Abstract
Background
Advanced cardiac imaging permits optimal targeting of cardiac treatment but needs to be faster, cheaper, and easier for global delivery. We aimed to pilot rapid cardiac magnetic resonance (CMR) with contrast in a developing nation, embedding it within clinical care along with training and mentoring.
Methods and Results
A cross‐sectional study of CMR delivery and clinical impact assessment performed 2016–2017 in an upper middle‐income country. An International partnership (clinicians in Peru and collaborators from the United Kingdom, United States, Brazil, and Colombia) developed and tested a 15‐minute CMR protocol in the United Kingdom, for cardiac volumes, function and scar, and delivered it with reporting combined with training, education and mentoring in 2 centers in the capital city, Lima, Peru, 100 patients referred by local doctors from 6 centers. Management changes related to the CMR were reviewed at 12 months. One‐hundred scans were conducted in 98 patients with no complications. Final diagnoses were cardiomyopathy (hypertrophic, 26%; dilated, 22%; ischemic, 15%) and 12 other pathologies including tumors, congenital heart disease, iron overload, amyloidosis, genetic syndromes, vasculitis, thrombi, and valve disease. Scan cost was $150 USD, and the average scan duration was 18±7 minutes. Findings impacted management in 56% of patients, including previously unsuspected diagnoses in 19% and therapeutic management changes in 37%.
Conclusions
Advanced cardiac diagnostics, here CMR with contrast, is possible using existing infrastructure in the developing world in 18 minutes for $150, resulting in important changes in patient care.
Keywords: cardiac magnetic resonance, effectiveness, impact on patient management, outcome, rapid cardiac magnetic resonance
Subject Categories: Magnetic Resonance Imaging (MRI), Imaging, Diagnostic Testing
Clinical Perspective
What Is New?
Cardiac Magnetic Resonance imaging improves the care of cardiac patients in high income countries by improving diagnostic accuracy and better targeting expensive treatments.
It is, however, too expensive for developing countries.
We developed and tested a rapid cardiac magnetic resonance protocol focusing on just measuring cardiac volumes and scar for deployment on existing scanners in the developing world.
What Are the Clinical Implications?
In a trial deployment at 2 sites in a capital city of an upper middle‐income country, rapid cardiac magnetic resonance could be delivered faster, cheaper, and easier than is conventionally done.
The rapid Cardiac Magnetic Resonance protocol was embedded in clinical care with training and education, which resulted in important and frequent patient management changes that appear beneficial for both patients and the healthcare system.
The impression is that resource scarcity is not a justification for the absence of key diagnostic tests in the developing world.
This protocol is focused on the essential: cardiac volumes and scar. It can be implemented in a capital city with existing infrastructure, and it has a major impact for sustainability if it is delivered with quality, education and training.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in developed countries, accounting for 17.9 million deaths per year causing 30% of global deaths.1 However, CVD disease and death rates are highest in low‐ and middle‐income countries, despite there being a lower burden of classical risk factors.2, 3, 4 For example, in Peru, CVD affects 3.2 million (16% of the adult population), leading to a significant loss of well‐being, estimated at 281 829 Disability Adjusted Life Years. The age‐standardized death rate is 142 per 100 000 inhabitants, resulting in productivity losses of 0.69 bn USD and health costs of 0.24 bn USD, representing 2.1% of total health spending, which the country can ill afford.5, 6
A key aspect of cardiovascular care is diagnostic testing. Without this, clinical care is inefficient at best, inaccurate or mistaken at worst. The hierarchy of diagnostic testing is used optimally by employing guidance and care pathways, taking into account local resource availability and cost constraints. Cardiac magnetic resonance (CMR) imaging is an emerging imaging technique with the capacity to transform cardiology in an analogous way to the impact of brain MRI in neurology.7, 8 Multiple different techniques are available using CMR (function, scar, perfusion, flow, mapping, angiography), delivering valuable clinical insights with the potential of a high impact.9, 10, 11 However, these varied techniques may make it slow (typically 45 minutes), expensive and complex, potentially out of reach for most inhabitants in the developing world. In Peru, although MRI scanners are available for other indications, just 2 public hospitals undertake CMR, performing a total of 6 scans per week (≈1 scan per million population per year), at a cost of $400 to $800. This compares to the United Kingdom where there are ≈60 UK centers performing ≈1000 scans per million population per year, at a cost of $640.12 However, we contend that CMR could be made faster, cheaper, and easier, which would increase its utility in the developing world. The core incremental diagnostic utility of CMR over other methods is left ventricular (LV) function and late gadolinium enhancement (LGE) imaging for scar. Previously, we implemented an ultrafast CMR protocol without contrast in Thailand for the relatively uncommon indication of iron assessment in a specific patient cohort (Thalassemia). Scanning was performed in 8 minutes, reducing costs 4‐fold.13 The purpose of the INCA (Impact of Non‐Invasive CMR Assessment) Peru study was to assess the potential of faster, cheaper, easier rapid contrast‐enhanced CMR program for wider cardiology indications in an upper middle‐income country.
Methods
The imaging data, analytic methods and study materials of this study are all anonymized and are available to other researchers upon (reasonable) request.
Ethics
The research received ethical approval in the United Kingdom: REC reference: 14/LO/1948, amendment: 3.0, IRAS ID: 142036; University College London REC reference: 11255/002 and Peru: Edgardo Rebagliati Martins Hospital, Institutional Ethics Committee: IRB No 00003285/Arch 1047, NIT 832–2016–1066. Participants gave written informed consent.
