Abstract
Background
Statins are commonly used for the prevention of cardiovascular events; however, statins are underutilized in patients with noncoronary atherosclerosis. We sought to establish the rates of statin use in patients with carotid artery disease and to examine the association between statin therapy and outcomes after carotid revascularization.
Methods and Results
In this population‐level retrospective cohort study, we identified all individuals aged ≥66 years who underwent carotid endarterectomy or stenting in Ontario, Canada (2002–2014). The primary outcome was a composite of 1‐year stroke, myocardial infarction, or death (major adverse cardiac and cerebrovascular events). Five‐year risks were also examined. Adjusted hazard ratios were computed using inverse probability of treatment weighting based on propensity scores. A total of 7893 of 10 723 patients (73.6%) who underwent carotid revascularization were on preprocedural statin therapy; moderate‐ or high‐dose therapy was utilized by 7384 patients (68.9%). The composite rate of 1‐year major adverse cardiac and cerebrovascular events was lower among statin users (adjusted hazard ratio: 0.76; 95% confidence interval, 0.70–0.83). Patients who were on persistent long‐term statin therapy after the carotid procedure continued to experience significantly lower risk of major adverse cardiac and cerebrovascular events at 5 years (adjusted hazard ratio: 0.75, 95% confidence interval, 0.71–0.80). The beneficial associations with statin use were observed regardless of type of carotid revascularization procedure, carotid artery symptom status, or statin dose.
Conclusions
Continuous statin therapy was associated with a 25% lower risk of long‐term adverse cardiovascular events in patients with significant carotid disease. Along with other supportive evidence, statins should be considered in patients undergoing carotid revascularization, and efforts are required to increase statin use in this undertreated population.
Keywords: carotid artery stenting, carotid endarterectomy, carotid revascularization, carotid stenosis, statins
Subject Categories: Cardiovascular Surgery, Vascular Disease, Cerebrovascular Disease/Stroke, Cerebrovascular Procedures, Peripheral Vascular Disease
Clinical Perspective
What Is New?
Only two thirds of patients aged ≥66 years with significant carotid artery stenosis undergoing carotid revascularization were on moderate‐ or high‐dose statin therapy at the time of the carotid procedure.
Patients with isolated carotid artery disease were less likely to be on statin therapy compared with those who had concomitant coronary artery disease.
Consistent use of statin therapy after carotid artery revascularization was associated with a sustained 25% reduction in the risk of stroke, myocardial infarction, or death at up to 5 years of follow‐up.
What Are the Clinical Implications?
Improving statin utilization among patients with significant carotid artery stenosis should be a key component of quality programs and may translate to better long‐term outcomes.
Introduction
Statins are competitive inhibitors of 3‐hydroxy 3‐methylglutaryl coenzyme A reductase, an enzyme responsible for making cholesterol in the liver, resulting in a 25% to 50% reduction in circulating LDL (low‐density lipoprotein) and cholesterol levels. In addition, statins have important pleiotropic cardiovascular effects including reduction of inflammation and atherosclerotic plaque stabilization.1, 2, 3 Clinically, statins have been shown to reduce rates of stroke, myocardial infarction, and death in patients with cardiovascular disease. To that end, major clinical practice guidelines recommend statin therapy for secondary prevention in most patients with established cardiovascular disease and for primary prevention in high‐risk patients.4, 5
Unfortunately, despite clear guidelines, a disproportionately high gap in care exists for patients with noncoronary atherosclerosis involving the peripheral and carotid circulations. Several studies have reported underutilization of established risk‐reduction therapies such as statins for peripheral artery disease relative to coronary artery disease,6, 7, 8 which may lead to poor cardiovascular and limb outcomes.9 Nevertheless, few data exist on the utilization of statin therapy among patients with carotid artery disease. In the multicenter randomized controlled EVA‐3S (Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis) and SPACE (Stent‐Supported Percutaneous Angioplasty of the Carotid Artery Versus Endarterectomy) trials, only 49% of patients were on lipid‐lowering therapy at the time of carotid revascularization.10, 11 This is particularly concerning, given data from the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial that showed carotid stenosis patients derive the greatest benefit from intense statin therapy with respect to adverse cerebro‐ and cardiovascular events.12
We set out to answer 2 specific questions in this study. First, we sought to establish the pre‐ and postprocedural rates of statin use in patients with established carotid artery disease (defined as those undergoing carotid artery revascularization) at a population level. Second, we sought to examine the association between statin use and long‐term outcomes in patients with carotid artery disease.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results based on institutional privacy policies.
Study Design and Sources of Data
We designed a retrospective population‐level cohort study using administrative healthcare databases in Ontario, Canada, between April 1, 2002, and March 31, 2015. These databases are linked using unique identifiers and capture all healthcare system interactions for 13.6 million Ontario residents with access to universal health care. The data sets we used contain information on admissions to acute care hospitals, ambulatory and emergency department visits, physician and healthcare‐provider billing claims, demographic and vital statistics records, and medication prescription claims data. See Table S1 and Figure S1 for more information on the databases used in this study. These databases are routinely used for health services and pharmacoepidemiological research,13, 14, 15 and validation studies have shown them to be of high quality.16, 17, 18, 19 The institutional review boards at St. Michael's Hospital and Sunnybrook Health Science Center approved this study. The need for patient consent to use these databases for research purposes was waived under the Personal Health Information Privacy Act. The first and last authors had full access to all data in the study and take responsibility for its integrity and the data analysis.
Study Population
All patients aged ≥66 years who underwent carotid artery revascularization (endarterectomy or stenting) between April 1, 2002, and March 31, 2014, were eligible for this study. We used Canadian Classification of Health Intervention procedure codes JE57Lx and 1JE50x to identify patients who underwent endarterectomy and stenting, respectively. A previous validation study showed that this approach accurately captures carotid revascularization patients in our databases (positive predictive value: 87–99%; sensitivity: 90–93%).16 We excluded patients who had multiple carotid procedures during the same hospital admission and those who underwent combined coronary and carotid revascularization.
