Abstract
Background
The American Heart Association Life's Simple 7 metric defines ideal cardiovascular health (CVH) on 7 factors: smoking, diet, physical activity, body mass index, blood sugar, blood pressure, and cholesterol. This metric has been used to define optimal brain health, but data relative to subclinical imaging biomarkers of brain aging are lacking. This study examines the association between Life's Simple 7 with white matter hyperintensity volume, silent brain infarcts, and cerebral volume.
Methods and Results
A subsample of stroke‐free participants from the population‐based Northern Manhattan Study underwent brain magnetic resonance imaging an average of 7 years after baseline. Linear and logistic regression models were constructed to estimate associations between the number of ideal CVH metrics achieved with imaging biomarkers of brain aging, adjusting for sociodemographics. Among 1031 participants (mean age at magnetic resonance imaging=72±8, 40% men, 19% black, 16% white, and 65% Hispanic), no one had ideal status in all 7 factors, 1% had ideal status in 6 factors, 18% in 4 to 5 factors, 30% in 3 factors, 33% in 2 factors, and 18% in 0 to 1 factors. The number of ideal CVH factors achieved was inversely associated with white matter hyperintensity volume (beta per factor=−0.047; P=0.04) and silent brain infarct (odds ratio per factor=0.84; 95% confidence interval=0.72–0.97) and positively associated with cerebral volume (beta per factor=0.300; P=0.002).
Conclusions
An increasing ideal CVH score was associated with less white matter hyperintensity volume and silent brain infarcts and greater cerebral volumes, supporting the Life's Simple 7 metric as a useful measure to quantify optimal brain health. Monitoring and promoting achievement of Life's Simple 7 ideal CVH factors may improve subclinical and clinical brain health outcomes.
Keywords: cardiovascular health, cerebral volume, magnetic resonance imaging, silent brain infarcts, smoking, white matter hyperintensities
Subject Categories: Risk Factors, Lifestyle, Epidemiology, Cardiovascular Disease, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
This is a racially and ethnically diverse, community‐based study examining the relationship between ideal cardiovascular health as defined by the Life's Simple 7 metric and several subclinical brain imaging biomarkers.
What Are the Clinical Implications?
The results show that having a greater number of ideal cardiovascular health factors is associated with a reduced burden of biomarkers of brain aging, including silent brain infarcts, white matter hyperintensity volume, and brain atrophy, which supports the American Heart Association's recommended use of the Life's Simple 7 metric in adulthood as an important predictor of optimal brain health.
In 2010, the American Heart Association (AHA) developed a metric known as Life's Simple 7 to define ideal cardiovascular health (CVH) based on 7 health factors for cardiovascular disease. Ideal levels for the following risk factors were defined: smoking, diet, physical activity, body mass index, blood sugar, blood pressure, and cholesterol. The AHA's 2020 goal to improve the CVH of all Americans by 20% while reducing deaths from cardiovascular disease and stroke by 20% can be realized if the number of ideal levels achieved across these 7 categories of health behaviors and risk factors increases in the population. It has been suggested that achieving ideal CVH could not only reduce heart disease and stroke, but also improve brain health.1 The AHA's 2017 presidential advisory on brain health2 described cognitive impairment and dementia as largely modifiable, and argued that achieving and maintaining ideal CVH in midadulthood, as defined by the Life's Simple 7 metric, may reduce the risk of cognitive impairment and dementia.
The Life's Simple 7 metric is appealing from a public health perspective given that it can easily be measured, monitored, and modified, and several epidemiological studies have shown its strong predictive relationship with vascular disease morbidity and mortality, cognitive decline, and dementia.3, 4, 5, 6, 7, 8 For example, in the NOMAS (Northern Manhattan Study), we found that participants who achieved more ideal CVH metrics had better cognitive performance and less decline over time,5 in addition to a lower risk of stroke, myocardial infarction, and vascular‐related death.6 However, although the AHA has now defined optimal brain health by the Life's Simple 7 metric, there is very limited evidence regarding its impact on biomarkers of subclinical brain aging. Subclinical brain aging biomarkers, including white matter hyperintensities, silent brain infarct (SBI), and brain atrophy, are common in the population and important risk factors for stroke as well as cognitive decline and dementia.9, 10, 11 The potential association of Life's Simple 7 and these important subclinical imaging biomarkers of brain aging is largely unknown and the motivation for the current analysis.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The NOMAS is a population‐based longitudinal cohort study created to determine the incidence and risk factors for stroke, dementia, and cognitive decline in an urban racially and ethnically diverse population. Northern Manhattan represents a well‐defined area of New York City with a unique population that was 63% Hispanic, 20% non‐Hispanic black, and 15% non‐Hispanic white during our random sampling (1993–2001). Study details have been published.12 Briefly, participants were identified using random‐digit dialing between 1993 and 2001 with the following eligibility criteria: (1) had never been diagnosed with a stroke; (2) were aged >40 years; and (3) resided in Northern Manhattan for ≥3 months, in a household with a telephone. Following telephone recruitment, the enrollment response rate with an in‐person baseline interview and assessment was 75% (overall was 68%). Starting in 2003, NOMAS participants who remained clinically stroke free were recruited sequentially during annual follow‐up into the magnetic resonance imaging (MRI) subcohort if they were aged >50 and had no contraindications to MRI (N=1091). Mean interval from baseline enrollment to MRI was 7.2 (±2.4) years. For this analysis, we excluded participants of “other” race (ie, not classified as non‐Hispanic white, non‐Hispanic black, or Hispanic) and those with missing baseline data for cardiovascular disease health metrics (final N=1031). The study was approved by the institutional review boards of Columbia University and the University of Miami, and all subjects provided written informed consent.
