Abstract
Background
Dietary micronutrient deficiencies have been shown to predict event‐free survival in other countries but have not been examined in patients with heart failure living in the United States. The purpose of this study was to determine whether number of dietary micronutrient deficiencies in patients with heart failure was associated with shorter event‐free survival, defined as a combined end point of all‐cause hospitalization and death.
Methods and Results
Four‐day food diaries were collected from 246 patients with heart failure (age: 61.5±12 years; 67% male; 73% white; 45% New York Heart Association [NYHA] class III/IV) and analyzed using Nutrition Data Systems for Research. Micronutrient deficiencies were determined according to methods recommended by the Institute of Medicine. Patients were followed for 1 year to collect data on all‐cause hospitalization or death. Patients were divided according to number of dietary micronutrient deficiencies at a cut point of ≥7 for the high deficiency category versus <7 for the no to moderate deficiency category. In the full sample, 29.8% of patients experienced hospitalization or death during the year, including 44.3% in the high‐deficiency group and 25.1% in the no/moderate group. The difference in survival distribution was significant (log rank, P=0.0065). In a Cox regression, micronutrient deficiency category predicted time to event with depression, NYHA classification, comorbidity burden, body mass index, calorie and sodium intake, and prescribed angiotensin‐converting enzyme inhibitors, diuretics, or β‐blockers included as covariates.
Conclusions
This study provides additional convincing evidence that diet quality of patients with heart failure plays an important role in heart failure outcomes.
Keywords: diet, heart failure, nutrition, risk factor, survival analysis
Subject Categories: Diet and Nutrition, Heart Failure, Mortality/Survival
Clinical Perspective
What Is New?
High dietary micronutrient deficiency was a strong independent predictor of 1‐year hospitalization or death in a sample of patients from the United States.
Most common dietary nutrient deficiencies were in calcium, folate, magnesium, zinc, and vitamins C, D, E, and K.
Patients with comorbid depressive symptoms may be particularly vulnerable to dietary micronutrient deficiencies.
Patients with high body mass indexes were equally likely to have diets high in micronutrient deficiencies.
What Are the Clinical Implications?
Nutritional deficiency is an important risk factor for hospitalization and death in patients with heart failure.
Patients should be assessed for potential dietary deficiencies regardless of body mass index.
The wide range of micronutrient deficiencies suggests that a focus on promoting a varied diet is necessary to prevent patients having multiple micronutrient deficiencies.
Patients at high risk for dietary micronutrient deficiencies may benefit from consultation with a dietitian.
Introduction
Sodium has been the primary nutrient of interest in heart failure management for >60 years because of its relationship with fluid retention, symptoms, and hospitalizations.1, 2 Nevertheless, nutrition in heart failure is recognized to extend beyond sodium3 and may be equally important for management as drugs and devices.4 Accumulating evidence suggests that micronutrient deficiencies are common in patients with heart failure,5, 6, 7, 8, 9, 10 but less evidence shows the relationship of micronutrient deficiencies to heart failure outcomes.11, 12, 13 A recent study by Song and Kang14 demonstrated that micronutrient deficiencies independently predicted cardiac event‐free survival in patients with heart failure living in South Korea. Patients’ risk of hospitalization or death increased by 14% for each additional micronutrient that was deficient in the diet. The purpose of this study was to determine whether micronutrient deficiencies in patients with heart failure living in the United States were associated with similar risks.
Specific aims were (1) to determine dietary nutritional status based on 4‐day food diaries; (2) to examine associations between nutritional deficiencies and demographic and clinical variables; and (3) to assess whether the degree of nutritional deficiency was predictive of time to event (all‐cause hospitalization or death), controlling for demographic and clinical covariates.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure by the corresponding author upon reasonable request.
Study Design and Population
This multicenter longitudinal study included 274 patients with heart failure recruited from 3 heart failure clinics affiliated with academic medical centers located in Georgia, Indiana, and Kentucky. Inclusion criteria were (1) confirmed diagnosis of heart failure with either reduced or preserved ejection fraction, (2) New York Heart Association (NYHA) classification I–IV, (3) stability on medication regimen for 3 months before enrollment, (4) no history of hospitalization in the 3 months before enrollment, (5) ability to speak and write English, and (6) no obvious cognitive impairment. Exclusion criteria were (1) status 1 on a cardiac transplant list or the presence of a left ventricular assist device; (2) end‐stage kidney (on dialysis), liver, or pulmonary disease; (3) active cancer treatment or cancer likely to cause death within 12 months; and (4) any health condition that required dietary restrictions other than those for heart failure or diabetes mellitus. Eight participants withdrew and 14 participants whose average 4‐day kcal intake from food diary was <40% of estimated energy requirements were excluded from the analysis.10 Another 6 participants were omitted from this analysis because they were missing either days of follow‐up or a depressive symptoms score (ie, Beck Depression Inventory II [BDI‐II]). The final sample comprised 246 patients.