Identification of Statin Use and Intensity at Baseline and Follow‐up
We used the Ontario Drug Benefit program database to establish baseline and postprocedural statin use during follow‐up. This database, established in 1990 by the Ontario Ministry of Health and Health and Long Term Care, captures data on medication prescriptions filled by Ontario residents aged ≥65 years and those on social assistance. It includes quantitative prescription information such as drug identification number, dispensed date, number of days supplied, and cost. The coding error rate in this database for dispensed prescriptions is <1%.19
To establish baseline statin use, we first used a 150‐day look‐back window from the date of the index carotid procedure to identify any prescription claims for a statin. We used a 150‐day window because the maximum quantity of medications that can be dispensed at 1 time in Ontario is 100 days. In addition, we added a 50% grace period to avoid excluding patients who were admitted to the hospital or who experienced other unexpected delays in refilling their prescriptions, as conducted in previous studies.20, 21 We then checked for active statin therapy at the time of the procedure for all patients with at least 1 statin prescription claim within the 150‐day window. We defined patients as baseline statin users if the date of the last statin prescription plus 1.5 times the number of prescription days supplied crossed the carotid procedure date. For example, if a patient had last filled a prescription for a 60‐day supply of statin therapy, the patient must have had the carotid procedure within 90 days to be categorized as a baseline statin user. Patients who were not actively on statins at the time of the procedure were categorized as non–statin users. In addition, we classified statin intensity based on the 2013 American College of Cardiology/American Heart Association guidelines on the treatment of blood cholesterol (Table S2).5
We also used the Ontario Drug Benefit program database to capture postprocedural statin use during long‐term follow‐up. Among baseline statin users, we defined ongoing use as filling of the next statin prescription within the duration defined in the prescription plus a 50% grace period. We censored statin users who stopped using a statin at any time during follow‐up. We defined statin discontinuation as no repeated statin prescription dispensation within the aforementioned time windows. With respect to non–statin users, we censored those who started statin therapy during follow‐up at the time of prescription dispensation to allow for an “on‐treatment” analysis. Finally, we also censored patients who had a repeated carotid intervention and those who reached the end of the study period (March 31, 2015). All patients received a minimum of 1‐year follow‐up, and we followed patients for a maximum of 5 years.
Outcomes
The primary outcome was a 1‐year composite risk of any stroke, myocardial infarction, or death. As secondary outcomes, we also studied individual components of this composite outcome at 1 year. With respect to long‐term outcomes, we examined 5‐year composite and individual risks of any stroke, myocardial infarction, or death. We used validated coding algorithms based on the International Classification of Diseases, 10th Revision (ICD‐10) diagnosis codes to capture stroke18 and myocardial infarction17 as in‐hospital complications of the index carotid procedure and most responsible diagnoses for readmission during follow‐up.
Covariates
We measured several baseline covariates that could potentially confound the relationship between statin use and adverse cardiovascular events, including age, sex, rural residence, neighborhood income quintile (as a measure for socioeconomic status),22 overall comorbidity burden (as indicated by the Charlson Comorbidity Index),23 and health services utilization. We also used a 5‐year look‐back window to establish medical comorbidities and prior cardiovascular procedures and a 1‐year look‐back window to establish baseline medication use. Symptomatic carotid stenosis was defined as a previous hospital admission or emergency department visit within 6 months for ischemic stroke or transient ischemic attack. Finally, we established procedural and hospital characteristics, including year of procedure, elective versus emergent admission, and academic or specialized stroke center. See Table S3 for a complete list of the codes used to establish covariates and outcomes in this study and their accuracy.
Statistical Analysis
We compared baseline characteristics of baseline statin users versus non–statin users using standardized differences. Standardized differences, which reflect the mean difference as a percentage of the standard deviation, are more suitable for population‐level studies as they are not as sensitive to sample size as traditional testing.24 A standardized difference of >0.1 is typically felt to be significant.24
We conducted time‐to‐event analyses using Cox proportional hazards regression to compare 1‐ and 5‐year outcomes between statin users and non–statin users. We calculated unadjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for each outcome with non–statin users as the reference group. We then used propensity score methods to adjust for potential confounding and to reduce selection bias. All variables listed in Table 1 were used to build multivariable logistic regression models to calculate propensity scores, including age, sex, rural residence, neighborhood income quintile, Charlson Comorbidity Index, health services utilization (mean outpatient physician visits in the past year and mean emergency department visits and hospital visits in the past 3 years), carotid artery symptom status, comorbid conditions (coronary artery disease, acute myocardial infarction, heart failure, peripheral artery disease, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, chronic kidney disease), prior procedures (carotid endarterectomy, coronary revascularization, and peripheral revascularization), year of procedure (2002–2006, 2007–2010, or 2011–2014), urgent admission, academic center, stroke center, and baseline medication use (acetylsalicylic acid, dipyridamole, clopidogrel, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, β‐blocker, diuretic, calcium channel blocker, oral antidiabetic drug, insulin, warfarin, and novel oral anticoagulant). The variable any antiplatelet agent was removed from the model due to collinearity (defined as a variance inflation factor >5).
Table 1.
