Abstract
Background
Infective endocarditis (IE) after transcatheter aortic valve replacement is a devastating complication associated with a high mortality. Our objective was to determine the impact of cardiac surgery (CS) and antibiotics (IE‐CS) compared with medical treatment with antibiotics only (IE‐ABx) on 1‐year mortality in patients developing IE after transcatheter aortic valve replacement.
Methods and Results
Patients developing IE after transcatheter aortic valve replacement were included in this retrospective analysis. All‐cause 1‐year mortality was the primary end point. A total of 20 patients underwent IE‐CS compared with 44 patients treated by IE‐ABx. In this unmatched cohort, patients treated by IE‐ABx were older (P=0.006), had a higher Society of Thoracic Surgeons score (P=0.029), and more often had severe chronic kidney disease (P=0.037). One‐year mortality was not different between groups (IE‐CS versus IE‐ABx, 65% versus 68.2%; P=0.802). The rate of any complication during treatment was higher in the IE‐CS group (P=0.024). In a matched cohort, baseline characteristics were not significantly different. All‐cause 1‐year mortality was not different between groups (IE‐CS versus IE‐ABx, 65% versus 75%; P=0.490). A Cox regression analysis revealed any indication for surgery (hazard ratio, 6.20; 95% confidence interval, 1.80–21.41; P=0.004), sepsis on admission (hazard ratio, 4.03; 95% confidence interval, 1.97–8.24; P<0.001), and mitral regurgitation ≥2 (hazard ratio, 2.91; 95% confidence interval, 1.33–6.37) as factors associated with 1‐year mortality.
Conclusions
In patients developing IE after transcatheter aortic valve replacement, mortality was predicted by the severity of IE and concomitant mitral regurgitation. In this small, and therefore statistically limited, but high‐risk patient cohort, CS provided no significant mortality benefit compared with medical therapy. Individual decision making by a “heart and endocarditis team” is necessary to offer those patients the most reasonable treatment option.
Keywords: antibiotic, cardiac valvular surgery, infective endocarditis, outcome, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation
Clinical Perspective
What Is New?
In infective endocarditis after TAVR, which develops in 0.67% to 3.4% per patient year, both cardiac surgery and antibiotics only are associated with a devastating high 1‐year mortality without a statistically significant advantage of one treatment form over the other in this small high‐risk patient group.
Mortality was predicted by the severity of infective endocarditis and concomitant mitral regurgitation but not the treatment form.
What Are the Clinical Implications?
Individual decision making by a “heart and endocarditis team” is necessary to offer those patients the most reasonable treatment option.
A future randomized study will have to determine the role of cardiac surgery in those high‐risk patients.
Introduction
Infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) occurs in 0.67% to 3.4% of patients per patient year.1, 2, 3, 4, 5, 6, 7 According to a recent multicenter registry, it is associated with an in‐hospital mortality of 41.8% and a 2‐year mortality rate of 66.7%,7 which is ≈2‐fold higher compared with contemporary surgical cohorts with prosthetic valve endocarditis (PVE).8 In those cohorts, surgery is performed in 50% of cases8 in contrast to the 10.8% rate of surgical valve explantation observed in the multicenter registry,7 despite a high rate (>80%) of patients with at least 1 indication for surgery, according to current guidelines.9 Early surgery in patients with native valve endocarditis and severe valve dysfunction or large vegetations reduces the risk of the combined end point of in‐hospital death and embolic events.10 In PVE, the best therapeutic option is still debated, with studies showing improved survival after early valve replacement11 and those showing no benefit of surgery compared with medical treatment after adjustment for differences in clinical characteristics and survival bias.12 Treatment of high‐risk patients undergoing TAVR developing IE is much more uncertain and data are rare. Surgery during initial hospitalization for IE did not reduce in‐hospital mortality in the Infectious Endocarditis After TAVR International Registry.7
The aim of this matched analysis was to evaluate the impact of cardiac surgery (CS) and antibiotics (IE‐CS) compared with medical treatment with antibiotics only (IE‐ABx) on 1‐year mortality in patients developing IE after TAVR.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Cohort and Definitions
Consecutive patients receiving TAVR between June 2008 and April 2017 and afterwards developing IE, which was treated in our tertiary center, were included this analysis. Diagnosis of endocarditis was verified applying the modified Duke criteria.9 The main inclusion criterion was the echocardiographic evidence of IE with or without the evidence of continuous bacteremia in at least 3 consecutive blood cultures, with the first and last sample taken ≥1 hour apart, or in 2 blood samples drawn >12 hours apart (Figure 1).
Figure 1.
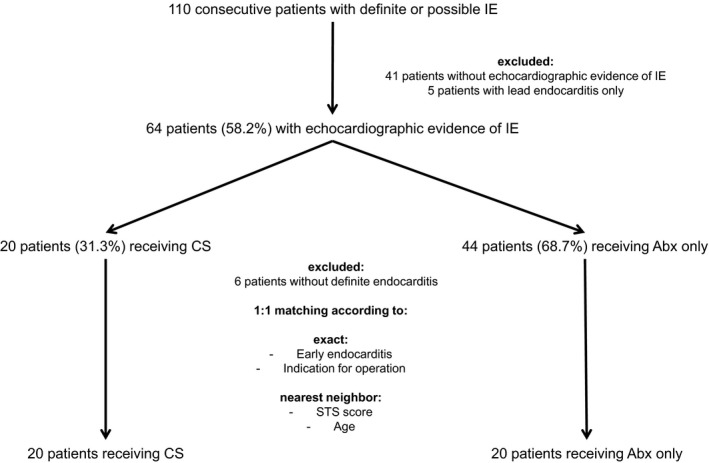
Flowchart of the study showing the formation of the unmatched and matched cohort. ABx indicates antibiotics only; CS, cardiac surgery; IE, infective endocarditis; STS, Society of Thoracic Surgeons.
Decision to perform surgery or not was made after careful discussion of all findings among the members of the TAVR heart team and the “endocarditis team,” including cardiologists, cardiac surgeons, imaging specialists, and microbiologists.
Definite and possible IE were classified according to current guidelines.9 Early and late endocarditis were defined by occurring within the first year and >1 year after TAVR, respectively.