Data Collection
At study baseline, interviews were conducted with trained bilingual research assistants in English or Spanish. Physical and neurological examinations were conducted by study physicians. Participants self‐identified their race/ethnicity using a series of questions modeled after the US Census and conforming to standard definitions outlined by Directive 15.13 Standardized questions adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control and Prevention were used to identify vascular risk factors, including smoking behavior.14, 15 Smoking was categorized using self‐reported age of starting and quitting smoking. Leisure‐time physical activity was calculated using a questionnaire based on the National Health Interview Survey.16 This questionnaire records the duration and frequency of various leisure time and recreational activities for the 2 weeks preceding the interview. Blood pressure was measured twice from the right brachial artery after a 10‐minute rest in a supine position and averaged (Dinamap Pro100; Critikon Inc, Tampa, FL). Fasting blood specimens were collected to measure glucose and lipid profiles, as described previously.17 Plasma levels of fasting total cholesterol were obtained using standardized enzymatic procedures with a Hitachi 705 automated spectrophotometer (Boehringer Mannheim, Mannheim, Germany). Diet was assessed by trained bilingual research assistants using a modified Block National Cancer Institute food frequency questionnaire.18, 19 This validated food frequency questionnaire includes 207 foods, modified slightly to include specific dietary items frequently consumed by Hispanic populations, and is intended to represent typical food consumption over the previous year.
The 7 CVH factors that comprise the Life's Simple 7 metric were classified into ideal, intermediate, and poor based on the definitions set forth by the AHA: (1) smoking: ideal (never or quit >1 year), intermediate (quit ≤1 year), and poor (current); (2) body mass index: ideal (<25 kg/m2), intermediate (25 to <30 kg/m2), and poor (≥30 kg/m2); (3) physical activity: ideal (≥150 min/week moderate intensity, ≥75 min/week vigorous intensity, or equivalent combination), intermediate (1–149 min/week moderate intensity, 1–74 min/week vigorous intensity, or equivalent combination), and poor (no moderate and vigorous activity); (4) diet: ideal (4–5 healthy components), intermediate (2–3 healthy components), and poor (0–1 healthy component) based on 5 health dietary metrics (≥4.5 cups of fruits and vegetables a day, 2 or more 3.5‐oz servings of fish a week, 3 or more 1‐oz equivalent servings of fiber‐rich whole grains per day, <1500 mg of sodium/day, and ≤450 kcal of sugar‐sweetened beverages a week); (5) total cholesterol: ideal (untreated and <200 mg/dL), intermediate (treated to <200 or 200–239 mg/dL), and poor (≥240 mg/dL); (6) blood pressure: ideal (untreated and <120/<80 mm Hg), intermediate (treated to <120/<80 or 120–139/80–89 mm Hg), and poor (≥140/90 mm Hg); and (7) fasting plasma glucose: ideal (untreated and <100 mg/dL), intermediate (treated to <100 or 100–125 mg/dL), and poor (≥126 mg/dL).
Brain MRI
Imaging was performed on a 1.5‐Tesla MRI system (Philips Medical Systems, Best, The Netherlands) at the Hatch Research Center at Columbia University. Processing of MRI scans to calculate total intracranial volume (ICV), cerebral volume, and white matter hyperintensity volumes (WMHV), has been previously described.20 Semiautomated measurements of pixel distributions using mathematical modeling of pixel‐intensity histograms for cerebrospinal fluid and brain white and gray matter were used to identify the optimal pixel‐intensity threshold to distinguish cerebrospinal fluid from brain matter, using a custom‐designed image analysis package (QUANTA 6.2 using a Sun Microsystems Ultra 5 workstation). To correct for head size, both WMHV and cerebral volume were calculated as a percent of total ICV (eg white matter hyperintensity/ICV×100), and WMHV was log‐transformed to create a normal distribution. Proportion of total cerebral volume to total ICV×100 is labeled as cerebral volume (percent ICV).
Methods to identify and classify MRI‐defined infarcts have been described in detail.21 Two independent raters used a superimposed image of the subtraction, proton density, and T2‐weighted images at 3× magnified view for interpretation of lesion characteristics. Agreement among raters has been generally good (previously published kappa values, 0.73–0.90).22 For this analysis, we examined the presence of SBI (versus absence).
