Abstract
Background
The optimal treatment for critical limb ischemia remains controversial owing to conflicting conclusions from previous studies.
Methods and Results
We obtained administrative claims on Medicare beneficiaries with initial critical limb ischemia diagnosis in 2011. Clinical outcomes and healthcare costs over 4 years were estimated among all patients and by first treatment (endovascular revascularization, surgical revascularization, or major amputation) in unmatched and propensity‐score–matched samples. Among 72 199 patients with initial primary critical limb ischemia diagnosis in 2011, survival was 46% (median survival, 3.5 years) and freedom from major amputation was 87%. Among 9942 propensity‐score–matched patients (8% rest pain, 26% ulcer, and 66% gangrene), survival was 38% with endovascular revascularization (median survival, 2.7 years), 40% with surgical revascularization (median survival, 2.9 years), and 23% with major amputation (median survival, 1.3 years; P<0.001 for each revascularization procedure versus major amputation). Corresponding major amputation rates were 6.5%, 9.6%, and 10.6%, respectively (P<0.001 for all pair‐wise comparisons). The cost per patient year during follow‐up was $49 700, $49 200, and $55 700, respectively (P<0.001 for each revascularization procedure versus major amputation).
Conclusions
Long‐term survival and cost in critical limb ischemia management is comparable between revascularization techniques, with lower major amputation rates following endovascular revascularization. Primary major amputation results in shorter survival, higher risk of subsequent major amputation, and higher healthcare costs versus revascularization. Results from this observational research may be susceptible to bias because of the influence of unmeasured confounders.
Keywords: amputation, cost, critical limb ischemia, Medicare, peripheral artery disease, revascularization
Subject Categories: Peripheral Vascular Disease, Mortality/Survival
Clinical Perspective
What Is New?
No study has reported long‐term outcomes and costs by first major critical limb ischemia treatment with adjustment for patient characteristics.
Long‐term survival and cost are comparable between endovascular and surgical revascularization, with lower major amputation rates following endovascular revascularization.
Compared with each revascularization approach, primary major amputation is associated with shorter survival time, higher risk of subsequent major amputation, and higher healthcare costs.
What Are the Clinical Implications?
Considerable efforts are needed to raise disease awareness, implement coding to better define and identify the disease, refine diagnostic algorithms, establish evidence‐based treatment pathways, and address the high mortality rates associated with this diagnosis.
Introduction
Critical limb ischemia (CLI) represents the most advanced manifestation of peripheral artery disease and is categorized as ischemic rest pain, nonhealing ischemic ulceration, or gangrene. Patients with CLI often present with multilevel peripheral artery disease that prevents the arterial supply from meeting the metabolic demands of tissue at rest. Prompt revascularization by endovascular or open surgical procedures is indicated following the diagnosis of CLI diagnosis to preserve the limb and maintain limb function.1 However, management of CLI remains highly controversial, particularly when selecting an initial revascularization strategy. In an analysis of 7900 CLI patients from the Vascular Quality Initiative, 3‐year survival was lower with endovascular versus surgical revascularization strategies (70% versus 78%).2 In the First‐Line Treatments in Patients With Critical Limb Ischemia (CRITISCH) registry of 1200 CLI patients, there was no difference in 1‐year mortality or major amputation between revascularization methods.3 The randomized BASIL (Bypass versus Angioplasty in Severe Ischaemia of the Leg) study also found no differences in long‐term mortality or major amputation when comparing endovascular versus surgical revascularization.4 Although primary major amputation for CLI is associated with impaired mobility, high cost, high risk of contralateral limb amputation, and poor prognosis,5, 6 this procedure may be indicated in some patients with cognitive impairment, nonambulatory status, extensive comorbidities, extensive gangrene, or infection.7 Overall, the optimal treatment for CLI and determinants of long‐term results remain controversial, and the total costs of care are unclear to the vascular specialist. The purpose of this study was to report long‐term outcomes and costs following initial CLI diagnosis, with comparisons among endovascular revascularization, surgical revascularization, or major amputation as first‐line treatment among a contemporaneous cohort of Medicare beneficiaries.
Methods
Data Sources
We obtained administrative claims from 2010 to 2015 on all fee‐for‐service Medicare beneficiaries available from the Centers for Medicare & Medicaid Services. Medicare is the primary payer in nearly 75% of CLI‐related hospitalizations8; thus, data derived from Medicare claims are representative of nation‐wide CLI outcomes. Data for the current analysis included claims from Medicare fee‐for‐service parts A (hospital inpatient) and B (hospital outpatient). Cost analyses additionally utilized a 5% sample from the Carrier file, which contains final action fee‐for‐service claims deriving mainly from noninstitutional providers, such as physicians, physician assistants, clinical social workers, and nurse practitioners. The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results. Metro Health Hospital Institutional Review Board (Wyoming, MI) reviewed and approved this research, and the requirement for informed consent was waived.
Patient Population
The patient population included adult Medicare beneficiaries with a first‐time CLI diagnosis (incident cases) arising from in‐ or outpatient care at a participating hospital. We identified patients with CLI using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes. Critical limb ischemia diagnosis date was defined as the date of the first claim with primary diagnosis of atherosclerosis of native arteries of the extremities with rest pain (ICD‐9‐CM code 440.22), ulceration (ICD‐9‐CM code 440.23), or gangrene (ICD‐9‐CM code 440.24) or the date of the first CLI‐related procedure (ie, endovascular revascularization, surgical revascularization, major [above ankle] amputation, or minor [below ankle] amputation) occurring up to 10 days preceding the first claim to allow for delayed diagnosis claim reporting following CLI intervention. Determination of primary CLI diagnosis was made if the diagnosis code was first‐ or second‐listed to minimize the influence of unrelated confounding conditions. Patients were included if CLI diagnosis was made between January 1, 2011 and December 31, 2011, they had continuous coverage from January 1, 2010 to December 31, 2011, and they did not have a CLI diagnosis code in 2010 (eg, patients had no CLI diagnosis during at least the past 12 months). Utilization of a 12‐month stable diagnosis period is common in Medicare claims analyses of CLI patients.9, 10 Patients were followed through September 30, 2015, which corresponds to the date of the transition from ICD‐9‐CM to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes.