Nosocomial infection was defined as IE developing in a patient hospitalized for >48 hours before the onset of signs or symptoms consistent with IE. Healthcare‐associated infection was defined as IE diagnosed within 48 hours of admission in an outpatient with extensive healthcare contact, as reflected by any of the following criteria: (1) received intravenous therapy, wound care, or specialized nursing care at home within the 30 days before the onset of IE; (2) attended a hospital or hemodialysis clinic or received intravenous chemotherapy within the 30 days before the onset of IE; or (3) resided in a nursing home or long‐term care facility.8
Chronic obstructive lung disease and peripheral artery disease were diagnosed according to the logistic EuroScore I definitions.13 Chronic kidney disease was defined according to Kidney Disease: Improving Global Outcomes.14
Clinical, microbiological, and imaging findings as well as treatment options and outcome of patients diagnosed with IE were retrospectively collected.
The study was approved by the Ethics Committee of the University of Leipzig (registration no. 047‐17‐23012017), and patients gave written informed consent.
End Points
All‐cause 1‐year mortality (after diagnosis of IE) was the primary end point of this analysis. In‐hospital mortality was a secondary end point. Complication rates during IE treatment were collected and defined as follows: (1) Need for hemodialysis: any need for hemodialysis during IE hospitalization attributable to oliguria/anuria, hyperkalemia, hypervolemia, metabolic acidosis, uremic symptoms, or elevated blood urea concentration. (2) Cerebral embolism: every new cerebral lesion with or without neurological symptoms in computed tomography and/or magnetic resonance imaging. (3) Peripheral embolism: every new embolism in a peripheral organ (eg, spleen or kidney) with or without clinical symptoms detected by appropriate imaging modalities. (4) Limb ischemia: clinically relevant limb ischemia attributable to embolism, arterial obstructive disease, or other mechanisms. (5) Bowel ischemia: acute bowel ischemia attributable to arterial embolism or venous thrombosis detected clinically or by computed tomography and/or explorative laparotomy. (6) Cardiopulmonary resuscitation: any need for mechanical cardiopulmonary resuscitation. (7) Transfusion: need for transfusion of >6 packed red blood cells within 24 hours. (8) Acute respiratory distress syndrome: acute respiratory distress syndrome according to current definitions.15 (9) Low cardiac output: any clinical condition that is caused by a transient decrease in systemic perfusion secondary to myocardial dysfunction. (10) Critical illness polyneuropathy/myopathy: severe limb and respiratory muscle weakness caused by damage to sensorimotor axons and skeletal muscles. (11) Seizures: any focal or generalized seizure detected clinically or by electroencephalogram.
Statistical Analysis
Numbers (percentages) are given for categorical variables, and mean±SD and median (25th–75th percentile) are given for continuous variables. The effect measures standardized mean difference and odds ratio, together with their 95% confidence interval (CI), were calculated before and after matching.
Frequencies were compared by χ2 test or Fisher's exact test, as appropriate. Groups were compared with respect to continuous variables by means of the Wilcoxon‐Mann‐Whitney U test.
Twenty patients of the IE‐ABx group were manually matched 1:1 with 20 patients of the IE‐CS group by the variables early endocarditis, any indication for operation, and nearest neighbor, according to Society of Thoracic Surgeons score and age. The effect sizes between the matched groups were compared with those in the unmatched ones to check balance.
In‐hospital mortality and all‐cause 1‐year mortality were calculated by the Kaplan‐Meier method, applying the log‐rank test for group comparison. Time do death was censored at December 15, 2017, or 1 year, whichever was earlier. Standard Cox regression was performed for the unmatched cohort, and conditional Cox regression was performed for the matched cohort. Looking for covariates multiply associated with 1‐year mortality, we calculated bivariate Cox regression in the unmatched cohort. Variables with P<0.05 were included in blockwise stepwise backward Cox regression. A combined model with the remaining variables of all blocks was reduced by backward selection. Further nonsignificant and nonrelevant variables were excluded to get a final model.
Significance was accepted as P<0.05. All analysis was performed with the use of SPSS, version 22 (IBM, Armonk, NY).
Results
Characteristics and Outcome of the Unmatched Population
We identified 64 patients meeting the inclusion criterion of IE with echocardiographic evidence of vegetation, abscess, and/or new dehiscence of the prosthetic or another valve. Twenty patients (31.3%) were referred to surgery (IE‐CS), and 44 patients (68.7%) were treated with antibiotics only (IE‐ABx) (Figure 1).
Baseline, IE‐associated, and TAVR characteristics of the unmatched cohort are summarized in Table 1 and Table S1. Compared with patients in the IE‐CS group, those in the IE‐ABx group were older (P=0.006), had a higher Society of Thoracic Surgeons score for reoperation (P=0.029), more often had severe chronic kidney disease (P=0.037), and more often had received a self‐expandable valve (P=0.013) via a transfemoral approach (P=0.026). IE‐related features were balanced between groups, except for initial symptoms, with a higher rate of sepsis (P=0.044) and a lower rate of patients having at least 1 formal indication for operation in the IE‐ABx group (P=0.047) (Table 1 and Table S2).
Table 1.