Statistical Analysis
The primary independent variable is the number of ideal CVH factors achieved, represented by a score with a range of 0 to 7. This score was examined continuously (per factor) and divided into 4 categories: 0 to 1 (reference), 2, 3, and 4 to 7.5 We also examined the CVH score as a continuous measure with a range of 0 to 14 (per point), as the sum of the 7 components given a score of 0 for poor, 1 for intermediate, and 2 for ideal. The primary dependent variables of interest were SBI (presence versus absence), WMHV, and cerebral volume, the latter 2 outcomes calculated as a proportion of ICV and examined continuously. Multivariable linear regression models were constructed to examine associations between the CVH metric scores and logWMHV and cerebral volume (percent ICV), and multivariable logistic regression models were constructed for SBI. These models were adjusted for the time from baseline to MRI, age at MRI, sex, race/ethnicity, education, and insurance status. Next, the 7 components of the ideal CVH score were included as independent variables in these multivariable‐adjusted linear and logistic regression models, each categorized as ideal versus not ideal, and they were mutually adjusted. The latter analysis was conducted to identify the components that were independently associated with the 3 MRI markers and driving associations observed with the CVH scores. Last, we examined effect modification by age, sex, education, and race/ethnicity by including interaction terms between these 3 variables with the score representing the number of ideal factors in separate models predicting the 3 outcomes. We have presented stratified analyses where effect modification was suggested (P for interaction, <0.10). Analyses were performed with SAS (version 9.4; SAS Institute Inc, Cary, NC).
Results
Among 1031 NOMAS participants included in this study, mean age at MRI was 72±8 years, 30% were men, 19% non‐Hispanic black, 16% non‐Hispanic white, 65% Hispanic, 51% uninsured/Medicaid, and median years of education was 10 (interquartile range [IQR]=5–13). Sociodemographic characteristics of the study population overall and stratified by the number of ideal CVH factors achieved are shown in Table 1. The breakdown of the number of ideal CVH factors achieved is shown in Figure 1A, and the distribution of the 14‐point CVH score is shown in Figure 1B. No one had all 7 ideal factors, 1% had 6 factors, 18% had 4 to 5 factors, 30% had 3 factors, 33% had 2 factors, and 18% had 0 to 1 ideal factors. Achieving a greater number of ideal CVH factors was more common among men, those who were insured, and those with more years of education. Median WMHV (% of ICV) was 0.39% (interquartile range=0.22–0.82), mean cerebral volume (% ICV) was 72.0% (SD=4.2), and 16.5% had an SBI.
Table 1.
Demographics Stratified by Number of Ideal Cardiovascular Health Factors
| Variables | All (N=1031) | 0 to 1 Ideal Factors (N=188) | 2 Ideal Factors (N=339) | 3 Ideal Factors (N=311) | 4 to 7 Ideal Factors (N=193) |
|---|---|---|---|---|---|
| Male sex, N (%) | 411 (40) | 54 (29) | 119 (35) | 140 (45) | 101 (52) |
| Race/ethnicity, N (%) | |||||
| Black | 197 (19) | 43 (23) | 56 (17) | 51 (16) | 48 (25) |
| White | 161 (16) | 27 (14) | 38 (11) | 50 (16) | 46 (24) |
| Hispanic | 675 (65) | 118 (63) | 245 (72) | 211 (68) | 99 (51) |
| Medicaid/uninsured, N (%) | 500 (49) | 108 (57) | 172 (51) | 150 (48) | 70 (36) |
| Age, mean (SD), y | 71.7 (8.3) | 71.1 (7.6) | 71.6 (8.1) | 71.9 (8.3) | 72.2 (9.3) |
| Education (y), mean (SD) | 9.6 (5.1) | 9.2 (4.7) | 8.9 (5.1) | 9.3 (5.2) | 11.5 (5.2) |
Figure 1.
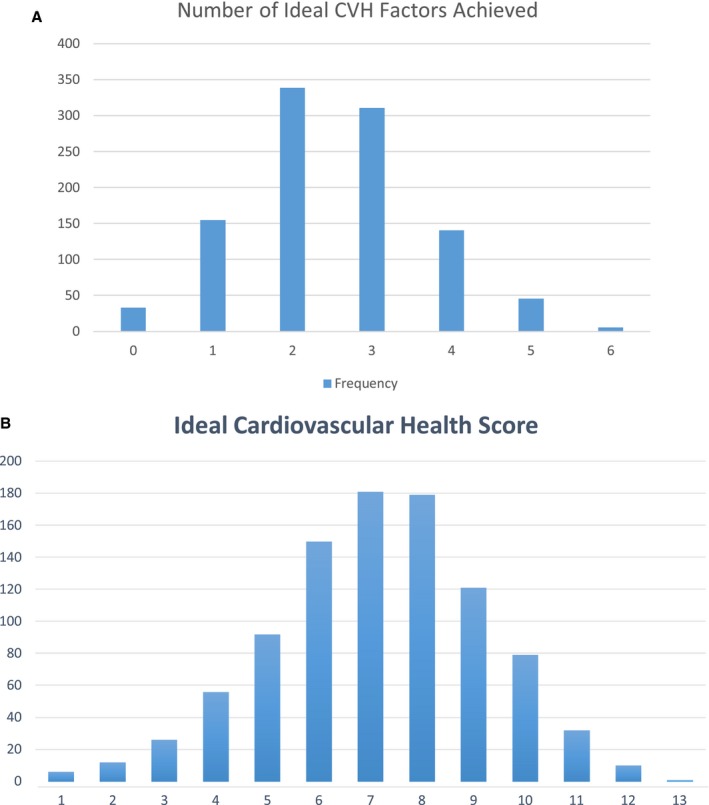
A, Number of ideal CVH factors achieved in the study population. B, Distribution of the 14‐point CVH score in the study population. CVH indicates cardiovascular health.