Measures
Demographic factors
Data on age, sex, and race were collected by patient interview. Body mass index (BMI) defined as weight in kilograms divided by height in square meters (kg/m2) was calculated for inclusion in the analysis.
Heart failure classification
The NYHA functional classification was used to summarize heart failure severity. This variable was dichotomized into III/IV versus I/II before analysis. NYHA functional class was determined by careful patient interview using a standardized form. Reproducibility both among different raters (interrater reliability) and for the same rater (intrarater reliability) at each site was ensured by training raters and testing them with sample patients until interrater agreement was 100%.
Prescription drugs
Prescribed medication regimens (ie drugs, doses, frequencies) were obtained from patient interview and medical record review to confirm patient report. Four indicator variables were created, one each for angiotensin‐converting enzyme inhibitor prescription (yes/no), angiotensin receptor blocker prescription (yes/no), diuretic prescription (yes/no), and β‐blocker prescription (yes/no).
Comorbidities
Comorbidity burden was determined by the Charlson Comorbidity Index interview format.15 Total scores could range from 1 to 34. Reliability and validity have been demonstrated.15, 16 The total comorbidity score was used in the analysis.
Depressive symptoms
The BDI‐II17, 18, 19 was used to assess level of depressive symptoms in the sample. This 21‐item instrument measures the degree of depressive symptoms, such as pessimism, agitation, and loss of energy, with most items scored on a 0 to 3 scale; 2 of the items are scored on a 0 to 6 scale and then rescaled to range from 0 to 3 before inclusion in the total. Higher scores on each item indicate greater depressive mood, and the total score is the sum of all 21 items. Scores >13 are indicative of clinically significant depressive symptoms.20 Cronbach's α for this sample is 0.91.
Laboratory values
Blood was drawn for NT‐proBNP (N‐terminal probrain natriuretic peptide), serum sodium, and uric acid during the baseline visit to the clinical research center at each site. Serum sodium and uric acid were measured at each participating site's hospital laboratory using standard laboratory methods. Blood for NT‐proBNP was shipped to the primary site. Plasma levels of NT‐proBNP were measured by the core laboratory at the primary site clinical research center using a commercially available ELISA kit. Assays were run in duplicate, and when the inter‐ and intra‐assay coefficients of variation (reflecting accuracy and reproducibility) were unacceptable (coefficient of variation >10%), the assay was redone.
Seattle Heart Failure Model
The Seattle Heart Failure Model score was included in the analysis to control for covariates associated with event‐free survival. The model score was calculated using the Windows version of the Seattle Heart Failure Model calculator for the web (https://depts.washington.edu/shfm/?width=1366&height=768) and included age, sex, ischemic origin of heart failure, systolic blood pressure, ejection fraction, medication (angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, aldosterone blocker, β‐blocker, statins, diuretic type and daily dose, and allopurinol), laboratory values (serum sodium, total cholesterol, hemoglobin, percent lymphocytes, and uric acid), body weight, and NYHA functional class. The presence of an implantable device (pacemaker, implantable cardioverter‐defibrillator, or cardiac resynchronization therapy) was also included.21 Default values for systolic blood pressure (120 mm Hg) and lymphocyte percentage (24%) were used because they were not collected at baseline. Data on presence of left bundle‐branch block and QRS >150 ms were also missing and subsequently not included in score calculation. Seattle Heart Failure Model scores are strong predictors of hosptializtion22 and both shorter (1‐year) and longer (2‐ and 3‐year) survival.23
Number of nutritional deficiencies
Nutritional intake averaged over the 4 days was determined using Nutritional Data Systems‐R software (Nutrition Coordinating Center, University of Minnesota). Dietary adequacy of 17 micronutrients (11 vitamins and 6 minerals) was defined according to recommendations outlined by the Food and Nutrition Board of the Institute of Medicine.24 For 7 micronutrients (magnesium, folate, vitamin B6, niacin, thiamin, riboflavin, and phosphorus), sufficient population‐based data were available for the Food and Nutrition Board to establish parameters to calculate a probability ratio that a patient's diet was habitually high or low in a micronutrient based on a 4‐day food diary. A ratio <−1 indicated a high probability that the patient's diet was habitually low in that micronutrient. For 8 micronutrients (calcium, iron, selenium, zinc, and vitamins B12, C, D, and E), diet adequacy was based on estimated average requirement because probability formulas have not been established. Micronutrient deficiency was defined as intake below the estimated average requirement for each micronutrient. For 2 vitamins (vitamin K and pantothenic acid), only the recommended daily allowance has been established. Diet deficiency for these micronutrients was defined as intake <50% of the recommended daily allowance for each micronutrient. The number of micronutrient deficiencies was summed. Because this variable was skewed to the right, we created a binary variable using the cut point of ≥7 for the high deficiency category. This value was chosen because 25% of the sample had a score at or above this level; this type of dichotomy has been used previously with skewed clinical predictors of heart failure outcomes.25 Participants with fewer micronutrient deficiencies were classified as no to moderate deficiency.