Baseline Characteristics of the Unmatched Cohort
| Characteristics | IE‐CS (n=20) | IE‐ABx (n=44) | OR or SMD (95% CI) | P Value |
|---|---|---|---|---|
| Baseline | ||||
| Age, y | 77.3±5.1 | 81.5±5.7 | 0.71 (0.20 to 1.30) | 0.006 |
| Male sex | 13/20 (65.0) | 25/44 (56.8) | 0.71 (0.20 to 2.38) | 0.537 |
| Body mass index, kg/m² | 28.2 (24.4–33.1) | 28.2 (24.1–30.4) | −0.14 (−0.43 to 0.27) | 0.454 |
| STS score, % | 17.2 (9.7–21.6) | 23.3 (14.6–35.0) | 0.53 (0.06 to 1.08) | 0.029 |
| NYHA class III/IV | 16/20 (80.0) | 30/41 (73.2) | 0.65 (0.13 to 2.62) | 0.754 |
| CAD | 8/20 (40.0) | 24/44 (54.5) | 1.78 (0.54 to 1.63) | 0.281 |
| Diabetes mellitus | 9/20 (45.0) | 21/44 (47.7) | 1.11 (0.34 to 3.72) | 0.839 |
| Atrial fibrillation | 14/20 (70.0) | 27/43 (62.8) | 0.73 (0.19 to 2.54) | 0.576 |
| Previous stroke | 2/20 (10.0) | 4/44 (9.1) | 0.64 (0.13 to 3.49) | 0.712 |
| PAD | 3/20 (15.0) | 13/44 (29.5) | 2.35 (0.54 to 14.62) | 0.213 |
| COPD | 8/20 (40.0) | 10/44 (22.7) | 0.45 (0.12 to 1.63) | 0.154 |
| CKD stage ≥3b | 7/20 (35.0) | 26/41 (63.4) | 3.15 (0.93 to 11.62) | 0.037 |
| Immunosuppressive therapy | 2/20 (10.0) | 9/44 (20.5) | 2.29 (0.41 to 23.97) | 0.479 |
| LV ejection fraction, % | 53±13 | 54±12 | 0.06 (−0.29 to 0.53) | 0.63 |
| Pressure mean aortic valve prosthesis, mm Hg | 13 (7–21) | 10 (7–19) | −0.09 (−0.43 to 0.40) | 0.677 |
| AR ≥2 | 4/19 (21.1) | 1/40 (2.5) | 0.10 (0.00 to 1.12) | 0.033 |
| MR ≥2 | 5/19 (26.3) | 6/40 (15.0) | 0.50 (0.11 to 2.44) | 0.308 |
| TR ≥2 | 3/19 (15.8) | 6/39 (15.4) | 0.97 (0.18 to 6.77) | 1.000 |
| Endocarditis features | ||||
| Definite endocarditis | 20/20 (100) | 38/44 (86.4) | NA | 0.165 |
| Early endocarditis | 12/20 (60.0) | 32/44 (72.7) | 1.76 (0.49 to 6.17) | 0.309 |
| Time from TAVR, d | 233 (60–578) | 139 (23–412) | −0.21 (−0.46 to 0.16) | 0.252 |
| Nosocomial/health care associated | 8/20 (40.0) | 18/44 (40.9) | 1.04 (0.31 to 3.57) | 0.945 |
| Initial symptoms | ||||
| Predisposition | 20/20 (100) | 44/44 (100) | NA | NA |
| Fever >38.0°C | 18/20 (90.0) | 36/43 (83.7) | 0.57 (0.11 to 3.04) | 0.706 |
| Vascular phenomena | 5/20 (25.0) | 7/44 (15.9) | 0.57 (0.03 to 2.07) | 0.492 |
| Heart failure | 13/20 (65.0) | 25/43 (58.1) | 0.75 (0.21 to 2.53) | 0.604 |
| Sepsis | 4/20 (20.0) | 20/43 (46.5) | 3.41 (0.90 to 16.36) | 0.044 |
| Indication for cardiac surgery | 19/20 (95.0) | 31/43 (72.1) | 0.14 (0.00 to 1.09) | 0.047 |
| Microbiological findings, n (%) | ||||
| All Staphylococcus | 8/20 (40.0) | 15/44 (34.1) | 0.78 (0.23 to 2.71) | 0.648 |
| Coagulase‐positive Staphylococcus | 6/20 (30.0) | 11/44 (25.0) | 0.78 (0.24 to 2.52) | 0.675 |
| Coagulase‐negative Staphylococcus | 2/20 (10.0) | 4/44 (9.1) | 0.90 (0.15 to 5.37) | 1.000 |
| Enterococcus | 8/20 (40.0) | 16/44 (36.4) | 0.86 (0.29 to 2.54) | 0.781 |
| Streptococcus | 3/20 (15.0) | 4/44 (9.1) | 0.57 (0.11 to 2.81) | 0.483 |
| Fungi | 1/20 (5.0) | 1/44 (2.3) | 0.44 (0.03 to 7.44) | 0.531 |
| Others | 0/20 (0) | 3/44 (6.8) | NA | 0.546 |
| BCNIE | 0/20 (0) | 5/44 (11.4) | NA | 0.314 |
| Echocardiographic findings | ||||
| PVE±other valve/lead | 19/20 (95.0) | 34/44 (77.4) | 0.18 (0.02 to 1.51) | 0.150 |
| Periannular abscess | 7/20 (35.0) | 11/44 (25.0) | 0.62 (0.20 to 1.94) | 0.410 |
| Other valve, no PVE | 1/20 (5.0) | 7/44 (15.9) | 3.60 (0.41 to 31.39) | 0.417 |
| Other valve+lead, no PVE | 0/20 (0) | 3/44 (6.8) | NA | 0.546 |
Variables are expressed as numbers/totals (percentages), means±SDs, or medians (25th–75th percentiles), as appropriate. Treatment effect is given as OR and SMD. AR indicates aortic regurgitation; BCNIE, blood culture–negative infective endocarditis; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive lung disease; IE‐ABx, infective endocarditis treated by antibiotics only; IE‐CS, infective endocarditis treated by cardiac surgery (and antibiotics); LV, left ventricular; MR, mitral regurgitation; NA, not applicable; NYHA, New York Heart Association; OR, odds ratio; PAD, peripheral artery disease; PVE, prosthetic valve endocarditis; SMD, standardized mean difference; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; TR, tricuspid regurgitation.
The outcome of the unmatched cohort is summarized in Table 2. In‐hospital (IE‐CS versus IE‐ABx, 50% versus 50%; P=1.000) and 1‐year (IE‐CS versus IE‐ABx, 65% versus 68.2%; P=0.802) mortality did not differ between groups (Figure 2A). In the particular group of patients with periannular abscess, in‐hospital (IE‐CS versus IE‐ABx, 57.1% versus 72.7%; P=0.627) and 1‐year (IE‐CS versus IE‐ABx, 71.4% versus 81.8%; P=1.000) mortality was not different. The rate of any complication was higher among patients in the IE‐CS group compared with those in IE‐ABx group (P=0.024), in particular with a higher rate of >6 red packed blood cells within 24 hours (P=0.003) and seizures (P=0.009).
