Abstract
Background
Anemia is considered to increase the risk of mortality in high‐risk populations, but its effect has not been examined among young populations. This study aimed to determine the effect of hemoglobin (Hb) concentration and its changes on the risk of acute myocardial infarction (AMI), stroke, cerebrovascular disease and all‐cause mortality among young women.
Methods and Results
We analyzed data from the Korean National Health Information Database on 808 143 women aged 20 to 39 years without any cardiocerebrovascular disease. A 1‐time Hb concentration and changes in Hb over a 2‐year period were calculated as exposures. Participants were followed for a median of 10 years to determine the risk of AMI, stroke, cerebrovascular disease, and all‐cause mortality. There were U‐ or J‐shaped associations between Hb concentration or change in Hb and AMI, stroke, cerebrovascular disease, and all‐cause mortality. Increasing the Hb concentration from normal to high increased the risk for AMI (hazard ratio [95% confidence interval]: 1.49 [1.08‐2.04]). With regard to the risk for stroke, increasing the Hb concentration from a normal to a high range increased the risk (hazard ratio [95% confidence interval]: 1.10 [1.02‐1.35]), and decreasing the Hb concentration from a high to a normal range decreased this risk (hazard ratio [95% confidence interval]: 0.80 [0.60‐0.97]). Improving anemia to the normal Hb range decreased all‐cause mortality (hazard ratio [95% confidence interval]: 0.81 [0.69‐0.94]); however, overcorrection of Hb concentration (Hb≥14.0 g/dL) was not significant.
Conclusions
These findings suggest that regular Hb analysis may assist in identifying young women who are at risk of AMI, stroke, cerebrovascular disease, and all‐cause mortality.
Keywords: acute myocardial infarction, cerebrovascular disease, hemoglobin, mortality, stroke
Subject Categories: Cardiovascular Disease
Clinical Perspective
What Is New?
There was a U‐ or J‐shaped association between hemoglobin (Hb) concentration and stroke or all‐cause mortality after a 10‐year follow‐up among more than 800 000 healthy premenopausal women.
Improving anemia via achieving the normal Hb range decreased all‐cause mortality, but overcorrection of Hb concentration was not significant.
What Are the Clinical Implications?
These findings can be used by physicians as evidence to administer a gradual course of treatment and conduct more frequent follow‐ups on changes in Hb concentration because overcorrection of Hb does not confer any benefit.
Introduction
The associations between hemoglobin (Hb) concentrations and cardiocerebrovascular diseases have been well investigated, primarily among high‐risk groups, including patients with chronic kidney disease or cardiovascular disease and older adults.1, 2, 3, 4, 5, 6, 7, 8 However, it is possible that an abnormal Hb concentration is a consequence of preclinical cardiocerebrovascular disease rather than a predisposing factor. In such patients, there are diverse possible causes of anemia, including iron deficiency anemia, anemia due to chronic diseases, and hemoglobinopathy. Thus, Hb concentration has not been considered as a risk factor in calculating cardiovascular disease risks. Additionally, in prior studies,1, 2, 3, 4, 5, 6 Hb has generally been examined only once, making it possible to misclassify a patient's Hb status and making it difficult to estimate the duration of an abnormal Hb status.
There are few studies on the associations between Hb status and cardiocerebrovascular diseases among healthy young adults. In particular, young women are predisposed to anemia due to regular bleeding in menstruation.9 Of the several types of anemia, iron‐deficiency anemia is the most common cause of anemia overall10 and accounts for up to 95% of anemias in nonpregnant women aged 18 to 35 years.11 The diagnosis of iron‐deficiency anemia is well established, and it is relatively easy to treat with oral iron medication.12, 13, 14 If the effects of abnormal Hb status increase the risk for disease in young women, its early detection is pivotal.
In this study we aimed to investigate the associations of Hb concentrations and its changes with cardiocerebrovascular disease risks and all‐cause mortality among nonpregnant premenopausal women by using information from the Korean National Health Insurance Service (NHIS) National Health Information Database (NHID).
Methods
The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Overview
We analyzed data from a customized retrospective cohort from the NHID managed by the NHIS, which is the Korean single insurer. The Korean National Health examination is conducted biannually; individuals who were born in an even year undergo screening in even years, whereas those who were born in an odd year undergo screening in odd years. Individuals aged 20 to 39 years who are employed or insured as a self‐employee are applicable. The NHID covers insurance claims, health care utilization, sociodemographic variables, health screenings, and information on diagnosis for the whole population of South Korea.15 The NHIS databases including the NHID are regarded as a world leader among population‐based study platforms, and their integrated information technology and validity have been established.16
Study Population
Among the possible participants, 838 457 women aged 20 to 39 in 2002 who had undergone medical examinations and had Hb concentrations available at both the baseline (2002 or 2003) and second (2004 or 2005) health examinations were selected. Six participants who died and 1591 participants diagnosed with ischemic heart disease (I20‐I25) or cerebrovascular disease (I60‐I69) based on diagnoses coded according to the International Classification of Disease, 10th revision (ICD‐10) before the index date (January 1, 2006) were excluded. Finally, 27 495 individuals were excluded due to a lack of information for important covariates, and 808 143 women were included in our study.