Tables 2 and 3 show the relationships between the CVH metrics and measures of subclinical brain aging. An increasing number of ideal CVH factors was associated with smaller ln(WMHV) (Table 2), greater cerebral volume (Table 2), and a lower odds of having SBI (Table 3; Figure 2). However, linear dose‐response trends were not apparent. The protective associations for these outcomes were apparent for participants who achieved 3 or more ideal CVH factors at baseline, given that those with 3 ideal factors and those with 4 to 6 ideal factors had lower WMHV and greater cerebral volume (% ICV) versus those with 0 to 1 factors. Tables 2 and 3 also show the associations between each of the individual CVH components in relation to the 3 measures of subclinical brain aging in mutually adjusted models. Nonsmoking was the driving protective component for ln(WMHV) and SBI, and the only component that was statistically significant for these outcomes. For cerebral volume, the components that were driving the positive association with ideal CVH were nonsmoking and ideal blood glucose (P<0.05).
Table 2.
Life's Simple 7 in Relation to White Matter Hyperintensity Volume and Cerebral Volume (N=1031)
| White Matter Hyperintensity Volume Beta (95% Confidence Interval), P Valuea | Cerebral Volume/Intracranial Volume Beta (95% Confidence Interval), P Valuea | |
|---|---|---|
| Ideal CVH score (continuous, range 0–14) | −0.029 (−0.055, −0.002), 0.03 | 0.144 (0.039, 0.249), 0.01 |
| No. of ideal CVH factors achieved (continuous per factor) | −0.047 (−0.093, −0.002), 0.04 | 0.300 (0.114, 0.485), 0.002 |
| No. of ideal CVH factors | P=0.15 | P=0.06 |
| 0 to 1 | Ref | Ref |
| 2 | −0.079 (−0.228, 0.069), 0.30 | 0.595 (−0.003, 1.193), 0.05 |
| 3 | −0.168 (−0.319, −0.016), 0.03 | 1.106 (0.496, 1.715), 0.0004 |
| 4 to 7 | −0.135 (−0.305, 0.036), 0.12 | 0.902 (0.215, 1.589), 0.01 |
| Ideal CVH components | ||
| Blood pressure ideal | 0.028 (−0.180, 0.235), 0.79 | −0.099 (−0.949, 0.751), 0.82 |
| BMI ideal | −0.028 (−0.147, 0.091), 0.64 | 0.319 (−0.169, 0.806), 0.20 |
| Total cholesterol ideal | −0.041 (−0.148, 0.067), 0.46 | 0.070 (−0.371, 0.512). 0.75 |
| Smoking ideal | −0.203 (−0.333, −0.073), 0.002 | 0.778 (0.245, 1.310), 0.004 |
| Physical activity ideal | 0.014 (−0.101, 0.129), 0.81 | −0.038 (−0.510, 0.434), 0.87 |
| Blood glucose ideal | −0.044 (−0.154, 0.067), 0.44 | 0.673 (0.221, 1.124), 0.004 |
| Diet ideal | −0.554 (−1.714, 0.605), 0.35 | −1.520 (−6.271, 3.231), 0.53 |
BMI indicates body mass index; CVH, cardiovascular health.
Adjusted for time from baseline to magnetic resonance imaging, age at magnetic resonance imaging, sex, race/ethnicity, education, and insurance status. Individual components were included in a mutually adjusted model.
Table 3.
Life's Simple 7 and SBI (N=1031)
| Odds Ratio for ≥1 SBI (95% Confidence Interval), P Valuea | |
|---|---|
| Ideal CVH score (continuous, range 0–14) | 0.91 (0.83, 0.99), 0.03 |
| Number of ideal CVH factors achieved (continuous per factor) | 0.84 (0.72, 0.97), 0.02 |
| Number of ideal CVH factors | P=0.18 |
| 0 to 1 | Ref |
| 2 | 1.01 (0.63, 1.65), 0.95 |
| 3 | 0.85 (0.51, 1.40), 0.51 |
| 4 to 7 | 0.57 (0.32, 1.04), 0.07 |
| Ideal CVH components | |
| Blood pressure ideal | 0.59 (0.26, 1.36), 0.22 |
| BMI ideal | 0.93 (0.63, 1.38), 0.71 |
| Total cholesterol ideal | 0.94 (0.66, 1.35), 0.75 |
| Smoking ideal | 0.59 (0.39, 0.88), 0.01 |
| Physical activity ideal | 0.86 (0.58, 1.28), 0.46 |
| Blood glucose ideal | 0.93 (0.65, 1.34), 0.69 |
| Diet ideal | b |
CVH indicates cardiovascular health; SBI, silent brain infarct.
