· | +++ |
| Nakashima22 | 6.0 | 151 | 40 | ··· | ··· | ++ |
| Nishiura23 | 3.5a | 99 | 27 | ··· | ··· | +++ |
| Speer24 | 2.6 | 98 | 23 | ··· | ··· | +++ |
| Yilmaz25 | 3.0d | 291 | 87 | ··· | ··· | +++ |
| Populations with preexisting heart disease at baseline | ||||||
| CLARICOR26, b | 2.6a | 4063 | 623 | 303 | 146 | +++ |
| CORONA27, b | 3.0 | 1464 | 318 | 255 | ··· | +++ |
| Jono28 | 5.1a | 225 | 101 | ··· | ··· | +++ |
| PEACE29, b | 7.0 | 3767 | 1290 | ··· | NR | Unadjusted/+++c |
| Pedersen (1)30 | 6.1d | 1025 | 60 | 103 | ··· | +++ |
| Populations with recent acute coronary syndromes at baseline | ||||||
| MERLIN‐TIMI3631, b | 0.9d | 4463 | 544 | 336 | ··· | +++ |
| OPTIMAAL32 | 2.3a | 234 | 26 | ··· | ··· | +++ |
| PLATO33, b | 2.4 | 5123 | 432 | ··· | ··· | ++ |
| PRACSIS34 | 10.1 | 897 | 150 | 107 | 43 | +++ |
| Pedersen (2)35 | 2.3d | 716 | ··· | 51 | ··· | +++ |
| Total | 4.2 | 27 450 | 4066 | 1592 | 260 | |
Summary statistics are weighted means or sums, as appropriate. ++ indicates adjusted for age, sex, and non–blood‐based risk factors; +++, further adjusted for blood‐based risk factors; ALERT, Assessment of Lescol in Renal Transplantation Study; CHD, coronary heart disease; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; CVD, cardiovascular disease; FINNDIANE, Finnish Diabetic Nephropathy Study; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; NR, study did not report the number of stroke events (despite reporting hazard ratios for stroke); OPTIMAAL, Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan; PLATO, Platelet Inhibition and Patient Outcomes Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
Mean.
Nested in clinical trial.
Median.
Study reported unadjusted risk ratios for the outcome cardiovascular events and multiple adjustment for the outcome stroke.
Overall Association of Osteoprotegerin With Cardiovascular Events
Figure 2 shows the forest plot of the association of baseline osteoprotegerin concentration with incident CVD events. The pooled relative risk for CVD events was 1.30 (95% CI, 1.12–1.50; P<0.001) for a comparison of individuals in the top versus the bottom tertile of baseline osteoprotegerin concentration. There was a high degree of between‐study heterogeneity (I2=68.3%; P<0.001). In comparison, a fixed‐effect meta‐analysis yielded a pooled risk ratio of 1.15 (95% CI, 1.10–1.21; P<0.001).
Figure 2.
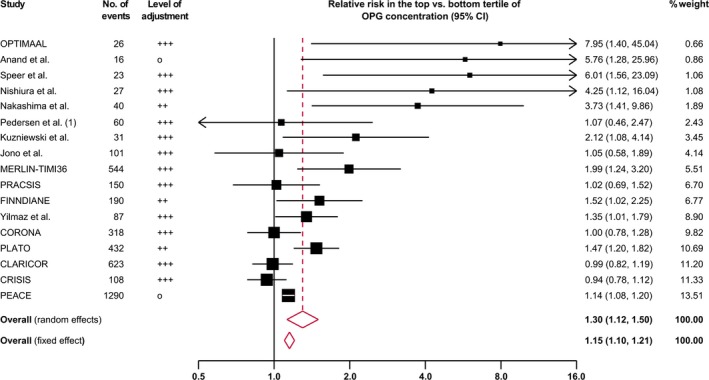
Combined relative risk for cardiovascular events in the top vs the bottom tertile of osteoprotegerin (OPG) concentration. Sizes of data markers indicate the weight of each study in the analysis. The I2 value was 68.3% (P<0.001). CI indicates confidence interval; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CORONA, Controlled Rosuvastatin Multinational Trial; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; FINNDIANE, Finnish Diabetic Nephropathy Study; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; OPTIMAAL, Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PLATO, Platelet Inhibition and Patient Outcomes Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
There was evidence for publication bias, as indicated by the funnel plot (Figure 3) and a significant Egger's asymmetry test (P=0.013). Using the trim‐and‐fill method, 5 additional artificial studies were included into the meta‐analysis to generate a symmetric funnel plot (Figure S1). This correction for publication bias yielded a relative risk of 1.21 (95% CI, 1.03–1.42; P=0.020). Reestimated pooled risk ratios by omitting each study in turn remained significant for all omissions (Figure 4).
Figure 3.