A Peru‐International partnership at academic and political levels was established: UK‐ UCL and Overseas Development Office; Peru—ESSALUD, The Peruvian Scientific, Technological Development and Technological Innovation (FONDECYT), Peruvian Society of Cardiology; International: Society for Cardiovascular Magnetic Resonance (SCMR) and Inter‐American Society of Cardiology (SIAC).
Rapid CMR Protocol Development
The protocol (cardiac volumes, function and scar via LGE) was developed and assessed in 45 participants (33 patients with cardiomyopathy, 12 healthy volunteers) at Heart Hospital, London, using a 1.5T scanner (Avanto, SIEMENS Healthineers, Erlangen Germany), Figure 1. All volunteers had no history of cardiovascular disease, hypertension or diabetes mellitus, and were not on regular medications. The protocol included:
Localizers, pilot 2 chamber, 3 slice short axis stack, and a transverse dark blood single shot fast spin echo stack for anatomic evaluation.
Volume and cardiac structure assessment: four, two, three chamber and aortic valve segmented k‐space cine acquisitions.
Hand‐contrast injection: gadolinium‐based contrast agent Gadoterate meglumine (Dotarem. Guerbet S.A France, 0.1 mmol/kg).
Short axis cine stack (7‐mm slice thickness, 3 mm inter‐slice gap).
Scar imaging: repeating cine views as needed, followed by an optional sequence to determine the optimal inversion time and segmented k‐space late gadolinium enhancement acquisitions in multiple planes with phase sensitive and magnitude reconstructions.
Figure 1.

Fifteen minutes rapid cardiac magnetic resonance protocol in the United Kingdom. AoV indicates Aortic Valve; CH, Chamber; HASTE, Half‐Fourier acquisition single‐shot turbo spin‐echo; LOC, Localizer; SAX, Short Axis.
Protocol Implementation
We embedded scanning into clinical care and within an education and mentoring program: 4 professionals from Peru visited the United Kingdom (2 doctors, 1 technologist, and 1 physicist) for 1 month. Preliminary scanning in Peru was mentored via live webcasting. A 2‐day training course (190 attendees) delivered in Peru for cardiologists, radiologists and technologists before scanning, followed by a reporting course (35 radiologists and cardiologists attending) after scanning. In total, 14 international faculty were involved from Europe, the United States, and Latin America.
Patients
On the 26th and 27th of November 2016, a total of 100 patients, referred by 10 doctors from 6 centers, underwent rapid CMR at 2 centers in Lima, Peru: Edgardo Rebagliati Martins National Hospital (1.5T Avanto, Siemens), and Delgado Private Hospital (1.5T Aera, Siemens).
CMR Complications
Major complications were predefined as death, resuscitation, complication requiring admission for at least 1 night. Mild complications were defined as any others clinically significant events, such as dyspnea, chest pain, and allergic reactions without shock).
CMR Analysis
Images were analyzed using dedicated software (cvi42, Circle Cardiovascular Imaging, Calgary Canada). Scans were reported within 2 days of scanning, during the reporting training course, with draft reports prepared by fellows (all SCMR level 2 trained) and finalized by 1 of 4 experts (level 3 trained, J.C.M./H.L./M.W./J.F.). They were translated into Spanish and incorporated into the medical records. Scan time was defined from the timestamp of first to the last image acquired.
Image Quality
Images that did not answer the clinical question were considered poor; those with artifact but still interpretable as moderate, and images with optimal quality allowing definitive assessment of the clinical question were graded as good.
Patient Outcomes
After 12 months, management changes related to CMR results were reviewed in consultation with general cardiologists or other physicians (hematologists) in the 6 referral centers. A change of patient management was reported if CMR resulted in (1) new diagnosis (eg cardiac amyloidosis diagnosed in a hypertrophic cardiomyopathy patient) (2) medication changes (eg suspension of secondary prevention drugs after detection of myocarditis and nonischemic disease; commencement of anticoagulation for thrombus) (3) interventional treatment (eg coronary artery bypass grafting) or (4) new testing (eg, ordering invasive angiography, biopsy). Many of these resulted in (5) hospital admission/discharge (eg a large apical thrombus in a patient with previous RCA infarction).
Cost Evaluation
Reference CMR costs in Peru are difficult to determine given the complex healthcare structure (public—coverage of 84% of the population in three sectors; private covering 4% with multiple insurance companies; 12% uncovered). We took as reference the average cost from the 2 public and 2 private hospitals offering CMR in Lima.14, 15 To determine the cost of rapid CMR, we included the following parameters: hospital charges, contrast and cannula costs, and physician, nurse and technologist fees, but not opportunity cost (eg, displaced MRI scanning for other organs) or training costs.
Statistical Analysis
Absolute numbers and percentages expressed as mean±SD if they had a normal distribution, and range, if not. Normal distribution formally tested using Shapiro–Wilk test. Association of two categorical variables was analyzed by Chi‐square test. To predict the response of two categories and ordinal level dependent variables with a set of categorical independent variable, binary and ordinal logistic regression analysis were used, respectively. Values of P<0.05 were considered statistically significant.
Results
The baseline patient characteristics are shown in Table 1.
Table 1.
