Abstract
Background
In patients with vascular disease, risk models may support decision making on novel risk reducing interventions, such as proprotein convertase subtilisin/kexin type 9 inhibitors or anti‐inflammatory agents. We developed and validated an innovative model to estimate life expectancy without recurrent cardiovascular events for individuals with coronary, cerebrovascular, and/or peripheral artery disease that enables estimation of preventive treatment effect in lifetime gained.
Methods and Results
Study participants originated from prospective cohort studies: the SMART (Secondary Manifestations of Arterial Disease) cohort and REACH (Reduction of Atherothrombosis for Continued Health) cohorts of 14 259 (REACH Western Europe), 19 170 (REACH North America) and 6959 (SMART, The Netherlands) patients with cardiovascular disease. The SMART‐REACH model to estimate life expectancy without recurrent events was developed in REACH Western Europe as a Fine and Gray competing risk model incorporating cardiovascular risk factors. Validation was performed in REACH North America and SMART. Outcomes were (1) cardiovascular events (myocardial infarction, stroke, cardiovascular death) and (2) noncardiovascular death. Predictors were sex, smoking, diabetes mellitus, systolic blood pressure, total cholesterol, creatinine, number of cardiovascular disease locations, atrial fibrillation, and heart failure. Calibration plots showed high agreement between estimated and observed prognosis in SMART and REACH North America. C‐statistics were 0.68 (95% confidence interval, 0.67–0.70) in SMART and 0.67 (95% confidence interval, 0.66–0.68) in REACH North America. Performance of the SMART‐REACH model was better compared with existing risk scores and adds the possibility of estimating lifetime gained by novel therapies.
Conclusions
The externally validated SMART‐REACH model could be used for estimation of anticipated improvements in life expectancy without recurrent cardiovascular events in individual patients with cardiovascular disease in Western Europe and North America.
Keywords: life expectancy, prognosis, risk stratification, secondary prevention, treatment effect
Subject Categories: Cardiovascular Disease, Epidemiology, Secondary Prevention, Risk Factors
Clinical Perspective
What Is New?
In the present study we developed and validated the innovative SMART‐REACH (Secondary Manifestations of Arterial Disease‐Reduction of Atherothrombosis for Continued Health) model to estimate life expectancy without recurrent cardiovascular events for individuals with coronary, cerebrovascular, and/or peripheral artery disease that enables estimation of preventive treatment effect in terms of lifetime gained.
The SMART‐REACH model was developed and validated in the prospective SMART and REACH cohorts of 14 259 (REACH Western Europe), 19 170 (REACH North America) and 6959 (SMART, The Netherlands) patients with cardiovascular disease.
What Are the Clinical Implications?
The externally validated SMART‐REACH lifetime model can estimate both 10‐year cardiovascular event risk and anticipated improvements in life expectancy without recurrent cardiovascular events in individual patients with cardiovascular disease in Western Europe and North America, for example using the calculator on http://www.U-Prevent.com.
Clinicians should be aware of the discrepancy in anticipated benefit of treatment using 10‐year versus life expectancy without recurrent cardiovascular events, as these may result in different clinical decisions about the appropriate preventive strategy for the individual with cardiovascular disease.
Introduction
Patients with a clinical manifestation of cardiovascular disease show substantial variation in cardiovascular prognosis.1 Similar to the primary prevention setting, decisions on initiation or intensification of preventive treatment should be based on anticipated clinical benefit derived from prediction models, rather than based on the level of individual cardiovascular risk factors. In particular with the emergence of novel therapeutic options such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, novel anticoagulants, or anti‐inflammatory agents, tools to predict recurrent cardiovascular events are needed.2, 3 Recently, 2 risk scores have been developed for the prediction of recurrent cardiovascular events based on the observational REACH (Reduction of Atherothrombosis for Continued Health) and SMART (Secondary Manifestations of Arterial Disease) cohort studies.4, 5, 6, 7, 8, 9 These scores estimate the 20‐month (REACH) and 10‐year (SMART) risk of recurrent major cardiovascular events in patients with established cardiovascular disease. The external validity of these scores needs to be established before widespread use is considered.1
The ability to estimate risk in patients with cardiovascular disease is a first step toward personalized secondary prevention of cardiovascular events.10 In addition, recent studies have shown that estimating cardiovascular prognosis from a lifetime perspective may have some advantages over 10‐year risk estimation, including a potentially better selection of patients for preventive treatment by accounting for remaining life expectancy and competing events.11, 12, 13, 14, 15, 16, 17 For example, the QRISK lifetime model in the primary prevention setting identifies patients with an unfavorable prognosis at a much younger age than the traditional 10‐year risk approach.11 In addition, recent data demonstrate that treatment benefit estimated in terms of gain in life expectancy was highest in younger patients with otherwise high risk factor levels and was limited in older patients with relatively low risk factor levels in whom remaining survival may be inadequate for meaningful cardiovascular risk reduction to occur.17, 18, 19
In this article, we aimed to develop, validate, and evaluate the innovative SMART‐REACH model for life expectancy without recurrent cardiovascular events for individual patients with clinically manifest coronary, cerebrovascular, and/or peripheral artery disease in Western Europe and North America.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Both the REACH and SMART data are property of the REACH and SMART study groups, respectively. The Methods, Results, and Supplemental sections provide a detailed description of the applied statistical methods and the formula of the REACH‐SMART algorithm.