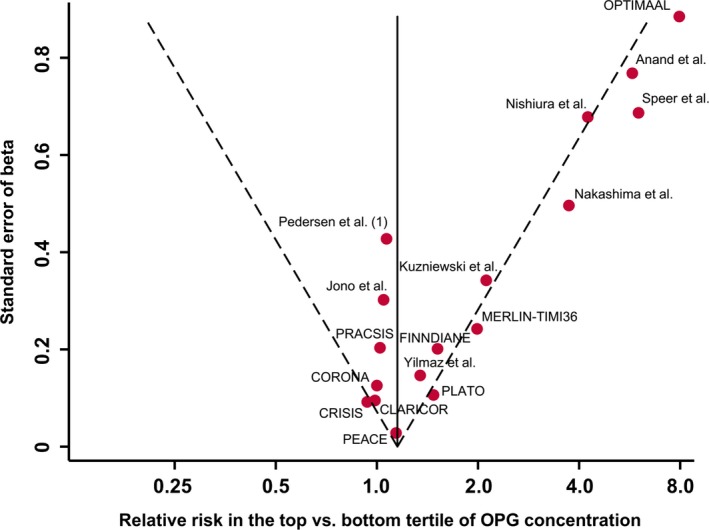
Funnel plot of reported associations between osteoprotegerin (OPG) concentration and risk of cardiovascular events. The dotted lines show pseudo 95% confidence intervals around the overall pooled estimate. The P value from Egger's asymmetry test of associations was 0.013. CLARICOR indicates Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CORONA, Controlled Rosuvastatin Multinational Trial; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; FINNDIANE, Finnish Diabetic Nephropathy Study; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; OPTIMAAL, Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PLATO, Platelet Inhibition and Patient Outcomes Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
Figure 4.
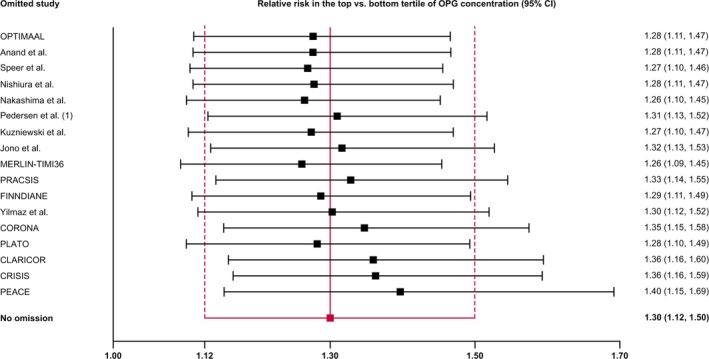
Reestimated pooled risk ratios for cardiovascular outcomes omitting one study in each turn. CI indicates confidence interval; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CORONA, Controlled Rosuvastatin Multinational Trial; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; FINNDIANE, Finnish Diabetic Nephropathy Study; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; OPG, osteoprotegerin; OPTIMAAL, Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PLATO, Platelet Inhibition and Patient Outcomes Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
A subset of studies had published risk ratios separately on the secondary outcomes CHD and stroke. When comparing the top versus the bottom tertile of baseline osteoprotegerin concentration, the risk ratio was 1.24 (95% CI, 0.94–1.64; 8 studies; 1592 events; P=0.128) for CHD and 1.21 (95% CI, 0.97–1.50; 4 studies; 260 events; P=0.090) for stroke (Figure 5). The I2 value for between‐study heterogeneity was high for CHD (71.7%; P=0.001) and low for stroke (0%; P=0.661). Corresponding risk ratios using fixed‐effect meta‐analysis were 1.14 (95% CI, 0.99–1.32; P=0.063) for CHD and 1.21 (95% CI, 0.97–1.50; P=0.090) for stroke.
Figure 5.
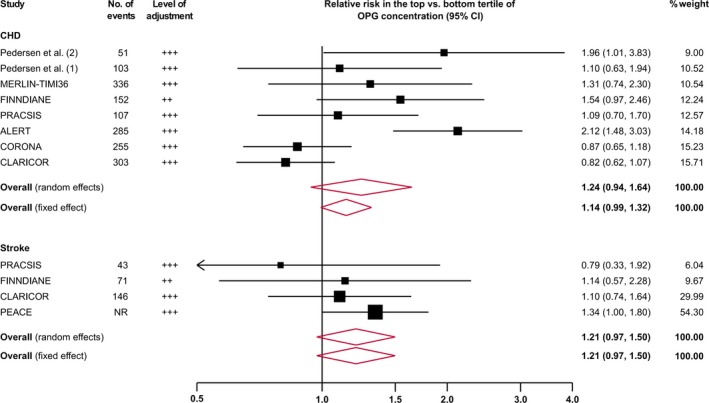
Combined relative risk for future coronary heart disease (CHD) and stroke in the top vs the bottom tertile of osteoprotegerin (OPG) concentration. Sizes of data markers indicate the weight of each study in the analysis. The I2 value was 71.7% (P=0.001) for CHD and 0% (P=0.661) for stroke. ALERT indicates Assessment of Lescol in Renal Transplantation Study; CI, confidence interval; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CORONA, Controlled Rosuvastatin Multinational Trial; FINNDIANE, Finnish Diabetic Nephropathy Study; MERLIN‐TIMI36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Elevation Acute Coronary Syndromes Trial; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PRACSIS, Prognosis and Risk in Acute Coronary Syndrome in Sweden.