Adjusted for time from baseline to magnetic resonance imaging, age at magnetic resonance imaging, sex, race/ethnicity, education, and insurance status. Individual components were included in a mutually adjusted model.
Estimates were not generated because of the small number of participants with ideal diet.
Figure 2.
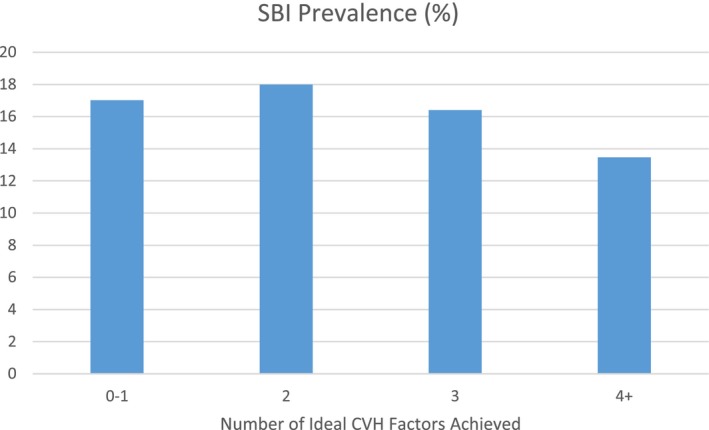
Prevalence of SBI across categories of ideal CVH. CVH indicates cardiovascular health; SBI, silent brain infarct.
Similarly, the CVH score with a range of 0 to 14 representing the sum of poor (0 points)/intermediate (1 point)/ideal (2 points) factors was also associated with lower WMHV, greater cerebral volume, and a lower odds of having an SBI (Tables 2 and 3). Model fit statistics, including the R‐squared and Akaike information criterion values, across all 3 outcomes were consistent for models that included the 7‐point score representing the number of ideal CVH factors achieved and the 14‐point CVH score (data not shown), suggesting that neither scale was superior in relation to the 3 outcomes. The value in examining the 14‐point scale in addition to the number of ideal CVH factors achieved is highlighted by the fact that many participants with only 0 to 1 ideal factors achieved intermediate status for several factors. Specifically, among those with 0 to 1 ideal factors, on the 14‐point CVH scale nobody had a score of 0, 3% had a score of 1, 7% had a score of 2, 15% had a score of 3, 27% had a score of 4, 28% had a score of 5, 15% had a score of 6, 4% had a score of 7, and 1% had a score of 8.
No effect modification by sex or education was suggested for the relationship between the number of ideal CVH factors achieved and any of the 3 outcomes (interactions terms, P>0.10). Effect modification by age was suggested for the association between ideal CVH score and both WMHV and cerebral volume (P<0.10). As shown in the stratified analyses presented in Figure 3A, the protective association between increased number of ideal CVH factors and ln(WMHV) was stronger among those who were aged <60 at baseline (β [95% confidence interval {CI}]=−0.08 [−0.16, −0.005]) compared with those aged ≥60 years (β [95% CI]=−0.03 [−0.08, 0.03]). Similarly, the positive association between the number of ideal CVH factors achieved and cerebral volume (% ICV) was stronger among those who were aged <60 at baseline (β [95% CI]=0.58 [0.28, 0.89]) compared with those aged ≥60 years (β [95% CI]=0.21 [−0.02, 0.43]). Effect modification by race/ethnicity was only suggested for the association between ideal CVH score and cerebral volume, and analyses stratified by race/ethnicity are shown in Figure 3B. The positive association between the number of ideal CVH factors achieved and cerebral volume was observed among blacks and Hispanics (blacks, β [95% CI]=0.51 [−0.02, 0.43]; Hispanics, β [95% CI]=0.36 [0.15, 0.58]), but not among whites (β [95% CI]=−0.05 [−0.57, 0.47]).
Figure 3.