Patient Baseline and Scan Characteristics
| All patients (%) | 98 (100%) |
| Age, mean (range), y | 52 (16–93) |
| Male (%) | 39 (40%) |
| Female (%) | 59 (60%) |
| Height, mean (SD), m | 1.62 (0.1) |
| Weight, mean (SD), kg | 69 (11.7) |
| BMI, mean (SD), kg/m2 | 24.8 (3.7) |
| Cardiology referral (%) | 93 (95%) |
| Reader | |
| Cardiologist (%) | 69 (70%) |
| Radiologist (%) | 29 (30%) |
| MRI Scanning duration, mean (SD) | 18 (7) |
| CMR cost | $150 |
| CMR exam | |
| Contrast enhanced (%) | 93 (95%) |
| Non‐contrast (%) | 3 (3%) |
| Repeated scans (%) | 2 (2%) |
BMI indicates bone mass index; CMR, cardiac magnetic resonance; MRI, magnetic resonance imaging.
Patients
One hundred patients were referred, 95 by cardiologists, 5 by hematologists. 98 were scanned, mean age 52 years (36–65 years), 60% female. Two underwent repeat scanning (1 due to failure of contrast delivery, 1 to confirm unexpected cardiac amyloidosis). Indications were: cardiomyopathy (hypertrophic 26%; non‐ischemic dilated 22%; ischemic including viability 15%). There were 12 other indications, Figure 2. Average scan time was 18±7 minutes, Table 1. Scan duration was not influenced by age (P=0.3).
Figure 2.
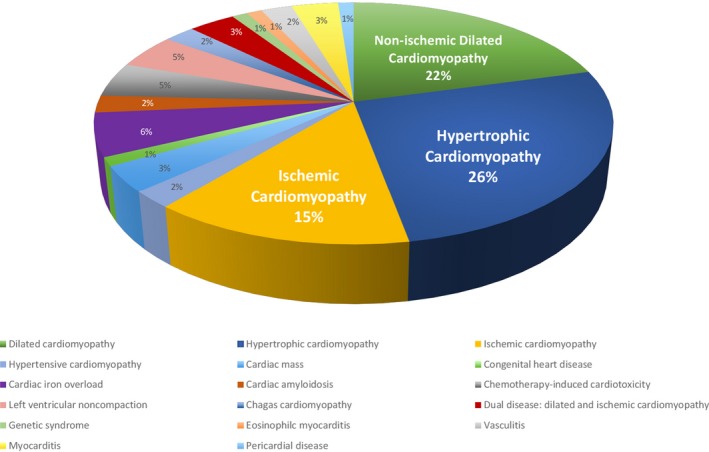
Cardiac pathologies evaluated in the INCA (Impact of Non‐Invasive CMR Assessment) Study. CMR indicates cardiac magnetic resonance.
Before CMR scanning, 94% of patients had undergone transthoracic echocardiogram, 14% cardiac catheterization, 7% Single‐photon emission computed tomography, 3% cardiac computed tomography; none had had prior CMR.
Scanning
Contrast was administered in 95% of cases with 5 being evaluated for cardiac iron so not requiring contrast. The contrast dose was 0.1 mmol/kg (0.2 mL/kg). All studies were performed without major complication. There was 1 minor complication (panic attack).
Good image quality was reported in 91% of cases, moderate in 7%. Images were non‐diagnostic in 2% and therefore graded as poor. In reality, the image quality was good but the LGE was so abnormal that we repeated scanning to confirm that contrast had actually been administered and that the cases were indeed cardiac amyloidosis (which they were). Image quality of exam was not influenced by age (trend assessed across 4 age categories: <44, 45–59, 60–74 and >75 year‐old, P>0.05). However, impact on patient care (new diagnosis and change of medication) was more likely with age (P<0.05), Table 2.
Table 2.
Image Indication, Quality, and Impact of Rapid CMR Findings in Accordance to Patient Age Group
| Age Group | ||||
|---|---|---|---|---|
| <44 Years (n=34) | 45 to 59 Years (n=22) | 60 to 74 Years (n=32) | >75 Years (n=10) | |
| Indication | ||||
| Non‐ischemic cardiomyopathy (%) | 28 (82%) | 14 (64%) | 24 (75%) | 5 (50%) |
| Ischemic cardiomyopathy (%) | 1 (3%) | 4 (18%) | 3 (9%) | 4 (40%) |
| Others (%) | 5 (15%) | 4 (18%) | 5 (16%) | 1 (10%) |
| Image quality | ||||
| Good (%) | 32 (94%) | 21 (95%) | 27 (85%) | 9 (90%) |
| Moderate (%) | 2 (6%) | 1 (5%) | 3 (9%) | 1 (10%) |
| Poor (%) | 0 (0%) | 0 (0%) | 2 (6%) | 0 (0%) |
| P value compared with age group >75 y | 0.66 | 0.57 | 0.62 | ··· |
| New diagnosis (%) | 6 (18%) | 3 (14%) | 5 (16%) | 5 (50%) |
| P value compared with age group >75 y | 0.047 | 0.037 | 0.035 | ··· |
| Therapeutic consequences | ||||
| Change of medication (%) | 7 (21%) | 4 (18%) | 6 (19%) | 6 (60%) |
| P value compared with age group >75 y | 0.023 | 0.025 | 0.018 | ··· |
CMR indicates cardiac magnetic resonance.
Extra‐Cardiac Findings
Incidental extra‐cardiac findings were found in 21% of cases but were mainly not incrementally important compared with existing clinical knowledge—2% were judged new important findings (example: malignant liver mass). The most common findings were: dilated great vessels (13%) and lung parenchymal abnormalities (3%).