Findings According to Study‐Level Characteristics
Figure 6 presents risk ratios pooled according to study‐level characteristics. There were no significant differences in the strength of association according to population type, geographical region, statistical adjustment, sample type, and assay type (all P>0.05). Furthermore, there was no evidence that the strength of association differed according to mean age, sex distribution, or length of follow‐up of the study population (P values from meta‐regression: 0.354, 0.170, and 0.564, respectively).
Figure 6.
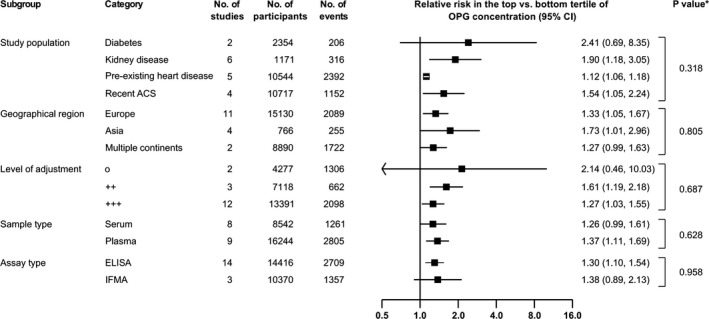
Relative risks for cardiovascular outcomes in individuals in the top vs bottom tertile of osteoprotegerin (OPG) concentration, according to categories of study‐level characteristics. *P values were calculated from meta‐regression. Levels of adjustment: o, unadjusted; ++, adjusted for age, sex, and non–blood‐based risk factors; +++, additionally adjusted for at least 1 blood‐based risk factor. ACS indicates acute coronary syndrome; CI, confidence interval; IFMA, immunofluorometric assay.
Discussion
In this literature‐based meta‐analysis, we analyzed 19 high‐risk population studies involving a total of 27 450 participants recruited between 1996 and 2013. Our analysis identified positive associations between osteoprotegerin concentrations and cardiovascular risk. Individuals with a high osteoprotegerin concentration (ie, in the top tertile of baseline osteoprotegerin distribution) had a relative risk of 1.30 (95% CI, 1.12‐1.50) for CVD events when compared with individuals with osteoprotegerin levels in the bottom tertile. This relative risk remained stable under multivariable adjustment and across various study‐level characteristics. The between‐study heterogeneity was high (I2=68.3%). Although studies varied in terms of population type, geographical region, level of adjustment, sample type, assay type, proportion of men, mean age, and length of follow‐up, none of these characteristics significantly influenced the strength of association of osteoprotegerin with future CVD risk. However, our analysis identified significant publication bias resulting from predominantly strong positive results in small studies. After correcting for publication bias, the relative risk was reduced to 1.21, but remained significant, with a 95% CI ranging from 1.03 to 1.42. In addition, we confirmed with the leave‐one‐out cross‐validation method that our overall result was not driven by a single study, highlighting the robustness of our finding.
We have previously demonstrated in a literature‐based meta‐analysis that osteoprotegerin is associated with incident CVD in people recruited from the general community.16 A combination of findings from 9 general population studies yielded a pooled relative risk for CVD of 1.83 (95% CI, 1.46‐2.30) for a comparison of extreme osteoprotegerin tertiles. In comparison, the present meta‐analysis of studies involving individuals at high CVD risk yielded an association directionally concordant but significantly weaker (Figure S2).
Three distinct features of high‐risk populations may contribute to this weaker association. First, most high‐risk individuals received (multi‐)drug treatment. It has been demonstrated that in vivo treatment with antidiabetic medication,43, 44, 45, 46 statins,47, 48 heparins,49, 50 or glucocorticoids51 and in vitro treatment with irbesartan52 or different immunosuppressive therapies53 affect circulating osteoprotegerin levels. Second, circulating osteoprotegerin levels differ in people with preexisting diseases. For instance, increased osteoprotegerin levels can be found in patients with preexisting CVD, such as severe peripheral artery disease,54 heart failure,55 and ST‐segment–elevation acute myocardial infarction.56 Moreover, serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease.57 Patients with type 1 or type 2 diabetes mellitus exhibit elevated osteoprotegerin levels.43, 44 High osteoprotegerin values have also been linked to poor glycemic control58, 59 and severity of diabetic nephropathy.60, 61 In patients with chronic renal failure, levels of osteoprotegerin are higher compared with healthy controls,62 are inversely correlated with glomerular filtration rate,63 and correlate with time on maintenance hemodialysis in patients with end‐stage renal disease.64 Third, associations of osteoprotegerin may be attenuated because of the dominance of other factors more relevant to CVD risk in high‐risk patients, including highly prevalent traditional CVD risk factors as well as factors related to quality of clinical care, treatment response, or medication adherence.65 Altogether, differences in medical treatment, patient histories, disease severity, multimorbidities, and clinical course of disease among high‐risk patients may obscure associations of osteoprotegerin levels with risk for future CVD and might result in reverse causation bias.