Study Populations
REACH and SMART are prospective cohort studies of patients with established cardiovascular disease or cardiovascular risk factors. Study details have been described elsewhere.6, 7, 20 In the present study, we included patients with stable clinical coronary artery disease, cerebrovascular disease and/or peripheral artery disease from both cohorts. From REACH, we used patient data from Western Europe (n=14 259) and North America (n=19 170). In the international, prospective REACH cohort, participants were enrolled between 2003 and 2004 from physician outpatient practices in several countries in Western Europe and North America. Participants were followed for a maximum of 4 years for the occurrence of cardiovascular events and mortality. Medical history, physical and laboratory measurements were collected with a standardized international case report form at baseline and then (bi)annually. Outcomes of patients were annually reported by the local investigator and not adjudicated.20 From the ongoing prospective SMART cohort we used data from 6959 patients with a history of cardiovascular disease enrolled between 1996 and 2014 at the University Medical Center Utrecht, The Netherlands. At inclusion, all patients completed a questionnaire, underwent standardized physical examination and fasting blood samples were collected. Follow‐up for cardiovascular events and mortality was performed every 6 months by sending letters to every participant and checking medical files. An outcome committee assessed whether outcomes occurred.7
Detailed definitions of risk factors at baseline, established cardiovascular disease and clinical outcomes are provided in Table S1. Both studies comply with the Declaration of Helsinki, both studies were approved by an institutional review committee and that the subjects gave informed consent.
The 20‐month REACH score and the 10‐year SMART risk score were developed in these REACH and SMART populations respectively. The external validity of the SMART score in the REACH data and of the REACH score in the SMART data was evaluated and is shown in Data S1.
Development of the SMART‐REACH Model for Estimating Life‐Expectancy Without Recurrent Cardiovascular Events
We developed the SMART‐REACH model in REACH Western Europe using statistical methods that were previously described in detail.17, 21 In short, 2 Fine and Gray competing risk models (Data S2) were fitted for cause specific estimates of the cumulative incidence, 1 for recurrent cardiovascular events and 1 for noncardiovascular mortality. Age was used as the underlying time function (ie, left truncation).20 This enables lifetime predictions across the age range from the youngest age at study entry to the highest age at study exit. Predictors were selected based on the original SMART and REACH scores.4, 5 Because not all of these predictors were available in both the SMART and REACH cohorts, further selection was based on availability of the predictors in both data sets. This resulted in the following nine predictors that were used for both models: sex, current smoking (yes/no), diabetes mellitus (yes/no), systolic blood pressure (mm Hg), total cholesterol (mmol/L), creatinine (µmol/L), number of locations of cardiovascular disease (ie, coronary artery disease, cerebrovascular disease, and peripheral artery disease), history of atrial fibrillation (yes/no) and history of congestive heart failure (yes/no). Linearity of the relation between continuous predictors and the outcomes was tested with restricted cubic splines, and transformation was applied when this improved model fit on the basis of Akaike's Information Criterion. Continuous predictors were truncated at the 1st and 99th percentiles to limit the effect of outliers. The proportional hazards assumption was assessed by testing the correlations between scaled Schoenfeld residuals for the various predictors and age.
Missing data (<1% of variables in SMART, and in REACH 20% creatinine, 21% total cholesterol, 3% current smoking, 2% atrial fibrillation and heart failure, and <1% for other variables) were reduced by single imputation using predictive mean matching (aregImpute‐algorithm in R, Hmisc‐package).22 Analyses were conducted with R statistical software V.3.2.2 (http://www.r-project.org; packages mstate, survival, cmprsk, pec, rms, Hmisc).
Estimating Life‐Expectancy Without Recurrent Cardiovascular Events for Individual Patients
Based on the newly developed SMART‐REACH models, life expectancy without recurrent cardiovascular events was estimated for all individual patients in the pooled populations (REACH Western Europe, SMART and REACH North America, n=34 841). Beginning at the starting age of each individual, the cumulative survival without recurrent cardiovascular events was estimated for each subsequent year. Therefore, the estimated survival at the beginning of each life‐year was multiplied by the survival probability during that year. The survival probability was obtained by subtracting cardiovascular risk and noncardiovascular mortality risk from 1. This was repeated up to the maximum age of 90, as the number of observations beyond the age of 90 was limited in the study populations.17 Life expectancy without recurrent cardiovascular events of an individual person was defined as the median estimated survival, which is the age where the predicted individual survival curve equals 50%. In addition, the SMART‐REACH model can be used to estimate 10‐year cardiovascular risk, adjusted for noncardiovascular mortality, which is calculated as the cumulative cause‐specific cardiovascular risk truncated at 10 years after the starting age.
To enable use of the SMART‐REACH lifetime model in daily clinical practice, we developed a calculator that allows estimation of life expectancy without recurrent cardiovascular events for an individual as well as 10‐year cardiovascular risk. Also, the calculator can be used to estimate potential gain in life expectancy by initiating additional therapy, including increasing the statin dose or adding ezetimibe or a PCSK9 inhibitor, anticoagulants, antihypertensives, or the novel inflammation‐targeting Canakinumab.23 The calculations and assumed hazard ratios on which the estimations in the calculator are based are explained in Data S3. Two individual patient examples are shown in the main manuscript.
Model Validation
External validity of the SMART‐REACH model was tested in the SMART population at 10‐year follow‐up and in REACH North America at 2‐year follow‐up. Calibration (the agreement between predicted and observed events) was assessed for the total survival without recurrent cardiovascular events as well as for the cardiovascular model and the noncardiovascular death models separately. Discrimination was expressed with C‐statistics based on the models' 1‐year predictions.24 We used 1‐year predictions instead of solely the linear predictor to incorporate age in the estimation of discriminative power. To adjust for geographic differences in underlying event rates, the ratio between expected and observed events in the SMART and North American REACH populations was used to update the models to the population of interest. Continuous variables were truncated on the basis of the limits of these values in the Western European REACH development population. In SMART, no information was available on heart failure; therefore, heart failure was assumed to be absent for all SMART participants.