Patient Characteristics
Demographic patient data included age, sex, and race. Comorbidities included hypertension, diabetes mellitus, coronary artery disease, chronic kidney disease, hyperlipidemia, and tobacco use history. Geography was reported by region (South, Midwest, Northeast, West, or Puerto Rico) and population density (urban/rural) of the center initially caring for the patient. Clinical presentation was categorized as ischemic rest pain, ischemic ulcer, or gangrene.
Determination of First Major Treatment
Major treatment for CLI was defined as endovascular revascularization, surgical revascularization, or major (above the ankle) amputation. Utilization of major treatments following CLI diagnosis was determined with a combination of ICD‐9‐CM and Current Procedural Terminology (CPT) procedure codes (Table S1). The first major treatment was reported as the first endovascular revascularization, surgical revascularization, or major amputation procedure, regardless of subsequent treatments. In patients who underwent multiple first major treatments during the same encounter, the most invasive treatment was used for classification where major amputation was considered most invasive and endovascular revascularization was considered least invasive.
Outcomes
Main clinical outcomes were survival and major amputation through 4 years of follow‐up. Healthcare costs were estimated from Medicare reimbursements for hospital inpatient, hospital outpatient, and physician/supplier services. Costs were adjusted to 2016 US dollars using the Medical Care component of the Consumer Price Index. In order to account for mortality rate differences among groups, costs were reported in patients who were alive at the start of each follow‐up year regardless of vital status during that year.
Statistical Analysis
Baseline characteristics were reported as mean and SD for continuous variables and counts and percentages for categorical variables. Time to first‐event outcomes were analyzed using Kaplan–Meier methods, Cox proportional hazards regression, and the cumulative incidence function in the presence of competing risks. The cumulative event hazard was estimated with the Anderson and Gill extension to the Cox proportional‐hazards model for recurrent events.11 The Hochberg procedure was used to control the family‐wise type I error rate at 5% for multiple comparisons. In each analysis, patients with no events were censored after 4 years’ follow‐up; thus, the maximum possible follow‐up duration for each patient ranged from 3.75 to 4.0 years, depending on actual diagnosis date. The hazard ratio for survival and freedom from major amputation was estimated in a multivariable model where variable selection used backward elimination. Owing to the large sample size of this study, the model was further reduced using the generalized R2 statistic to identify the strongest predictors of outcomes.12 Variables were retained in this reduced multivariable model if R2 decreased by at least 0.01 when removed. In order to compare patient outcomes and costs by first major treatment, propensity scores were estimated using multinomial logistic regression, which represented the probability of receiving each major treatment for each patient. The covariates used to estimate propensity scores included all variables specified in the Patient Characteristics section and listed in Table 1. The trio of propensity scores were used to match like patients using a nearest neighbor approach without replacement, where caliper width was set at 0.09 (0.25 SDs of the propensity score).13, 14 Costs were adjusted for censoring using partitioned estimator methods.15 Data were analyzed using SAS (v9.4; SAS Institute, Cary, NC) and Stata Statistical Software (Release 13.1; StataCorp LP, College Station, TX).
Table 1.
Baseline Characteristics of Medicare Eligible Patients Diagnosed With Critical Limb Ischemia in 2011 Categorized by First Major Treatmenta
| Characteristic | All Patients | Patient Status By First Major Treatment | ||||
|---|---|---|---|---|---|---|
| Alive w/o Major Treatment | Endovascular | Surgical | Major Amputation | Died w/o Major Treatment | ||
| No. of patients | 72 199 (100) | 11 465 (16) | 28 530 (40) | 14 283 (20) | 3982 (6) | 13 939 (19) |
| Geographyb | ||||||
| Region | ||||||
| South | 29 721 (42) | 4349 (40) | 12 250 (44) | 5713 (41) | 2057 (53) | 5352 (41) |
| Midwest | 17 000 (24) | 2637 (24) | 7177 (26) | 3390 (24) | 703 (18) | 3093 (23) |
| Northeast | 13 802 (20) | 2427 (22) | 4756 (17) | 3037 (22) | 653 (17) | 2929 (22) |
| West | 9204 (13) | 1356 (13) | 3795 (14) | 1916 (14) | 365 (9) | 1772 (13) |
| Puerto Rico | 340 (<1) | 50 (<1) | 93 (<1) | 39 (<1) | 90 (2) | 68 (<1) |
| Population density | ||||||
| Urban | 62 623 (89) | 9643 (89) | 25 339 (90) | 12 715 (90) | 3345 (86) | 11 581 (88) |
| Rural | 7444 (11) | 1176 (11) | 2732 (10) | 1380 (10) | 523 (14) | 1633 (12) |
| Demographics | ||||||
| Male sex | 37 681 (52) | 5479 (48) | 14 773 (52) | 8281 (58) | 2107 (53) | 7041 (51) |
| Age, y | 74±12 | 71±12 | 74±11 | 72±11 | 76±12 | 78±11 |
| Race | ||||||
| White | 54 617 (76) | 8601 (75) | 21 374 (75) | 11 290 (79) | 2426 (61) | 10 926 (79) |
| Black | 13 476 (19) | 2164 (19) | 5346 (19) | 2352 (17) | 1340 (34) | 2274 (16) |
| Other/unknown | 4106 (6) | 649 (6) | 1723 (6) | 599 (4) | 209 (5) | 698 (5) |
| Medical history | ||||||
| Hypertension | 53 046 (73) | 8217 (72) | 21 205 (74) | 10 333 (72) | 2937 (74) | 10 354 (74) |
| Diabetes mellitus | 38 823 (54) | 5420 (47) | 16 776 (59) | 6798 (48) | 2268 (57) | 7561 (54) |
| Coronary artery disease | 34 570 (48) | 4059 (35) | 14 440 (51) | 7027 (49) | 1854 (47) | 7190 (52) |
| Chronic kidney disease | 23 672 (33) | 2378 (21) | 10 162 (36) | 3853 (27) | 1507 (38) | 5772 (41) |
| Hyperlipidemia | 19 134 (27) | 3064 (27) | 7775 (27) | 4059 (28) | 808 (20) | 3428 (25) |
| Smoking | 14 481 (20) | 1753 (15) | 5754 (20) | 4180 (29) | 777 (20) | 2017 (14) |
| Clinical presentation | ||||||
| Rest pain | 21 298 (29) | 4339 (38) | 8365 (29) | 5977 (42) | 276 (7) | 2341 (17) |
| Ulcer | 32 493 (45) | 5568 (49) | 13 625 (48) | 4786 (34) | 906 (23) | 7608 (55) |
| Gangrene | 18 408 (25) | 1558 (14) | 6540 (23) | 3520 (25) | 2800 (70) | 3990 (29) |
w/o indicates without.