Table 2.
Outcome of the Unmatched Cohort
| Outcome | IE‐CS (n=20) | IE‐ABx (n=44) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Mortality | ||||
| In‐hospital mortality | 10/20 (50.0) | 22/44 (50.0) | 1.00 (0.35–2.88) | 1.000 |
| 1‐y mortality | 13/20 (65.0) | 30/44 (68.2) | 1.15 (0.38–3.52) | 0.802 |
| Complications during IE treatment | ||||
| Any complication | 17/20 (85.0) | 24/43 (55.8) | 0.22 (0.06–0.88) | 0.024 |
| Need for hemodialysis | 9/20 (45.0) | 10/43 (23.3) | 0.37 (0.12–1.15) | 0.08 |
| Cerebral embolism | 3/20 (15.0) | 3/42 (7.1) | 0.44 (0.08–2.38) | 0.377 |
| Peripheral embolism | 4/20 (20.0) | 7/42 (16.7) | 0.80 (0.21–3.13) | 0.735 |
| Limb ischemia | 2/20 (10.0) | 0/42 (0) | 0.30 (0.20–0.44) | 0.100 |
| Bowel ischemia | 1/20 (5.0) | 1/42 (2.4) | 0.46 (0.03–7.81) | 0.545 |
| CPR | 4/20 (20.0) | 3/42 (7.1) | 0.31 (0.06–1.53) | 0.199 |
| >6 RBCs within 24 h | 6/20 (30.0) | 1/42 (2.4) | 0.06 (0.01–0.52) | 0.003 |
| ARDS | 1/20 (5.0) | 0/42 (0) | 0.31 (0.21–0.45) | 0.323 |
| LCO | 4/20 (20.0) | 3/42 (7.1) | 0.31 (0.06–1.53) | 0.199 |
| Critical illness PNP | 1/20 (5.0) | 1/42 (2.4) | 0.46 (0.03–7.81) | 0.545 |
| Seizures | 4/20 (20.0) | 0/42 (0) | 0.28 (0.18–0.42) | 0.009 |
Variables are expressed as numbers/totals (percentages). Treatment effect is given as OR. ARDS indicates acute respiratory distress syndrome; CI, confidence interval; CPR, cardiopulmonary resuscitation; IE, infective endocarditis; IE‐ABx, IE treated by antibiotics only; IE‐CS, IE treated by cardiac surgery (and antibiotics); LCO, low cardiac output syndrome; OR, odds ratio; PNP, polyneuropathy; RBC, red packed blood cell.
Figure 2.
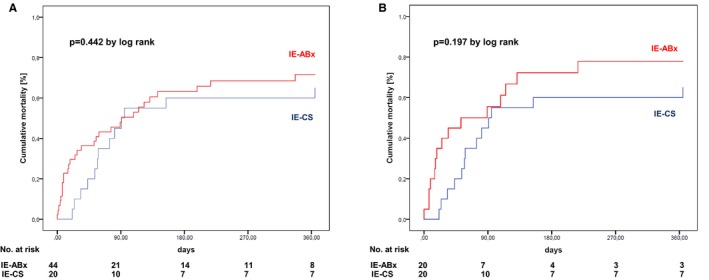
Time‐to‐event curves showing all‐cause 1‐year mortality in the unmatched (A) and matched (B) cohorts. IE‐ABx indicates infective endocarditis treated by antibiotics only; IE‐CS, infective endocarditis treated by cardiac surgery (and antibiotics).
Characteristics and Outcome of the Matched Population
Table 3 and Tables S2 and S3 summarize baseline, IE‐associated, and TAVR characteristics after matching 20 patients in the IE‐ABx group to the 20 patients in the IE‐CS group. More important, all patients had definite endocarditis, and at least 1 formal indication for operation was given in 95% of the patients in each group. According to the P value, all baseline and IE‐associated parameters were well balanced between the IE‐CS and IE‐ABx groups. However, the odds ratios and standardized mean differences still revealed differences between the groups, in particular in regard to severe chronic kidney disease and sepsis at initial presentation.
Table 3.
Baseline Characteristics of the Matched Cohort
| Characteristics | IE‐CS (n=20) | IE‐ABx (n=20) | OR or SMD (95% CI) | P Value |
|---|---|---|---|---|
| Baseline | ||||
| Age, y | 77.3±5.1 | 79.4±5.4 | 0.32 (−0.17 to 0.96) | 0.214 |
| Male sex | 13/20 (65.0) | 12/20 (60.0) | 0.81 (0.18 to 3.49) | 0.744 |
| Body mass index, kg/m² | 28.2 (24.4–33.1) | 28.9 (24.4–36.4) | 0.04 (−0.36 to 0.62) | 0.866 |
| STS score, % | 17.2 (9.7–21.6) | 17.6 (13.7–30.0) | 0.29 (−0.19 to 0.92) | 0.267 |
| NYHA class III/IV | 16/20 (80.0) | 13/20 (65.0) | 0.59 (0.10 to 3.10) | 0.288 |
| CAD | 8/20 (40.0) | 10/20 (50.0) | 1.48 (0.29 to 5.16) | 0.525 |
| Diabetes mellitus | 9/20 (45.0) | 11/20 (55.0) | 1.48 (0.36 to 6.20) | 0.527 |
| Atrial fibrillation | 14/20 (70.0) | 12/20 (60.0) | 0.65 (0.14 to 2.86) | 0.507 |
| Previous stroke | 2/20 (20.0) | 1/20 (5.0) | 0.45 (0.04 to 3.66) | 1.000 |
| PAD | 3/20 (15.0) | 4/20 (20.0) | 1.40 (0.20 to 11.11) | 1.000 |
| COPD | 8/20 (40.0) | 4/20 (20.0) | 0.38 (0.07 to 1.86) | 0.168 |
| CKD stage ≥3b | 7/20 (35.0) | 12/20 (60.0) | 2.71 (0.65 to 12.20) | 0.113 |