Event‐free survival
Patients kept a diary of all hospitalizations and were called monthly by a trained research assistant to obtain hospitalization data for 1 year following baseline data collection using a well‐validated life history method.26, 27 All hospitalizations were verified by medical record review. When patient recall varied from the medical records, the medical record was considered the gold standard. With patient or family assistance, hospital discharge and other medical records were obtained from identified sources of care outside of the study‐site healthcare systems. We have >20 years of experience collecting these data and have successfully obtained hospitalization data on all rural patients in our current and previous studies.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 All‐cause mortality data (date and cause of death) were collected for 1 year from baseline by a combination of medical record review, electronic clinical record review, interviews with patients’ healthcare providers and family, and public death record review.30, 31, 40, 41, 42, 43
Procedure
The study was approved by the institutional review board at each site before start of study. All participants gave signed informed consent before data collection, which occurred between June 2006 and March 2009. Patients were referred by nurse practitioners to a research assistant who verified eligibility. For those who agreed to participate, appointments were made by a trained research nurse to visit them in their homes to complete all questionnaires and to provide the equipment (food diary, digital food scale, food portion models, and food portion pictures) and detailed instructions for filling out the 4‐day food diary. Patients did a return demonstration so investigators could verify understanding and provide an opportunity to clarify procedures. Patients recorded all foods, liquids, and dietary supplements consumed for 1 weekend day and 3 weekdays. The research nurse called the morning of the first recording day to again verify understanding and answer any questions about recording the food diary. Patients who consumed packaged foods were instructed to bring the empty packages with them with the food diary. The morning following completion of food diary, patients visited the clinical research center at each site for measurement of height and weight and for review of the food diary. A dietician at each site carefully reviewed the food diaries with the patients, and spouses if appropriate, to clarify information regarding ingredients, verify portion sizes, and obtain any missing data (eg, preparation methods). The interviews averaged 30 to 45 minutes. The completed food diaries from all sites were entered into the Nutritional Data Systems‐Research software package (Nutrition Coordinating Center, Minneapolis, MN) at the primary site by a trained research assistant. Data entry was verified by the primary investigator (T.A.L.). Patients, or family members if patients were not available, were followed for 1 year by monthly phone calls to collect data on hospitalizations and mortality. Data were verified by reviewing hospital records.
Data Analysis
Descriptive statistics, including means and standard deviations or frequency distributions, were used to summarize the study variables. Both arithmetic and geometric means and their 95% confidence intervals (CIs) were used to summarize micronutrient intake. Comparisons between the no‐ to moderate‐deficiency group and the high‐deficiency group were accomplished using the 2‐sample t test or χ2 test of association. Although other clinical variables had distributions that were approximately normal, NT‐proBNP was log‐transformed before group comparison because of a right‐skewed distribution. Predictors of event‐free survival were assessed using Cox regression. Several Cox regression models were run, and the proportional hazards assumption was tested by introducing time‐dependent covariates in the fitted main effects models. The first model, based on the full sample (N=246), included nutrient deficiencies as the predictor of event‐free survival, with race, total comorbidities, depressive symptoms, sodium intake/1000 kcal, and Seattle Heart Failure Model score as covariates. The second model included all the same variables plus log‐transformed NT‐proBNP as an additional covariate. The sample size for this model was smaller (n=222) because NT‐proBNP was not available for all patients. Third, because kcal intake was lower in the high‐deficiency group, we ran a sensitivity analysis with kcal/kg added to the Cox model that included the full sample. For each Cox regression, the proportionality test evaluated whether the required condition of proportional hazards was met, whereas variance inflation factors were used to assess the presence of multicollinearity. Finally, the bivariate association between deficiency group status and time to event (all‐cause hospitalization or death) was evaluated with the log rank test and graphed using a Kaplan–Meier plot. Data analysis was done using SAS v9.3 (SAS Institute); an α level of 0.05 was used throughout.