Cardiac Findings and Impact
CMR directly impacted clinical management at 12 months in 56% of patients, either by revealing an unsuspected new diagnosis (19%) and/or leading to a change in management with therapeutic consequences (37%), Table 3. In 5%, a change in management was suggested but not delivered due to access barriers (cardiac surgery or device therapy)—we did not count these patients in the 56% (ie rapid CMR could have changed care in 61%).
Table 3.
Impact of Non‐Invasive CMR Assessment on Patient Management
| Impact of INCA (Peru) | |
|---|---|
| All patients | 98 (100%) |
| New diagnosis | |
| Completely new diagnosis not suspected before (%) | 19 (19%) |
| Therapeutic consequences | |
| Change/addition in medication (%) | 23 (23%) |
| Intervention/surgery (%) | 6 (6%) |
| Invasive angiography/biopsy (%) | 4 (4%) |
| Hospital discharge/admission (%) | 3 (3%) |
| Impact on patient management (%) (new diagnosis and therapeutic consequences) | 55 (56%) |
| Non‐invasive imaging ordered after CMR | |
| Transthoracic—transesophageal echocardiogram (%) | 6 (6%) |
| Cardiac computed tomography (%) | 3 (3%) |
CMR indicates cardiac magnetic resonance; INCA; Impact of Non‐Invasive CMR Assessment.
Figure 3 shows the impact of the rapid CMR on patient management by the 3 main indications: non‐ischemic cardiomyopathy (hypertrophic cardiomyopathy, dilated cardiomyopathy); ischemic cardiomyopathy, and other.
Figure 3.
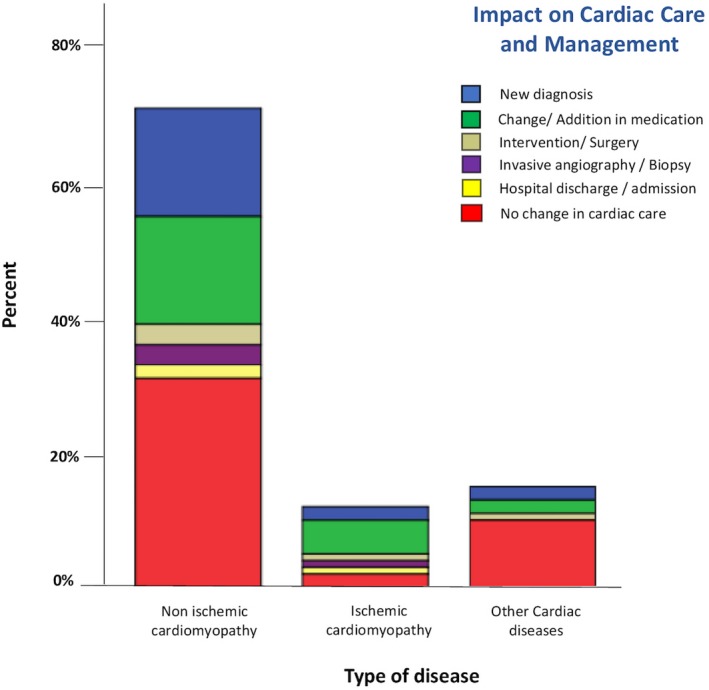
Impact of rapid cardiac magnetic resonance on cardiac care and management by indication.
Rapid CMR satisfied all imaging needs in 89% of patients. In 7%, where CMR was the first imaging technique performed, no further non‐invasive imaging was needed. Figures 4 and 5 show 2 cases demonstrating the impact of CMR on the management. Although non‐blinded, CMR did not miss any diagnoses initially found by echocardiography.
Figure 4.
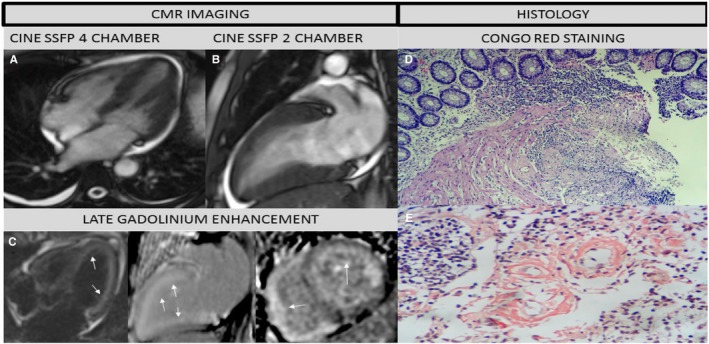
Fifty‐two‐year‐old personal trainer who complained of shortness of breath and fatigue. Previous echocardiogram showed hypertrophy. Cardiologist referral suspicious of Fabry's Disease. CMR study: (A) 4 chamber, (B) 2 chamber cine steady free precession revealed pleural and pericardial effusion, left ventricle hypertrophy and mild systolic dysfunction and (C) Contrast CMR (LGE: 4, 2, and short axis views) showed diffuse sub‐endocardial enhancement in both ventricles (white arrows), suggestive of Cardiac Amyloidosis (most likely type AL). Labial salivary gland biopsy revealed (D) Hematoxylin‐eosin stain, panoramic view: amyloid tissue infiltrating the muscularis mucosae and blood vessels, (E) Congo red stain, original magnification ×8: Type AL positive Congo red staining. Currently patient is receiving chemotherapy and continues follow‐up in heart failure clinic. Biopsy images shared by courtesy of Dr Jose Luis Arenas Gamio, Pathology Service, Guillermo Almenara Irigoyen Hospital, Lima—Peru. AL indicates amyloid light‐chain; CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Figure 5.