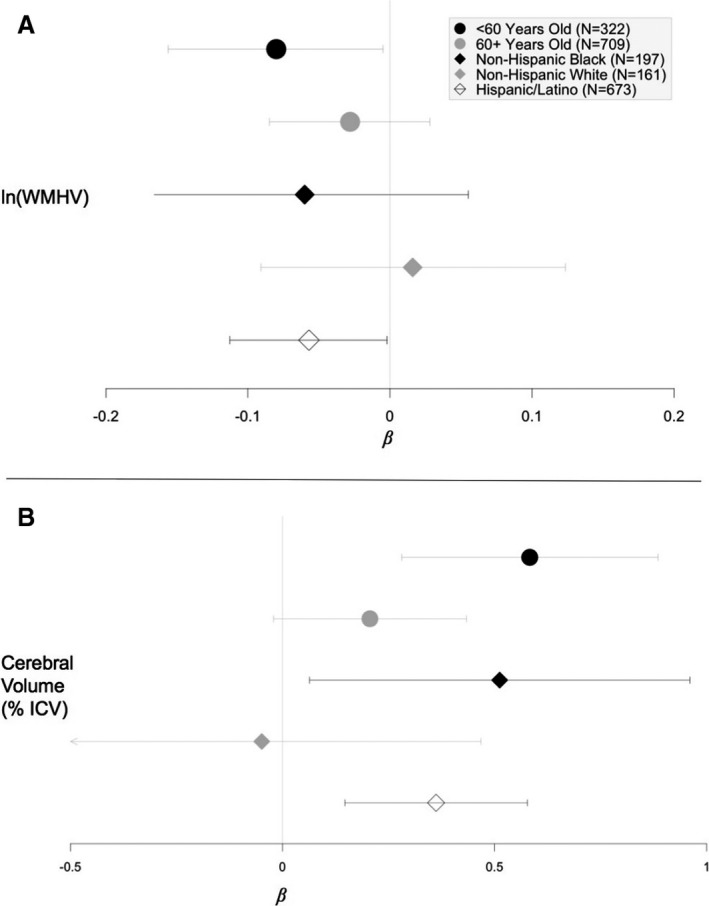
Forest plot of adjusted beta estimates and 95% confidence intervals for the association of ideal cardiovascular health (CVH) score with white matter lesion load and cerebral volume (percent ICV).* A, In(WMHV)=log‐transformed value of white mattter hyperintensity volume as a percentage of total intracranial volume. B, Cerebral volume=cerebral volume as a percentage of total intracranial volume. Models were adjusted for baseline to magnetic resonance imaging, age at baseline, sex, race/ethnicity, education, and insurance status. Note: different scales for ln(WMHV) and cerebral volume. Beta estimates represent the mean expected change in ln(WMHV) or cerebral volume (% ICV) for every 1‐point increase in ideal CVH score. ICV indicates total intracranial volume. *This figure was generated with R Studio (https://www.R-project.org/) using the forestplot package (https://CRAN.R-project.org/package=forestplot). WMHV indicates white matter hyperintensity volumes.
Discussion
Results of this study show that having a greater number of ideal CVH factors is associated with a reduced burden of several biomarkers of brain aging. Nonsmoking was a particularly strong driving factor for this association across all 3 biomarkers. The protective associations with WMHV and brain atrophy were particularly apparent among participants whose baseline CVH was measured before age 60, and the association with brain atrophy was most apparent among black and Hispanic participants.
In 2010, it was suggested that achieving Life's Simple 7 could be instrumental in reducing heart disease, stroke, and improving brain health.1 The AHA recently released a presidential advisory in which optimal brain health was operationally defined according to the Life's Simple 7 metric.2 In other words, the Life's Simple 7 metric at midlife was an important predictor of optimal brain health. This presidential advisory was supported by a strong and growing body of literature showing that the Life's Simple 7 metric was positively associated with cognitive health and protective against cognitive decline, dementia, and stroke in observational studies. In the NOMAS, we have previously shown strong positive associations between the Life's Simple 7 metric and several brain health outcomes. First, we showed a graded relationship between ideal CVH and stroke incidence over a mean follow‐up of 11 years.6 Next, we found a positive association between ideal CVH score and functional status 5 and 10 years after study baseline using the Barthel Index, and this observation was independent of incident stroke and myocardial infarction.23 Most recently, we reported a positive association between ideal CVH score and brain processing speed, and found that those with a higher ideal CVH score had less decline over time in processing speed, executive function, and episodic memory.5 Associations between ideal CVH and cognitive performance and decline in NOMAS persisted after adjustment for the subclinical imaging biomarkers examined in the current study. However, as we stressed previously, that finding could still be consistent with these imaging biomarkers partially mediating a relationship between ideal CVH and cognitive health.5 In fact, our finding that processing speed was the cognitive domain most sensitive to the effects of ideal CVH supports the hypothesis that subclinical imaging biomarkers involving intra‐ and interhemispheric connections were relevant underlying mechanisms involved.
The current study fills an important research gap, given that data are lacking on the relationship between ideal CVH as defined by the Life's Simple 7 metric and subclinical imaging biomarkers. Participants in the CARDIA (Coronary Artery Risk Development in Young Adults) brain MRI substudy who achieved more ideal CVH at ages 18 to 30 had larger brain volumes 25 years later, but they observed no associations with normal gray or white matter volume or abnormal white matter volume.24 WMHV, brain atrophy, and SBI are all important imaging biomarkers of brain aging and predictive of future risk of stroke and dementia across study populations.9, 10, 11 These brain changes have potential as brain health markers that can be used both clinically and in research studies as intermediate or surrogate outcomes to help identify at‐risk patients. The results of the current study lend further support and validation for the AHA's presidential advisory relating optimal brain health to the Life's Simple 7 metric. As explained by the AHA's presidential advisory panel, the Life's Simple 7 metric is an attractive tool for public health purposes because it can be measured, modified, and monitored easily and inexpensively over the life course.2 The results of this study also underscore the importance of continued measuring and monitoring of these 7 factors by primary care physicians in their patients throughout adulthood. Communication with patients about these 7 factors, including diet and physical activity, decades before clinical brain health impairments become apparent may represent an opportunity to improve and preserve both heart and brain health.