Values are mean±SD or count (percentage).
Data available in 70 067 patients.
Results
Patient Characteristics and Major Treatments
Of approximately 36.5 million Medicare beneficiaries enrolled in 2011, 116 031 received a CLI diagnosis (0.32% prevalence), of which 96 628 had no CLI‐related claim over the previous year (0.26% incidence). After excluding 24 429 cases without primary CLI diagnosis (ie, CLI not first‐ or second‐listed diagnosis within claim), 72 199 incident cases of primary CLI were included in this study.
Baseline patient characteristics among the entire sample and according to first major treatment are described in Table 1. Mean patient age was 74±12 years, 52% were male, and predominant races were white (76%) and black (19%). Hypertension (73%), diabetes mellitus (54%), and coronary artery disease (48%) were the most common comorbidities. Clinical presentation was characterized by rest pain in 29%, ulcer in 45%, and gangrene in 25% of patients. Among patients undergoing primary major amputation, 30% did not receive a diagnosis of gangrene. The percentage of patients with gangrene was 36% for black, 36% for other race, and 22% for white (P<0.001 for white versus black and other races). Primary major amputation was performed more frequently (P<0.001) in patients of black race (10%) versus white (4%) and other races (5%).
A total of 46 795 (65%) patients received a major treatment during follow‐up (median, 9 days from CLI diagnosis), where primary procedures were endovascular revascularization (40%), surgical revascularization (20%), or major amputation (6%). Multiple primary major treatments were performed during the same encounter in 2670 patients, most commonly endovascular and surgical revascularization (2541 patients). Subsequent major treatments were relatively common in patients first treated with endovascular (50% of patients) or surgical (44% of patients) revascularization, but not major amputation (21% of patients). The cumulative number of subsequent major treatments received during follow‐up was 1.4 per patient with primary endovascular revascularization, 1.2 per patient with surgical revascularization, and 0.5 for major amputation. Throughout the follow‐up period, 33% of patients underwent a single revascularization procedure, 27% underwent multiple revascularizations, 9% received a single major amputation, and 1% received multiple major amputations. Among patients who underwent major amputation at any time in follow‐up, 51% did not undergo previous revascularization, 25% underwent 1 previous revascularization procedure, and 24% underwent multiple previous revascularization procedures. Among patients not receiving a major treatment, 45% were alive throughout the follow‐up period and 55% died during follow‐up.
Clinical Outcomes
Over 4 years follow‐up, survival was 46% (median survival, 3.5 years) and freedom from major amputation was 87% (Figures S1 and S2). In multivariate models of the association of baseline variables on risk of mortality and major amputation, statistically significant associations were observed with mortality among all 13 baseline variables and with major amputation among 9 of 13 baseline variables. In a reduced multivariate model, older age (hazard ratio, 5.1 for age ≥90 versus <50 years), greater clinical presentation severity (hazard ratio, 2.4 for gangrene, 1.6 for ulcer versus rest pain), and chronic kidney disease (hazard ratio, 1.8) had the strongest associations with mortality risk. Greater clinical presentation severity (hazard ratio, 5.6 for gangrene, 1.4 for ulcer versus rest pain) was the only significant predictor of major amputation in the reduced model; patient race was not a significant explanatory variable in the final model (Table 2).
Table 2.