Results
Baseline characteristics of the study populations are shown in Table 1. Different age groups were well represented in the 3 cohorts. Risk factor distribution was similar across the 3 populations, although SMART included more current smokers (32% versus 16% and 13% in REACH Western Europe and North America, respectively), and in REACH more patients had diabetes mellitus: 33% (Western Europe) and 42% (North America) versus 18% in SMART. Loss of follow‐up was 8% in REACH Western Europe, 6% in SMART, and 14% in REACH North America. In REACH Western Europe, a total of 1555 cardiovascular events (32% stroke, 20% myocardial infarction, 48% cardiovascular death) and 490 noncardiovascular deaths were observed during a median follow‐up of 1.8 years (quartiles, 1.5–2.2). In SMART, 1077 cardiovascular events (25% stroke, 34% myocardial infarction, 41% cardiovascular death) and 554 noncardiovascular deaths occurred during 6.5 (quartiles, 3.4–9.9) years, and in REACH North America 1743 cardiovascular events (22% stroke, 26% myocardial infarction, 52% cardiovascular death) and 679 noncardiovascular deaths occurred during a median follow‐up of 1.8 (quartiles, 1.5–1.8) years.
Table 1.
Baseline Characteristics of the REACH and SMART Populations
| REACH Western Europe (n=14 259) | SMART Cohort (n=6959) | REACH North America (n=19 170) | |
|---|---|---|---|
| Age, y | 68 (10) | 60 (10) | 70 (10) |
| <55 y | 1481 (10) | 2093 (30) | 1658 (9) |
| 55 to 65 y | 3525 (25) | 2382 (34) | 4325 (23) |
| 65 to 75 y | 5509 (39) | 2005 (29) | 6413 (33) |
| ≥75 y | 3744 (26) | 479 (7) | 6774 (35) |
| Male sex | 10 270 (72) | 5098 (73) | 11 861 (62) |
| Current smoking | 2283 (16) | 2195 (32) | 2546 (13) |
| Systolic blood pressure, mm Hg | 140 (18) | 140 (21) | 132 (18) |
| Diastolic blood pressure, mm Hg | 80 (10) | 81 (11) | 75 (11) |
| Diabetes mellitus | 4771 (33) | 1227 (18) | 8118 (42) |
| Cardiovascular history | |||
| Congestive heart failure | 2208 (15) | ··· | 3692 (19) |
| Atrial fibrillation | 1629 (11) | 79 (1) | 2605 (14) |
| Coronary artery disease | 9860 (69) | 4367 (63) | 15 512 (81) |
| Cerebrovascular disease | 4451 (31) | 2124 (31) | 5348 (28) |
| Peripheral artery disease | 3343 (23) | 1377 (20) | 2329 (12) |
| Laboratory values | |||
| Total cholesterol, mmol/L | 5.1 (1.2) | 4.8 (1.2) | 4.6 (1.1) |
| Creatinine, μmol/L | 93 (28) | 88 (77) | 100 (35) |
| Medication use | |||
| Statin | 10 176 (71) | 4683 (67) | 14 787 (77) |
| Acetylsalicylic acid | 9529 (67) | 4022 (68) | 14 459 (75) |
| Antihypertensive medication | 12 900 (90) | 5183 (74) | 17 933 (94) |
All data are displayed as mean (standard deviation) or n (%). REACH indicates Reduction of Atherothrombosis for Continued Health; SMART, Secondary Manifestations of Arterial Disease.
Development and Validation of the REACH‐SMART Lifetime Model
Table 2 shows the coefficients and subdistribution hazard ratios of both the cardiovascular and noncardiovascular death models. The age‐specific baseline survivals are presented in Table S2. Table S3 provides the calculation formulas of cause‐specific survivals on which the SMART‐REACH predictions were built. The proportional hazard assumption was met for the cardiovascular event model. In the noncardiovascular death model, nonproportionality was observed for current smoking, with a decreasing effect with increasing age. Therefore, an interaction between age and smoking status was included in this model. We included quadratic terms for systolic blood pressure and total cholesterol in the cardiovascular event model and for creatinine in the noncardiovascular death model.
Table 2.
Coefficients and Subdistribution Hazard Ratios of the SMART‐REACH Lifetime Models
| Coefficient | sHR (95% CI) | P Value | |
|---|---|---|---|
| Model 1 (cardiovascular events) | |||
| Male sex | 0.0720 | 1.07 (0.96–1.21) | 0.23 |
| Current smoking | 0.4309 | 1.54 (1.34–1.77) | <0.01 |
| Diabetes mellitus | 0.4357 | 1.55 (1.39–1.71) | <0.01 |
| Systolic blood pressure (per 10 mm Hg) | −0.2814 | 0.07 | |
| Systolic blood pressure squared (per 10 mm Hg) | 0.0010 | 0.07 | |
| Total cholesterol (mmol/L) | −0.3671 | 0.02 | |
| Total cholesterol squared (mmol/L) | 0.0356 | 0.01 | |
| Creatinine (per 10 μmol/L) | 0.0612 | 1.06 (1.05–1.08) | <0.01 |
| Nr. of locations of cardiovascular disease: 1 | ref | 1 (ref) | |
| Nr. of locations of cardiovascular disease: 2 | 0.3176 | 1.37 (1.22–1.54) | <0.01 |
| Nr. of locations of cardiovascular disease: 3 | 0.2896 | 1.34 (1.03–1.73) | 0.03 |
| Atrial fibrillation | 0.2143 | 1.24 (1.08–1.42) | <0.01 |
| Congestive heart failure | 0.4447 | 1.56 (1.38–1.76) | <0.01 |
| Model 2 (other causes of mortality) | |||
| Male sex | 0.5986 | 1.82 (1.45–2.29) | <0.01 |
| Current smoking | 4.2538 | <0.01 | |
| Current smoking×age | −0.0486 | <0.01 | |
| Diabetes mellitus | 0.4065 | 1.50 (1.25–1.80) | <0.01 |
| Systolic blood pressure (per 10 mm Hg) | −0.0741 | 0.93 (0.88–0.98) | <0.01 |
| Total cholesterol (mmol/L) | −0.0030 | 1.00 (0.92–1.09) | 0.95 |
| Creatinine (per 10 μmol/L) | −0.1886 | <0.01 | |
| Creatinine squared (per 10 μmol/L) | 0.0008 | <0.01 | |
| Nr. of location of cardiovascular disease: 1 | ref | 1 (ref) | |
| Nr. of location of cardiovascular disease: 2 | 0.1442 | 1.16 (0.93–1.44) | 0.19 |
| Nr. of location of cardiovascular disease: 3 | 0.5694 | 1.77 (1.17–2.68) | <0.01 |
| Atrial fibrillation | 0.3213 | 1.38 (1.09–1.75) | <0.01 |
| Congestive heart failure | 0.2061 | 1.23 (0.98–1.55) | 0.08 |
Model 1: competing risk model for recurrent cardiovascular events. The model contains squared terms for systolic blood pressure and total cholesterol. For these terms only, coefficients were provided as the sHRs cannot be interpreted independently. Model 2: competing risk model for noncardiovascular mortality. The model contains squared terms for creatinine and an interaction between smoking and age. For these terms only, coefficients were provided as the sHRs cannot be interpreted independently. CI indicates confidence interval; REACH, Reduction of Atherothrombosis for Continued Health; sHR, subdistribution hazard ratio; SMART, Secondary Manifestations of Arterial Disease.