Results
On average, the participants were aged 61 years (range: 24–97 years; see Table 1). They were predominantly male and white, with NYHA classification of I or II. Most participants were prescribed angiotensin‐converting enzyme inhibitors, diuretics, and/or β‐blockers; a fifth were prescribed an angiotensin receptor blocker. The mean comorbidity score was 3 (range: 1–8). The majority of participants were overweight or obese (24% normal weight, 29% overweight, 24% obese class 1, and 23% obese class 2 or 3).44
Table 1.
Sociodemographic and Clinical Characteristics of the Study Sample by Micronutrient Deficiency Status, With Group Comparisons Using 2‐Sample t Tests or χ2 Tests of Association
| Full Sample (N=246) | Micronutrient Deficiency Status | P Value | ||
|---|---|---|---|---|
| No to Moderate Deficiency (n=185) | High Deficiency (n=61) | |||
| Age,a | 61.4 (12.0); 24–97 | 62.6 (12.1); 32–97 | 57.9 (11.1); 24–83 | 0.007 |
| Sex | 0.40 | |||
| Male, n (%) | 164 (67) | 126 (68) | 38 (62) | |
| Race, n (%) | 0.001 | |||
| White | 180 (73) | 145 (78) | 35 (57) | |
| Black or other minority | 66 (27) | 40 (22) | 26 (43) | |
| NYHA classification, n (%) | 0.22 | |||
| I/II | 133 (54) | 104 (57) | 29 (48) | |
| III/IV | 112 (46) | 80 (43) | 32 (52) | |
| LVEF <50%, n (%) | 212 (86) | 160 (86) | 52 (85) | 0.81 |
| Serum sodium, mmol/dLa | 138.9 (2.4); 129–145 | 138.9 (2.4); 129–145 | 139.2 (2.5); 132–145 | 0.38 |
| Uric acid, mg/dLa | 6.9 (1.8); 2.3–16.0 | 6.9 (1.8); 2.3–16.0 | 6.9 (1.9); 3.4–12.3 | 0.83 |
| NT‐proBNP, fmol/mL, mean; 95% CIa, b | 610.7; 553.0–674.4c | 638.7; 567.2–719.2c | 529.4; 445.6–628.9c | 0.08 |
| ACEI, yes, n (%) | 164 (69) | 124 (69) | 40 (67) | 0.71 |
| ARB, yes, n (%) | 50 (21) | 35 (19) | 15 (26) | 0.28 |
| Diuretic, yes, n (%) | 184 (76) | 138 (76) | 46 (75) | 0.95 |
| Β‐blocker, yes, n (%) | 216 (89) | 160 (87) | 56 (92) | 0.35 |
| Number of comorbiditiesa | 3.0 (1.8); 1–8 | 3.0 (1.7); 1–8 | 2.9 (2.0); 1–8 | 0.92 |
| BMIa | 30.4 (7.1); 16.8–61.3 | 30.1 (6.9); 16.8–61.3 | 31.2 (7.4); 18.1–51.3 | 0.31 |
| Depressive symptoms (BDI‐II)a | 10.5 (8.3); 0–41 | 9.8 (8.3); 0–41 | 12.8 (8.2); 0–38 | 0.02 |
| Calorie intakea | 1852 (639); 716–4363 | 1970 (656); 716–4363 | 1493 (417); 775–2870 | <0.001 |
| Sodium intakea | 3198 (1306); 888–9138 | 3309 (1353); 888–9138 | 2861 (1091); 890–6003 | 0.01 |
| Sodium intake/1000 kcala | 1.8 (0.5); 0.4–3.8 | 1.7 (0.4); 0.4–3.4 | 1.9 (0.6); 0.6–3.8 | 0.005 |
| Seattle Heart Failure Model scorea | 11.3 (4.6); 2.6–27.4 | 11.3 (4.7); 2.6–27.4 | 11.3 (4.3); 3.7–23.4 | 0.99 |
Data are shown as mean (SD); range, except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BDI‐II, Beck Depression Inventory II; BMI, body mass index; CI, confidence interval; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal probrain natriuretic peptide; NYHA, New York Hospital Association.