Multivariate Analysis of Mortality and Major Amputation Risk Following Diagnosis of Critical Limb Ischemiaa
| Characteristic | Mortality | Major Amputation |
|---|---|---|
| Multivariate model | ||
| Age, y | ||
| <50 | 1.0 (Ref.) | 1.0 (Ref.) |
| 50 to 59 | 1.33 (1.22, 1.44) | 1.49 (1.28, 1.73) |
| 60 to 69 | 1.53 (1.41, 1.65) | 1.34 (1.17, 1.54) |
| 70 to 79 | 2.02 (1.87, 2.18) | 1.39 (1.21, 1.61) |
| 80 to 89 | 3.22 (2.98, 3.49) | 1.70 (1.48, 1.97) |
| ≥90 | 5.49 (5.05, 5.97) | 2.12 (1.81, 2.49) |
| Clinical presentation | ||
| Rest pain | 1.0 (Ref.) | 1.0 (Ref.) |
| Ulcer | 1.55 (1.51, 1.59) | 1.41 (1.31, 1.52) |
| Gangrene | 2.35 (2.28, 2.42) | 5.21 (4.87, 5.57) |
| Medical history | ||
| Chronic kidney disease | 1.71 (1.68, 1.75) | 1.14 (1.09, 1.20) |
| Coronary artery disease | 1.27 (1.24, 1.30) | 1.09 (1.04, 1.14) |
| Smoking | 1.18 (1.15, 1.22) | 1.34 (1.26, 1.41) |
| Diabetes mellitus | 1.09 (1.06, 1.11) | b |
| Renal insufficiency | 1.08 (1.05, 1.12) | b |
| Male sex | 1.06 (1.04, 1.08) | b |
| Rural geography | 1.06 (1.02, 1.09) | 1.19 (1.11, 1.28) |
| Hypertension | 0.90 (0.88, 0.92) | b |
| Hyperlipidemia | 0.86 (0.84, 0.88) | 0.85 (0.81, 0.90) |
| Region | ||
| Midwest | 1.0 (Ref.) | 1.0 (Ref.) |
| West | 0.99 (0.96, 1.03) | 0.90 (0.83, 0.99) |
| Northeast | 0.98 (0.95, 1.01) | 0.99 (0.92, 1.07) |
| South | 1.06 (1.03, 1.09) | 1.29 (1.22, 1.37) |
| Puerto Rico | 1.30 (1.13, 1.49) | 3.25 (2.66, 3.97) |
| Race | ||
| Other | 1.0 (Ref.) | 1.0 (Ref.) |
| Black | 1.05 (1.00, 1.11) | 1.60 (1.44, 1.79) |
| White | 1.24 (1.18, 1.30) | 0.97 (0.88, 1.08) |
| Reduced multivariate model | ||
| Age, y | ||
| <50 | 1.0 (Ref.) | c |
| 50 to 59 | 1.37 (1.26, 1.49) | |
| 60 to 69 | 1.57 (1.45, 1.70) | |
| 70 to 79 | 2.04 (1.89, 2.20) | |
| 80 to 89 | 3.13 (2.90, 3.38) | |
| ≥90 | 5.13 (4.73, 5.57) | |
| Clinical presentation | ||
| Rest pain | 1.0 (Ref.) | 1.0 (Ref.) |
| Ulcer | 1.56 (1.52, 1.60) | 1.41 (1.32, 1.52) |
| Gangrene | 2.35 (2.28, 2.41) | 5.60 (5.24, 5.97) |
| Chronic kidney disease | 1.82 (1.79, 1.86) | c |
Values are hazard ratio (95% confidence interval).
Variable not retained in multivariate model.
Variable not retained in reduced multivariate model.
Patient survival and freedom from major amputation through 4 years were lower in patients with gangrene (Figures 1 and 2). Among patients who underwent a major treatment, 4‐year survival was 45% for endovascular revascularization, 51% for surgical revascularization, and 21% for major amputation (all pair‐wise comparisons statistically significant at P<0.001; Figure S3). Patients undergoing primary major amputation had the worst prognosis across each clinical presentation category, with 4‐year survival of 34% in those with rest pain, 22% in those with ulcer, and 20% in those with gangrene (Figure S4). Major amputation rates in follow‐up were 3.9% for endovascular revascularization, 6.0% for surgical revascularization, and 11.0% for primary major amputation (P<0.001 for each revascularization procedure versus major amputation). Comparing patients of black, other, and white races, black patients had the highest major amputation rates in each clinical presentation category. Corresponding major amputation rates were 10.3%, 6.0%, and 5.4% in patients presenting with rest pain, 15.5%, 9.5%, and 8.9% in patients presenting with ulcer, and 35.5%, 22.6%, and 24.8% in patients presenting with gangrene (P<0.001 for black versus each race among each clinical presentation category).
Figure 1.
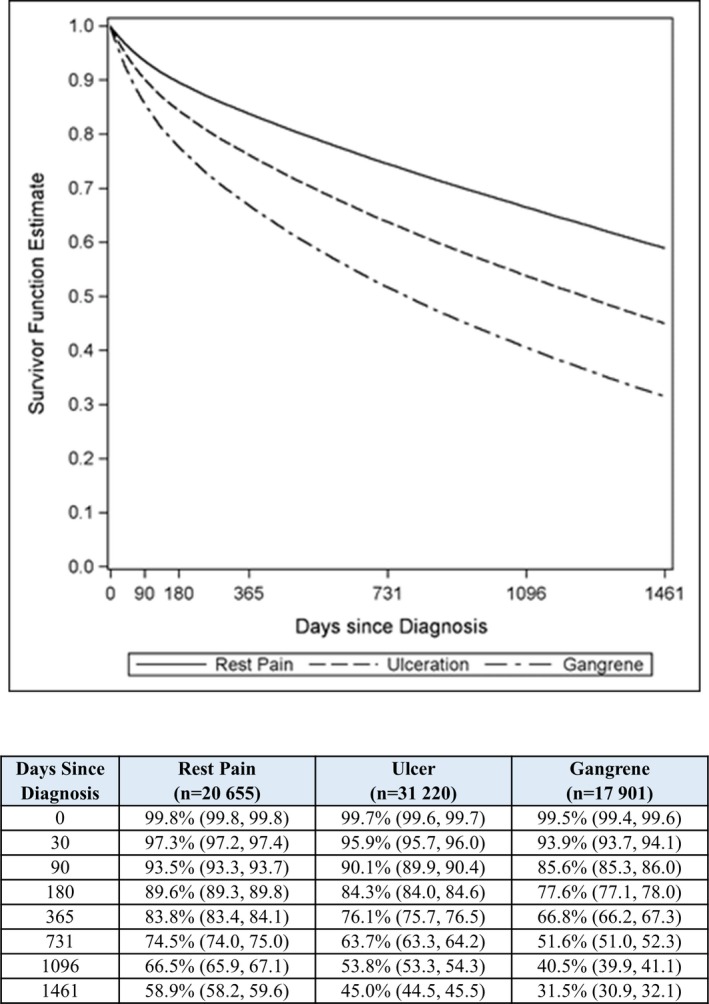
Patient survival over 4 years following diagnosis of critical limb ischemia by clinical presentation in entire sample. *P<0.001 vs ulcer; † P<0.001 vs gangrene; ‡ P<0.001 vs rest pain.
Figure 2.