Discrimination of the estimated survivals showed an overall C‐statistic of 0.68 (95% confidence interval, 0.67–0.70) in SMART and 0.67 (95% confidence interval, 0.66–0.68) in REACH North America. The expected/observed ratios in SMART were 1.53 for cardiovascular risk and 0.88 for noncardiovascular death. In REACH North America, the expected/observed ratios were 0.86 for cardiovascular risk and 0.66 for noncardiovascular death.
The agreement between the estimated survival without recurrent cardiovascular events and the observed survival in both SMART and REACH North America is shown in Figure 1, after correction for differences in geographic event rates (Figure S1, Table S3).
Figure 1.
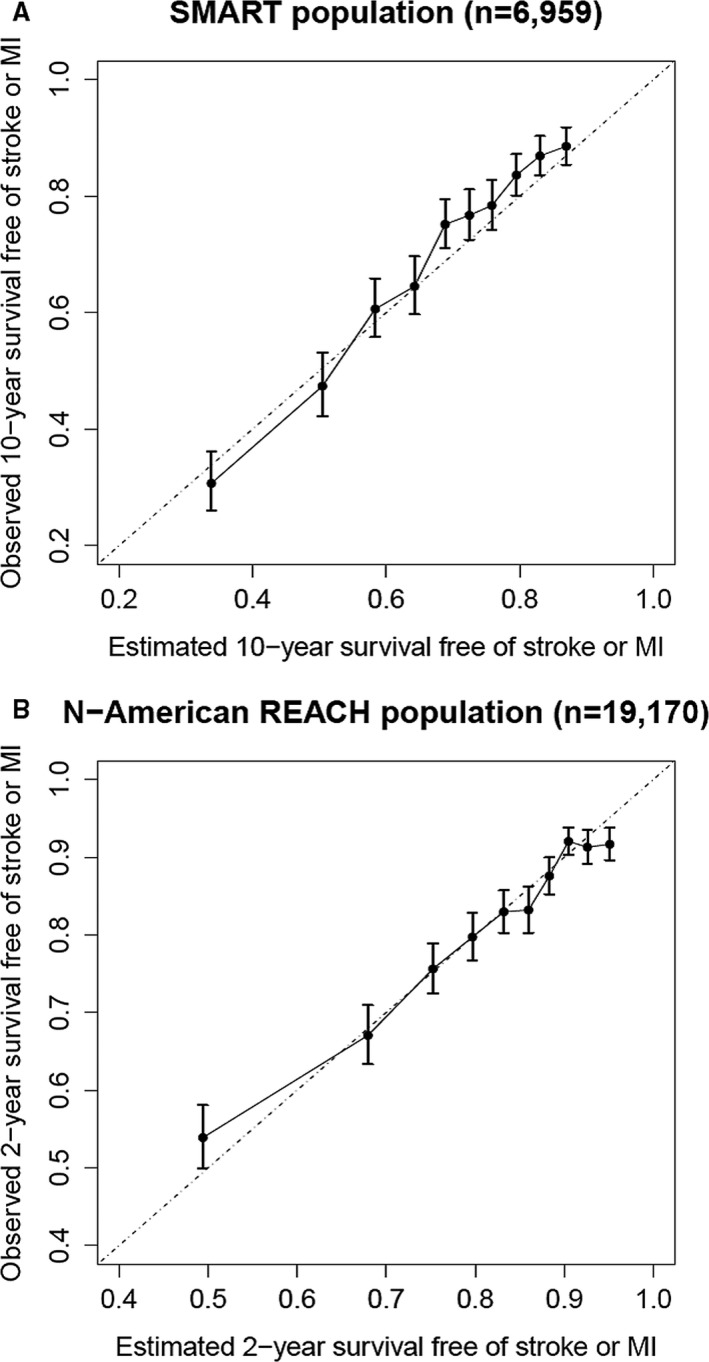
External calibration of estimated survival with the SMART‐REACH model. A, Estimated vs observed 10‐year survival without recurrent cardiovascular events in the SMART population (after correction for geographic differences in event rates). B, Estimated vs observed 2‐year survival without recurrent cardiovascular events in North American REACH (after correction for geographic differences in event rates). MI indicates myocardial infarction; REACH, Reduction of Atherothrombosis for Continued Health; SMART, Secondary Manifestations of Arterial Disease.