| Immunosuppressive therapy | 2/20 (10.0) | 4/20 (20.0) | 2.21 (0.27 to 27.48) | 0.661 |
| LV ejection fraction, % | 53±13 | 51±13 | −0.13 (−0.46 to 0.39) | 0.725 |
| Pressure mean aortic valve prosthesis, mm Hg | 13 (7–21) | 10 (8–20) | −0.04 (−0.43 to 0.57) | 0.901 |
| AR ≥2 | 4/19 (21.1) | 0/19 (0) | NA | 0.105 |
| MR ≥2 | 5/19 (26.3) | 3/19 (15.8) | 0.53 (0.07 to 3.34) | 0.693 |
| TR ≥2 | 3/19 (15.8) | 2/18 (11.1) | 0.67 (0.05 to 6.74) | 1.000 |
| Endocarditis features | ||||
| Definite endocarditis | 20/20 (100) | 20/20 (100) | NA | NA |
| Early endocarditis | 12/20 (60.0) | 12/20 (60) | 1.00 (0.28 to 3.54) | 1.000 |
| Time from TAVR, d | 233 (60–578) | 198 (71–740) | 0.08 (−0.33 to 0.65) | 0.903 |
| Nosocomial/health care associated | 8/20 (40.0) | 6/20 (30.0) | 0.65 (0.14 to 2.86) | 0.507 |
| Initial symptoms | ||||
| Predisposition | 20/20 (100) | 20/20 (100) | NA | NA |
| Fever >38.0°C | 18/20 (90.0) | 16/20 (80.0) | 0.44 (0.07 to 2.76) | 0.661 |
| Vascular phenomena | 5/20 (25.0) | 6/20 (30.0) | 1.29 (0.32 to 5.18) | 0.723 |
| Heart failure | 13/20 (65.0) | 12/20 (60.0) | 0.81 (0.18 to 3.49) | 0.744 |
| Sepsis | 4/20 (20.0) | 8/20 (40.0) | 2.60 (0.54 to 14.76) | 0.168 |
| Indication for cardiac surgery | 19/20 (95.0) | 19/20 (95.0) | 1.00 (0.06 to 17.18) | 1.000 |
| Microbiological findings | ||||
| All Staphylococcus | 8/20 (40.0) | 10/20 (50.0) | 1.50 (0.43 to 5.25) | 0.525 |
| Coagulase‐positive Staphylococcus | 6/20 (30.0) | 7/20 (35.0) | 1.26 (0.33 to 4.73) | 0.736 |
| Coagulase‐negative Staphylococcus | 2/20 (10.0) | 3/20 (15.0) | 1.59 (0.24 to 10.70) | 1.000 |
| Enterococcus | 8/20 (40.0) | 7/20 (35.0) | 0.81 (0.22 to 2.91) | 0.744 |
| Streptococcus | 3/20 (15.0) | 2/20 (10.0) | 0.63 (0.09 to 4.24) | 1.000 |
| Fungi | 1/20 (5.0) | 0/20 (0) | NA | 1.000 |
| Others | 0/20 (0) | 1/20 (5.0) | NA | 1.000 |
| BCNIE | 0/20 (0) | 0/20 (0) | NA | NA |
| Echocardiographic findings | ||||
| PVE±other valve/lead | 19/20 (95.0) | 18/20 (90.0) | 0.47 (0.04 to 5.69) | 1.000 |
| Periannular abscess | 7/20 (35.0) | 8/20 (40.0) | 1.24 (0.34 to 4.46) | 0.744 |
| Other valve, no PVE | 1/20 (5.0) | 0/20 (0) | NA | 1.000 |
| Other valve+lead, no PVE | 0/20 (0) | 2/20 (10.0) | NA | 0.487 |
Variables are expressed as numbers/totals (percentages), means±SDs, or medians (25th–75th percentiles), as appropriate. Treatment effect is given as OR and SMD. AR indicates aortic regurgitation; BCNIE, blood culture–negative infective endocarditis; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive lung disease; IE‐ABx, infective endocarditis treated by antibiotics only; IE‐CS, infective endocarditis treated by cardiac surgery (and antibiotics); LV, left ventricular; MR, mitral regurgitation; NA, not applicable; NYHA, New York Heart Association; OR, odds ratio; PAD, peripheral artery disease; PVE, prosthetic valve endocarditis; SMD, standardized mean difference; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; TR, tricuspid regurgitation.
The outcome of the matched cohort is summarized in Table 4. In‐hospital (IE‐CS versus IE‐ABx, 50% versus 55%; P=0.752) and 1‐year (IE‐CS versus IE‐ABx, 65% versus 75%; P=0.490) mortality did not differ significantly between groups (Figure 2B). In the particular group of patients with periannular abscess, in‐hospital (IE‐CS versus IE‐ABx, 57.1% versus 42.9%; P=1.000) and 1‐year (IE‐CS versus IE‐ABx, 71.4% versus 71.4%; P=1.000) mortality was not different. The rate of any complication was still higher among patients in the IE‐CS group compared with those in the IE‐ABx group (P=0.077), in particular with a higher rate of >6 red packed blood cells within 24 hours (P=0.02).
Table 4.