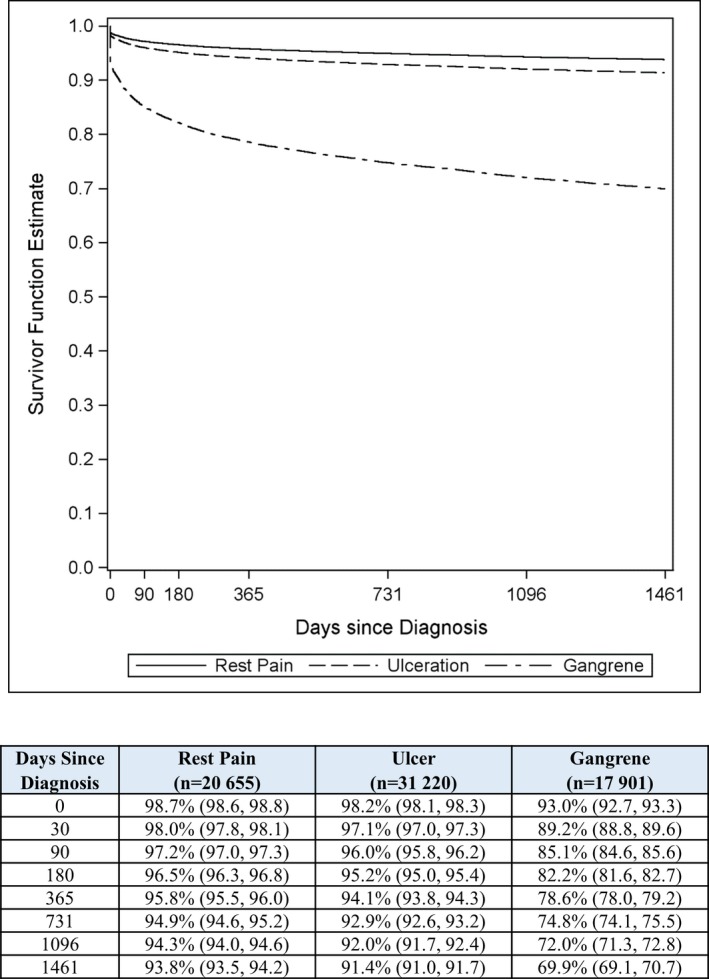
Freedom from major amputation over 4 years following diagnosis of critical limb ischemia by clinical presentation in entire sample. *P<0.001 vs ulcer; † P<0.001 vs gangrene; ‡ P<0.001 vs rest pain.
Healthcare Costs
Complete cost data were available in 70 160 (97%) patients in which Medicare was the primary payer. During the 12 months before CLI diagnosis, average cost was $25 100 per patient. Average cost per patient over the follow‐up period was $93 800, of which 62% was attributable to hospital inpatient costs, 20% to hospital outpatient costs, and 18% to physician/supplier costs. Average per‐patient costs during follow‐up based on initial clinical presentation were $78 300 for rest pain, $91 200 for ulcer, and $116 400 for gangrene (all pair‐wise comparisons statistically significant at P<0.001). When adjusting for follow‐up duration, cost per patient‐year after CLI diagnosis was $35 700. Among the entire sample, total healthcare costs were $6.5 billion over the study period.
Propensity‐Matched Comparison of Primary Endovascular Revascularization, Surgical Revascularization, and Major Amputation
After propensity‐score matching, 9942 patients were available for comparison of primary endovascular revascularization, surgical revascularization, or major amputation (3314 per group). Propensity‐score matching resulted in comparable baseline characteristics among these groups. The propensity‐matched sample presented with greater clinical presentation severity than in the unmatched sample (66% versus 25% with gangrene; Table S2), which was attributable to the overlap in propensity score distribution among the 3 groups that allowed a 1:1:1 match in most patients treated with primary major amputation. The 4‐year survival estimates were 38% with endovascular revascularization (median survival, 2.7 years), 40% with surgical revascularization (median survival, 2.9 years), and 23% with major amputation (median survival, 1.3 years). Survival was higher when comparing each revascularization approach to major amputation (P<0.001), but was not different comparing endovascular to surgical revascularization. The higher relative mortality risk associated with major amputation versus revascularization was mainly observed in the first 6 months, after which the mortality rate was comparable (Figure 3). Patients undergoing primary major amputation had the worst prognosis, regardless of clinical presentation severity, with 4‐year survival estimates of 34% in those with rest pain, 21% in those with ulcer, and 22% in those with gangrene (Figure 4). Major amputation rates in follow‐up were 6.5% for endovascular revascularization, 9.6% for surgical revascularization, and 10.6% for primary major amputation (P<0.001 for all pair‐wise comparisons).
Figure 3.
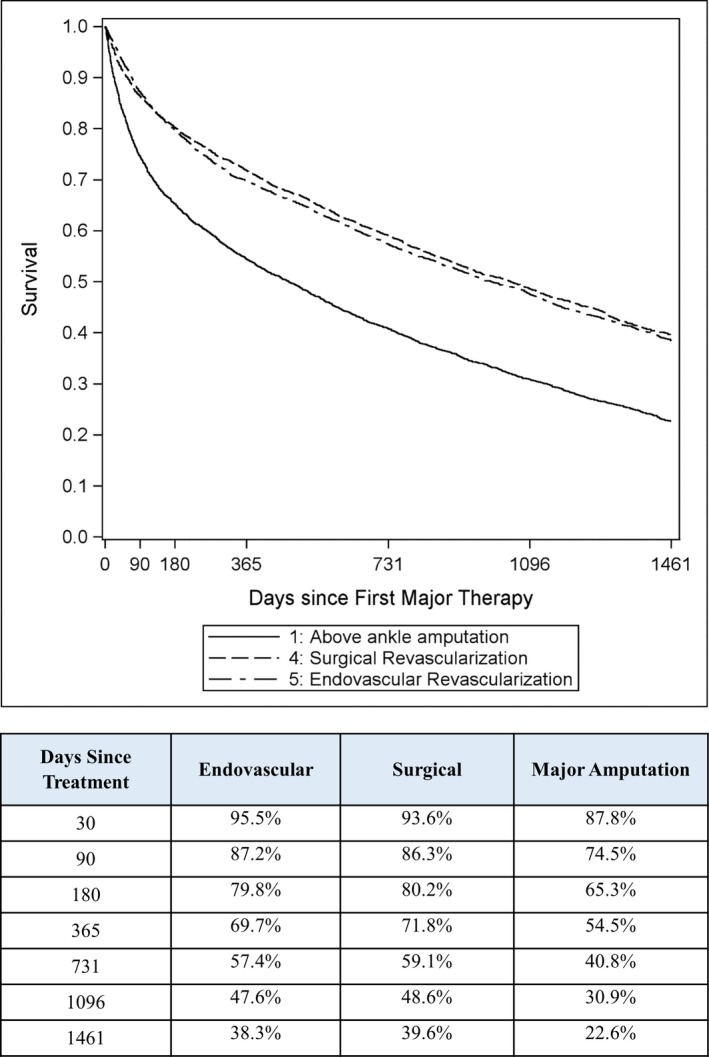
Patient survival over 4 years following first major therapy for critical limb ischemia in matched patients. *P<0.001 vs major amputation.