Estimated Life Expectancy Without Recurrent Cardiovascular Events Versus 10‐Year Risk
The potential benefit of using life expectancy in addition to estimated 10‐year absolute risk is illustrated by individual patient examples in Figure 2 as well as with the Supplemental Calculator, or the online calculator on http://www.U-Prevent.com, in which estimations can be made for real patient data. Figure 2 illustrates the use of estimated 10‐year risk versus estimated life expectancy without recurrent cardiovascular events for making treatment decisions for 2 patient examples. In these examples, the potential benefit of intensifying lipid‐lowering treatment by raising atorvastatin 10 to 80 mg is considered. Patient A has a lower estimated 10‐year risk than patient B (26.7% versus 32.8%). As patient A is 55 years old, her 10‐year risk is driven by her risk factors. Patient B's risk is mainly driven by his age of 75. Note that, due to her higher risk factor levels, patient A has a lower estimated life expectancy without recurrent cardiovascular events than patient B (70.0 versus 84.3). Importantly, different prognostic estimates may result in different clinical decisions (Figure 2): based on their estimated 10‐year risks and 10‐year absolute risk reduction, intensification of secondary prevention is deemed more necessary for patient B than for patient A. However, from a lifetime perspective, patient A is likely to benefit more from intensifying preventive secondary prevention than patient B: when atorvastatin 10 mg would be raised to atorvastatin 80 mg, patient A has an estimated gain of 2.0 years versus 0.9 years for patient B. This is because patient A has several risk factors in combination with longer remaining life expectancy in which she can benefit from treatment compared with patient B. Similar estimations can be made for several therapeutic options such as novel anticoagulants, PCSK9‐inhibitors, or anti‐inflammatory agents (Supplemental Calculator; http://www.U-Prevent.com; Data S3).
Figure 2.
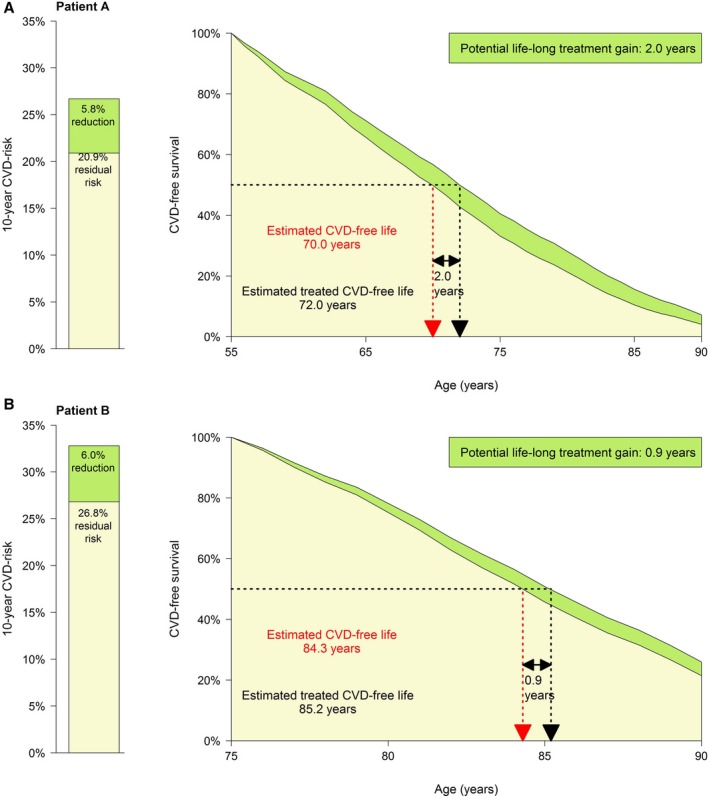
Patient examples. Patient A is a 55‐year‐old woman. She is a current smoker and has no diabetes mellitus. Her systolic blood pressure is 145 mm Hg. Her laboratory values are total cholesterol, 6.0 mmol/L (LDL‐c 4.0 mmol/L); and creatinine, 70 μmol/L. She has a history of 1 location of cardiovascular disease as well as atrial fibrillation, and she has no congestive heart failure. As lipid‐lowering treatment, patient A currently takes atorvastatin 10 mg. The clinician considers raising the atorvastatin dose to 80 mg. Patient A wants to know what her expected benefit is from this change in therapy. The estimated 10‐year risk for patient A is 26.7%. Her life expectancy free from recurrent cardiovascular disease is 70.0 years. When she would take atorvastatin 80 mg instead of 10 mg, this would reduce her 10‐year risk to 20.9% (−5.8%, or 10‐year NNT, 17). The change in therapy would increase her estimated cerebrovascular disease–free life expectancy with 2.0 years to 72.0. Patient B is a 75‐year old male who does not smoke and has no diabetes mellitus. His systolic blood pressure is 140 mm Hg. His total cholesterol is 5.0 mmol/L and creatinine 80 μmol/L. He has a history of 1 location of cardiovascular disease, no atrial fibrillation, and no congestive heart failure. As lipid‐lowering treatment, patient B currently takes atorvastatin 10 mg. The clinician considers raising the atorvastatin dose to 80 mg. Patient B wants to know his expected benefit from this change in therapy. The estimated 10‐year risk for patient B is 32.8%. His life expectancy free from recurrent cardiovascular disease is 84.3 years. When he would take atorvastatin 80 mg instead of 10 mg, this would reduce his 10‐year risk to 26.8% (−6.0%, or 10‐year NNT, 17). The change in therapy would increase his estimated cerebrovascular disease–free life expectancy with 0.9 years to 85.2. CVD indicates cardiovascular disease; NNT, number needed to treat.
Discussion
In this study, we demonstrate the development and external validation of the SMART‐REACH model for estimating life expectancy without recurrent cardiovascular events in patients with established cardiovascular disease that is applicable to patients in Western Europe and North America. Using an online calculator as can be downloaded as supplemental material, and found on http://www.U-Prevent.com, the SMART‐REACH model can be used to estimate an individual's potential gain in life expectancy without a recurrent cardiovascular event for several intended therapies.