Outcome of the Matched Cohort
| Outcome | IE‐CS (n=20) | IE‐ABx (n=20) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Mortality | ||||
| In‐hospital mortality | 10/20 (50.0) | 11/20 (55.0) | 1.22 (0.35–4.24) | 0.752 |
| 1‐y mortality | 13/20 (65.0) | 15/20 (75.0) | 1.62 (0.41–6.34) | 0.490 |
| Complications during IE treatment | ||||
| Any complication | 17/20 (85.0) | 12/20 (60.0) | 0.27 (0.06–1.21) | 0.077 |
| Need for hemodialysis | 9/20 (45.0) | 4/20 (20.0) | 0.31 (0.08–1.25) | 0.091 |
| Cerebral embolism | 3/20 (15.0) | 2/20 (10.0) | 0.63 (0.09–4.24) | 1.000 |
| Peripheral embolism | 4/20 (20.0) | 6/20 (30.0) | 1.71 (0.40–7.34) | 0.465 |
| Limb ischemia | 2/20 (10.0) | 0/20 (0) | NA | 1.000 |
| Bowel ischemia | 1/20 (5.0) | 0/20 (0) | NA | 1.00 |
| CPR | 4/20 (20.0) | 1/20 (5.0) | 0.21 (0.02–2.08) | 0.342 |
| >6 RBCs within 24 h | 6/20 (30.0) | 0/20 (0) | NA | 0.020 |
| ARDS | 1/20 (5.0) | 0/20 (0) | NA | 1.000 |
| LCO | 4/20 (20.0) | 2/20 (10.0) | 0.44 (0.07–2.76) | 0.661 |
| Critical illness PNP | 1/20 (5.0) | 0/20 (0) | NA | 1.000 |
| Seizures | 4/20 (20.0) | 0/20 (0) | NA | 0.106 |
Variables are expressed as numbers/totals (percentages). Treatment effect is given as OR. ARDS indicates acute respiratory distress syndrome; CI, confidence interval; CPR, cardiopulmonary resuscitation; IE, infective endocarditis; IE‐ABx, IE treated by antibiotics only; IE‐CS, IE treated by cardiac surgery (and antibiotics); LCO, low cardiac output syndrome; NA, not applicable; OR, odds ratio; PNP, polyneuropathy; RBC, red packed blood cell.
Operative Techniques
CS was performed with a median time of 17 days (25th–75th percentile, 4–35 days) after confirmation of IE diagnosis, all within the first IE hospitalization. It was the first reoperation in 19 patients (95%) and the second reoperation in 1 patient (5%) after a valve‐in‐valve procedure. Isolated valve replacement was performed in 6 patients (30%); in the remaining 14 patients (70%), additional procedures were mandatory, including coronary artery bypass grafting (n=3), pacemaker extraction (n=3), replacement of the ascending aorta (n=3), and reconstruction of the aortic root with a bovine pericardial patch (n=6). In addition, complex reconstruction of the intervalvular fibrous body was necessary in 2 patients because of excessive abscess formation. Two patients required aortic root replacement (ie, Bentall procedure). Double valve replacement/reconstruction was performed in 7 patients (35%).
Predictors of Mortality
Characteristics of patients with and without 1‐year mortality are shown in Table S4. Predictors of 1‐year mortality were evaluated in the unmatched population (Table S5). Factors associated with 1‐year mortality were any indication for surgery (hazard ratio [HR], 6.20; 95% CI, 1.80–21.41; P=0.004), sepsis on admission (HR, 4.03; 95% CI, 1.97–8.24; P<0.001), and mitral regurgitation ≥2 (HR, 2.91; 95% CI, 1.33–6.37). Therapy decision (IE‐CS versus IE‐ABx) did not affect 1‐year mortality (HR, 1.66; 95% CI, 0.77–3.59; P=0.196) (Table 5).
Table 5.
Factors Associated With 1‐Year Mortality
| Parameter | HR (95% CI) | P Value |
|---|---|---|
| Any indication for cardiac surgery | 6.20 (1.80–21.41) | 0.004 |
| Initial sepsis | 4.03 (1.97–8.24) | <0.001 |
| Mitral regurgitation ≥2 | 2.91 (1.33–6.37) | 0.008 |
| Antibiotics vs surgery | 1.66 (0.77–3.59) | 0.196 |
CI indicates confidence interval; and HR, hazard ratio.
Risk of Reendocarditis
After a first successful IE treatment, reendocarditis was observed in 2 patients in the IE‐CS group (20.0% of the patients surviving the first IE hospitalization), occurring after 71 and 454 days; and in 3 patients in the IE‐ABx group (13.6% of the patients surviving the first IE hospitalization), occurring after 210, 406, and 1117 days.
Discussion
This analysis compared the effect of CS during initial IE hospitalization with medical treatment on 1‐year mortality in patients developing IE after TAVR. The main findings of the study are as follows: (1) Neither an unadjusted nor an adjusted analysis revealed a statistically significant mortality benefit of CS compared with medical therapy in those high‐risk patients developing IE after TAVR. (2) Mortality was predicted by the severity of IE (eg, sepsis on admission or formal indication for CS) and concomitant mitral regurgitation (at the time of IE diagnosis) rather than by treatment choice.
Treatment options in IE after TAVR
The rate of CS in contemporary cohorts of patients with PVE is ≈50%,8, 12 which contrasts with the 10.8% rate of surgical valve explantation observed in the Infectious Endocarditis After TAVR International Registry.7 In this analysis, selected patients with echocardiographic evidence of IE were considered for CS in about one third of all cases, which is somewhat higher than in other studies in the field.3, 7 However, compared with 72% of patients having at least 1 indication for surgery, according to current guidelines,9 the rate of CS performed in this cohort is still low. This discrepancy is mainly caused by the high operative risk and age of the patients considered inoperable or at high surgical risk, even for the initial TAVR procedure. In the International Collaboration on Endocarditis–Prospective Cohort Study,12 patients with PVE were ≈60 years of age, which is 20 years younger than in our cohort. However, the decision to perform surgery or not was made by the same TAVR and endocarditis team during the whole study time, providing stability in personal judgement and readiness to assume risk. Noteworthy, the decision by the heart team might be an important bias in this analysis. However, typically in such a scenario, younger and “healthier” patients are sent to surgery, whereas older and “futile” patients are denied high‐risk and invasive procedures.
The in‐hospital and 1‐year mortality was high in our cohort but is comparable to the multicenter cohort of patients undergoing TAVR in the Infectious Endocarditis After TAVR International Registry.7 Compared with surgical patients with PVE, mortality rates are ≈2‐fold higher in our patient population.12 This might be explained by several patient‐ and disease‐related factors known to affect survival in IE: older age, high operative risk because of comorbidities, and a high rate of nosocomial/healthcare‐associated IE, with Staphylococcus as the main causative microorganism.16
Both unmatched and matched analyses revealed no significant difference between IE‐CS and IE‐ABx on in‐hospital and 1‐year mortality. This is comparable to the findings in the Infectious Endocarditis After TAVR International Registry,7 which stated that surgery during IE hospitalization was not associated with a reduced risk of in‐hospital death (29.7% for surgery versus 37.1% for no surgery; odds ratio, 0.72 [95% CI, 0.33–1.53]; P=0.39). However, no data beyond the initial IE hospitalization are available from this registry and, because it was not the aim of this registry, the analysis did not adjust for any difference in baseline and IE‐related factors.