Figure 4.
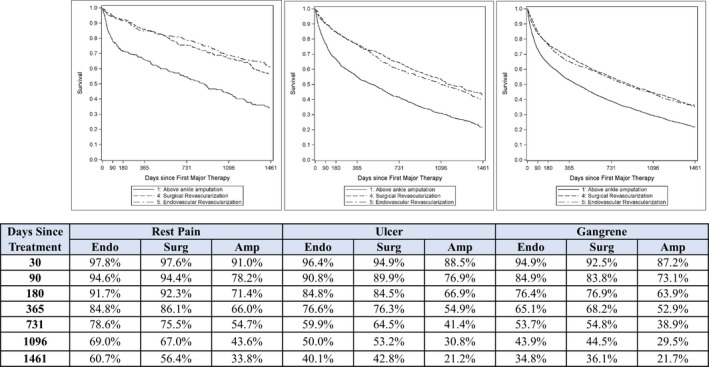
Patient survival over 4 years following first major therapy for critical limb ischemia by clinical presentation (A, rest pain; B, ulcer; C, gangrene) in matched patients. Amp indicates major amputation; endo, endovascular; surg, surgical. *P<0.001 vs major amputation within each clinical presentation category.
Complete cost data in which Medicare was primary payer were available in 9219 (93%) of matched patients. During the 12 months before CLI diagnosis, the average cost was $27 500 in patients ultimately treated with endovascular revascularization, $26 100 in those treated with surgical revascularization, and $38 300 in those treated with major amputation (P<0.001 comparing each revascularization approach to major amputation). Average cost per patient over the follow‐up period was $117 800, of which 68% was attributable to hospital inpatient costs, 14% to hospital outpatient costs, and 17% to physician/supplier costs. Average cost per patient over the follow‐up period according to initial major treatment was $121 900 for endovascular revascularization, $122 700 for surgical revascularization, and $107 500 for major amputation (P<0.001 comparing each revascularization approach with major amputation). The lower cost associated with major amputation was attributed to lower survival rates. When adjusting for follow‐up duration, cost per patient‐year was $49 700 for endovascular revascularization, $49 200 for surgical revascularization, and $55 700 for major amputation (P<0.001 comparing each revascularization approach to major amputation).
Discussion
Results of this claims analysis of the US Medicare population highlight the considerable clinical burden and high cost in patients following an initial diagnosis with CLI. Long‐term survival and cost are comparable between revascularization techniques, with lower major amputation rates following endovascular revascularization. Primary major amputation portends a poor prognosis even when adjusting for demographics, medical history, and disease severity. Compared with revascularization, primary major amputation is associated with shorter survival time, increased risk of second major amputation, and higher healthcare costs. These results were generally consistent regardless of patient characteristics and clinical presentation.
This study showed that among incident cases of CLI, 29% will die or undergo major amputation in the first year. Furthermore, the typical patient will endure multiple revascularization procedures over a median survival of only 3.5 years. For comparison, the estimated additional life expectancy among age‐ and sex‐matched adults is 13.4 years.16 Survival following CLI diagnosis is lower than that of heart failure,17 stroke,18 and most cancers.19 Given that CLI is underdiagnosed, increasing in prevalence, and responsible for significant risk to life and limb, considerable efforts are needed to raise disease awareness, refine diagnostic algorithms, and establish evidence‐based treatment pathways. Assuming an annual CLI incidence of 0.26%, 49.2 million adults aged ≥65 years in the United States in 2016, and estimated 4‐year costs of $93 800, this yields ≈$12 billion in annual costs attributable to incident cases of CLI.
Consensus recommendations cite revascularization as the optimal treatment for patients with CLI20 given the poor prognosis21 and functional impairment22 following major amputation. Allie et al23 reported that 51% of CLI patients had no diagnostic vascular evaluation preceding primary amputation. Goodney et al10 reported that 54% of patients with CLI had no vascular procedures in the year before undergoing amputation. In the current study, 51% of patients undergoing primary major amputation received no previous revascularization. These data highlight the heterogenous approach to CLI management and suggest that many patients may not undergo adequate diagnostic evaluation with imaging at initial presentation, potentially leading to unnecessary amputations and associated morbidity. On balance, primary major amputation may be appropriate in select patients, such as nonambulatory nursing home residents, where there is little chance of improved function or survival with revascularization.7 We noted important racial differences in major amputation rates in this study. Frequency of primary major amputation and subsequent major amputations was disproportionately higher in patients of black race versus white and other races that were not fully explained by differences in patient characteristics. Additional study is warranted to elucidate the factors that result in the striking racial disparities observed in clinical presentation and CLI management strategies.