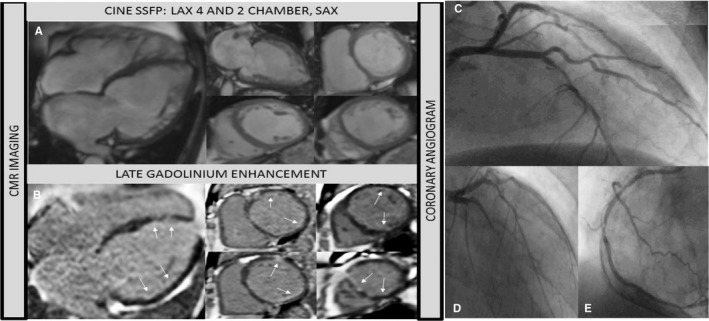
Sixty‐five‐year‐old man. History of chronic heart disease and heart failure. Chest pain. No previous angiogram. CMR to assess viability. A, Cine steady free precession (4 chamber, 2 chamber and short axis views) showed bi‐ventricular dilatation and severe systolic dysfunction. B, Contrast CMR (LGE: 4 and short axis views) revealed sub‐endocardial enhancement in the ventricle (see arrows). CMR study suggests possible multi‐vessel CA disease with a low overall infarcted burden: global hibernation or dilated cardiomyopathy as differential diagnosis. Angiogram study (C through E) revealed triple vessel disease. Pending Coronary Artery Bypass Graft surgery. Angiogram images shared courtesy of Dr Milder Granados, Cayetano Heredia Hospital, Lima—Peru. CA indicates coronary artery; CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Cost
The rapid CMR strategy was 3 to 5 times less expensive than current CMR exams in Peru: costing 62%, 75%, and 81% less compared with existing public/private (for public) or private (for insurer) fees.
Discussion
Cardiac MRI is a key diagnostic imaging technology that helps target often expensive treatments, but uptake is limited in middle‐income countries because, although cardiac enabled systems are present, barriers exist to delivery. This may result in the poor targeting of already rationed treatments such as drugs, surgery, stenting, and device therapy. We did 3 things to make CMR suitable for major centers in developing world healthcare systems, using Peru as the exemplar: firstly, created national/international support structures for a service at the political and healthcare system levels; secondly, created an education/mentoring system for both those delivering the technology and referrers;and thirdly, by focusing on the core utility of CMR, volumes and scar imaging, we made CMR faster, cheaper, and easier. We delivered high quality CMR at 2 (capital city) sites in 2 parts of the healthcare system on patients from 10 clinicians at 6 sites that was 2 to 3 times faster and 3 to 5 times cheaper than usual and changed the care in 56% of subjects scanned.
The morbidity and mortality from cardiovascular disease in the developing world is high.4 Healthcare systems need to be cost appropriate, accessible, and capable of making a diagnosis, implementing appropriate therapy and monitoring that therapy. In cardiology, the key diagnostic tests are the ECG, echocardiogram, angiography, cardiac CT, and cardiac MRI, typically embedded in a framework of education, training, and guidelines/protocols for their delivery and quality control. In middle‐income countries, this hierarchy is typically truncated with absence of the more expensive, technically demanding tests such as CMR. Echocardiogram is still the first imaging technique option for cardiac patients as it is portable, inexpensive, and recommended by international guidelines.16, 17 This exam remains the first line exam to assess cardiac structure, but this is not sufficient to get critical information on causation, risk, therapeutic strategy, and reversibility across a wide range of diseases. CMR tissue characterization using the late gadolinium enhancement technique adds major value in many clinical scenarios, providing information that cannot be obtained any other way (myocarditis,18 amyloidosis,19 takotsubo, small apical thrombi, sarcoid20 for example), risk stratification21 and prognostic information22, 23, 24 and is becoming essential in several key clinical scenarios (chest pain, troponin rise, normal coronaries, to confirm/refute history of ischemic scarring, some cardiomyopathies). With forthcoming developments in cardiac personalized care, an appreciation of inflammation and scarring is likely to prove essential to arbitrate novel therapies.
With expensive technology, usage patterns should reflect the ratio of capital costs to labor costs that the payee can bear: so, an MRI scanner operational day in the United Kingdom may be 12 hours, 6 days a week but 20 hours, 7 days a week in Argentina, for example. Modern MRI scanners can all do cardiac MRI with the addition of ECG gating and proper sequences, the basic examples being supplied routinely with scanner purchase. However, CMR is perceived as slow, expensive, and complex, not only for imager to analyze and interpret, but also to the clinician “gatekeepers”, who decide whom to refer.
We had previously shown that CMR could be performed quickly in Thailand13 for a single indication, evaluation of cardiac iron, in a cohort where the clinical value for mortality and morbidity of this measurement is established.25 However, in this first pilot project, an approach was taken focusing on technology delivery with less investment in education and mentoring. In Peru, a broader landscape was emphasized in a generalize cohort of patients, where the evaluation of cardiac function, volumes and scar are important diagnostic criteria.