The pathophysiological role of osteoprotegerin in CVD development is multifaceted and not completely understood. It is considered to reflect the overall activity of the osteoprotegerin/RANK/RANKL signaling pathway and regulate calcification in both the bone and the vasculature.9, 10 Osteoprotegerin is expressed in a variety of human tissues1; in the vessel wall, it is mainly secreted by endothelial66 and vascular smooth muscle cells.67 Beneficial effects of osteoprotegerin on the cardiovascular system were reported by several earlier studies. For instance, osteoprotegerin deficiency in mice led to early‐onset osteoporosis and arterial calcification.68 Furthermore, osteoprotegerin inactivation in apolipoprotein E–deficient knockout mice increased plaque calcification.69 In in vitro studies, osteoprotegerin was found to inhibit calcification in vascular smooth muscle cells70 and act as a survival factor in endothelial cells.71 In contrast, several lines of evidence from experimental studies in animals and cell cultures suggested harmful effects of osteoprotegerin in agreement with the positive association with CVD risk in our meta‐analysis.6 Osteoprotegerin not only contributes to systemic inflammation,72 but also to vasculature‐specific inflammation by increasing macrophage infiltration73 and leukocyte adhesion to endothelial cells.74, 75 Moreover, it promotes vascular medial fibrosis76 and may exert indirect proatherosclerotic effects by blocking TNF‐related apoptosis‐inducing ligand.77 Atherosclerotic plaques that highly express osteoprotegerin exhibit more calcification,8 but studies yielded conflicting results about its relevance to plaque stability and conversion to a symptomatic plaque.52, 73, 78, 79 These inconsistent reports emphasize the wide‐ranging aspects of osteoprotegerin in the complexity of regulatory processes in atherogenesis and call for more experimental studies to improve our understanding of this pathway in human disease.
Although our meta‐analysis shows positive associations of baseline osteoprotegerin concentration and CVD risk, its incorporation in clinical routine entails some analytical issues. First, available commercial kits for osteoprotegerin measurement use different reference standards of different molecular weight and may, therefore, produce different absolute osteoprotegerin values for the same sample.80 Second, previous findings underline the importance of standardized preanalytical and analytical conditions and the need of establishing valid reference ranges for both serum‐ and plasma‐derived blood samples, because osteoprotegerin levels were found lower in serum than in plasma samples.81 In analogy to other emerging biomarkers, such as troponin I,82 addressing these analytical issues will be an important next step for any use of osteoprotegerin assessment in clinical practice, including the definition of risk thresholds and potential use in risk prediction.
Strengths of the current review include the systematic and comprehensive search of the literature and the standardization of different reported parameters. We rescaled the reported relative risks to reflect a uniform scale (top versus bottom tertile), thereby enabling a direct comparison between the study estimates.39 A weakness of the present analysis is that we relied on published information when combining effect estimates from the different studies. A meta‐analysis of individual‐participant data would allow a more consistent approach in defining CVD outcomes and adjusting effect estimates for potential confounding factors. Also, most of the CVD outcomes in our primary analysis were related to CHD and less to stroke. In addition, further investigations on the different components of the osteoprotegerin/RANK/RANKL/TNF‐related apoptosis‐inducing ligand pathway could provide useful pathogenic insight into the role of osteoprotegerin in CVD.
In conclusion, osteoprotegerin is associated with the risk of future CVD in high‐risk populations. The magnitude of association appears weaker than in general population studies.
Author Contributions
Tschiderer, Klingenschmid, and P. Willeit conducted the systematic literature search, analyzed data, and wrote the article. Nagrani, J. Willeit, Schett, Kiechl, and Laukkanen contributed to writing the discussion and reviewing the article. P. Willeit is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This work was supported by a K‐project grant from the Austrian Research Promotion Agency (“VASCage,” grant 843536).
Disclosures
None.
Supporting information
Table S1. Search Terms Used to Identify Relevant Articles
Table S2. Detailed Baseline Conditions of Contributing Studies
Figure S1. Funnel plot including artificial studies generated with the “trim and fill” method.
Figure S2. Comparison of combined relative risk for cardiovascular events in the top vs the bottom tertile of osteoprotegerin concentration of high‐risk populations and general population results.20
(J Am Heart Assoc. 2018;7:e009012 DOI: 10.1161/JAHA.118.009012.)