Group comparisons done with 2‐sample t test; all other comparisons accomplished using χ2 test of association.
Whereas all other study variables were missing for at most 7 participants, this one was missing for 24.
As a correction for skewness, a log transformation was used before the group comparison of NT‐proBNP; the values shown in the table for this variable are the geometric mean and corresponding 95% CI.
The median number of micronutrient deficiencies in patients’ diets was 4 (range: 0–14). Comparing those with high micronutrient deficiency to those with no to moderate deficiency, the high‐deficiency group was younger, more likely to be black or other minority, and other minority (Asian, Hispanic, or Native American) had higher depressive symptoms scores. The percentage of participants in the high‐deficiency group was relatively evenly distributed across BMI: 22% were normal weight, 29% were overweight, 26% were obese class 1, and 23% were obese class 2 or 3. Those in the high‐deficiency group had lower kcal and sodium intake than the no‐ to moderate‐deficiency group. The 2 groups also differed on the ratio of sodium per 1000 kcal, with the average for the high‐deficiency group being 12% higher than the no‐ to moderate‐deficiency group. The 2 deficiency groups did not differ by sex; NYHA classification group; left ventricular ejection fraction <50%; serum sodium, uric acid, or NT‐proBNP; prescribed angiotensin‐converting enzyme inhibitors, angiotensin receptor blocker, diuretics, or β‐blockers; number of comorbidities; BMI; or Seattle Heart Failure Model score.
The summary of deficiency status for each micronutrient is given in Table 2. The table entries are ordered from greatest percentage of participants with a micronutrient deficiency to least, with recommended daily micronutrient requirements for men and women aged 51 to 70 provided as reference values. Both the arithmetic and geometric means are given for the level of consumption of each micronutrient, along with the CIs for each. The micronutrients most often deficient included calcium, magnesium, and vitamins D and E, whereas phosphorus, niacin, thiamin, and riboflavin were less frequently deficient.
Table 2.
Summary of the Micronutrients, Including the Reference of Lowest Micronutrient Intake/Day for Men and Women Aged 51–70 Years, Percentage of the Sample With Deficiency, and Arithmetic and Geometric Means and Their Corresponding 95% CIs (N=246)
| Variable | Reference | % Deficient | Untransformed Data | Log‐Transformed Data | ||
|---|---|---|---|---|---|---|
| Micronutrients/Day, Men; Women | Mean (Arithmetic) | 95% CI | Mean (Geometric) | 95% CI | ||
| Calcium, mga | 1.0; 1.2 | 67.1 | 862.1 | 801.5–922.6 | 747.9 | 699.1–800.2 |
| Magnesium, mgb | 420; 320 | 62.2 | 301.9 | 284.6–319.3 | 275.3 | 260.9–290.6 |
| Vitamin D, qgc | 15; 15 | 60.6 | 9.2 | 7.9–10.6 | 5.8 | 5.1–6.6 |
| Vitamin E, mga | 15; 15 | 59.4 | 82.0 | 44.8–119.2 | 13.0 | 10.8–15.6 |
| Zinc, mga | 11; 8 | 45.5 | 15.5 | 14.0–17.0 | 12.8 | 11.8–13.8 |
| Vitamin C, mga | 90; 75 | 39.4 | 185.8 | 123.0–248.5 | 83.7 | 72.5–96.8 |
| Vitamin K, qgc | 120; 90 | 29.7 | 109.4 | 97.1–121.8 | 81.5 | 74.0–89.7 |
| Folate, qgb | 400; 400 | 27.2 | 548.7 | 502.6–594.9 | 463.7 | 431.5–498.3 |
| Vitamin B12, qga | 2.4; 2.4 | 9.4 | 50.6 | 4.5–96.7 | 7.5 | 6.4–8.7 |
| Pantothenic acid, mgc | 5; 5 | 4.1 | 9.7 | 7.7–11.7 | 6.7 | 6.1–7.4 |
| Vitamin B6, mgb | 1.7; 1.5 | 3.3 | 3.4 | 2.5–4.3 | 2.3 | 2.1–2.5 |
| Selenium, qgc | 55; 55 | 2.0 | 117.3 | 110.2–124.4 | 107.7 | 102.4–113.4 |
| Iron, mga | 8; 8 | 2.0 | 20.9 | 16.9–24.8 | 16.4 | 15.2–17.6 |
| Phosphorus, mgb | 700; 700 | 1.6 | 1200.0 | 1145.4–1254.6 | 1129.1 | 1080.8–1179.6 |
| Niacin, mg3 | 16; 14 | 1.6 | 54.7 | 33.6–75.9 | 29.3 | 26.6–32.1 |
| Thiamin, mgb | 1.2; 1.1 | 0.4 | 4.8 | 2.3–7.2 | 2.1 | 1.9–2.3 |
| Riboflavin, mgb | 1.3; 1.1 | 0 | 3.6 | 2.6–4.5 | 2.5 | 2.3–2.7 |
CI indicates confidence interval.