In PVE after surgical valve replacement, the best therapeutic option is still debated, with nonrandomized studies showing improved survival after early valve replacement11 and those showing no benefit of surgery compared with medical treatment after adjustment for differences in clinical characteristics and survival bias.12 There is only 1 randomized study in patients with native valve endocarditis showing that early surgery (within 48 hours) compared with conventional treatment (77% of patients receiving surgery beyond 48 hours) reduced the risk of the composite end point of embolic events and in‐hospital mortality within 6 weeks, with no difference in all‐cause mortality after 6 months.10 In our study, the median time from diagnosis to CS was 17 days (25th–75th percentile, 4–35 days). This prolonged time period was caused by several factors, including transfer from another hospital, difficult decision making by both the patient and the treating physicians, and failure of an initial medical treatment approach. Those factors might have diminished the positive effects of CS.
No randomized clinical trials evaluating the role of CS in PVE have been performed. Therefore, treatment recommendations are only based on the conflicting results of the above mentioned studies.11, 12 Subgroup analyses show that surgery was beneficial in patients with the greatest need for surgery, including those with valve regurgitation, vegetation, and dehiscence or paravalvular abscess/fistula,9 reflecting the indications for CS in current guidelines.9 In our matched analysis, the need for CS according to current guidelines was equally distributed. Despite this, there was no strong signal for an improvement in 1‐year mortality.
In IE after TAVR, which is still a relatively new treatment option for patient with severe aortic stenosis, data about treatment options and predictors of mortality are still rare. In our analysis, prognosis was not determined by the choice of treatment (eg, CS versus ABx), but by disease characteristics (eg, sepsis on admission or a formal indication for CS). We interpret the latter one as a sign of disease severity rather than a factor implicating a certain therapy. The overall complication rate during IE treatment was significantly higher in IE‐CS, which may, in part, be explained by the necessity to perform an extensive surgical procedure. Hypothetically, this higher complication rate may have outweighed the potential benefit of cankerous tissue removal.
Potential Reasons for the Missing Mortality Benefit of CS
First, there are statistical reasons. This is an observational nonrandomized study in a small patient population treated in a single center. Patients were selected according to the echocardiographic evidence of IE. Patients with negative imaging did not undergo 18F‐fluorodeoxyglucose positron emission tomography/computed tomography and computed tomography angiography on a regular basis, leading to a potential bias of missing definite IE in those patients. In a former analysis,3 we were able to show that there was no difference in mortality between patients having echocardiographic evidence of IE and those with negative echocardiography (HR, 0.77; 95% CI, 0.32–1.88; P=0.576). Manual matching and multivariable testing were applied to adjust for relevant baseline and IE‐associated factors. However, because of the small sample size, adjustment was only possible for some parameters. Moreover, P values may not tell the whole truth in such a small cohort because there was a 10% absolute risk reduction by CS in the matched analysis and in the multivariate analysis; the HR was 1.66 (95% CI, 0.77–3.59) for ABx compared with CS. Last, but not least, the retrospective design of a registry renders it susceptible to confounders.
Second, the optimal time point of CS in PVE is uncertain and, as discussed above, the prolonged time from diagnosis to CS may have diminished the positive effects of surgery in the examined cohort.
Third, most of the patients were already at high risk for the initial TAVR procedure, with even more pronounced risk by developing IE afterwards. Therefore, projecting the future expansion to more low‐ and intermediate‐risk patients receiving TAVR, these results may not be transferable, and surgery could be an excellent option in low‐ and intermediate‐risk patients developing IE after TAVR. This is supported by the observation that there was a higher complication rate in IE‐CS compared with IE‐ABx. Hypothetically, the complication rate should be reduced in lower‐risk patients undergoing surgery.
Fourth, additional procedures (eg, coronary artery bypass grafting) prolong operation time and are associated with early mortality.17 Hypothetically, skipping procedures that are not absolutely necessary may be beneficial for patient outcome.
Overall, our results should be interpreted cautiously and in the understanding of hypothesis‐generating means. A future randomized multicenter study will have to determine the role of CS in patients developing IE after TAVR.
Conclusion
In patients developing IE after TAVR, mortality was predicted by the severity of IE and concomitant mitral regurgitation. In this small but high‐risk patient cohort, CS provided no statistically significant mortality benefit compared with medical therapy. Individual decision making by a “heart and endocarditis team” is necessary to offer those patients the most reasonable treatment option.
Sources of Funding
This work was supported by a research grant to Mangner, funded by the Leipzig Heart Institute.
Disclosures
Mangner reports speaker's honoraria from Edwards and Medtronic and consultant honoraria from Biotronik, outside the submitted work. Leontyev reports speaker's honoraria from St Jude Medical and Medtronic, outside the submitted work. M. Mende reports personal fees from Leipzig Heart Institute (Leipzig, Germany) during the conduct of the study. Borger reports personal fees from Edwards Lifesciences, personal fees from Medtronic, and personal fees from CryoLife, outside the submitted work. Holzhey reports speaker's honoraria from Symetis and Medtronic, outside the submitted work. Linke reports grants and personal fees from Medtronic, personal fees from St Jude Medical, grants from Claret Medical, personal fees and other from Claret Medical, personal fees from Boston Scientific, personal fees from Bard, and personal fees from Edwards, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1. Procedural Characteristics for TAVR in the Unmatched Cohort
Table S2. Different Indications for Cardiac Surgery in the Unmatched and Matched Cohort
Table S3. Procedural Characteristics for TAVR in the Matched Cohort
Table S4. Characteristics According to 1‐Year Mortality (Unmatched Cohort)
Table S5. Predictors of 1‐Year Mortality (Unmatched Cohort)
(J Am Heart Assoc. 2018;7:e010027 DOI: 10.1161/JAHA.118.010027.)