References
- 1. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw‐Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. [DOI] [PubMed] [Google Scholar]
- 2. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. [DOI] [PubMed] [Google Scholar]
- 3. Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast‐mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. [DOI] [PubMed] [Google Scholar]
- 5. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. [DOI] [PubMed] [Google Scholar]
- 6. Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4:801–811. [DOI] [PubMed] [Google Scholar]
- 7. Schoppet M, Al‐Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor‐related apoptosis‐inducing ligand, and receptor activator of nuclear factor‐kappaB ligand in Monckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–4112. [DOI] [PubMed] [Google Scholar]
- 8. Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. [DOI] [PubMed] [Google Scholar]
- 9. Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. [DOI] [PubMed] [Google Scholar]
- 10. Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. [DOI] [PubMed] [Google Scholar]
- 11. Biscetti F, Giovannini S, Straface G, Bertucci F, Angelini F, Porreca C, Landolfi R, Flex A. RANK/RANKL/OPG pathway: genetic association with history of ischemic stroke in Italian population. Eur Rev Med Pharmacol Sci. 2016;20:4574–4580. [PubMed] [Google Scholar]
- 12. Song D‐H, Zhou P‐Z, Xiu X‐L, Zhou G‐H, Sun Y‐X, Song C. Relationships of OPG genetic polymorphisms with susceptibility to cardiovascular disease: a meta‐analysis. Med Sci Monit. 2016;22:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Straface G, Biscetti F, Pitocco D, Bertoletti G, Misuraca M, Vincenzoni C, Snider F, Arena V, Stigliano E, Angelini F, Iuliano L, Boccia S, de Waure C, Giacchi F, Ghirlanda G, Flex A. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke. 2011;42:3022–3028. [DOI] [PubMed] [Google Scholar]
- 14. Ueland T, Wilson SG, Amirul Islam FM, Mullin B, Devine A, Bollerslev J, Zhu K, Prince RL. A cohort study of the effects of serum osteoprotegerin and osteoprotegerin gene polymorphisms on cardiovascular mortality in elderly women. Clin Endocrinol (Oxf). 2009;71:828–833. [DOI] [PubMed] [Google Scholar]
- 15. Zhao H, Cao Y, Chen H, Xu W, Sun X, Pan X. The association between OPG rs3102735 gene polymorphism, microembolic signal and stroke severity in acute ischemic stroke patients. Gene. 2017;613:25–29. [DOI] [PubMed] [Google Scholar]
- 16. Tschiderer L, Willeit J, Schett G, Kiechl S, Willeit P. Osteoprotegerin concentration and risk of cardiovascular outcomes in nine general population studies: literature‐based meta‐analysis involving 26,442 participants. PLoS One. 2017;12:e0183910 DOI: 10.1371/journal.pone.0183910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anand DV, Lahiri A, Lim E, Hopkins D, Corder R. The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol. 2006;47:1850–1857. [DOI] [PubMed] [Google Scholar]
- 18. Gordin D, Soro‐Paavonen A, Thomas MC, Harjutsalo V, Saraheimo M, Bjerre M, Forsblom C, Flyvbjerg A, Groop P‐H. Osteoprotegerin is an independent predictor of vascular events in Finnish adults with type 1 diabetes. Diabetes Care. 2013;36:1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svensson M, Dahle DO, Mjoen G, Weihrauch G, Scharnagl H, Dobnig H, Marz W, Jardine A, Fellstrom B, Holdaas H. Osteoprotegerin as a predictor of renal and cardiovascular outcomes in renal transplant recipients: follow‐up data from the ALERT study. Nephrol Dial Transplant. 2012;27:2571–2575. [DOI] [PubMed] [Google Scholar]
- 20. Alderson HV, Ritchie JP, Middleton R, Larsson A, Larsson TE, Kalra PA. FGF‐23 and osteoprotegerin but not fetuin‐A are associated with death and enhance risk prediction in non‐dialysis chronic kidney disease stages 3‐5. Nephrology. 2016;21:566–573. [DOI] [PubMed] [Google Scholar]
- 21. Kuzniewski M, Fedak D, Dumnicka P, Stepien E, Kusnierz‐Cabala B, Cwynar M, Sulowicz W. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv Med Sci. 2016;61:269–275. [DOI] [PubMed] [Google Scholar]
- 22. Nakashima A, Carrero JJ, Qureshi AR, Hirai T, Takasugi N, Ueno T, Taniguchi Y, Lindholm B, Yorioka N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos Int. 2011;22:1695–1701. [DOI] [PubMed] [Google Scholar]
- 23. Nishiura R, Fujimoto S, Sato Y, Yamada K, Hisanaga S, Hara S, Nakao H, Kitamura K. Elevated osteoprotegerin levels predict cardiovascular events in new hemodialysis patients. Am J Nephrol. 2009;29:257–263. [DOI] [PubMed] [Google Scholar]