The results of the current study and others show that achievement of all 7 ideal factors is not necessary to see measurable benefits to brain health. In fact, NOMAS participants who only achieved 3 ideal factors had significantly greater cerebral volumes and lower WMHV than those who only achieved 1 or no ideal factors. No participants in our study population had all 7 ideal CVH factors, which was not unexpected given that our study population represents an older, low socioeconomic, urban population, with a high percentage of minority immigrants, and at high risk for stroke. Still, though cross‐sectional, our data are consistent with the idea that their cerebrovascular health may be modified by the Life's Simple 7 metric, particularly at middle age, in this at‐risk population with overall poor CVH health.
Most previous studies on the Life's Simple 7 metric have either examined it as a score ranging from 0 to 7 reflecting the number of ideal factors achieved or as the full 14‐point score.7, 8, 25, 26 We chose to analyze the metric in both ways. The consistency in results for both approaches is expected because of their common derivation from a single fundamental scale, and the results of our study are unable to elucidate which approach better reflects the relationship of this construct with brain health. The 14‐point score has the advantage of incorporating intermediate values of the 7 components, reflecting the fact that these CVH factors likely have a graded relationship with brain health that is not fully captured using a dichotomous variable. However, a disadvantage of the 14‐point scale is the heterogeneity of underlying CVH within each score. For example, an individual with a score of 6 on the 14‐point scale could have intermediate values on 6 factors and no ideal status (ie, a score of 0 on the 7‐point score) or could have achieved ideal status on 3 of the factors and poor status on the remaining 4 (ie, a score of 3 on the 7‐point score). These 2 scenarios may, in fact, confer very different associations with brain health markers, though they cannot easily be discerned using this scoring system.
We observed effect modification by age for both white matter hyperintensities and brain atrophy, such that the protective association of ideal CVH was stronger among NOMAS participants who were aged <60 at baseline when the risk factors were assessed. This finding is consistent with other studies showing that CVH in midlife is more predictive of brain health decades later than CVH in later life. Evidence is growing that the sensitive period for modifying late‐life cognitive and brain health is most likely at or before midlife, and that patients and physicians need to start addressing these health factors before midlife, given that waiting until late life may be too late.2 Whether modifying these risk factors later in life can effectively mitigate the detrimental effects of early‐ and mid‐adult poor CVH remains poorly understood. Further research in longitudinal studies is needed to better understand how the risk trajectory changes over the life course.
We also observed effect modification by race/ethnicity, such that the positive relationship between ideal CVH score and cerebral volume was only apparent in blacks and Hispanics. In a previous publication in the full NOMAS cohort, we observed a significantly greater prevalence of having 5 to 6 ideal CVH factors among whites (7.7%) compared with blacks (4.3%) and Caribbean Hispanics (3.2%), which persisted after adjustment for age and sex.6 Despite these race/ethnic disparities in the prevalence of ideal CVH, we previously observed that the relationships between ideal CVH with stroke and cognitive impairment were similar across race/ethnic groups.5, 6 The findings regarding race/ethnicity, ideal CVH score, and cerebral volume will require further study.
The strong relationship between ideal CVH factors and WMHV, brain atrophy, and SBIs suggests the role of metabolic processes influencing brain health. Long‐term exposure to vascular risk factors through adulthood, including diabetes mellitus, hypertension, hypercholesterolemia, and obesity, are likely to impair brain health by altering the structure and function of cerebral blood vessels, limiting blood flow, and therefore reducing the availability of oxygen and glucose to the brain. Additionally, the relationship between vascular risk factors and neurodegenerative processes has been suggested in recent epidemiological studies that show an association between vascular risk factors and markers of neurodegeneration.27 All together, these data suggest that modifiable vascular risk factors may impact neurodegenerative processes. The extent of this interaction is yet unknown, but the potential for ideal CVH to positively impact both vascular and neurodegenerative processes is promising.
The race/ethnically diverse, population‐based cohort is a strength of this study. However, this analysis was conducted within the MRI subcohort, which was slightly healthier than the overall NOMAS study population attributable to survival bias and a healthy cohort effect because MRI participants were enrolled an average of 7 years after baseline. The MRI subcohort was younger at baseline and more likely to be Hispanic, be insured, have hypercholesterolemia, and have completed high school and were less likely to be obese, smoke, and have diabetes mellitus. Participants in the MRI subcohort had a higher number of ideal CVH factors at baseline as compared with those not included. We are unable to rule out the potential for selection bias in this analysis. Also, because diet was only assessed at study baseline, that was the only time point available at which to calculate the complete ideal CVH score. Baseline cardiovascular risk factors and the MRI outcomes were each measured only once, and therefore we are unable to draw conclusions about temporality and causality. Future studies are needed to examine how changes in CVH profile over time impact the progression of imaging biomarkers over time, and determine whether and when behavioral modifications may be most beneficial for preserving brain health and function. Additionally, we had a relatively smaller number of younger and non‐Hispanic participants in our study; thus, our tests for interactions may have been underpowered to detect associations. Larger studies to examine age and race/ethnicity as potential moderators for this association are warranted. As in any observational epidemiologic study, residual confounding by unmeasured risk factors remains a potential source of bias.