Baseline Characteristics of Patients
| No Statin (n=2830) | Statin (n=7893) | SDiffa (Unadjusted Comparison) | SDiffa (After IPTW Adjustment) | |
|---|---|---|---|---|
| Age, y | ||||
| Mean±SD | 75.6±6.2 | 74.7±5.6 | 0.15 | 0.00 |
| Range, n (%) | ||||
| 66–75 | 1462 (51.7) | 4530 (57.4) | 0.12 | 0.01 |
| ≥76 | 1368 (48.3) | 3363 (42.6) | 0.12 | 0.01 |
| Female sex, n (%) | 976 (34.5) | 2607 (33.0) | 0.03 | 0.00 |
| Rural residence, n (%)b | 558 (19.7) | 1434 (18.2) | 0.04 | 0.02 |
| Neighborhood income quintile, n (%)b | ||||
| 1 (lowest) | 580 (20.5) | 1499 (19.0) | 0.04 | 0.00 |
| 2 | 606 (21.4) | 1718 (21.8) | 0.01 | 0.00 |
| 3 | 546 (19.3) | 1605 (20.3) | 0.03 | 0.00 |
| 4 | 530 (18.7) | 1574 (19.9) | 0.03 | 0.01 |
| 5 (highest) | 559 (19.8) | 1473 (18.7) | 0.03 | 0.01 |
| Charlson comorbidity index, n (%)b | ||||
| 0 | 824 (29.1) | 2123 (26.9) | 0.05 | 0.00 |
| 1 | 597 (21.1) | 1737 (22.0) | 0.02 | 0.01 |
| ≥2 | 829 (29.3) | 2720 (34.5) | 0.11 | 0.02 |
| Health service utilization | ||||
| Outpatient physician visits in past year, mean±SD | 13.9±8.4 | 15.8±8.6 | 0.22 | 0.03 |
| Emergency department visits in past 3 y, mean±SD | 2.8±3.7 | 2.6±3.1 | 0.05 | 0.01 |
| Hospital admissions in past 3 y, mean±SD | 1.9±1.4 | 2.0±1.3 | 0.02 | 0.01 |
| Comorbid conditions, n (%) | ||||
| Symptomatic carotid stenosis | 1301 (46.0) | 3449 (43.7) | 0.05 | 0.00 |
| Coronary artery disease | 442 (15.6) | 2018 (25.6) | 0.25 | 0.02 |
| Acute MI | 89 (3.1) | 510 (6.5) | 0.16 | 0.01 |
| Congestive heart failure | 115 (4.1) | 378 (4.8) | 0.04 | 0.02 |
| Peripheral arterial disease | 137 (4.8) | 414 (5.2) | 0.02 | 0.02 |
| Diabetes mellitus | 787 (27.8) | 3013 (38.2) | 0.22 | 0.02 |
| Hypertension | 2305 (81.4) | 6998 (88.7) | 0.20 | 0.01 |
| COPD | 897 (31.7) | 2456 (31.1) | 0.01 | 0.02 |
| Chronic kidney disease | 90 (3.2) | 325 (4.1) | 0.05 | 0.01 |
| Prior procedures, n (%) | ||||
| Carotid endarterectomy | 101 (3.6) | 319 (4.0) | 0.02 | 0.01 |
| Coronary revascularization | 24 (0.8) | 172 (2.2) | 0.11 | 0.01 |
| Peripheral revascularization | 86 (3.0) | 220 (2.8) | 0.02 | 0.01 |
| Procedural and hospital characteristics | ||||
| Year of procedurec | ||||
| 2002–2006 | 1433 (50.6) | 3024 (38.3) | 0.25 | 0.00 |
| 2007–2010 | 779 (27.5) | 2765 (35.0) | 0.16 | 0.00 |
| 2011–2014 | 618 (21.8) | 2104 (26.7) | 0.11 | 0.00 |
| Urgent admission | 857 (30.3) | 1437 (18.2) | 0.28 | 0.03 |
| Academic center | 1278 (45.2) | 3741 (47.4) | 0.05 | 0.00 |
| Stroke center | 2155 (76.1) | 5771 (73.1) | 0.07 | 0.00 |
| Medication use, n (%) | ||||
| Any antiplatelet agentd | 923 (32.6) | 3947 (50.0) | 0.36 | 0.03 |
| Acetylsalicylic acidd | 501 (17.7) | 1794 (22.7) | 0.13 | 0.04 |
| Dipyridamole | 229 (8.1) | 966 (12.2) | 0.14 | 0.02 |
| Clopidogrel | 477 (16.9) | 2453 (31.1) | 0.34 | 0.04 |
| ACEI or ARB | 1260 (44.5) | 5262 (66.7) | 0.46 | 0.03 |
| β‐Blocker | 715 (25.3) | 3192 (40.4) | 0.33 | 0.03 |
| Diuretic | 766 (27.1) | 2706 (34.3) | 0.16 | 0.01 |
| Calcium channel blocker | 823 (29.2) | 2949 (37.4) | 0.18 | 0.01 |
| Oral antidiabetic | 330 (11.7) | 1671 (21.2) | 0.26 | 0.02 |
| Insulin | 104 (3.7) | 478 (6.1) | 0.11 | 0.02 |
| Warfarin | 177 (6.3) | 609 (7.7) | 0.06 | 0.02 |
| NOAC | 7 (0.2) | 53 (0.7) | 0.06 | 0.00 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; IPTW, inverse probability treatment weighting; MI, myocardial infarction; NOAC, novel oral anticoagulant; SDiff, standardized difference.
SDiff >0.1 indicates significant difference.
Missing values: ≤5 rural residence; 33 neighborhood income quintile (0.3%); 1893 Charlson comorbidity (17.7%).
For 2002, only procedures performed after March 31, 2002, are included. For 2014, procedures performed after March 31, 2014 are not included.
Acetylsalicylic acid use is underreported because over‐the‐counter purchases of this drug were not captured.
Inverse probability of treatment weighting (IPTW) analysis was then used to adjust for differences between the 2 groups based on the propensity scores.25, 26 We used the methods described by Austin and Stuart27 to calculate standardized differences post‐IPTW adjustment to ensure all baseline covariates were equally distributed in the adjusted cohorts. We then built IPTW‐adjusted Cox proportional hazards regression models to calculate adjusted HRs for the risk of each outcome and estimated IPTW‐adjusted survival curves using the approach of Cole and Hernán.28 Finally, we conducted subgroup analyses for the primary composite outcome at 1 and 5 years by type of carotid procedure (endarterectomy or stenting), carotid artery symptom status (symptomatic or asymptomatic), and statin dose (high or moderate/low). We combined moderate‐ and low‐dose statin groups because of the relatively small sample size of the low‐dose statin group. We also used interaction terms to test for heterogeneity between subgroups.
We visually inspected log−log survival curves and examined the statistical significance of time‐dependent covariates to test the proportional hazards assumption of all our models. All analyses were robust with the proportional hazards assumptions. All P values are 2‐sided, and P<0.05 was considered statistically significant. All statistical analyses were conducted using SAS Enterprise Guide v7.1 (SAS Institute).