- 24. Speer G, Fekete BC, El Hadj Othmane T, Szabo T, Egresits J, Fodor E, Kiss I, Logan AG, Nemcsik J, Szabo A, Nemeth ZK, Szathmari M, Tisler A. Serum osteoprotegerin level, carotid‐femoral pulse wave velocity and cardiovascular survival in haemodialysis patients. Nephrol Dial Transplant. 2008;23:3256–3262. [DOI] [PubMed] [Google Scholar]
- 25. Yilmaz MI, Siriopol D, Saglam M, Unal HU, Karaman M, Gezer M, Kilinc A, Eyileten T, Guler AK, Aydin I, Vural A, Oguz Y, Covic A, Ortiz A, Kanbay M. Osteoprotegerin in chronic kidney disease: associations with vascular damage and cardiovascular events. Calcif Tissue Int. 2016;99:121–130. [DOI] [PubMed] [Google Scholar]
- 26. Bjerre M, Hilden J, Kastrup J, Skoog M, Hansen JF, Kolmos HJ, Jensen GB, Kjoller E, Winkel P, Flyvbjerg A, Gluud C. Osteoprotegerin independently predicts mortality in patients with stable coronary artery disease: the CLARICOR trial. Scand J Clin Lab Invest. 2014;74:657–664. [DOI] [PubMed] [Google Scholar]
- 27. Ueland T, Dahl CP, Kjekshus J, Hulthe J, Bohm M, Mach F, Goudev A, Lindberg M, Wikstrand J, Aukrust P, Gullestad L. Osteoprotegerin predicts progression of chronic heart failure: results from CORONA. Circ Heart Fail. 2011;4:145–152. [DOI] [PubMed] [Google Scholar]
- 28. Jono S, Otsuki S, Higashikuni Y, Shioi A, Mori K, Hara K, Hashimoto H, Ikari Y. Serum osteoprotegerin levels and long‐term prognosis in subjects with stable coronary artery disease. J Thromb Haemost. 2010;8:1170–1175. [DOI] [PubMed] [Google Scholar]
- 29. Omland T, Cristophi C, Flyvbjerg A, Rice MM, Jablonski KA, Rasmussen L, Rouleau JL, Sabatine MS, Pfeffer MA, Braunwald E. Abstract P653: circulating osteoprotegerin levels are associated with peripheral vascular intervention and stroke in patients with stable coronary artery disease: the PEACE trial. Eur Heart J. 2008;29:85. [Google Scholar]
- 30. Pedersen ER, Ueland T, Seifert R, Aukrust P, Schartum‐Hansen H, Ebbing M, Bleie O, Igland J, Svingen G, Nordrehaug JE, Nygard O. Serum osteoprotegerin levels and long‐term prognosis in patients with stable angina pectoris. Atherosclerosis. 2010;212:644–649. [DOI] [PubMed] [Google Scholar]
- 31. Roysland R, Bonaca MP, Omland T, Sabatine M, Murphy SA, Scirica BM, Bjerre M, Flyvbjerg A, Braunwald E, Morrow DA. Osteoprotegerin and cardiovascular mortality in patients with non‐ST elevation acute coronary syndromes. Heart. 2012;98:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. [DOI] [PubMed] [Google Scholar]
- 33. Ueland T, Åkerblom A, Ghukasyan T, Michelsen AE, Aukrust P, Becker RC, Bertilsson M, Himmelmann A, James SK, Siegbahn A, Storey RF, Kontny F, Wallentin L. Abstract 12851: osteoprotegerin is associated with major bleeding but not with cardiovascular outcomes in patients with acute coronary syndromes: insights from the PLATelet inhibition and patient outcomes (PLATO) trial. Circulation. 2016;134:A12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Omland T, Ueland T, Jansson AM, Persson A, Karlsson T, Smith C, Herlitz J, Aukrust P, Hartford M, Caidahl K. Circulating osteoprotegerin levels and long‐term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–633. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen S, Mogelvang R, Bjerre M, Frystyk J, Flyvbjerg A, Galatius S, Sorensen TB, Iversen A, Hvelplund A, Jensen JS. Osteoprotegerin predicts long‐term outcome in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Cardiology. 2012;123:31–38. [DOI] [PubMed] [Google Scholar]
- 36. Tschiderer L, Klingenschmid G, Willeit P. Meta‐analyses: clinical epidemiology. Medical University of Innsbruck; https://clinicalepi.i-med.ac.at/research/meta-analyses/. Published June 11, 2018. Accessed July 16, 2018. [Google Scholar]
- 37. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Department of Epidemiology and Community Medicine, University of Ottawa. The Ottawa Hospital Research Institute; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 19, 2018. [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C‐reactive protein, albumin, or leukocyte count with coronary heart disease: meta‐analyses of prospective studies. JAMA. 1998;279:1477–1482. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta‐analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 43. Xiang GD, Sun HL, Zhao LS. Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients. Diabetes Res Clin Pract. 2007;76:199–206. [DOI] [PubMed] [Google Scholar]
- 44. Xiang GD, Xu L, Zhao LS, Yue L, Hou J. The relationship between plasma osteoprotegerin and endothelium‐dependent arterial dilation in type 2 diabetes. Diabetes. 2006;55:2126–2131. [DOI] [PubMed] [Google Scholar]