Exposures and Covariates
Two types of exposures were evaluated (Figure 1). First, once‐off Hb concentrations before the index date were categorized into 5 groups based on the World Health Organization criteria for anemia (<12.0 g/dL).17 Second, the change in Hb concentration was determined over 2 health examinations using a 3‐category system.
Figure 1.
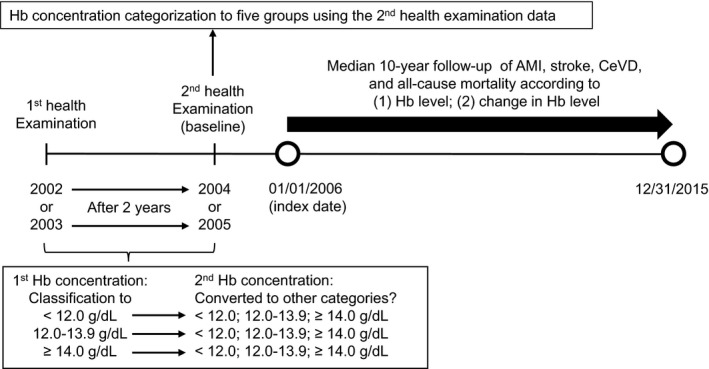
Timeline of the study. AMI indicates acute myocardial infarction; CeVD, cerebrovascular disease; Hb, hemoglobin.
Covariates were from the second health examination, which included age, socioeconomic status, smoking status (never and ever), alcohol use (none, 1‐2 times per week, and ≥3 times per week), regular exercise (none, 1‐2 times per week, and ≥3 times per week), body mass index (BMI; <18.5, 18.5‐22.9, and ≥23.0 kg/m2), systolic and diastolic blood pressure (mm Hg), fasting serum glucose (mg/dL), total cholesterol (mg/dL), and the Charlson Comorbidity Index (0, 1, and ≥2). The Charlson Comorbidity Index is well established for predicting mortality.18 Cancer and chronic kidney disease prevalence were analyzed because anemia is regarded as a comorbidity of these diseases.
The main outcomes were hospitalizations for ≥2 days due to acute myocardial infarction (AMI), stroke, or cerebrovascular disease that occurred from January 1, 2006 to December 31, 2015. ICD‐10 codes were used to identify these outcomes: AMI (I21), stroke (I60‐I64), and cerebrovascular disease (I60‐I69). The identification of all‐cause mortality was based on a notification of death provided to the government between January 1, 2006 and December 31, 2015.
Statistical Analysis
We used Cox proportional hazards regression models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of outcomes of interest according to each Hb concentration category or change in Hb adjusted for cardiocerebrovascular risk factors, including age, socioeconomic status, smoking status, alcohol use, regular exercise, BMI, blood pressure, fasting serum glucose, total cholesterol, and the Charlson Comorbidity Index. A change in Hb was determined as the change (increasing or decreasing) between 2 health examinations after the Hb concentrations had been divided into 3 categories: <12.0, 12 to 13.9, and ≥14.0 g/dL. The reference groups for the evaluation of changes in Hb consisted of those whose Hb concentrations were unchanged from the first to the second health examination (persistent low Hb, persistent high Hb, and persistent normal Hb). A sensitivity analysis was performed by excluding patients with cancer or chronic kidney disease. Data curation was performed with SAS 9.3 (SAS Institute, Cary, NC), and statistical analyses were performed with STATA 15.0 (StataCorp LP, College Station, TX).
Ethics
This study was conducted according to the guidelines in the Declaration of Helsinki, and all procedures involving human subjects (patients) were approved by the institutional review board of the Seoul National University (no. 1703‐039‐863). All participants were informed regarding the objective of the survey and provided consent. The NHIS database was anonymized according to strict confidentiality guidelines.
Results
The study population of 808 143 women was observed for a median 10‐year follow‐up. The mean Hb concentration was 12.8 g/dL (SD 1.1), and 17.2% and 0.2% of participants were anemic (Hb<12.0 g/dL) and had Hb concentrations >16.0 g/dL, respectively. Baseline characteristics according to Hb concentration before the index date health examination are shown in Table 1. Cardiovascular risk of participants was generally low. The mean age of participants at baseline was 32.5 years (SD 6.3). Most were never smokers (96.3%) and drank alcohol less than 3 times per week (98.6%). Participants without any comorbidities accounted for 46.8% of the study population. The mean BMI was 21.4 kg/m2 (SD 2.9). Patients with chronic kidney disease and cancer accounted for 0.2% and 1.9% of the total population, respectively. During follow‐up, hospitalization for AMI, stroke, and cerebrovascular disease, and all‐cause mortality occurred in 455 (0.1%), 2573 (0.3%), 5478 (0.7%), and 3878 (0.5%) individuals, respectively.