Results
Patient Cohort
We identified a total of 10 723 patients, of which 7893 (73.6%) were on statin therapy and 2830 (26.4%) were not at baseline. Statin use was highest among those with concomitant coronary artery disease (82.0% versus 71.1%; P<0.0001; Figure 1). With respect to the intensity of statins, 4843 patients (45.2%) were on moderate‐dose therapy, 2541 (23.7%) were on high‐dose therapy, and 509 (4.8%) were on low‐dose therapy (Figure 1). The most commonly prescribed statin was atorvastatin (54.9%), followed by rosuvastatin (24.3%) and simvastatin (13.8%) (Figure S2).
Figure 1.
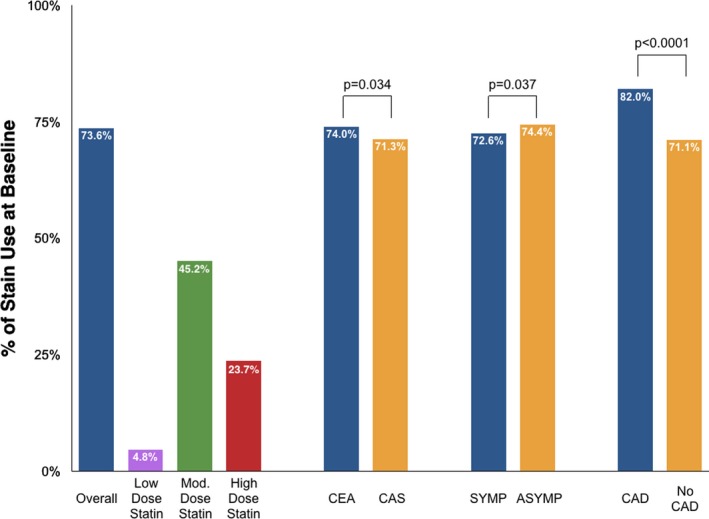
Proportion of patients on preprocedural statin therapy. ASYMP indicates asymptomatic carotid stenosis; CAD, coronary artery disease; CAS, carotid artery stenting; CEA, carotid endarterectomy; Mod., moderate; SYMP, symptomatic carotid stenosis.
Statin users were slightly younger than non–statin users (mean age: 74.7 versus 75.4 years). Overall, about a third were female, and 44% to 46% had symptomatic carotid stenosis. Statin users were more likely to have greater overall comorbidity burden (including a history of coronary disease, myocardial infarction, diabetes mellitus, hypertension, and prior coronary revascularization); tended to utilize more health services; and were more likely to be on antiplatelet, antihypertensive, or antidiabetic medications at baseline. Non–statin users were more likely to have urgent admission and undergo the carotid procedure earlier in the study period. After IPTW adjustment, all baseline variables were equally distributed between the statin and non–statin user groups (Table 1).
1‐Year Outcomes
The risk‐adjusted rate of the primary composite outcome of 1‐year stroke, myocardial infarction, or death was 26% lower among statin users (9.6% versus 11.2% for non–statin users; adjusted HR: 0.76; 95% CI, 0.70–0.83). Statin use was also associated with lower rates of 1‐year stroke or death (7.4% versus 9.1% for non–statin users; adjusted HR: 0.75; 95% CI, 0.68–0.82), stroke (4.4% versus 5.6% for non–statin users; adjusted HR: 0.76; 95% CI, 0.67–0.86), death (4.2% versus 4.7% for non–statin users; adjusted HR: 0.76; 95% CI, 0.67–0.87), and myocardial infarction (2.7% versus 2.9% for non–statin users; adjusted HR: 0.81; 95% CI, 0.69–0.95; Table 2).
Table 2.
One and 5‐Year Outcomes After Carotid Revascularization by Statin Therapy
| Outcome | 1‐Year Period | 5‐Year Period | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | IPTW‐Adjusted HR (95% CI) | Unadjusted HR (95% CI) | IPTW‐Adjusted HR (95% CI) | |
| Stroke, MI, or death | 0.69 (0.60–0.79) | 0.76 (0.70–0.83) | 0.71 (0.65–0.78) | 0.75 (0.71–0.80) |
| Stroke or death | 0.65 (0.56–0.75) | 0.75 (0.68–0.82) | 0.69 (0.62–0.76) | 0.75 (0.71–0.80) |
| Stroke | 0.69 (0.57–0.83) | 0.76 (0.67–0.86) | 0.72 (0.61–0.86) | 0.80 (0.72–0.89) |
| Death | 0.62 (0.51–0.76) | 0.76 (0.67–0.87) | 0.68 (0.61–0.77) | 0.73 (0.68–0.79) |
| MI | 0.92 (0.76–1.13) | 0.81 (0.69–0.95) | 0.84 (0.69–1.02) | 0.83 (0.73–0.93) |
Values are presented as n (%). CI indicates confidence interval; HR, hazard ratio; IPTW, inverse probability treatment weighting; MI, myocardial infarction.
5‐Year Outcomes
The median follow‐up for the 5‐year outcome analysis was 3.0 years (interquartile range: 0.48–5.0 years). We found that continuous statin use was associated with a 25% lower composite risk of stroke, myocardial infraction, or death at 5 years (adjusted HR: 0.75; 95% CI, 0.71–0.80). Individual rates of 5‐year stroke (adjusted HR: 0.80; 95% CI, 0.72–0.89), death (adjusted HR: 0.73; 95% CI, 0.68–0.79), and myocardial infarction (adjusted HR: 0.83; 95% CI, 0.73–0.93) were consistently lower among statin users. See Table 2 for 5‐year outcomes and Figure 2 for IPTW‐adjusted survival curves for the 5‐year composite outcome.
Figure 2.