- 45. Park JS, Cho MH, Nam JS, Yoo JS, Ahn CW, Cha BS, Kim KR, Lee HC. Effect of pioglitazone on serum concentrations of osteoprotegerin in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nybo M, Preil SR, Juhl HF, Olesen M, Yderstraede K, Gram J, Henriksen JE, Rasmussen LM. Rosiglitazone decreases plasma levels of osteoprotegerin in a randomized clinical trial with type 2 diabetes patients. Basic Clin Pharmacol Toxicol. 2011;109:481–485. [DOI] [PubMed] [Google Scholar]
- 47. Kadoglou NPE, Gerasimidis T, Moumtzouoglou A, Kapelouzou A, Sailer N, Fotiadis G, Vitta I, Katinios A, Kougias P, Bandios S, Voliotis K, Karayannacos PE, Liapis CD. Intensive lipid‐lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur J Vasc Endovasc Surg. 2008;35:661–668. [DOI] [PubMed] [Google Scholar]
- 48. Mori K, Jono S, Emoto M, Kawagishi T, Yasumoto H, Konishi T, Furumitsu Y, Shioi A, Shoji T, Inaba M, Nishizawa Y. Effects of pravastatin on serum osteoprotegerin levels in patients with hypercholesterolemia and type 2 diabetes. Angiology. 2010;61:86–91. [DOI] [PubMed] [Google Scholar]
- 49. Vik A, Brodin E, Sveinbjornsson B, Hansen J‐B. Heparin induces mobilization of osteoprotegerin into the circulation. Thromb Haemost. 2007;98:148–154. [PubMed] [Google Scholar]
- 50. Klejna K, Naumnik B, Koc‐Żórawska E, Myśliwiec M. Effect of unfractionated and low‐molecular‐weight heparin on OPG, sRANKL, and von Willebrand factor concentrations during hemodialysis. Clin Appl Thromb Hemost. 2014;20:433–441. [DOI] [PubMed] [Google Scholar]
- 51. Sasaki N, Kusano E, Ando Y, Yano K, Tsuda E, Asano Y. Glucocorticoid decreases circulating osteoprotegerin (OPG): possible mechanism for glucocorticoid induced osteoporosis. Nephrol Dial Transplant. 2001;16:479–482. [DOI] [PubMed] [Google Scholar]
- 52. Golledge J, McCann M, Mangan S, Lam A, Karan M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–1641. [DOI] [PubMed] [Google Scholar]
- 53. Hofbauer LC, Shui C, Riggs BL, Dunstan CR, Spelsberg TC, O'Brien T, Khosla S. Effects of immunosuppressants on receptor activator of NF‐kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem Biophys Res Commun. 2001;280:334–339. [DOI] [PubMed] [Google Scholar]
- 54. Ziegler S, Kudlacek S, Luger A, Minar E. Osteoprotegerin plasma concentrations correlate with severity of peripheral artery disease. Atherosclerosis. 2005;182:175–180. [DOI] [PubMed] [Google Scholar]
- 55. Ueland T, Yndestad A, Oie E, Florholmen G, Halvorsen B, Froland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. [DOI] [PubMed] [Google Scholar]
- 56. Crisafulli A, Micari A, Altavilla D, Saporito F, Sardella A, Passaniti M, Raffa S, D'anneo G, Luca F, Mioni C, Arrigo F, Squadrito F. Serum levels of osteoprotegerin and RANKL in patients with ST elevation acute myocardial infarction. Clin Sci (Lond). 2005;109:389–395. [DOI] [PubMed] [Google Scholar]
- 57. Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. [DOI] [PubMed] [Google Scholar]
- 58. Rasmussen LM, Tarnow L, Hansen TK, Parving H‐H, Flyvbjerg A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006;154:75–81. [DOI] [PubMed] [Google Scholar]
- 59. Altinova AE, Toruner F, Akturk M, Bukan N, Yetkin I, Cakir N, Arslan M. Relationship between serum osteoprotegerin, glycemic control, renal function and markers of atherosclerosis in type 2 diabetes. Scand J Clin Lab Invest. 2011;71:340–343. [DOI] [PubMed] [Google Scholar]
- 60. Wang ST, Xu JM, Wang M, Chen FL, Ding G. Increased plasma osteoprotegerin concentrations in type 1 diabetes with albuminuria. Clin Nephrol. 2013;79:192–198. [DOI] [PubMed] [Google Scholar]
- 61. Chang YH, Lin KD, He SR, Hsieh MC, Hsiao JY, Shin SJ. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing‐ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism. 2011;60:1064–1069. [DOI] [PubMed] [Google Scholar]
- 62. Shaarawy M, Fathy SA, Mehany NL, Hindy OW. Circulating levels of osteoprotegerin and receptor activator of NF‐kappaB ligand in patients with chronic renal failure. Clin Chem Lab Med. 2007;45:1498–1503. [DOI] [PubMed] [Google Scholar]
- 63. Morena M, Jaussent I, Dupuy AM, Bargnoux AS, Kuster N, Chenine L, Leray‐Moragues H, Klouche K, Vernhet H, Canaud B, Cristol J‐P. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrol Dial Transplant. 2015;30:1345–1356. [DOI] [PubMed] [Google Scholar]
- 64. Moe SM, Reslerova M, Ketteler M, O'neill K, Duan D, Koczman J, Westenfeld R, Jahnen‐Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int. 2005;67:2295–2304. [DOI] [PubMed] [Google Scholar]