Some less apparent aspects of the study merit attention. Firstly, CMR has new technologies (sequences) that make scanning easier—for example motion corrected (MOCO) late enhancement sequences. We wanted to install these in Peru (and test their incremental utility), but the industry waiver form stipulations made these onerous for the local hospitals to sign (eg, all liability for sequence use was laid on the hospital not the manufacturer). Secondly, cultural aspects are important: we were able to scan on Saturday and Sunday at both sites, and both cardiologists and radiologists worked together, but at 1 site, a 1 hour lunch‐break was mandated. Thirdly, we could have delivered 15‐minute scanning, focusing on core utility, but the clinical disease stopped this—we had decided not to do complex imaging particularly congenital heart disease, but clinician demand meant that we had several such cases, increasing scan‐length (but also adding to yield). At one stage, we dropped the aortic valve short axis view—but we soon realized that this was a step too far as there are high levels of congenital (bicuspid) and acquired (stenosis/regurgitation) aortic valve disease in clinical care. Fourthly, we were not expecting the high clinical utility we encountered. Focusing on 1 familiar disease, hypertrophic cardiomyopathy is illuminating: the hypertrophic cardiomyopathy referred in Peru was far more extreme than in the United Kingdom (more hypertrophy, more syndromic, more scar, and more obstruction). We believe that this reflects not a different disease profile, but the test scarcity—only the most concerning patients were referred by clinicians.
The images from this rapid CMR had routine and conventional volumes, mass and ejection fraction quantified. Other measurements are also possible. There is indeed mileage in alternative and incremental, sometimes superior, techniques for assessing cardiac function. Feature tracking for quantification of global left ventricular strain in patients with Global Longitudinal Strain (GLS) is a fast and robust technique, carrying important clinical information in many circumstances26, 27 and the myocardial contraction fraction (stroke volume over myocardial volume) is an underused indexless measure that imports more prognostic information than the ejection fraction in many clinical scenarios.28, 29 The cine images acquired in the rapid protocol are conventional and may also be used for more advanced analysis even without the use of contrast. However, specialist software requirements and the incomplete development of clinical utilities means this was outside the key priorities for this study. Development over time would help its implementation and it would make CMR even easier to implement.
The clinical utility found in Peru of 56% having changed care after CMR is somewhat less than demonstrated in international registries, as European/US registries have reported that CMR changed care in 61.8% of patients.30, 31 However, we would have reached these levels in our study by including the 5% of patients in which a change in management was appropriate but not possible due to access barriers.
This study was structured to result in a sustainable service, however, this requires multiple aspects: Local champions: The project conducted as a Peruvian fellow (KM) came to learn CMR in the United Kingdom and wanted to translate CMR to Peru—her PhD title is “the $100, 15‐minute CMR scan”. Political support: was from the UK Overseas Development Office via the embassy to one of five the sectors which administers healthcare in Peru: EsSalud, with scanning in both the public and private sector. We also were careful to include software solutions so analysis was not perceived as a barrier. Education/training in depth: Four Peruvians came to the United Kingdom to train (a cardiologist, radiologist, technologist, and physicist). We implemented a 2‐day training course for cardiologists and radiologists with a specific technologist track—including lectures delivered by technologists. Partnership: all scanning conducted in partnership (day 1 mainly overseas technologists; day 2 mainly Peruvian); slots for attendance at scanning were available to clinicians and all reporting made by overseas level 3 reporters (expertise is known to be important32) but within a level 1 reporting course as a training exercise in partnership, and subsequent free 1‐year membership to an international society. Quality: The rapid CMR protocol was tested in advance using freely available video conferencing software. We used low‐dose gadolinium (0.1 mmol/L per kg) for cost and so the blood‐pool was not bright at the time of LGE imaging.33 The CMR studies were conducted within a safe environment, with only one minor complication which was a panic attack. The images of the protocol answered the clinical question in 91% of studies—we used criteria for quality based not directly on artifact, but on ability of the scan to answer the clinical questions. Follow‐up: the 12‐month outcome adjudication was to ensure outcomes were not just technical but clinical (care changed in 56%), to make sure there were not major added investigations triggered (91% of patients) and to engender engagement. Since the project ended, a clinical service at the public hospital is continuing, 2 more public hospitals in Lima have adopted the rapid CMR protocol (1 of them had never done CMR before), 2 of them initially scanned 4 to 6 patients per week and included 2 research programs (1 on HIV cardiomyopathy, and the other on the effect of chronic altitude exposure during gestation).
Study Limitations
This protocol did not include advanced magnetic resonance sequences (there was no mapping, no perfusion, and flow only for selected cases). Perfusion CMR is a safe, accurate, and cost‐efficient test for inducible ischemia34 with superior or similar performance compared with SPECT35 or invasive Fractional flow reserve36 However, as alternatives and delivery are complex, it is outside the scope of rapid CMR with currently available infrastructure, although this may change with the latest sequences and developments over time.37
Finally, outcomes were clinical change in management rather than objective gold standards (not easily available when CMR is often that standard). The study is described as assessing CMR in the developing world, but it is perhaps better described as in the capital city/leading centers in the developing world, and we have not unequivocally demonstrated sustainability.
Conclusions
The advanced diagnostic technique cardiac MRI can be made faster, cheaper, and easier for deployment in the developing world. This can be done safely with high diagnostic quality on existing infrastructure and it changes patients care in most of cases. Finally, this rapid CMR protocol includes an educational and support package in order to ensure sustainability.
Sources of Funding
This study was funded by the United Kingdom Foreign & Commonwealth Office and The Peruvian Scientific, Technological Development and Technological Innovation (FONDECYT). Dr Malcolm Walker was supported by University College London Biomedical Research Centre and University College London, Special Trustees Charity.
Disclosures
None.