References
- 1. Amat‐Santos IJ, Messika‐Zeitoun D, Eltchaninoff H, Kapadia S, Lerakis S, Cheema A, Gutierrez‐Ibanes E, Munoz‐Garcia A, Pan M, Webb JG, Herrmann H, Kodali S, Nombela‐Franco L, Tamburino C, Jilaihawi H, Masson JB, de Sandoli BF, Ferreira MC, Lima VC, Mangione JA, Iung B, Durand E, Vahanian A, Tuzcu M, Hayek SS, Angulo‐Llanos R, Gomez‐Doblas JJ, Castillo JC, Dvir D, Leon MB, Garcia E, Cobiella J, Vilacosta I, Barbanti M, Makkar R, Barbosa RH, Urena M, Dumont E, Pibarot P, Lopez J, San RA, Rodes‐Cabau J. Infective endocarditis following transcatheter aortic valve implantation: results from a large multicenter registry. Circulation. 2015;131:1566–1745. [DOI] [PubMed] [Google Scholar]
- 2. Latib A, Naim C, De Bonis M, Sinning JM, Maisano F, Barbanti M, Parolari A, Lorusso R, Testa L, Actis Dato GM, Miceli A, Sponga S, Rosato F, De Vincentiis C, Werner N, Fiorina C, Bartorelli A, Di Gregorio O, Casilli F, Muratori M, Alamanni F, Glauber M, Livi U, Nickenig G, Tamburino C, Alfieri O, Colombo A. TAVR‐associated prosthetic valve infective endocarditis: results of a large, multicenter registry. J Am Coll Cardiol. 2014;64:2176–2178. [DOI] [PubMed] [Google Scholar]
- 3. Mangner N, Woitek F, Haussig S, Schlotter F, Stachel G, Hollriegel R, Wilde J, Lindner A, Holzhey D, Leontyev S, Mohr FW, Schuler G, Linke A. Incidence, predictors, and outcome of patients developing infective endocarditis following transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2907–2908. [DOI] [PubMed] [Google Scholar]
- 4. Martinez‐Selles M, Bouza E, Diez‐Villanueva P, Valerio M, Farinas MC, Munoz‐Garcia AJ, Ruiz‐Morales J, Galvez‐Acebal J, Antorrena I, de la Hera Galarza JM, Navas E, Munoz P. Incidence and clinical impact of infective endocarditis after transcatheter aortic valve implantation. EuroIntervention. 2016;11:1180–1187. [DOI] [PubMed] [Google Scholar]
- 5. Olsen NT, De Backer O, Thyregod HG, Vejlstrup N, Bundgaard H, Sondergaard L, Ihlemann N. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e001939. [DOI] [PubMed] [Google Scholar]
- 6. Puls M, Eiffert H, Hunlich M, Schondube F, Hasenfuss G, Seipelt R, Schillinger W. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single‐centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention. 2013;8:1407–1418. [DOI] [PubMed] [Google Scholar]
- 7. Regueiro A, Linke A, Latib A, Ihlemann N, Urena M, Walther T, Husser O, Herrmann HC, Nombela‐Franco L, Cheema AN, Le BH, Stortecky S, Kapadia S, Bartorelli AL, Sinning JM, Amat‐Santos I, Munoz‐Garcia A, Lerakis S, Gutierrez‐Ibanes E, Abdel‐Wahab M, Tchetche D, Testa L, Eltchaninoff H, Livi U, Castillo JC, Jilaihawi H, Webb JG, Barbanti M, Kodali S, de Brito FSJ, Ribeiro HB, Miceli A, Fiorina C, Dato GM, Rosato F, Serra V, Masson JB, Wijeysundera HC, Mangione JA, Ferreira MC, Lima VC, Carvalho LA, Abizaid A, Marino MA, Esteves V, Andrea JC, Giannini F, Messika‐Zeitoun D, Himbert D, Kim WK, Pellegrini C, Auffret V, Nietlispach F, Pilgrim T, Durand E, Lisko J, Makkar RR, Lemos PA, Leon MB, Puri R, San RA, Vahanian A, Sondergaard L, Mangner N, Rodes‐Cabau J. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in‐hospital death. JAMA. 2016;316:1083–1092. [DOI] [PubMed] [Google Scholar]
- 8. Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Pare C, Almirante B, Munoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton‐Suty C, Jones SB, Casabe J, Morris A, Corey GR, Cabell CH. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354–1361. [DOI] [PubMed] [Google Scholar]
- 9. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del ZF, Dulgheru R, El KG, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska‐Gosciniak E, Price S, Roos‐Hesselink J, Snygg‐Martin U, Thuny F, Tornos MP, Vilacosta I, Zamorano JL. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 10. Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH, Yun SC, Song JM, Choo SJ, Chung CH, Song JK, Lee JW, Sohn DW. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. [DOI] [PubMed] [Google Scholar]
- 11. Kiefer T, Park L, Tribouilloy C, Cortes C, Casillo R, Chu V, Delahaye F, Durante‐Mangoni E, Edathodu J, Falces C, Logar M, Miro JM, Naber C, Tripodi MF, Murdoch DR, Moreillon P, Utili R, Wang A. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA. 2011;306:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante‐Mangoni E, Fowler VG Jr, Gordon D, Grossi P, Hannan M, Hoen B, Munoz P, Rizk H, Kanj SS, Selton‐Suty C, Sexton DJ, Spelman D, Ravasio V, Tripodi MF, Wang A. In‐hospital and 1‐year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013;173:1495–1504. [DOI] [PubMed] [Google Scholar]
- 13. Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–822. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De ZD, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 15. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 16. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893. [DOI] [PubMed] [Google Scholar]
- 17. Grubitzsch H, Tarar W, Claus B, Gabbieri D, Falk V, Christ T. Risks and challenges of surgery for aortic prosthetic valve endocarditis. Heart Lung Circ. 2018;27:333–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Procedural Characteristics for TAVR in the Unmatched Cohort
Table S2. Different Indications for Cardiac Surgery in the Unmatched and Matched Cohort
Table S3. Procedural Characteristics for TAVR in the Matched Cohort
Table S4. Characteristics According to 1‐Year Mortality (Unmatched Cohort)
Table S5. Predictors of 1‐Year Mortality (Unmatched Cohort)