Deficiency status based on estimated average requirement.
Deficiency status based on probability formula.
Deficiency status based on <50% recommended daily allowance.
The Cox regression model to assess predictors of time to event was significant overall (likelihood ratio, χ2=26.6, P<0.001). With demographic and clinical variables included as covariates, number of comorbidities, depressive symptoms, and high micronutrient deficiency status were significant predictors of time to all‐cause hospitalization or death, whereas race, BMI, sodium intake per 1000 kcal, comorbidity score, and Seattle Heart Failure Model score were not significant (model 1; Table 3). For every 5‐U increase in BDI‐II score, the hazard of having a hospitalization or death increased by 15%. Compared with those with no or moderate micronutrient deficiency, those with high deficiency had 92% greater hazard of hospitalization for or death from any cause.
Table 3.
Cox Proportional Hazards Models for Time to All‐Cause Hospitalization or Death
| Variable | Parameter Estimate | SE | HR | 95% CI | χ2 (P Value) |
|---|---|---|---|---|---|
| Model 1 (N=246) | |||||
| White | 0.1721 | 0.2739 | 1.19 | 0.69 to 2.03 | 0.4 (0.39) |
| Comorbidity score | 0.1142 | 0.0649 | 1.21 | 0.99 to 1.27 | 3.1 (0.08) |
| BMI | 0.0265 | 0.0175 | 1.03 | 0.99 to 1.06 | 2.3 (0.13) |
| BDI‐II | 0.0324* | 0.0131* | 1.03* | 1.01 to 1.06* | 6.1 (0.01) |
| Sodium intake/1000 kcal | −0.1878 | 0.2567 | 0.83 | 0.50 to 1.37 | 0.5 (0.46) |
| Seattle Heart Failure Model score | −4.326 | 2.303 | 0.01 | 0.00 to 1.21 | 3.5 (0.06) |
| Have high deficiency | 0.6519* | 0.2621* | 1.92* | 1.15 to 3.21* | 6.2 (0.012) |
| Model 2 (n=222) | |||||
| White | 0.2322 | 3182 | 1.27 | 0.68 to 2.35 | 0.5 (0.46) |
| Comorbidity score | 0.0883 | 0.0684 | 1.09 | 0.96 to 1.25 | 1.7 (0.20) |
| BMI | 0.0312 | 0.0201 | 1.03 | 0.99 to 1.07 | 2.6 (0.11) |
| BDI‐II | 0.0427* | 0.0143* | 1.04* | 1.02 to 1.07* | 8.9 (0.003) |
| Sodium intake/1000 kcal | 0.1178 | 0.2548 | 1.12 | 0.68 to 1.85 | 0.2 (0.64) |
| Seattle Heart Failure Model score | 2.206 | 3.594 | 9.08 | 0.01 to 10 416 | 0.4 (0.54) |
| NT‐proBNP | 1.014* | 0.2228* | 2.76* | 1.78 to 4.27* | 21 (<0.001) |
| Have high deficiency | 0.7021* | 0.2892* | 2.02* | 1.14 to 3.58* | 5.9 (0.01) |
BDI‐II indicates Beck Depression Inventory‐II; BMI, body mass index; CI, confidence interval; HR, hazard ratio; NT‐proBNP, N‐terminal probrain natriuretic peptide.