Table 1.
General Characteristics of Women According to Hemoglobin Level
| Total | Hemoglobin Concentration | |||
|---|---|---|---|---|
| <12.0 | 12.0 to 13.9 | ≥14.0 | ||
| Total, n | 808 143 | 138 973 | 564 876 | 104 294 |
| Total, % | 100 | 17.2 | 69.9 | 12.9 |
| Age, y | 32.5 (6.3) | 33.6 (6.2) | 32.3 (6.3) | 31.7 (6.4) |
| 20 to 24 | 9.1 | 6.4 | 9.3 | 11.7 |
| 25 to 29 | 30.5 | 25.3 | 31.3 | 33.5 |
| 30 to 34 | 22.6 | 23.5 | 22.6 | 21.5 |
| ≥35 | 37.8 | 44.8 | 36.9 | 33.3 |
| Socioeconomic status | ||||
| First (lowest) | 18.0 | 18.7 | 17.8 | 18.4 |
| Second | 29.9 | 27.8 | 29.9 | 32.5 |
| Third | 33.0 | 32.5 | 33.3 | 32.1 |
| Fourth (highest) | 19.1 | 21.0 | 19.0 | 17.0 |
| Smoking status | ||||
| Never | 96.3 | 97.5 | 96.3 | 94.4 |
| Ever | 3.7 | 2.5 | 3.7 | 5.6 |
| Alcohol (per wk) | ||||
| None | 61.2 | 67.3 | 60.5 | 56.7 |
| 1 to 2 times | 37.4 | 31.7 | 38.1 | 41.3 |
| ≥3 times | 1.4 | 1.0 | 1.4 | 2.0 |
| Regular exercise (per wk) | ||||
| None | 64.9 | 65.3 | 64.6 | 65.6 |
| 1 to 2 times | 21.6 | 20.8 | 21.8 | 21.9 |
| ≥3 times | 11.7 | 12.1 | 11.7 | 10.9 |
| Underlying diseases | ||||
| Cancer | 1.9 | 2.2 | 1.8 | 1.8 |
| CKD | 0.2 | 0.2 | 0.2 | 0.2 |
| CCI | ||||
| 0 | 46.8 | 46.1 | 46.9 | 47.4 |
| 1 | 37.0 | 36.8 | 37.1 | 36.8 |
| ≥2 | 16.2 | 17.1 | 16.1 | 15.8 |
| BMI (kg/m2) | 21.4 (2.9) | 21.4 (2.7) | 21.3 (2.8) | 21.5 (3.3) |
| BMI<18.5 | 12.5 | 10.6 | 12.7 | 14.3 |
| 18.5≤BMI<23.0 | 52.2 | 52.9 | 52.6 | 49.0 |
| BMI≥23.0 | 35.2 | 36.5 | 34.7 | 36.6 |
| sBP (mm Hg) | 112.4 (12.2) | 111.5 (12.2) | 112.2 (12.0) | 114.3 (12.8) |
| dBP (mm Hg) | 71.0 (9.0) | 70.9 (8.9) | 72.7 (9.2) | 72.7 (9.2) |
| FSG (mg/dL) | 87.0 (14.8) | 86.9 (14.3) | 87.5 (18.1) | 87.5 (18.1) |
| TC (mg/dL) | 177.4 (35.2) | 176.1 (37.2) | 176.8 (34.7) | 182.7 (35.0) |
Data are expressed as the mean (SD), or percentage. For each variable, if the sum is not 100%, the remainder is nonavailable. BMI indicates body mass index; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; dBP, diastolic blood pressure; FSG, fasting serum glucose; sBP, systolic blood pressure; TC, total cholesterol.
Association of Hb Concentration With Cardiocerebrovascular Disease Risks and All‐Cause Mortality
Figure 2 demonstrates the association between Hb concentration and incident AMI, stroke, cerebrovascular disease, and all‐cause mortality after adjustment for age, socioeconomic status, regular exercise, smoking status, alcohol use, BMI, blood pressure, fasting serum glucose, total cholesterol, and the Charlson Comorbidity Index after the 10‐year follow‐up among women without cardiocerebrovascular disease at index date. There was a U‐shaped association between Hb concentration and risk of cardiocerebrovascular disease and all‐cause mortality. In particular, stroke risk was significantly increased when the Hb concentration was lower than 12.0 g/dL and higher than 14.0 g/dL. All‐cause mortality was increased when the Hb concentration was lower than 12.0 g/dL; however, it was not significant when the Hb concentration was higher than 14.0 g/dL. AMI and cerebrovascular disease were not significantly related to Hb concentration, although the relationships were U‐shaped. Figure S1 shows the association between the categorized Hb concentration and risk of each outcome. There was a marginally U‐shaped association between Hb concentrations and AMI risks compared with that for those with Hb concentrations between 12.0 and 12.9 g/dL.