Compared to risk prediction in the primary prevention setting, estimating prognosis in patients with established cardiovascular disease is challenged by some typical characteristics of the population of interest. Because of shared risk factors, patients with cardiovascular risk are also at increased risk of other causes of death.25, 26 For example, smoking causes cardiovascular disease but also increases a patient's risk to die from cancer or chronic obstructive pulmonary disease. Risk scores that do not account for these competing risks, such as the original REACH and SMART scores,4, 5 assume that the patient remains alive until a recurrent cardiovascular event occurs. In reality, a patient may also die from something else in the meantime. Failure to account for these competing events may result in overestimation of cardiovascular risk, particularly in high‐risk patients, as was seen in the external performance of the SMART risk score (Figure S3).1
In the SMART‐REACH model for lifetime predictions, we applied methods accounting for competing events and using age as the time axis, which enabled us to make valid 10‐year predictions in the external SMART population despite more limited follow‐up in the REACH development set (median 1.8 years). As event rates vary between geographic areas,27, 28 recalibration to the population of interest is often necessary. This resulted in accurate estimates of the REACH‐SMART model in the external validation sets. The discriminatory ability of the 3 models was moderate, which we considered acceptable, as this is in line with previous studies on models in patients with cardiovascular disease.4, 5, 29, 30
Both the REACH and SMART risk scores as well as the SMART‐REACH lifetime model may be of value in daily clinical practice. The 20‐month REACH scores and 10‐year SMART risk score can be used to identify high‐risk patients for intensification of short‐term follow‐up or for motivating patients for medication adherence and adopting a healthier lifestyle.31 Another important application of risk estimates is to select patients for clinical trials that typically need limited follow‐up for occurrence of events of interest to improve study power and efficiency.2
For clinical decision making on treatment strategies for individuals, several studies have demonstrated the advantage of lifetime estimates over traditional risk estimation. In the primary prevention setting, lifetime estimates have been shown to identify patients most likely to benefit from treatment at a much earlier age.11, 13, 14, 17, 32, 33, 34 The present study demonstrates that this likely also applies to patients with established cardiovascular disease. Clinicians and guideline makers should be aware of the discrepancy between 10‐year risk and estimated life expectancy without recurrent cardiovascular events, as these may result in different therapeutic decisions for the individual with cardiovascular disease (Figure 2). The SMART‐REACH model, incorporated in an online calculator (eg, Supplemental Calculator and http://www.U-Prevent.com), may support clinical decision making on (novel) therapeutic options by estimating an individual's anticipated treatment benefit in terms of life expectancy without recurrent cardiovascular events. This may particularly be of value for novel effective but costly agents such as PCSK9 inhibitors, potent antithrombotics, and anti‐inflammatory agents.23, 35, 36, 37 Ideally, such treatment effect estimations are validated on the basis of the original trial data of such novel therapies.
As the SMART and REACH participants originate from daily clinical practice with limited selection criteria, the models presented in this study are broadly applicable to patients with a clinical manifestation of cardiovascular disease. When applying the SMART‐REACH model in practice, the physician should consider whether the available literature applies to the individual patient in question. For example, for patients with moderate to severe heart failure, evidence on several preventive therapies is limited, as these patients are often excluded from trials such as was the case for the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial.35 The presented SMART‐REACH model can be applied to patients from Western Europe in general. For patients similar to the Dutch SMART population or the North American REACH population, the geographic correction factors can be applied (Table S3).
Strengths of this study are the observational cohort design representing clinical practice, geographic variation, and the use of a lifetime model that accounts for competing events, which can directly be applied in clinical practice (http://www.U-Prevent.com). A limitation is that risk factors were measured at baseline and were thus considered to remain constant the rest of a patient's life. A second limitation is that lifetime estimates often go beyond the 10 years of follow‐up in which we validated the SMART‐REACH model. In a previous study, it was shown that lifetime predictions based on the applied methods are valid for survival up to 17 years.17 Nevertheless, this type of modeling does not account for survival up to the year of observation, which theoretically may result in biased estimates toward healthier survivors in very long‐term predictions. Third, the limited discriminatory ability of the SMART‐REACH model is comparable to previous risk scores for patients with clinically manifest vascular disease.4, 5, 29, 30 Previous studies have shown that additional risk factors are unlikely to result in relevant improvement.4, 38 This discriminatory ability may be due to the fact that selecting patients on the basis of a certain disease (vascular disease) results in a relatively homogenous population, in which discrimination becomes more difficult. Notably, the predictive ability of the SMART‐REACH model is still a major improvement compared with the current criteria for identification of very high‐risk patients with vascular disease, as recommended by the American College of Cardiology/American Heart Association guidelines (C‐statistics, 0.53 and 0.54).39 Finally, the present study focuses on the development and validation of the SMART‐REACH score. Further studies may be undertaken to evaluate the actual potential clinical impact of the SMART‐REACH model.
In conclusion, for patients with established cardiovascular disease, the risk of recurrent cardiovascular events can be estimated with the 20‐month REACH scores, or the recalibrated 10‐year SMART risk score. In addition, (anticipated improvements in) life‐expectancy without recurrent cardiovascular events can be estimated with the externally validated REACH‐SMART model for individuals with cardiovascular disease in North America and Western Europe. Clinicians should be aware of the discrepancy in anticipated benefit of treatment using 10‐year cardiovascular event risk versus life expectancy without recurrent cardiovascular events, as these may result in different clinical decisions about the appropriate preventive strategy for the individual with cardiovascular disease.