The second model included the demographic and clinical characteristics of the first with the addition of log‐transformed NT‐proBNP (model 2; Table 3). The significant predictors in this model included depressive symptoms, NT‐proBNP, and deficiency status, and the overall model was significant (likelihood ratio, χ2=39.5, P<0.001). This model was largely consistent with the prior model except that number of comorbidities no longer met the threshold for significance, and the sample size was diminished by 24 (the number of participants for whom NT‐proBNP was not measured). In this model, a 5‐point increase in BDI‐II was associated with a 22% increase in the hazard of hospitalization or death. Those with high micronutrient deficiency had double the hazard of all‐cause events compared with the reference group of no to moderate deficiency. The hazard ratio for the log of NT‐proBNP suggested that those with increased values for this measure had greater hazard of experiencing an event (ie, 2.8 times the hazard for each 1‐U increase in the log of NT‐proBNP). For both models, the test of proportionality was not significant (P>0.68 for both), and the variance inflation factors were <1.2 for each independent variable in the model, suggesting that multicollinearity did not distort parameter estimates.
In the sensitivity analysis with kcal/kg added to the full‐sample model, deficiency status remained a significant predictor of event‐free survival (likelihood ratio, χ2=4.25, P=0.039). The CI for the hazard ratio for this predictor was relatively wide, suggesting increased variability related to the inclusion of kcal/kg, likely caused by the strong association between kcal and deficiency status. The estimated hazard ratio for deficiency status in this model was 4.87, and the corresponding 95% CI was 1.08 to 21.89. This model also had a nonsignificant proportionality of hazards test (P=0.83), and the variance inflation factors were all <1.7.
In the full sample, 30.1% of patients experienced hospitalization or death during the year. There were 2 deaths (3%) and 25 hospitalizations (41%) in the high‐deficiency group and 3 (2%) deaths and 44 hospitalizations (24%) in the no/moderate‐deficiency group. The Kaplan–Meier plot shown in the Figure provides a graphical representation of the survival distribution over 1‐year follow‐up for the 2 micronutrient deficiency status groups. At the end of the yearlong follow‐up period, both groups had >50% surviving event free, so the median event‐free survival time was not estimable; however, the upper quartile (ie, with 75% surviving event‐free) was 193 days (95% CI, 103–303) for the high‐deficiency group compared with 333 days for the no/moderate deficiency group (the lower 95% confidence limit was 225, and the upper confidence limit was not estimable given the limited number of events in this group). Mean survival was 266.5 days (SE: 14.1) and 312.9 days (SE: 7.7) for the high‐ and no/moderate‐deficiency groups, respectively. In Figure, the survival distribution for the group with high deficiency is below than the group with a more complete diet. Consistent with the observed difference in distributions, the log rank test for the group comparison was significant (χ2=7.6; P=0.0065).
Figure 1.
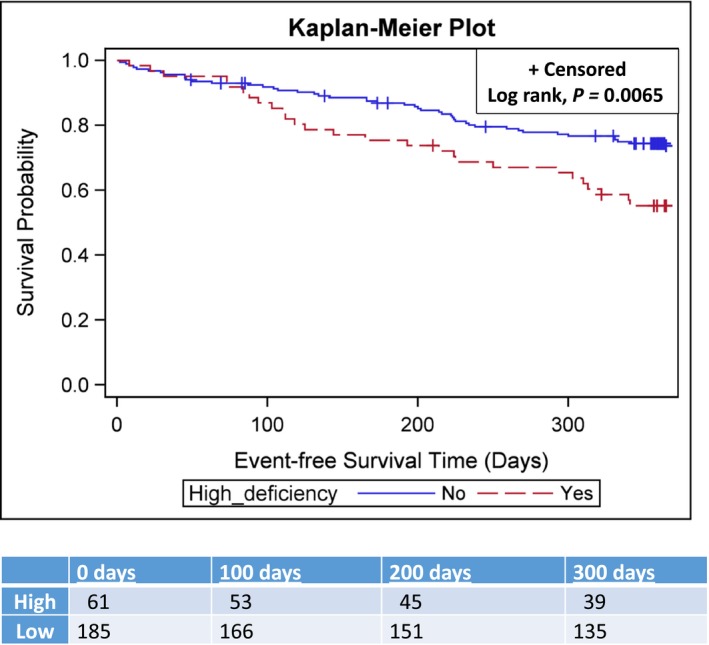
Kaplan–Meier plot of event‐free survival by micronutrient deficiency status.
Discussion
Our results using a sample of patients with heart failure from the United States were similar to those of Song and Kang14 using a sample of patients from South Korea. The Kaplan–Meier plot and log rank test demonstrate the shortest event‐free survival was among patients in the high micronutrient deficiency group. Their event hazard was an alarming 93% higher than patients with fewer micronutrient deficiencies.