Acknowledgments
Our sincere acknowledgments to the UK ambassador in Peru, Anwar Choudhury, and his team; Past Peruvian Social Secure President (ESSALUD), Del Castillo Mori Jorge; Radiology and Cardiology Service Rebagliati Public Hospital: Dr Miguel Reyes, Dr Gerardo Pacheco, David Uscamaita, Dr Mario Zubiate; Radiology and Cardiology Service Delgado Private Hospital: Dr Miguel Trelles, Dr Jorge Salinas; Cardiologist: Dr Martin Salazar, Dr Zoila Rodriguez, Dr Luisa Talledo, Dr Ivan Valdeiglesias, Dr Cesar Peralta, and Bart's Heart Centre—UCL team, London: radiographers Louise McGrath and Patricia Feuchter. Biopsy images shared by courtesy of Dr Jose Luis Arenas Gamio, Pathology Service, Guillermo Almenara Irigoyen Hospital, Lima—Peru.
(J Am Heart Assoc. 2018;7:e008981 DOI: 10.1161/JAHA.118.008981.)
References
- 1. Teo KK, Dokainish H. The emerging epidemic of cardiovascular risk factors and atherosclerotic disease in developing countries. Can J Cardiol. 2016;33:358–365. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2015 Mortality and Causes of Death Colaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lu F, Liu T, Yu L, Zhang P, Mony P, Swaminathan S, Mohan R, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez‐Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G. Cardiovascular risk and events in 17 low‐, middle‐, and high‐income countries. N Engl J Med. 2014;371:818–827. [DOI] [PubMed] [Google Scholar]
- 5. Pezullo L, Stevens B, Verdian L, Tomlinson J, Zegenhagen S. The economic burden of heart diseases in Peru. Proceeding of the World Congr Cardiol Cardiovasc Health; 2016, Jun 4–7; Mexico City, Mexico: GHEART Press; 2016:131–132. [Google Scholar]
- 6. World Health Organization . Peru—noncommunicable diseases country profile. 2014. Available at: http://www.who.int/gho/countries/per/country_profiles/en/. Accessed November 24, 2017.
- 7. Shah S, Luby M, Poole K. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurolgy. 2015;84:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grigoryan M, Tung C, Albers G. Role of diffusion and perfusion MRI in selecting patients for reperfusion therapies. Neuroimaging Clin N Am. 2011;21:247–257. [DOI] [PubMed] [Google Scholar]
- 9. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm S, Fogel M, Friedrich M, Kim R, Knobelsdorff‐Brenkenhoff F, Kramer C, Pennell D, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;1:15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedric MG, Ho VB, Jerosch‐Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moon J, Messroghli D, Kellman P, Piechnik S, Rodson M, Ugander M, Gatehouse P, Arai A, Friedrich M, Neubauer S, Schulz‐Menger J, Schelbert E. Myocardial T1 mapping and extracellular volume quantification: Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:15–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renjith A, Daghem M, McCann G, Daghem S, Moon J, Pnnell D, Neubauer S, Dargie H, Berry C, Payne J, Petrie M, Hawkins N. Cardiovascular magnetic resonance activity in the United Kingdom: a survey on behalf of the British Society of Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson. 2011;13:13–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdel‐Gadir A, Vorasettakarnkij Y, Ngamkasem H, Nordin S, Ako EA, Tumkosit M, Sucharitchan P, Uaprasert N, Kellman P, Piechnik S, Fontana M, Fernandes J, Manisty C, Westwood M, Walker M, Moon J. Ultrafast magnetic resonance imaging for iron quantification in thalassemia participants in the developing world: the TIC‐TOC Study (Thailand and UK International Collaboration in Thalassaemia Optimising Ultrafast CMR). Circulation. 2016;134:432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. INEN: Instituto Nacional Enfermedades neoplasicas 2012, Tarifario. 2012. Available at: http://www.inen.sld.pe/portal/documentos/pdf/tarifario_institucional/31012012_Tarif_Instit_2012.pdf. Accessed January 31, 2012.
- 15. Alcalde‐Rabanal JE, Lazo‐González O, Nigenda G. The health system of Peru. Salud Publica Mex. 2011;53:243–254. [PubMed] [Google Scholar]
- 16. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Alain A, Lafont A, Limongelli G, McKenna W, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper P, Pieske B, Rapezzi C, Rutten F, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voor AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzales‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope L, Ruschitzka F, Rutten FH, van der Meer P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 18. Biesbroek PS, Hirsch A, Zweerink A, Van de Ven PM, Beek AM, Groenik M, Windhausen F, Planken RN, van Rossum AC, Nijveldt R. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur Heart J Cardiovasc Imaging. 2017. Available at: https://academic.oup.com/ehjcimaging/advance-article-abstract/doi/10.1093/ehjci/jex308/4658823?redirectedFrom=fulltext. Accessed August 2, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Baroni M, Nava S, Quattrocchi G, Milazzo A, Giannattasio C, Roghi A, Pedrotti P. Role of cardiovascular magnetic resonance in suspected cardiac amyloidosis: late gadolinium patter as mortality predictor. Neth Heart J. 2018;26:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greulich S, Deluigi C, Glokler S, Wahl A, Zurn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein K, Gawaz M, Sechtem U, Bruder O, Mahrholdt H. CMR, imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. [DOI] [PubMed] [Google Scholar]