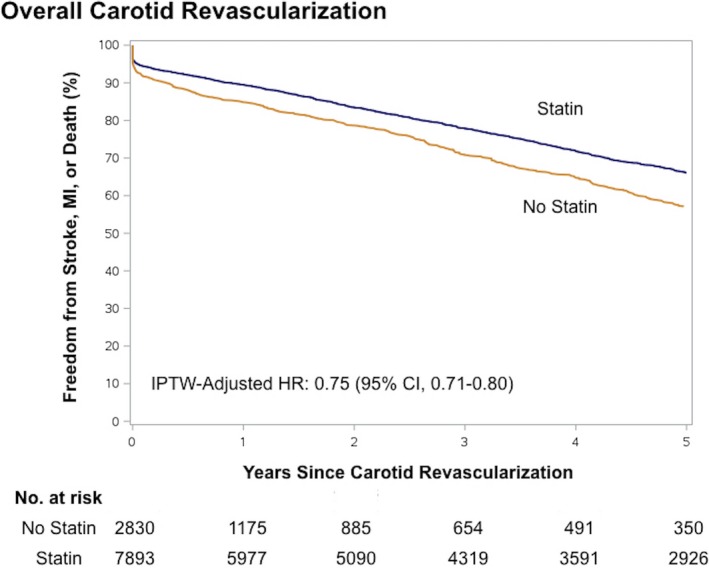
Adjusted Kaplan–Meier curves of 5‐year outcomes after carotid revascularization by statin therapy. Shown are the 5‐year adjusted Kaplan–Meier curves for freedom from any stroke, myocardial infarction, or death after carotid revascularization. CI indicates confidence interval; HR hazard ratio; IPTW, inverse probability of treatment weighting; MI, myocardial infarction.
Statin Use at Follow‐up
We examined postprocedural statin adherence among those patients alive at 1 and 5 years. More than half (53.6%, 1445/2696) of non–statin users at baseline were initiated on statin therapy by 1‐year follow‐up, and more than three quarters (76.2%, 1892/2483) were taking a statin by 5 years. With respect to baseline statin users, 13.1% (993/7559) were no longer adherent to a statin at 1 year—this number increased slightly to 16.0% (1047/6553) at 5 years. Overall, statin adherence increased from 73.6% at baseline to 78.1% at 1‐year follow‐up and 81.9% at 5‐year follow‐up.
Subgroup Analyses
For the composite risk of stroke, myocardial infarction, or death by carotid procedure, we conducted subgroup analyses for symptom status, and statin intensity (Figure 3). The 5‐year composite risk was lower among statin users who underwent carotid endarterectomy (adjusted HR: 0.73; 95% CI, 0.69–0.78) and carotid artery stenting (adjusted HR: 0.86, 95% CI, 0.75–0.98)—this benefit of statin therapy appeared to be greater in the endarterectomy group (P=0.002 for interaction). See Figure S3 for adjusted survival curves by type of carotid procedure. Of note, our previous work has shown that patients who receive carotid stenting in Ontario are more likely to have symptomatic carotid stenosis and a higher comorbidity burden compared with those who receive endarterectomy.29 With respect to carotid artery symptom status, patients on statin therapy, whether symptomatic (adjusted HR: 0.75; 95% CI, 0.69–0.81) or asymptomatic (adjusted HR: 0.80; 95% CI, 0.74–0.87), had lower 5‐year event rates, with the symptomatic group receiving a slightly greater benefit (P=0.033 for interaction). We did not observe a dose‐dependent effect of statins, as both high‐dose statins (adjusted HR: 0.74; 95% CI, 0.68–0.80) and moderate/low‐dose statins (adjusted HR: 0.76; 95% CI, 0.71–0.81) were associated with similar reductions in 5‐year events (P=0.76 for interaction). We also observed similar associations between statin use and the individual rates of stroke in these subgroups (Figure S4).
Figure 3.
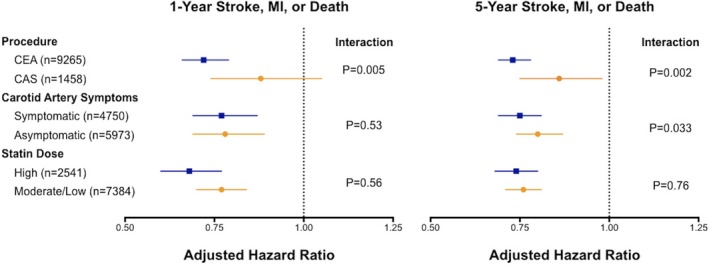
Risk of stroke, myocardial infarction, or death after carotid revascularization among subgroups by statin therapy. CAS indicates carotid artery stenting; CEA, carotid endarterectomy; MI, myocardial infarction.
Discussion
In the current analysis, we found that only three quarters of older adult patients undergoing carotid artery revascularization were on statin therapy at the time of the carotid procedure—postprocedural statin use increased modestly to 82% at 5‐year follow‐up. Patients with isolated carotid artery disease were less likely to be on statin therapy (71%) compared with those who had concomitant coronary artery disease (82%). Consistent use of statin therapy after carotid artery revascularization was associated with a sustained 25% reduction in the risk of stroke, myocardial infarction, or death at up to 5 years of follow‐up. Events rates were lower after both carotid endarterectomy and stenting with statin use, and the protective association with statin use was observed regardless of carotid artery symptom status and statin intensity. Given the significant morbidity and disability associated with stroke,30 our results may have important public health implications.
To the best of our knowledge, this study is the first to examine the association between statin use and long‐term outcomes after carotid artery revascularization while considering postprocedural statin use based on actual medication prescription claims. The few previous studies in this area have been limited by lack of data on statin use after the carotid procedure and/or lack of data on statin intensity. AbuRhama and colleagues conducted a retrospective analysis of 500 patients who underwent carotid endarterectomy over a 3‐year period and found that those on baseline statin therapy had a 50% reduction in the risk of death after a mean follow‐up of 2.3 years.31 Their study, however, was limited by a single‐center design and lacked information about the use of statin therapy after endarterectomy. In a retrospective analysis of 2127 carotid endarterectomies performed between 1989 and 1999, LaMuraglia and colleagues observed improved anatomic durability and survival among patients on lipid‐lowering drugs after mean follow‐up of 6.2 years.32 Despite longer follow‐up, their study also lacked detailed information about postprocedural statin use and intensity. A study of 1083 patients who underwent carotid stenting in Italy showed that statin use was associated with half the risk of mortality and borderline reduced risk of ischemic stroke at 5 years compared with non−statin use.33 The investigators, however, could not accurately capture details of postprocedural statin use and intensity in their cohort. Baseline statin use at the time of the carotid procedure ranged from 43% to 60% in these studies, which was lower than what we observed in the current study (74%)—this might be because our cohort was limited to older patients who are more likely to be treated with lipid‐lowering therapy.