Figure 2.
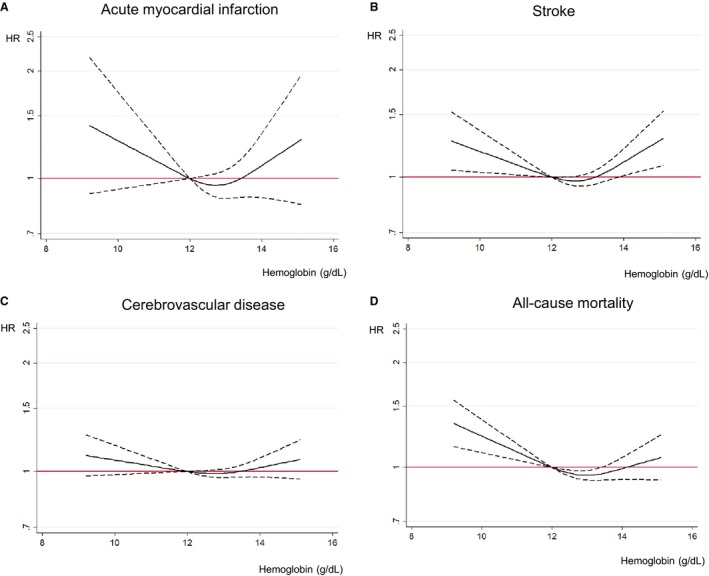
Association of hemoglobin concentration with cardiovascular risks and all‐cause mortality after 10‐year follow‐up among young women. A, Association between hemoglobin concentration and acute myocardial infarction after a 10‐year follow‐up among young women. B, Association between hemoglobin concentration and stroke after a 10‐year follow‐up among young women. C, Association between hemoglobin concentration and cerebrovascular disease after a 10‐year follow‐up among young women. D, Association between hemoglobin concentration and all‐cause mortality after a 10‐year follow‐up among young women. Hazard ratio analyzed by Cox proportional hazards regression analysis adjusted for age, socioeconomic status, physical activity, smoking, alcohol, body mass index, blood pressure, fasting plasma glucose, total cholesterol, and Charlson Comorbidity Index (95% confidence interval). HR indicates hazard ratio.
There was a U‐shaped association between Hb concentrations and stroke (HR [95% CI] for Hb <11.0 g/dL 1.24 [1.06‐1.44] and for Hb ≥14.0 g/dL 1.25 [1.12‐1.41], respectively) compared with those for Hb 12.0‐12.9 g/dL. The association between Hb concentrations and cerebrovascular disease risks was also U‐shaped. Anemic status was related to an increased risk of all‐cause mortality: (HR [95% CI] for Hb <11.0 g/dL 1.30 [1.15‐1.47], and for Hb 11.0‐11.9 g/dL 1.10 [0.99‐1.22], respectively) compared with that for those with Hb concentrations 12.0‐12.9 g/dL. These J‐ or U‐shaped associations between Hb concentrations and cardiocerebrovascular disease risks or all‐cause mortality were similar when stratified by BMI and the Charlson Comorbidity Index status (Table S1). The results of sensitivity analyses conducted by excluding patients with chronic kidney disease or cancer (Tables S2 and S3) were consistent with the main results.
Association Between Change in Hb Concentration and Cardiocerebrovascular Disease Risks and All‐Cause Mortality
Figure 3 depicts the associations between the change in Hb concentration over a 2‐year period and cardiocerebrovascular disease risks and all‐cause mortality after adjustment for cardiocerebrovascular risk factors. Among the interesting outcomes, AMI was significantly associated with the magnitude of change in Hb concentration. Other outcomes, including stroke, cerebrovascular disease, and all‐cause mortality, were not significant. Table 2 shows the associations between change in Hb concentrations over a 2‐year period and each outcome and mortality after adjustment for covariates, which were considered categorical changes. With regard to the risk of AMI, an increase in the Hb concentration from normal to high increased the risk (HR [95% CI] 1.49 [1.08‐2.04]); however, other changes in the Hb concentration were not significant. With regard to the risk of stroke, increasing the Hb concentration from a normal to a high range elevated the risk (HR [95% CI] 1.10 [1.02‐1.35]), and decreasing the Hb concentration from a high to a normal range decreased the risk (HR [95% CI] 0.80 [0.60‐0.97]). The risk of cerebrovascular disease was not significantly associated with any change in Hb. In terms of all‐cause mortality, improving anemia to the normal Hb range decreased all‐cause mortality (HR [95% CI] 0.81 [0.69‐0.94]); however, the effect of overcorrection of Hb concentration (Hb≥14.0 g/dL) was not significant. Change to anemic status from normal range increased all‐cause mortality (HR [95% CI] 1.13 [1.01‐1.26]).