- 65. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. [DOI] [PubMed] [Google Scholar]
- 66. Zannettino ACW, Holding CA, Diamond P, Atkins GJ, Kostakis P, Farrugia A, Gamble J, To LB, Findlay DM, Haynes DR. Osteoprotegerin (OPG) is localized to the Weibel‐Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol. 2005;204:714–723. [DOI] [PubMed] [Google Scholar]
- 67. Zhang J, Fu M, Myles D, Zhu X, Du J, Cao X, Chen YE. PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett. 2002;521:180–184. [DOI] [PubMed] [Google Scholar]
- 68. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin‐deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE‐/‐ mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. [DOI] [PubMed] [Google Scholar]
- 70. Zhou S, Fang X, Xin H, Li W, Qiu H, Guan S. Osteoprotegerin inhibits calcification of vascular smooth muscle cell via down regulation of the Notch1‐RBP‐Jκ/Msx2 signaling pathway. PLoS One. 2013;8:e68987 DOI: 10.1371/journal.pone.0068987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cross SS, Yang Z, Brown NJ, Balasubramanian SP, Evans CA, Woodward JK, Neville‐Webbe HL, Lippitt JM, Reed MWR, Coleman RE, Holen I. Osteoprotegerin (OPG): a potential new role in the regulation of endothelial cell phenotype and tumour angiogenesis? Int J Cancer. 2006;118:1901–1908. [DOI] [PubMed] [Google Scholar]
- 72. Bernardi S, Fabris B, Thomas M, Toffoli B, Tikellis C, Candido R, Catena C, Mulatero P, Barbone F, Radillo O, Zauli G, Secchiero P. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol Cell Endocrinol. 2014;394:13–20. [DOI] [PubMed] [Google Scholar]
- 73. Heymann MF, Herisson F, Davaine JM, Charrier C, Battaglia S, Passuti N, Lambert G, Goueffic Y, Heymann D. Role of the OPG/RANK/RANKL triad in calcifications of the atheromatous plaques: comparison between carotid and femoral beds. Cytokine. 2012;58:300–306. [DOI] [PubMed] [Google Scholar]
- 74. Zauli G, Corallini F, Bossi F, Fischetti F, Durigutto P, Celeghini C, Tedesco F, Secchiero P. Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood. 2007;110:536–543. [DOI] [PubMed] [Google Scholar]
- 75. Mangan SH, Campenhout AV, Rush C, Golledge J. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor‐alpha associated with induction of angiopoietin‐2. Cardiovasc Res. 2007;76:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toffoli B, Pickering RJ, Tsorotes D, Wang B, Bernardi S, Kantharidis P, Fabris B, Zauli G, Secchiero P, Thomas MC. Osteoprotegerin promotes vascular fibrosis via a TGF‐beta1 autocrine loop. Atherosclerosis. 2011;218:61–68. [DOI] [PubMed] [Google Scholar]
- 77. Forde H, Harper E, Davenport C, Rochfort KD, Wallace R, Murphy RP, Smith D, Cummins PM. The beneficial pleiotropic effects of tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL) within the vasculature: a review of the evidence. Atherosclerosis. 2016;247:87–96. [DOI] [PubMed] [Google Scholar]
- 78. Davaine JM, Quillard T, Brion R, Laperine O, Guyomarch B, Merlini T, Chatelais M, Guilbaud F, Brennan MA, Charrier C, Heymann D, Goueffic Y, Heymann M‐F. Osteoprotegerin, pericytes and bone‐like vascular calcification are associated with carotid plaque stability. PLoS One. 2014;9:e107642 DOI: 10.1371/journal.pone.0107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vik A, Mathiesen EB, Noto AW, Sveinbjornsson B, Brox J, Hansen JB. Serum osteoprotegerin is inversely associated with carotid plaque echogenicity in humans. Atherosclerosis. 2007;191:128–134. [DOI] [PubMed] [Google Scholar]
- 80. Clancy P, Oliver L, Jayalath R, Buttner P, Golledge J. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:2574–2576. [DOI] [PubMed] [Google Scholar]
- 81. Perez de Ciriza C, Lawrie A, Varo N. Influence of pre‐analytical and analytical factors on osteoprotegerin measurements. Clin Biochem. 2014;47:1279–1285. [DOI] [PubMed] [Google Scholar]
- 82. Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, Schimmel H, Wang L, Panteghini M. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010;42:402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Terms Used to Identify Relevant Articles
Table S2. Detailed Baseline Conditions of Contributing Studies
Figure S1. Funnel plot including artificial studies generated with the “trim and fill” method.
Figure S2. Comparison of combined relative risk for cardiovascular events in the top vs the bottom tertile of osteoprotegerin concentration of high‐risk populations and general population results.20