In conclusion, this study supports the AHA's recommended use of the Life's Simple 7 metric in adulthood as an important predictor of optimal brain health. Brain aging is an important mechanism underlying both stroke and cognitive decline, and we have shown that adults who achieve more‐ideal CVH factors have better brain health as defined by multiple brain imaging biomarkers. The goal of the AHA to improve the CVH of all Americans by 20% by 2020 is predicted to have measurable and impactful benefits for brain health in addition to heart health. The public health importance of reducing and reversing clinical and subclinical disease across the population is critical as our communities age and the consequences of suboptimal brain health increase over the next 2 decades.
Sources of Funding
This study was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS 29993) and the Evelyn F. McKnight Brain Institute.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009544 DOI: 10.1161/JAHA.118.009544.)
References
- 1. Sacco RL. Achieving ideal cardiovascular and brain health: opportunity amid crisis: presidential address at the American Heart Association 2010 scientific sessions. Circulation. 2011;123:2653–2657. [DOI] [PubMed] [Google Scholar]
- 2. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd‐Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, Girgus M, Howard VJ, Lazar RM, Seshadri S, Testai FD, van Gaal S, Yaffe K, Wasiak H, Zerna C; American Heart Association/American Stroke Association . Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR Jr, Zhu N, Lloyd‐Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MS, Sacco RL. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5:e002731 DOI: 10.1161/JAHA.115.002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, Post WS, Sacco RL, Szklo M, Lloyd‐Jones DM. Association of cardiovascular health with subclinical disease and incident events: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6:e004894 DOI: 10.1161/JAHA.116.004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 9. Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. [DOI] [PubMed] [Google Scholar]
- 10. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 11. Wright CB, Dong C, Perez EJ, De Rosa J, Yoshita M, Rundek T, DeCarli C, Gutierrez J, Elkind MSV, Sacco RL. Subclinical cerebrovascular disease increases the risk of incident stroke and mortality: the Northern Manhattan Study. J Am Heart Assoc. 2017;6:e004069 DOI: 10.1161/JAHA.116.004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacco RL, Anand K, Lee HS, Boden‐Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke. 2004;35:2263–2269. [DOI] [PubMed] [Google Scholar]
- 13. Wallman KK, Hodgdon J. Race and ethnic standards for Federal statistics and administrative reporting. Stat Report. 1977;77:450–454. [PubMed] [Google Scholar]
- 14. Sacco RL, Elkind M, Boden‐Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. [DOI] [PubMed] [Google Scholar]
- 15. Kargman DE, Sacco RL, Boden‐Albala B, Paik MC, Hauser WA, Shea S. Validity of telephone interview data for vascular disease risk factors in a racially mixed urban community: the Northern Manhattan Stroke Study. Neuroepidemiology. 1999;18:174–184. [DOI] [PubMed] [Google Scholar]
- 16. Willey JZ, Moon YP, Paik MC, Boden‐Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology. 2009;73:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardener H, Della Morte D, Elkind MS, Sacco RL, Rundek T. Lipids and carotid plaque in the Northern Manhattan Study (NOMAS). BMC Cardiovasc Disord. 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardener H, Wright CB, Gu Y, Demmer RT, Boden‐Albala B, Elkind MS, Sacco RL, Scarmeas N. Mediterranean‐style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr. 2011;94:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 20. Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, DeCarli C, Sacco RL, Stern Y, Wright CB. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 23. Dhamoon MS, Dong C, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts functional status independently of vascular events: the Northern Manhattan Study. J Am Heart Assoc. 2015;4:e001322 DOI: 10.1161/JAHA.114.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bancks MP, Allen NB, Dubey P, Launer LJ, Lloyd‐Jones DM, Reis JP, Sidney S, Yano Y, Schreiner PJ. Cardiovascular health in young adulthood and structural brain MRI in midlife: the CARDIA study. Neurology. 2017;89:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. González HM, Tarraf W, Rodríguez CJ, Gallo LC, Sacco RL, Talavera GA, Heiss G, Kizer JR, Hernandez R, Davis S, Schneiderman N, Daviglus ML, Kaplan RC. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J. 2016;176:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. González HM, Tarraf W, Gouskova N, Rodríguez CJ, Rundek T, Grober E, Pirzada A, González P, Lutsey PL, Camacho A, Daviglus ML, Wright C, Mosley TH. Life's Simple 7's cardiovascular health metrics are associated with Hispanic/Latino neurocognitive function: HCHS/SOL results. J Alzheimers Dis. 2016;53:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff‐Radford J, Murray ME, Roberts RO, Vassilaki M, Lowe VJ, Machulda MM, Jones DT, Petersen RC, Jack CR Jr. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. 2017;74:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]