Figure 3.
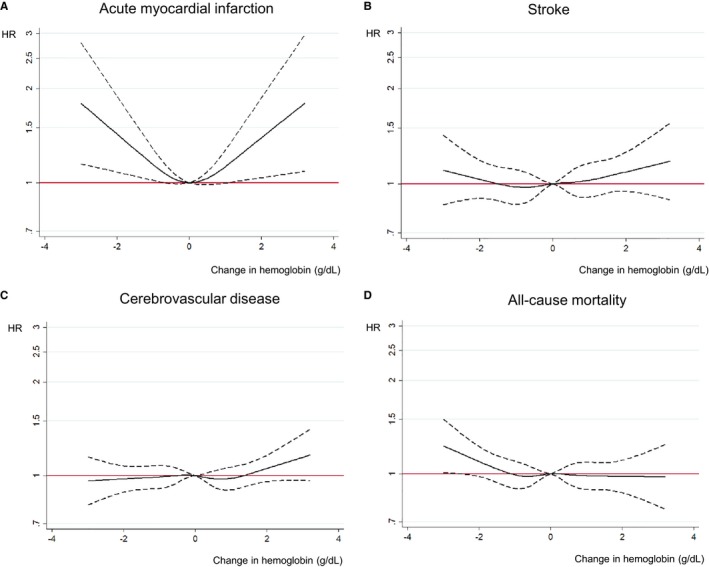
Association of change in hemoglobin concentration with cardiovascular risks and all‐cause mortality after a 10‐year follow‐up among young women. A, Association between change in hemoglobin concentration over 2 years and acute myocardial infarction after a 10‐year follow‐up among young women. B, Association between change in hemoglobin concentration over 2 years and stroke after a 10‐year follow‐up among young women. C, Association between change in hemoglobin concentration over 2 years and cerebrovascular disease after a 10‐year follow‐up among young women. D, Association between change in hemoglobin concentration over 2 years and all‐cause mortality after a 10‐year follow‐up among young women. Hazard ratio analyzed by Cox proportional hazards regression analysis adjusted for age, socioeconomic status, physical activity, smoking, alcohol, body mass index, blood pressure, fasting plasma glucose, total cholesterol, and Charlson Comorbidity Index (95% confidence interval). HR indicates hazard ratio.
Table 2.
Association of Change in Hemoglobin Status With Cardiovascular Outcome and Mortality Among Young Women
| Change in Hb (g/dL) | Events (n) | Follow‐Up Duration (Person‐Years) | HR | 95% CI |
|---|---|---|---|---|
| Risk of AMI | ||||
| Hb <12.0 at first examination | ||||
| <12.0 | 39 | 584 986 | 1 | Reference |
| 12.0 to 13.9 | 38 | 678 635 | 0.92 | 0.59 to 1.44 |
| ≥14.0 | 4 | 52 249 | 1.16 | 0.41 to 3.27 |
| Hb 12.0 to 13.9 at first examination | ||||
| <12.0 | 50 | 737 294 | 1.32 | 0.97 to 1.79 |
| 12.0 to 13.9 | 208 | 4 251 360 | 1 | Reference |
| ≥14.0 | 47 | 638 139 | 1.49 | 1.08 to 2.04 |
| Hb ≥14.0 at first examination | ||||
| <12.0 | 5 | 62 523 | 1.36 | 0.51 to 3.62 |
| 12.0 to 13.9 | 42 | 702 212 | 1.06 | 0.62 to 1.80 |
| ≥14.0 | 21 | 349 470 | 1 | Reference |
| Risk of stroke | ||||
| Hb <12.0 at first examination | ||||
| <12.0 | 206 | 584 243 | 1 | Reference |
| 12.0 to 13.9 | 223 | 677 811 | 1.06 | 0.87 to 1.28 |
| ≥14.0 | 26 | 52 148 | 1.45 | 0.96 to 2.18 |
| Hb 12.0 to 13.9 at first examination | ||||
| <12.0 | 238 | 736 582 | 1.07 | 0.93 to 1.23 |
| 12.0 to 13.9 | 1232 | 4 247 534 | 1 | Reference |
| ≥14.0 | 226 | 637 392 | 1.1 | 1.02 to 1.35 |
| Hb ≥14.0 at first examination | ||||
| <12.0 | 26 | 62 423 | 0.95 | 0.63 to 1.45 |
| 12.0 to 13.9 | 235 | 701 428 | 0.8 | 0.60 to 0.97 |
| ≥14.0 | 160 | 348 929 | 1 | Reference |
| Risk of CeVD | ||||
| Hb <12.0 at first examination | ||||
| <12.0 | 454 | 583 316 | 1 | Reference |
| 12.0 to 13.9 | 474 | 676 815 | 1.02 | 0.89 to 1.16 |
| ≥14.0 | 51 | 52 059 | 1.3 | 0.97 to 1.74 |
| Hb 12.0 to 13.9 at first examination | ||||
| <12.0 | 524 | 735 560 | 1.04 | 0.95 to 1.14 |
| 12.0 to 13.9 | 2759 | 4 242 089 | 1 | Reference |
| ≥14.0 | 427 | 636 618 | 1.01 | 0.92 to 1.12 |
| Hb ≥14.0 at first examination | ||||
| <12.0 | 47 | 62 330 | 0.95 | 0.70 to 1.30 |
| 12.0 to 13.9 | 463 | 700 501 | 0.87 | 0.75 to 1.02 |
| ≥14.0 | 279 | 348 432 | 1 | Reference |
| All‐cause mortality | ||||
| Hb <12.0 at first examination | ||||
| <12.0 | 355 | 585 220 | 1 | Reference |
| 12.0 to 13.9 | 312 | 678 798 | 0.81 | 0.69 to 0.94 |
| ≥14.0 | 21 | 52 265 | 0.65 | 0.42 to 1.01 |
| Hb 12.0 to 13.9 at first examination | ||||
| <12.0 | 394 | 737 588 | 1.13 | 1.01 to 1.26 |
| 12.0 to 13.9 | 1929 | 4 252 615 | 1 | Reference |
| ≥14.0 | 296 | 638 359 | 1.02 | 0.90 to 1.15 |