SPARCL was a randomized controlled trial that compared high‐dose statin therapy (atorvastatin 80 mg once daily) with placebo in 4731 patients with a history of stroke or transient ischemic attack.34 The 5‐year risk of adverse cardiovascular events was 20% lower with atorvastatin in the overall trial (17.2% versus 14.1% for atorvastatin; HR: 0.80; 95% CI, 0.69–0.92). Furthermore, a subsequent subanalysis of SPARCL showed that patients with carotid stenosis in the trial had a higher rate of cardiovascular events and also benefited more from high‐dose statin therapy (21.0% versus 14.2% for atorvastatin; HR: 0.64; 95% CI, 0.47–0.86).12 In the current analysis, the vast majority (94%) of statin users were on moderate‐ or high‐dose therapy at the time of the carotid procedure. Interestingly, we did not find a dose‐dependent relationship between statin therapy and long‐term outcomes, as both high‐ and moderate/low‐dose statins were associated with ≈25% reductions in cardiovascular events. In contrast, a recent retrospective registry‐based analysis of 397 patients undergoing carotid stenting showed a trend toward lower risk of 30‐day events among patients treated with high‐dose statins, suggesting a potential dose‐dependent benefit of statins in this population.35 Furthermore, clinical trials of patients with stable coronary artery disease, such as TNT (Treating to New Targets), suggest an incremental clinical benefit of high‐dose statin therapy over moderate‐dose therapy.36 Although it is unclear why we did not observe a similar dose response in the carotid stenosis population in this study, variations in study cohort and LDL levels may help explain this discrepancy.
Although the focus of the current analysis was on long‐term outcomes in patients with carotid artery disease, several studies have also observed an association between statin use and better periprocedural outcomes after carotid revascularization. Kennedy and colleagues reported a protective association with statins in 2031 symptomatic patients who underwent carotid endarterectomy in western Canada with respect to in‐hospital mortality (75% odds reduction) and in‐hospital ischemic stroke or death (45% odds reduction).37 Similarly, another single‐center retrospective study showed that statin use was associated with a 3‐fold lower risk of 30‐day stroke (1.2% versus 4.5%) and 5‐fold lower risk of 30‐day mortality (0.3% versus 2.1%) after carotid endarterectomy.38 More recent studies of carotid stenting have also reported similar results. In an analysis of 344 carotid stenting patients in Germany, the authors reported a lower composite risk of periprocedural ischemic stroke, myocardial infarction, or death (odds ratio: 0.31) with statin use.39 Data from smaller prospective studies of carotid artery stenting patients also suggest a protective effect of statin therapy on periprocedural ischemic complications.40, 41 Furthermore, Tadros and colleagues showed that preprocedural statin use is associated with less embolic debris during the carotid stenting procedure.42 In addition, accumulating observational evidence suggests that statin use is associated with reduced rates of periprocedural complications and mortality after other types of major noncardiac surgery procedures.43
Studies of populations with other manifestations of atherosclerosis have also demonstrated protective effects of statins. Kumbhani and colleagues used the Reduction of Atherothrombosis of Continued Health (REACH) registry to demonstrate a 17% lower rate of the 4‐year composite rate of cardiovascular death, myocardial infarction, or stroke among statin users with symptomatic peripheral arterial disease.44 In a study of patients undergoing percutaneous coronary interventions, Chan and colleagues reported 45% and 33% lower rates of all‐cause mortality with statin therapy during the periprocedural and 6‐month periods, respectively.45 Systematic reviews and meta‐analyses of patients undergoing cardiac surgery46 and abdominal aortic aneurysm repair47 have also reported similar associations with lower rates of periprocedural events in statin users. Finally, high‐quality multicenter randomized controlled trials of general populations at risk for cardiovascular events indicate that statin therapy is associated with a 20% to 30% reduction in long‐term cardiovascular event rates.48, 49 We observed similar reductions in rates of 5‐year events after carotid revascularization among statin users. Given these data, it is not surprising that major societal clinical practice guidelines recommend statin therapy in most patients undergoing carotid artery revascularization despite no specific randomized clinical trial data in this population.50, 51
Our study has some limitations. First, as with all nonrandomized studies, potential imbalances in unmeasured confounders, such as race, ethnicity, smoking history, and body mass index, may have biased our results; however, we used propensity score methods to ameliorate this potential bias. Second, although we used validated coding to capture patients, covariates, and outcomes as possible, inaccurate coding in our databases may have biased our results. Third, we could not differentiate between new versus long‐term statin users before cohort entry, and this may bias our findings.52 Fourth, our databases lacked laboratory information on lipid levels at baseline and follow‐up and medication data on those aged <65 years; the care gap of statin underuse may be larger among younger patients with carotid artery disease. Furthermore, we did not capture data on temporal changes in the types of preferred statins over the study period, and this could confound our results. Fifth, the proportion of patients receiving aspirin therapy is underestimated in our study, as aspirin is generally purchased as an over‐the‐counter drug in Ontario. Finally, the subanalysis of outcomes by carotid artery symptoms should be interpreted with caution because we were not able to capture minor neurological events for which patients did not seek hospital treatment; therefore, the proportion of symptomatic patients may have been underestimated. Despite the aforementioned limitations, our study is the largest evaluating the role of statins in the incident risk of cardiovascular disease among patients undergoing carotid revascularization with long‐term follow‐up.
Conclusions
In summary, this study showed that continuous statin use is associated with a 25% lower rate of adverse cardiovascular events after carotid artery revascularization with up to 5 years of follow‐up. Along with other supportive evidence, clinicians should consider statins for patients undergoing carotid artery revascularization. In addition, we found that statin therapy is underused before and after carotid artery revascularization. Improving statin utilization by these patients should be a key component of quality programs and may translate to better long‐term outcomes. Future research in this area should focus on exploring the influence of different lipid levels on carotid plaques and clinical outcomes.
Author Contributions
All authors made substantial contributions to conception and design of the study, interpretation of data, critical revision of the article, and approval of the final article. Hussain did the statistical analysis and wrote the first draft of the article. Hussain and Al‐Omran take overall responsibility for the analyses and the article.
Sources of Funding
The Physicians’ Services Inc Foundation and the King Saud University‐Li Ka Shing Collaborative Research Program funded this study. Dr Hussain is also supported by a University of Toronto Postgraduate Research Award (Joseph M. West Family Memorial Fund and the William S. Fenwick Research Fellowship) for this study.