Sources of Funding
The SMART study was financially supported by a grant from the University Medical Center Utrecht, The Netherlands. The REACH Registry was supported by Sanofi‐Aventis and Bristol‐Myers Squibb and is endorsed by the World Heart Federation. For the present study, the above supporting sources had no involvement in study design, analysis, interpretation, or writing of the results or the decision to submit the report for publication.
Disclosures
Bhatt declares the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI (Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran Versus Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor‐in‐Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Coinvestigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, and Takeda. Kappelle received fees for consultation and presentations from Boehringer Ingelheim, Bayer Health Care, and Bristol Meyers Squibb. Ph Gabriel Steg discloses the following relationships: research grant from Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, CSL‐Behring, Daiichi‐Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier, and The Medicines Company. The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental Methods.
Data S2. Supplemental Methods—Fine and Gray Competing Risk Model.
Data S3. Supplemental Methods.
Table S1. Inclusion and Exclusion Criteria of the REACH and SMART Cohorts and Definitions of History of Cardiovascular Disease and the Outcome Major Cardiovascular Events
Table S2. Age‐Specific Baseline Survivals for the REACH‐SMART Models
Table S3. REACH‐SMART Model Formulas
Figure S1. External calibration of the REACH‐SMART cardiovascular risk and non‐cardiovascular death models. A, Estimated versus observed 10‐year cardiovascular risk in the SMART population (left, E/O ratio 1.53) and after recalibration adjusting for the E/O ratio (right). B, Estimated versus observed 10‐year risk of noncardiovascular death in the SMART population (left, E/O ratio 0.88) and after recalibration adjusting for the E/O ratio (right). C, Estimated versus observed 2‐year cardiovascular risk in the North American REACH population (left, E/O ratio 0.86) and after recalibration adjusting for the E/O ratio (right). D, Estimated versus observed 2‐year risk of noncardiovascular death in the North American REACH population (left, E/O ratio 0.66) and after recalibration adjusting for the E/O ratio (right). E/O indicates estimated/observed ratio; REACH, Reduction of Atherothrombosis for Continued Health; and SMART, Secondary Manifestations of Arterial Disease.
Figure S2. SMART‐REACH score—Calculation Sheet. See Excel file.
Figure S3. A, Calibration of the REACH risk models in SMART. Calibration of the REACH recurrent event model (left) and REACH cardiovascular death model (right) in the SMART population. B, Calibration of the SMART risk score in the REACH cohort. Calibration of the SMART risk score in REACH North America before (left) and after (right) recalibration for the baseline survival (0.855 instead of 0.962) and mean linear predictor (1.142 instead of 2.099). Calibration of the SMART risk score in REACH Western Europe before (left) and after (right) recalibration for the baseline survival (0.882 instead of 0.962) and mean linear predictor (1.611 instead of 2.099). REACH indicates Reduction of Atherothrombosis for Continued Health; and SMART, Secondary Manifestations of Arterial Disease.
Acknowledgments
The authors gratefully acknowledge the contribution of the SMART research nurses, R. van Petersen (data manager) and B. Dinther (vascular manager), and the members of the SMART Study Group: P.A. Doevendans, Department of Cardiology; A. Algra; Graaf; D.E. Grobbee; G.E.H.M. Rutten, Julius Center for Health Sciences and Primary Care; Kappelle, Department of Neurology; T. Leiner, Department of Radiology; Borst, Department of Vascular Surgery; Visseren, Department of Vascular Medicine.
(J Am Heart Assoc. 2018;7:e009217 DOI: 10.1161/JAHA.118.009217.)
References
- 1. Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, Amarenco P, LaRosa JC, Cramer MJ, Westerink J, Kappelle LJ, de Borst GJ, Visseren FL. Distribution of estimated 10‐year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134:1419–1429. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Sipido KR, Cowie MR, Eschenhagen T, Fox KA, Katus H, Schroeder S, Schunkert H, Priori S. The continuum of personalized cardiovascular medicine: a position paper of the European Society of Cardiology. Eur Heart J. 2014;35:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson JG, Ray K. Counterpoint: low‐density lipoprotein cholesterol targets are not needed in lipid treatment guidelines. Arterioscler Thromb Vasc Biol. 2016;36:586–590. [DOI] [PubMed] [Google Scholar]
- 4. Dorresteijn JA, Visseren FL, Wassink AM, Gondrie MJ, Steyerberg EW, Ridker PM, Cook NR, van der Graaf Y. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99:866–872. [DOI] [PubMed] [Google Scholar]
- 5. Wilson PWF, D'Agostino R, Bhatt DL, Eagle K, Pencina MJ, Smith SC, Alberts MJ, Dallongeville J, Goto S, Hirsch AT, Liau CS, Ohman EM, Rother J, Reid C, Mas JL, Steg G. Reach registry. An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125:695–703. [DOI] [PubMed] [Google Scholar]
- 6. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 7. Simons PCG, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y; SMART Study Group . Second Manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15:773–781. [DOI] [PubMed] [Google Scholar]
- 8. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S; REACH Registry Investigators . One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 10. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 11. Hippisley‐Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leening MJ, Berry JD, Allen NB. Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease. JAMA. 2016;315:1449–1450. [DOI] [PubMed] [Google Scholar]
- 13. Lloyd‐Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24. [DOI] [PubMed] [Google Scholar]
- 14. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham heart study. Circulation. 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd‐Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorresteijn JA, Kaasenbrood L, Cook NR, van Kruijsdijk RC, van der Graaf Y, Visseren FL, Ridker PM. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ. 2016;352:i1548. [DOI] [PubMed] [Google Scholar]