| Hb ≥14.0 at first examination | ||||
| <12.0 | 34 | 62 557 | 0.96 | 0.67 to 1.39 |
| 12.0 to 13.9 | 341 | 702 433 | 0.9 | 0.76 to 1.08 |
| ≥14.0 | 197 | 349 600 | 1 | Reference |
Hazard ratio calculated by Cox proportional hazards regression analysis adjusted for age, socioeconomic status, physical activity, smoking status, alcohol habit, body mass index, blood pressure, fasting serum glucose, total cholesterol, and Charlson Comorbidity Index.
AMI indicates acute myocardial infarction; CeVD, cerebrovascular disease; CI, confidence interval; Hb, hemoglobin; HR, hazard ratio.
Discussion
In this large population of women aged 20 to 39 years who did not have underlying cardiocerebrovascular diseases at baseline, Hb concentrations showed a U‐ or J‐shaped association with both cerebrovascular disease risks and all‐cause mortality after a 10‐year follow‐up and after adjusting for traditional cardiovascular risk factors. Moving into the normal range of Hb concentration from a lower status reduced the risk of all‐cause mortality, and moving into the higher Hb status from the normal range of Hb concentration elevated the risk of AMI and stroke. Similar results were obtained after patients with chronic kidney disease or cancer had been excluded.
To our knowledge, this is the first study to investigate the association between 2‐year changes in Hb concentrations and cardiocerebrovascular disease after 10 years of follow‐up among more than 800 000 healthy premenopausal women. A few previous studies have examined the association between a change in Hb concentration and mortality. A prospective cohort study of patients with a mean age of 64 years from 45 countries with stable coronary artery disease reported after a 4‐year follow‐up that persistent anemia elevated the risk of mortality, although patients whose anemia normalized over time did not appear to have an increased mortality risk.7 More recently, we also reported the association between change in Hb and risks of cardiovascular diseases and mortality among individuals over 40 years, which revealed that reaching and maintaining Hb concentrations within the normal range were associated with decreased all‐cause mortality.8
However, the subjects in these studies included the elderly, which made it possible to include preclinical status of cardiovascular disease. Additionally, the causes of anemia among the elderly are very diverse, including iron deficiency anemia, anemia due to chronic diseases, and hemoglobinopathy. On the other hand, the cause of anemia among young adults, especially young women, is predominantly iron‐deficiency anemia,10 which is due to regular bleeding during menstruation.9 As noted previously, iron deficiency is the most common cause of anemia,10 accounting for up to 95% of nonpregnant women aged 18 to 35 years with anemia.11 Fortunately, iron deficiency is more easily treated than are other types of anemia. Furthermore, we found that overcorrection of Hb concentration (ie, from Hb<12 g/dL to Hb≥14.0 g/dL) was insignificant. Improving anemia to the normal range rather than overcorrection of the Hb concentration decreased all‐cause mortality. This finding is useful for physicians because it provides evidence supporting the administration of a gradual course of treatment and emphasizes the need to conduct more frequent follow‐ups on changes in Hb values, as overcorrection of Hb does not confer any benefit.