Support and Disclaimers
This study was also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. All data sets used in this study were linked using unique encoded identifiers and analyzed at ICES. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI); however, the analyses, conclusions, opinions, and statements expressed herein are those of the author and not necessarily those of CIHI.
Disclosures
Dr Saposnik is supported by the Heart and Stroke Foundation of Canada Career Award following an open peer‐reviewed competition. Dr Bhatt discloses the following relationships: advisory board for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; board of directors for Boston VA Research Institute, Society of Cardiovascular Patient Care; chair of the American Heart Association Quality Oversight Committee; data monitoring committee for the Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; honoraria received from American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), WebMD (CME steering committees); other relationships with Clinical Cardiology (deputy editor), NCDR‐ACTION Registry Steering Committee (chair), VA CART Research and Publications Committee (chair); research funding from Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site coinvestigator for Biotronik, Boston Scientific, St. Jude Medical (now Abbott); trustee of the American College of Cardiology; unfunded research for FlowCo, Merck, PLx Pharma, Takeda. None of the other authors report conflicts.
Supporting information
Table S1. Sources of Data for the Current Population‐Level Study
Table S2. Classification of Statin Intensity Based on the 2013 American College of Cardiology/American Heart Association Guidelines on the Treatment of Blood Cholesterol (1)
Table S3. Coding Definitions for Identifying Patients, Comorbid Conditions, and Outcomes
Figure S1. Overview of Ontario healthcare administrative databases.
Figure S2. Types of statins prescribed to patients at baseline who underwent carotid artery revascularization (n=7893).
Figure S3. Adjusted Kaplan–Meier curves of 5‐year outcomes after carotid endarterectomy (A) and stenting (B) by statin therapy.
Figure S4. Risk of stroke after carotid revascularization among subgroups by statin therapy.
Acknowledgments
We thank Dr Jiming Fang, PhD from the Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada, for his assistance with data set creation and management. In memoriam: Dr. Jack V. Tu, who unfortunately passed away on May 30, 2018 while this manuscript was under review for publication.
(J Am Heart Assoc. 2018;7:e009745 DOI: 10.1161/JAHA.118.009745.)
This study was presented at the American Heart Association's Scientific Sessions 2017, Anaheim, California, November 11 to 15, 2017.
References
- 1. Patel TN, Shishehbor MH, Bhatt DL. A review of high‐dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J. 2007;28:664–672. [DOI] [PubMed] [Google Scholar]
- 2. Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 suppl 1):III‐39–III‐43. [DOI] [PubMed] [Google Scholar]
- 3. Chan AW, Bhatt DL, Chew DP, Reginelli J, Schneider JP, Topol EJ, Ellis SG. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003;107:1750–1756. [DOI] [PubMed] [Google Scholar]
- 4. Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GB, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 6. Berger JS, Ladapo JA. Underuse of prevention and lifestyle counseling in patients with peripheral artery disease. J Am Coll Cardiol. 2017;69:2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, Berger J, Torp‐Pedersen C, Fosbol EL. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower‐extremity peripheral artery disease, underuse remains. Circulation. 2012;126:1345–1354. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 9. Hussain MA, Al‐Omran M, Mamdani M, Eisenberg N, Premji A, Saldanha L, Wang X, Verma S, Lindsay TF. Efficacy of a guideline‐recommended risk‐reduction program to improve cardiovascular and limb outcomes in patients with peripheral arterial disease. JAMA Surg. 2016;151:742–750. [DOI] [PubMed] [Google Scholar]
- 10. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lièvre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touzé E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X; EVA‐3S Investigators . Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. [DOI] [PubMed] [Google Scholar]
- 11. SPACE Collaborative Group , Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial. Lancet. 2006;368:1239–1247. [DOI] [PubMed] [Google Scholar]
- 12. Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM; Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators . Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–3302. [DOI] [PubMed] [Google Scholar]
- 13. Hussain MA, Mamdani M, Tu JV, Saposnik G, Khoushhal Z, Aljabri B, Verma S, Al‐Omran M. Impact of clinical trial results on the temporal trends of carotid endarterectomy and stenting from 2002 to 2014. Stroke. 2016;47:2923–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 15. Mamdani M, Juurlink DN, Lee DS, Rochon PA, Kopp A, Naglie G, Austin PC, Laupacis A, Stukel TA. Cyclo‐oxygenase‐2 inhibitors versus non‐selective non‐steroidal anti‐inflammatory drugs and congestive heart failure outcomes in elderly patients: a population‐based cohort study. Lancet. 2004;363:1751–1756. [DOI] [PubMed] [Google Scholar]
- 16. Hussain MA, Mamdani M, Saposnik G, Tu JV, Turkel‐Parrella D, Spears J, Al‐Omran M. Validation of carotid artery revascularization coding in Ontario health administrative databases. Clin Invest Med. 2016;39:73–78. [DOI] [PubMed] [Google Scholar]
- 17. Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto, ON: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 18. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 19. Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]
- 20. Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ. 2009;339:b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomes T, Juurlink DN, Mamdani MM. Comparative adherence to oxybutynin or tolterodine among older patients. Eur J Clin Pharmacol. 2012;68:97–99. [DOI] [PubMed] [Google Scholar]
- 22. Glazier RH, Creatore MI, Agha MM, Steele LS; Inner City Toronto Time Trends Working Group . Socioeconomic misclassification in Ontario's Health Care Registry. Can J Public Health. 2003;94:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 24. Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330:960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC. The performance of different propensity‐score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29:2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 29. Hussain MA, Mamdani M, Tu JV, Saposnik G, Aljabri B, Bhatt DL, Verma S, Al‐Omran M. Long‐term outcomes of carotid endarterectomy versus stenting in a multicenter population‐based Canadian study. Ann Surg. 2018;268:364–373. [DOI] [PubMed] [Google Scholar]