- 18. Ferket BS, van Kempen BJ, Heeringa J, Spronk S, Fleischmann KE, Nijhuis RL, Hofman A, Steyerberg EW, Hunink MG. Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study. PLoS Med. 2012;9:e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dehmer SP, Maciosek MV, Flottemesch TJ, LaFrance AB, Whitlock EP. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777–786. [DOI] [PubMed] [Google Scholar]
- 20. Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, Mas JL, Richard AJ, Rother J, Wilson PW; REACH Registry Investigators . The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events‐study design. Am Heart J. 2006;151:786.e1–786.e710. [DOI] [PubMed] [Google Scholar]
- 21. Geskus RB. Cause‐specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49. [DOI] [PubMed] [Google Scholar]
- 22. Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. [DOI] [PubMed] [Google Scholar]
- 23. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 24. Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Kruijsdijk RCM, van der Graaf Y, Koffijberg H, de Borst GJ, Nathoe HM, Kappelle LJ, Visseren FLJ; SMART Study Group . Cause‐specific mortality and years of life lost in patients with different manifestations of vascular disease. Eur J Prev Cardiol. 2016;23:160–169. [DOI] [PubMed] [Google Scholar]
- 26. vanKruijsdijk RCM , van der Graaf Y, Peeters PHM, Visseren FLJ; SMART Study Group . Cancer risk in patients with manifest vascular disease: effects of smoking, obesity, and metabolic syndrome. Cancer Epidemiol Biomarkers Prev. 2013;22:1267–1277. [DOI] [PubMed] [Google Scholar]
- 27. Ducrocq G, Bhatt DL, Labreuche J, Corbalan R, Porath A, Gao R, Panchenko E, Liau CS, Ikeda Y, Goto S, Amarenco P, Steg PG. Geographic differences in outcomes in outpatients with established atherothrombotic disease: results from the REACH Registry. Eur J Prev Cardiol. 2014;21:1509–1516. [DOI] [PubMed] [Google Scholar]
- 28. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2950–2959. [DOI] [PubMed] [Google Scholar]
- 29. Weimar C, Diener HC, Alberts MJ, Steg PG, Bhatt DL, Wilson PW, Mas JL, Rother J; REACH Registry Investigators . The Essen stroke risk score predicts recurrent cardiovascular events: a validation within the REduction of Atherothrombosis for Continued Health (REACH) registry. Stroke. 2009;40:350–354. [DOI] [PubMed] [Google Scholar]
- 30. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and validation of a protein‐based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. 2016;315:2532–2541. [DOI] [PubMed] [Google Scholar]
- 31. Sheridan SL, Viera AJ, Krantz MJ, Ice CL, Steinman LE, Peters KE, Kopin LA, Lungelow D. The effect of giving global coronary risk information to adults a systematic review. Arch Intern Med. 2010;170:230–239. [DOI] [PubMed] [Google Scholar]
- 32. Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd‐Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long‐term risk of cardiovascular and all‐cause mortality. JAMA. 2004;292:1588–1592. [DOI] [PubMed] [Google Scholar]
- 33. Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long‐term coronary, cardiovascular, and all‐cause mortality and to longevity. JAMA. 2000;284:311–318. [DOI] [PubMed] [Google Scholar]
- 34. Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk‐factor profile and long‐term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle‐aged men and women. JAMA. 1999;282:2012–2018. [DOI] [PubMed] [Google Scholar]
- 35. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 36. Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long‐term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 37. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez‐Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Stork S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp‐Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 38. Austin PC, Pencinca MJ, Steyerberg EW. Predictive accuracy of novel risk factors and markers: a simulation study of the sensitivity of different performance measures for the Cox proportional hazards regression model. Stat Methods Med Res. 2017;26:1053–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Berg MJ, Bhatt DL, Kappelle LJ, de Borst GJ, Cramer MJ, van der Graaf Y, Steg PG, Visseren FLJ; SMART Study Group, REACH Registry Investigators . Identification of vascular patients at very high risk for recurrent cardiovascular events: validation of the current ACC/AHA very high risk criteria. Eur Heart J. 2017;38:3211–3218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Data S2. Supplemental Methods—Fine and Gray Competing Risk Model.
Data S3. Supplemental Methods.
Table S1. Inclusion and Exclusion Criteria of the REACH and SMART Cohorts and Definitions of History of Cardiovascular Disease and the Outcome Major Cardiovascular Events
Table S2. Age‐Specific Baseline Survivals for the REACH‐SMART Models
Table S3. REACH‐SMART Model Formulas
Figure S1. External calibration of the REACH‐SMART cardiovascular risk and non‐cardiovascular death models. A, Estimated versus observed 10‐year cardiovascular risk in the SMART population (left, E/O ratio 1.53) and after recalibration adjusting for the E/O ratio (right). B, Estimated versus observed 10‐year risk of noncardiovascular death in the SMART population (left, E/O ratio 0.88) and after recalibration adjusting for the E/O ratio (right). C, Estimated versus observed 2‐year cardiovascular risk in the North American REACH population (left, E/O ratio 0.86) and after recalibration adjusting for the E/O ratio (right). D, Estimated versus observed 2‐year risk of noncardiovascular death in the North American REACH population (left, E/O ratio 0.66) and after recalibration adjusting for the E/O ratio (right). E/O indicates estimated/observed ratio; REACH, Reduction of Atherothrombosis for Continued Health; and SMART, Secondary Manifestations of Arterial Disease.
Figure S2. SMART‐REACH score—Calculation Sheet. See Excel file.
Figure S3. A, Calibration of the REACH risk models in SMART. Calibration of the REACH recurrent event model (left) and REACH cardiovascular death model (right) in the SMART population. B, Calibration of the SMART risk score in the REACH cohort. Calibration of the SMART risk score in REACH North America before (left) and after (right) recalibration for the baseline survival (0.855 instead of 0.962) and mean linear predictor (1.142 instead of 2.099). Calibration of the SMART risk score in REACH Western Europe before (left) and after (right) recalibration for the baseline survival (0.882 instead of 0.962) and mean linear predictor (1.611 instead of 2.099). REACH indicates Reduction of Atherothrombosis for Continued Health; and SMART, Secondary Manifestations of Arterial Disease.