We also assessed the association between once‐off Hb concentrations and cardiocerebrovascular disease, obtaining results consistent with those in diverse previous studies. The Framingham study, which had a 34‐year follow‐up period, reported a J‐shaped relationship between hematocrit and AMI and a U‐shaped relationship between hematocrit and all‐cause mortality after multivariable adjustment among women aged 35 to 64 years at baseline.19 Additionally, 1 prospective cohort of 62 763 Norwegian women aged 30 to 85 years with a 12‐year follow‐up period reported that anemic status (Hb<12.0 g/dL) significantly elevated AMI risk.20 A study from Japan reported a U‐shaped association between hematocrit concentration and stroke risks.21
Possible mechanisms relating to high Hb and cardiocerebrovascular diseases have been suggested. As a hemorheological parameter such as hematocrit increases, blood viscosity is reduced in both cerebral and coronary circulation.22, 23 High blood viscosity leads to increased peripheral resistance, thereby reducing cardiac output, blood flow, and perfusion.22 In addition, increased iron is related to inflammatory reactions, including oxidative stress via lipid peroxidation, which is regarded as a risk factor for coronary heart diseases.24 In our study a Hb concentration of >14 g/dL was a risk factor for AMI and stroke in young women.
Conversely, anemia is also regarded as a risk factor for cardiocerebrovascular diseases, and several mechanisms to explain this relationship have been proposed. If red blood cells, which play an important role in transporting oxygen from the lungs to other organs, are decreased, vascular resistance will be systemically reduced and affect cardiocerebrovascular systems.25 Under conditions of chronic anemia, compensation of reduced perfusion leads to increased cardiac output by increasing the preload and decreasing the afterload, thereby inducing left ventricular hypertrophy.26 Moreover, decreased hematocrit leads to changes in red blood cell–mediated nitric oxide metabolism, increasing cardiac output.25 Another proposal is that decreased hematocrit activates erythrocyte‐derived adenosine diphosphate, which stimulates platelet activation and increases platelet adhesiveness, thereby elevating the risk of atherosclerosis and thrombosis,27, 28 which can cause ischemic cardiocerebrovascular diseases.
Some limitations of this study bear mention. First, this customized retrospective cohort study from the NHID was not representative of all young adults in South Korea. Although enrollment in the NHIS is mandatory for all South Koreans, national health screening examinations for young adults cover only employed people or householders. Data from unemployed people and nonhouseholders were therefore not included, which could have caused an underestimation of anemia status. Second, it is also possible that we underestimated the incidence of AMI, stroke, or cerebrovascular disease by defining hospitalization as a period of 2 days or more for the relevant diseases, although prior studies reported that defining cardiovascular disease based on the ICD‐10 code is accurate in more than 80% of cases.29 Despite these possibilities, we found a significant association between Hb concentration or its change and cardiocerebrovascular disease among young women.
In conclusion, these findings show that there is a J‐ or U‐ shaped association between Hb concentration and both cardiocerebrovascular disease and all‐cause mortality. In addition, we found that both achieving and maintaining a normal Hb concentration were related to decreased all‐cause mortality among young women. Regular Hb analysis may assist in identifying young women who are at risk of AMI, stroke, cerebrovascular disease, and all‐cause mortality.
Sources of Funding
This research was supported by a grant of the Korea Health technology R&D Project through the Korea Health Industry Development Institute(KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No: 20170322652‐00).
Disclosures
None.
Supporting information
Figure S1. Association of hemoglobin concentration (as a categorical variable) with cardiovascular risks and all‐cause mortality after 10‐year follow‐up among young women. Hazard ratio analyzed by Cox proportional hazards regression analysis adjusted for age, socioeconomic status, physical activity, smoking, alcohol, body mass index, blood pressure, fasting plasma glucose, total cholesterol, and Charlson Comorbidity Index (95% confidence interval).
Table S1. Subgroup Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Stratified by Body Mass Index and the Charlson Comorbidity Index Status
Table S2. Sensitivity Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Excluding Women With Cancer
Table S3. Sensitivity Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Excluding Women With Chronic Kidney Disease
(J Am Heart Assoc. 2018;7:e008147 DOI: 10.1161/JAHA.117.008147.)
References
- 1. Kikuchi M, Inagaki T, Shinagawa N. Five‐year survival of older people with anemia: variation with hemoglobin concentration. J Am Geriatr Soc. 2001;49:1226–1228. [DOI] [PubMed] [Google Scholar]
- 2. Lawler PR, Filion KB, Dourian T, Atallah R, Garfinkle M, Eisenberg MJ. Anemia and mortality in acute coronary syndromes: a systematic review and meta‐analysis. Am Heart J. 2013;165:143–153.e145. [DOI] [PubMed] [Google Scholar]
- 3. Nissenson AR, Goodnough LT, Dubois RW. Anemia: not just an innocent bystander? Arch Intern Med. 2003;163:1400–1404. [DOI] [PubMed] [Google Scholar]
- 4. Panwar B, Judd SE, Warnock DG, McClellan WM, Booth JN III, Muntner P, Gutierrez OM. Hemoglobin concentration and risk of incident stroke in community‐living adults. Stroke. 2016;47:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah AD, Nicholas O, Timmis AD, Feder G, Abrams KR, Chen R, Hingorani AD, Hemingway H. Threshold haemoglobin levels and the prognosis of stable coronary disease: two new cohorts and a systematic review and meta‐analysis. PLoS Med. 2011;8:e1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker AM, Schneider G, Yeaw J, Nordstrom B, Robbins S, Pettitt D. Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. J Am Soc Nephrol. 2006;17:2293–2298. [DOI] [PubMed] [Google Scholar]
- 7. Kalra PR, Greenlaw N, Ferrari R, Ford I, Tardif J‐C, Tendera M, Reid CM, Danchin N, Stepinska J, Steg PG. Hemoglobin and change in hemoglobin status predict mortality, cardiovascular events, and bleeding in stable coronary artery disease. Am J Med. 2017;130:720–730. [DOI] [PubMed] [Google Scholar]
- 8. Lee G, Choi S, Kim K, Yun JM, Son JS, Jeong SM, Kim SM, Park SM. Association of hemoglobin concentration and its change with cardiovascular and all‐cause mortality. J Am Heart Assoc. 2018;7:e007723 DOI: 10.1161/JAHA.117.007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . The global prevalence of anaemia in 2011. Geneva: WHO; 2015.