- 30. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker‐Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group . Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. AbuRahma AF, Srivastava M, Stone PA, Richmond BK, AbuRahma Z, Jackson W, Dean LS, Mousa AY. Effect of statins on early and late clinical outcomes of carotid endarterectomy and the rate of post‐carotid endarterectomy restenosis. J Am Coll Surg. 2015;220:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LaMuraglia GM, Stoner MC, Brewster DC, Watkins MT, Juhola KL, Kwolek C, Dorer DJ, Cambria RP. Determinants of carotid endarterectomy anatomic durability: effects of serum lipids and lipid‐lowering drugs. J Vasc Surg. 2005;41:762–768. [DOI] [PubMed] [Google Scholar]
- 33. Verzini F, De Rango P, Parlani G, Giordano G, Caso V, Cieri E, Isernia G, Cao P. Effects of statins on early and late results of carotid stenting. J Vasc Surg. 2011;53:71–79. [DOI] [PubMed] [Google Scholar]
- 34. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 35. Hong JH, Sohn SI, Kwak J, Yoo J, Chang HW, Kwon OK, Jung C, Chung I, Bae HJ, Lee JS, Han MK. Dose‐dependent effect of statin pretreatment on preventing the periprocedural complications of carotid artery stenting. Stroke. 2017;48:1890–1894. [DOI] [PubMed] [Google Scholar]
- 36. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 37. Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005;36:2072–2076. [DOI] [PubMed] [Google Scholar]
- 38. McGirt MJ, Perler BA, Brooke BS, Woodworth GF, Coon A, Jain S, Buck D, Roseborough GS, Tamargo RJ, Heller J, Freischlag JA, Williams GM. 3‐Hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg. 2005;42:829–836. [DOI] [PubMed] [Google Scholar]
- 39. Reiff T, Amiri H, Rohde S, Hacke W, Ringleb PA. Statins reduce peri‐procedural complications in carotid stenting. Eur J Vasc Endovasc Surg. 2014;48:626–632. [DOI] [PubMed] [Google Scholar]
- 40. Takayama K, Taki W, Toma N, Nakahara I, Maeda M, Tanemura H, Kuroiwa T, Imai K, Sakamoto M, Nakagawa I, Masuo O, Myouchin K, Wada T, Suzuki H. Effect of pitavastatin on preventing ischemic complications with carotid artery stenting: a multicenter prospective study—EPOCH‐CAS study. Cardiovasc Intervent Radiol. 2014;37:1436–1443. [DOI] [PubMed] [Google Scholar]
- 41. Patti G, Tomai F, Melfi R, Ricottini E, Macrì M, Sedati P, Giardina A, Aurigemma C, Leporace M, D'Ambrosio A, Di Sciascio G. Strategies of clopidogrel load and atorvastatin reload to prevent ischemic cerebral events in patients undergoing protected carotid stenting. Results of the randomized ARMYDA‐9 CAROTID (Clopidogrel and Atorvastatin Treatment During Carotid Artery Stenting) study. J Am Coll Cardiol. 2013;61:1379–1387. [DOI] [PubMed] [Google Scholar]
- 42. Tadros RO, Vouyouka AG, Chung C, Malik RK, Krishnan P, Ellozy SH, Marin ML, Faries PL. The effect of statin use on embolic potential during carotid angioplasty and stenting. Ann Vasc Surg. 2013;27:96–103. [DOI] [PubMed] [Google Scholar]
- 43. London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med. 2017;177:231–242. [DOI] [PubMed] [Google Scholar]
- 44. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL; REACH Registry Investigators . Statin therapy and long‐term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan AW, Bhatt DL, Chew DP, Quinn MJ, Moliterno DJ, Topol EJ, Ellis SG. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation. 2002;105:691–696. [DOI] [PubMed] [Google Scholar]
- 46. Kuhn EW, Liakopoulos OJ, Stange S, Deppe AC, Slottosch I, Choi YH, Wahlers T. Preoperative statin therapy in cardiac surgery: a meta‐analysis of 90,000 patients. Eur J Cardiothorac Surg. 2014;45:17–26. [DOI] [PubMed] [Google Scholar]
- 47. Salata K, Syed M, Hussain MA, de Mestral C, Greco E, Tu JV, Forbes TL, Bhatt DL, Verma S, Al‐Omran M. Statins reduce abdominal aortic aneurysm growth, rupture, and peri‐operative mortality: a systematic review and meta‐analysis. JAHA. 2018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. US Preventive Services Task Force , Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FA, Gillman MW, Kemper AR, Krist AH, Kurth AE, Landefeld CS, LeFevre ML, Mangione CM, Phillips WR, Owens DK, Phipps MG, Pignone MP. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 49. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists’ (CTT) Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 50. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola‐Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; Document Reviewers , Widimsky P, Kolh P, Agewall S, Bueno H, Coca A, De Borst GJ, Delgado V, Dick F, Erol C, Ferrini M, Kakkos S, Katus HA, Knuuti J, Lindholt J, Mattle H, Pieniazek P, Piepoli MF, Scheinert D, Sievert H, Simpson I, Sulzenko J, Tamargo J, Tokgozoglu L, Torbicki A, Tsakountakis N, Tuñón J, de Ceniga MV, Windecker S, Zamorano JL. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:305–368. [DOI] [PubMed] [Google Scholar]
- 51. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ; American College of Cardiology Foundation; American Stroke Association; American Association of Neurological Surgeons; American College of Radiology; American Society of Neuroradiology; Congress of Neurological Surgeons; Society of Atherosclerosis Imaging and Prevention; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of NeuroInterventional Surgery; Society for Vascular Medicine; Society for Vascular Surgery . 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. [DOI] [PubMed] [Google Scholar]
- 52. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sources of Data for the Current Population‐Level Study
Table S2. Classification of Statin Intensity Based on the 2013 American College of Cardiology/American Heart Association Guidelines on the Treatment of Blood Cholesterol (1)
Table S3. Coding Definitions for Identifying Patients, Comorbid Conditions, and Outcomes
Figure S1. Overview of Ontario healthcare administrative databases.
Figure S2. Types of statins prescribed to patients at baseline who underwent carotid artery revascularization (n=7893).
Figure S3. Adjusted Kaplan–Meier curves of 5‐year outcomes after carotid endarterectomy (A) and stenting (B) by statin therapy.
Figure S4. Risk of stroke after carotid revascularization among subgroups by statin therapy.