Because most patients with CLI are elderly with multiple comorbidities, endovascular techniques have been adopted with increasing frequency.24 Although primary revascularization was superior to major amputation in every scenario in this study, whether an endovascular first strategy in CLI management improves long‐term patient outcomes remains unclear. In the current study, long‐term survival was comparable between revascularization approaches whereas major amputation risk was lower with endovascular revascularization. A large ongoing National Institutes of Health–sponsored multicenter, randomized controlled trial of endovascular versus surgical therapy in 2100 CLI patients was designed to address this question with greater rigor.25 Regardless of revascularization approach, an interdisciplinary care team skilled in wound healing, foot surgery, medical evaluation, and medical care should assist with patient care given the systemic involvement of the disease process.20, 26
Effective October 1, 2015, ICD‐10‐CM replaced ICD‐9‐CM for coding purposes in the United States. The new coding system offers greater detail and specificity for the purposes of diagnosing patients afflicted with CLI. The ICD‐10‐CM offers dozens of codes describing laterality, location of wounds, and disease burden. This CLI patient analysis would be more intensive using ICD‐10‐CM methodology and would lend itself to subset analyses at a greater investment of time and labor. This raises the question of whether this specific disease would be best served by the assignment of a Medicare Severity Diagnosis Related Group (MS‐DRG) code to facilitate the monitoring of this population moving forward. Such a code would allow for hospitals as well as public and private payers to prospectively track the incidence and burden of this population and make strategic investments to offset the effects of this disease.
Strengths of this research include long‐term follow‐up in a nation‐wide sample of patients initially diagnosed with CLI and managed under real‐world conditions. Furthermore, the comparison of 3 primary CLI treatment strategies in unmatched and matched samples is novel. There were also several limitations of this study inherent to administrative claims analysis that warrant further discussion. First, as a retrospective evaluation of claims records, potential exists for misclassification of important demographic, medical history, diagnostic, or procedural data. Second, CLI diagnosis in this study required a primary ICD‐9‐CM code of 440.22, 440.23, or 440.24. Other codes such as 707.14 (ulcer of heel and midfoot) or 785.4 (gangrene), with or without associated procedure codes, could have justifiably been used. However, the addition of procedure codes has been shown to reduce sensitivity and overall agreement when applied to well‐qualified CLI patients.27 Because there are no universally accepted ICD‐9‐CM codes to identify CLI and that the focus of this study was not to determine CLI incidence, but to characterize clinical outcome following diagnosis, a focused list of ICD‐9‐CM codes for CLI diagnosis was used to limit bias introduced by inclusion of cases with unrelated conditions. Third, we were unable to control for laterality where repeat procedures may have been performed on the contralateral leg. Fourth, the rationale for treatment decisions cannot be determined from this research. For example, although primary major amputation was performed in 6% of patients, whether amputation was performed because of standard institutional practice, extensive gangrene, inability to ambulate, or otherwise is unknown. Finally, despite the use of propensity‐score matching to adjust for selection bias among major treatments, the possibility that unmeasured patient characteristics were different among treatment groups and may have influenced outcomes cannot be discounted. For example, in the propensity‐matched sample, characteristics of patients initially treated with major amputation were comparable to those treated with revascularization; yet, healthcare costs in the year before CLI diagnosis were higher in those with major amputation as primary treatment. This suggests that patients initially managed with major amputation may have presented with greater unmeasured comorbidity that negatively influenced subsequent clinical outcomes. Finally, owing to the greater clinical severity in patients treated with primary major amputation, the propensity‐matched sample presented with gangrene more frequently than in unmatched cases. Still, the observation that primary major amputation resulted in the lowest survival rates in patients with rest pain, ulcer, and gangrene demonstrates the utility of the propensity‐matched results.
Conclusions
Patients initially diagnosed with CLI suffer poor long‐term prognosis and generate high healthcare costs. Long‐term survival and cost are comparable between revascularization techniques, with lower major amputation rates following endovascular revascularization. Compared with each revascularization approach, primary major amputation is associated with shorter survival time, higher risk of subsequent major amputation, and higher healthcare costs. Results from this observational research may be susceptible to bias attributable to the influence of unmeasured confounders. Considerable efforts are needed to raise disease awareness, implement coding to better define and identify the disease, refine diagnostic algorithms, establish evidence‐based treatment pathways, and address the high mortality rates associated with this diagnosis.
Sources of Funding
CLI Global Society provided financial support for this research.
Disclosures
Mustapha reports consultancy with Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Cardiovascular Systems, Cook Medical, Medtronic, Spectranetics, and Terumo. Katzen is a member of the Scientific Advisory Board of Boston Scientific, WL Gore, and Philips Healthcare. Neville is a member of the Scientific Advisory Board of WL Gore, Cormatrix, Graftworx, and Tissue Analytics; holds equity investment in Graftworx and Tissue Analytics; and received research grants from WL Gore and Medtronic. Lookstein reports consultancy with Boston Scientific and Medtronic; and is a member of the Scientific Advisory Board of Boston Scientific and Medtronic. Zeller received honoraria for speaking or moderating educational programs from 480 biomedical, Abbott Vascular, Biotronik, Boston Scientific Corp., Cordis, Medtronic, Shockwave Medical, Spectranetics, Veryan/Novate, Phillips‐Volcano, and WL Gore; reports consultancy with Boston Scientific Corp., Medtronic, Gore & Associates, Spectranetics, and Veryan/Novate; and holds equity investment in QT Medical and Veryan/Novate. Miller reports consultancy with CLI Global Society, Spectranetics, and TriReme Medical. Jaff is a noncompensated advisor to Abbott Vascular, Boston Scientific, Cordis, and Medtronic Vascular; reports consultancy with Micell, Philips/Volcano, Venarum, American Orthotics and Prosthetics Association, and Vactronix; holds equity investment in PQ Bypass, Vascular Therapies, Primacea, and Embolitech; and is a board member of Greenway Health.
Supporting information
Table S1. Coding Scheme to Identity Interventional Procedures in Medicare Eligible Patients Diagnosed With Critical Limb Ischemia in 2011
Table S2. Baseline Characteristics of Medicare Eligible Patients Diagnosed With Critical Limb Ischemia in 2011 Categorized by First Major Therapy in Propensity‐Matched Sample
Figure S1. Patient survival over 4 years following diagnosis of critical limb ischemia among entire sample.