- 10. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thankachan P, Muthayya S, Walczyk T, Kurpad AV, Hurrell RF. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr Bull. 2007;28:328–336. [DOI] [PubMed] [Google Scholar]
- 12. Camaschella C. Iron‐deficiency anemia. N Engl J Med. 2015;372:1832–1843. [DOI] [PubMed] [Google Scholar]
- 13. Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225–233. [DOI] [PubMed] [Google Scholar]
- 14. Lopez A, Cacoub P, Macdougall IC, Peyrin‐Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. [DOI] [PubMed] [Google Scholar]
- 15. Cheol Seong S, Kim Y‐Y, Khang Y‐H, Heon Park J, Kang H‐J, Lee H, Do C‐H, Song J‐S, Hyon Bang J, Ha S. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2016;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population‐based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, 2011 (who/nmh/nhd/mnm/11.1). 2017. Available at: http://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=3&isAllowed=y. Accessed August 7, 2018.
- 18. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. [DOI] [PubMed] [Google Scholar]
- 19. Gagnon DR, Zhang T‐J, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease—the Framingham study: a 34‐year follow‐up. Am Heart J. 1994;127:674–682. [DOI] [PubMed] [Google Scholar]
- 20. Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. High blood hemoglobin concentration as risk factor of major atherosclerotic cardiovascular events in 114,159 healthy men and women in the Apolipoprotein Mortality Risk Study (AMORIS). Ann Med. 2012;44:476–486. [DOI] [PubMed] [Google Scholar]
- 21. Gotoh S, Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Fukuhara M, Ikeda F, Ago T, Kitazono T. Hematocrit and the risk of cardiovascular disease in a Japanese community: the Hisayama study. Atherosclerosis. 2015;242:199–204. [DOI] [PubMed] [Google Scholar]
- 22. Wood JH, Kee D Jr. Hemorheology of the cerebral circulation in stroke. Stroke. 1985;16:765–772. [DOI] [PubMed] [Google Scholar]
- 23. Kesmarky G, Toth K, Habon L, Vajda G, Juricskay I. Hemorheological parameters in coronary artery disease. Clin Hemorheol Microcirc. 1998;18:245–251. [PubMed] [Google Scholar]
- 24. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in Eastern Finnish men. Circulation. 1992;86:803–811. [DOI] [PubMed] [Google Scholar]
- 25. Kuhn V, Diederich L, Keller TS IV, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M, Cortese‐Krott MM. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal. 2017;26:718–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. London GM. Left ventricular alterations and end‐stage renal disease. Nephrol Dial Transplant. 2002;17:29–36. [DOI] [PubMed] [Google Scholar]
- 27. Turitto V, Weiss H. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541–543. [DOI] [PubMed] [Google Scholar]
- 28. Karino T, Goldsmith H. Role of blood cell‐wall interactions in thrombogenesis and atherogenesis: a microrheological study. Biorheology. 1984;21:587–601. [DOI] [PubMed] [Google Scholar]
- 29. Park JK, Kim KS, Kim CB, Lee TY, Lee KS, Lee DH, Lee S, Jee SH, Suh I, Koh KW. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33:76–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association of hemoglobin concentration (as a categorical variable) with cardiovascular risks and all‐cause mortality after 10‐year follow‐up among young women. Hazard ratio analyzed by Cox proportional hazards regression analysis adjusted for age, socioeconomic status, physical activity, smoking, alcohol, body mass index, blood pressure, fasting plasma glucose, total cholesterol, and Charlson Comorbidity Index (95% confidence interval).
Table S1. Subgroup Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Stratified by Body Mass Index and the Charlson Comorbidity Index Status
Table S2. Sensitivity Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Excluding Women With Cancer
Table S3. Sensitivity Analysis of Association Between Hemoglobin Concentration and Cardiovascular Risks and All‐Cause Mortality Excluding Women With Chronic Kidney Disease