- 21. Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JM, Kellman P, Cook SC, Schwartzman DS, Simon MA, Mulukutla SR, Schelbert EB. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. J Cardiovasc Magn Reson. 2013;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuruvilla S, Adenaw N, Katwal AB, Lipinsi MJ, Kramer CM, Salermo M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systemic review and meta‐analysis. Circ Cardiovasc Imaging. 2014;7:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pontone G, Guaricci AI, Andreini D, Ferro G, Guglielmo M, Baggiano A, Fusini L, Muscogiuri G, Lorenzoni V, Mushtaq S, Conte E, Annoni A, Formenti A, Mancini ME, Carita P, Verdecchia M, Pical S, Fazzari F, Cosentino N, Marenzi G, Rabbat MG, Agostoni P, Bartorelli AL, Pepi M, Masci PG. Prognostic stratification of patients with ST‐segment–elevation myocardial infarction (PROSPECT). A cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2017;10:1–11. [DOI] [PubMed] [Google Scholar]
- 24. Pontone G, Guaricci AI, Andreini D, Solbiati A, Guglielmo M, Mushtaq S, Baggiano A, Beltrama V, Fusini L, Rota C, Segurini C, Conte E, Gripari P, Dello Russo A, Moltrasio M, Tundo F, Lombardi F, Muscogiuri G, Lorenzoni V, Tondo C, Agostoni P, Bartorelli Al, Pepi M. Prognostic benefit of cardiac magnetic resonance over transthoracic echocardiography for the assessment of ischemic and nonischemic dilated cardiomyopathy patients referred for the evaluation of primary prevention implanted cardioverter‐defibrillator therapy. Circ Cardiovasc Imaging. 2016;9:1–12. [DOI] [PubMed] [Google Scholar]
- 25. Pennell DJ, Udelson J, Arai AE, Bozkurt B, Cohen AR, Galanello R, Hoffman TM, Kiernan MS, Lerakis S, Piga A, Porter JB, Walker JM, Wood J. Cardiovascular function and treatment in beta‐thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128:281–308. [DOI] [PubMed] [Google Scholar]
- 26. Obakata M, Nagata Y, Wu VC, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging. 2016;17:525–532. [DOI] [PubMed] [Google Scholar]
- 27. Yoon YE, Kang SH, Choi HM, Jeong S, Sung JM, Lee SE, Choi I, Cho GY, Chang HJ, Chun EJ. Prediction of infarct size and adverse cardiac outcomes by tissue tracking‐ cardiac magnetic resonance imaging in ST segment elevation myocardial infarction. Eur Radiol. 2018;28:3454–3463. [DOI] [PubMed] [Google Scholar]
- 28. Arenja N, Fritz T, Andre F, Riffel J, Aus dem Siepen F, Ochs M, Paffhausen J, Hegenbart U, Schonland S, Muller‐Hennessen M, Giannitsis E, Kristen A, Katus HA, Friedrich MG, Buss SJ. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images‐reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur Heart J Cardiovasc Imaging. 2017;18:1414–1422. [DOI] [PubMed] [Google Scholar]
- 29. Arenja N, Riffel J, Fritz T, Andre F, Siepen F, Mueller‐Hennessen M, Giannitsis E, Katus HA, Friedrich MG, Buss SJ. Diagnostic and prognostic value of longitudinal axis strain and myocardial contraction fraction using standard cardiovascular magnetic resonance imaging in patients with non‐ischaemic dilated cardiomyopathies. Radiology. 2017;283:681–691. [DOI] [PubMed] [Google Scholar]
- 30. Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, Nothnagel D, Steen H, Petersen S, Nagel E, Prasad S, Schumm J, Greulich S, Cagnolo A, Monney P. European Cardiovascular Magnetic Resonance (EuroCMR) registry—multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwong RY, Petersen SE, Schulz‐Menger J, Arai E, Bingham E, Chen Y, Choi YL, Cury C, Ferreira VM, Flamm SD, Steel K, Bandettini WP, Martin ET, Nallamshetty L, Neubauer S, Raman SV, Schelbert EB, Valeti US, Cao JJ, Reichek N, Young AA, Fexon L, Pivovarov M, Ferrari VA, Simonetti OP. The global cardiovascular magnetic resonance registry (GCMR) of the society for cardiovascular magnetic resonance (SCMR): its goals, rationale, data infrastructure, and current developments. J Cardiovasc Magn Reson. 2017;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mavrogeni S, Bratis K, Papadopoulos G, Terrovitis J, Kitsiou A, Kattamis A, Papavassillio A, Ageli K, Kolovou G. The Greek cardiac magnetic resonance experience: a comparison with the EuroCMR Registry. Hellenic J Cardiol. 2013;54:355–361. [PubMed] [Google Scholar]
- 33. Kramer CM, Barkhausen J, Flamm SD, Kim R, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:15–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pontone G, Andreini D, Guaricci AI, Rota C, Guglielmo M, Mushtaq S, Baggiano A, Beltram V, Fusini L, Solbiati A, Segurini C, Conte E, Gripari P, Annoni A, Formenti A, Petulla M, Lombardi F, Muscogiuri G, Bartorelli AL, Pepi M. The STRATEGY Study (Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Re‐Vascularized Patients): resources and outcomes impact. Circ Cardiovasc Imaging. 2016;9:1–11. [DOI] [PubMed] [Google Scholar]
- 35. Greenwood JP, Maredia N, Younger JF, Brown JM, Noxon J, Everett CC, Bijsterveld P, Ridway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single‐photon emission computed tomography for diagnosis of coronary artery heart disease (CE‐MARC): a prospective trial. Lancet. 2012;379:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagel E. MR‐INFORM: stress perfusion imaging to guide the management with stable coronary artery disease. Paper presented at American College of Cardiology Annual Scientific Session ACC 2017 March 17th; Washington, USA. [Google Scholar]
- 37. Wollny G, Kellman P. Free breathing myocardial perfusion data sets for performance analysis of motion compensation algorithms. Gigascience. 2014;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