Figure S2. Freedom from major amputation over 4 years following diagnosis of critical limb ischemia among entire sample.
Figure S3. Patient survival over 4 years following first major therapy for critical limb ischemia among entire sample.
Figure S4. Patient survival over 4 years following first major therapy for critical limb ischemia by clinical presentation (A, rest pain; B, ulcer; C, gangrene) among entire sample.
Acknowledgments
The authors thank Noel Martinson for assistance in Medicare claims access and management, Teresa Nelson for assistance in data analysis, and Louise Anderson for assistance in cost analysis. We gratefully acknowledge the Board of the Critical Limb Ischemia Global Society for their role in study design conception and development.
(J Am Heart Assoc. 2018;7:e009724 DOI: 10.1161/JAHA.118.009724.)
References
- 1. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. [DOI] [PubMed] [Google Scholar]
- 2. Siracuse JJ, Menard MT, Eslami MH, Kalish JA, Robinson WP, Eberhardt RT, Hamburg NM, Farber A; Vascular Quality Initiative . Comparison of open and endovascular treatment of patients with critical limb ischemia in the Vascular Quality Initiative. J Vasc Surg. 2016;63:958–965.e1. [DOI] [PubMed] [Google Scholar]
- 3. Bisdas T, Borowski M, Stavroulakis K, Torsello G; CRITISCH Collaborators . Endovascular therapy versus bypass surgery as first‐line treatment strategies for critical limb ischemia: results of the interim analysis of the CRITISCH Registry. JACC Cardiovasc Interv. 2016;9:2557–2565. [DOI] [PubMed] [Google Scholar]
- 4. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H; BASIL trial participants . Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong EJ, Ryan MP, Baker ER, Martinsen BJ, Kotlarz H, Gunnarsson C. Risk of major amputation or death among patients with critical limb ischemia initially treated with endovascular intervention, surgical bypass, minor amputation, or conservative management. J Med Econ. 2017;20:1148–1154. [DOI] [PubMed] [Google Scholar]
- 6. Peacock JM, Keo HH, Duval S, Baumgartner I, Oldenburg NC, Jaff MR, Henry TD, Yu X, Hirsch AT. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis. 2011;8:A141. [PMC free article] [PubMed] [Google Scholar]
- 7. Oresanya L, Zhao S, Gan S, Fries BE, Goodney PP, Covinsky KE, Conte MS, Finlayson E. Functional outcomes after lower extremity revascularization in nursing home residents: a national cohort study. JAMA Intern Med. 2015;175:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agency for Healthcare Research and Quality . HCUP Databases. 2011. Available at: http://www.hcup-us.ahrq.gov/databases.jsp. Accessed October 9, 2017.
- 9. Baser O, Verpillat P, Gabriel S, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manage. 2013;10:26–36. [Google Scholar]
- 10. Goodney PP, Travis LL, Nallamothu BK, Holman K, Suckow B, Henke PK, Lucas FL, Goodman DC, Birkmeyer JD, Fisher ES. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 12. Cox DR, Snell EJ. The Analysis of Binary Data. 2nd ed London: Chapman & Hall; 1989. [Google Scholar]
- 13. Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24:401–409. [DOI] [PubMed] [Google Scholar]
- 14. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 15. Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]
- 16. Social Security Administration . Retirement & Survivors Benefits: Life Expectancy Calculator. Available at: https://www.ssa.gov/OACT/population/longevity.html. Accessed August 27, 2017.
- 17. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 18. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, Stewart‐Wynne EG. Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. 2000;31:2080–2086. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Institute . Cancer Stat Facts. Available at: https://seer.cancer.gov/statfacts/. Accessed: August 27, 2017.
- 20. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 21. Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, Peterson ED. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013;165:809–815, 815.e1. [DOI] [PubMed] [Google Scholar]
- 22. Suckow BD, Goodney PP, Cambria RA, Bertges DJ, Eldrup‐Jorgensen J, Indes JE, Schanzer A, Stone DH, Kraiss LW, Cronenwett JL; Vascular Study Group of New England . Predicting functional status following amputation after lower extremity bypass. Ann Vasc Surg. 2012;26:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allie DE, Hebert CJ, Lirtzman MD, Wyatt CH, Keller VA, Khan MH, Khan MA, Fail PS, Vivekananthan K, Mitran EV, Allie SE, Chaisson G, Stagg SJ, Allie AA, McElderry MW, Walker CM. Critical limb ischemia: a global epidemic. A critical analysis of current treatment unmasks the clinical and economic costs of CLI. EuroIntervention. 2005;1:75–84. [PubMed] [Google Scholar]
- 24. Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, Dake MD, Jaff MR, Kaufman JA, Powell RJ, Reid DM, Siami FS, Sopko G, White CJ, Rosenfield K. Design and Rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST‐CLI) Trial. J Am Heart Assoc. 2016;5:e003219 DOI: 10.1161/JAHA.116.003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bekwelem W, Bengtson LG, Oldenburg NC, Winden TJ, Keo HH, Hirsch AT, Duval S. Development of administrative data algorithms to identify patients with critical limb ischemia. Vasc Med. 2014;19:483–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Coding Scheme to Identity Interventional Procedures in Medicare Eligible Patients Diagnosed With Critical Limb Ischemia in 2011
Table S2. Baseline Characteristics of Medicare Eligible Patients Diagnosed With Critical Limb Ischemia in 2011 Categorized by First Major Therapy in Propensity‐Matched Sample
Figure S1. Patient survival over 4 years following diagnosis of critical limb ischemia among entire sample.
Figure S2. Freedom from major amputation over 4 years following diagnosis of critical limb ischemia among entire sample.
Figure S3. Patient survival over 4 years following first major therapy for critical limb ischemia among entire sample.
Figure S4. Patient survival over 4 years following first major therapy for critical limb ischemia by clinical presentation (A, rest pain; B, ulcer; C, gangrene) among entire sample.