A large number of patients in our study had diets deficient in calcium, folate, magnesium, zinc, and vitamins C, D, E, and K. This finding is similar to a study from Canada10 and 2 from South Korea14, 42 in which the same methods were used to measure nutritional intake and to define micronutrient deficiencies. Dietary deficiencies in calcium and magnesium measured by a variety of other methods have also been reported in studies from the United States,6, 45 Brazil,9, 46 Ireand,47 and Italy.7 Other commonly reported dietary deficiencies include vitamin D,45, 46, 47 vitamin E,6, 7, 45 and zinc.7, 9, 45 This high level of micronutrient deficiency is not confined to adults with heart failure. Diets of age‐ and sex‐matched adults without and with heart failure have been reported to be comparably poor in several studies.5, 7, 10 Nevertheless, because patients with heart failure have enhanced excretion of water‐soluble vitamins from diuretic therapy and evidence of increased metabolic demand,48 these deficiencies may have greater consequences for patients with heart failure than without.
A cause for the high number of micronutrient deficiencies in older adults may be diet monotony. Eating a varied diet has been shown to be positively correlated with dietary micronutrient adequacy49—that is, the greater the diversity of foods in the diet, the less likely there are to be dietary deficiencies. This is particularly true for calcium,49 the micronutrient most commonly deficient in the diets of patients with heart failure. Anecdotally, we observed that a large number of our patients consumed the same foods for multiple meals across all 4 days of the food diary. Consistent with this observation are data suggesting that older adults are more vulnerable to consuming a monotonous diet because of a decreased drive to consume varied foods. Pelchat and Shaefer50 compared food cravings in a group of young adults (mean age: 25 years) with a group of older adults (mean age: 73 years) before and while on a 5‐day, monotonous, nutritionally complete liquid diet. The number of cravings was similar in the groups at baseline. During the diet monotony period, young adults reported a significant increase in the number of cravings, whereas older adults reported no increase in cravings. The older adults also indicated a greater liking of the monotonous diet than the younger adults. Supporting our anecdotal observation, these results indicate that older adults with heart failure may be prone to eating diets with limited variability, placing them at risk for dietary micronutrient deficiencies.
In addition to diet monotony, other factors related to heart failure and aging may also affect food intake. We conducted a study comparing heart failure patients’ perceptions of factors influencing food intake with a similar group of healthy older adults.51 Factors with greater impact for patients with heart failure were dietary restrictions, lack of hunger, feeling full after eating only a small amount of food, shortness of breath, and limited energy. Factors common in both groups, and presumably more related to aging than heart failure, were decreased senses of taste and smell.
Patients attempting to follow a very low sodium diet (<2000 mg) have been reported to develop dietary micronutrient deficiencies, suggesting that sodium‐restricted diets may contribute to dietary deficiencies.52 Although the group with higher micronutrient deficiency in our study also had significantly lower daily sodium intake, 2 observations suggest that sodium restriction may not have played a role in dietary deficiencies in our study. First, sodium intake per 1000 kcal was not an independent predictor of event‐free survival. Second, sodium intake per 1000 kcal was greater in the high micronutrient deficiency group than in the lower deficiency group, indicating that the patients in the higher micronutrient group were not consuming foods lower in sodium but rather consuming smaller amounts of food.
The presence of depressive symptoms may be another factor contributing to micronutrient deficiencies. Patients in the high‐deficiency group had significantly higher depressive symptom scores than those in the lower deficiency group. The variable of depressive symptoms was the only other one that independently predicted event‐free survival in the first model and that remained a significant predictor in the second model. This is consistent with an earlier study of a large sample of patients in South Korea.42 Patients with depressive symptoms in that study also had significantly more dietary micronutrient deficiencies than patients with low depressive symptoms. Furthermore, a synergistic negative effect of depressive symptoms and nutrient deficiencies was demonstrated in that study. Those with the shortest event‐free survival were patients with both depressive symptoms and a high number of nutrient deficiencies, whereas those with the longest survival had low depressive symptoms and few micronutrient deficiencies.
It is important to highlight the relatively even distribution of participants in the high‐deficiency group across BMI categories. This finding is comparable to that of Shetty et al,53 who reported that the number of dietary micronutrient deficiencies was similar in normal weight and overweight/obese patients with heart failure. Many screening tools for malnutrition (eg Geriatric Nutritional Risk Index, Mini Nutrition Assessment, Nutritional Risk Index) include an anthropometric measure, most commonly BMI.54, 55 Although such indexes predict outcomes in patients with heart failure, they may have lower sensitivity for identifying malnutrition risk in patients with high BMI. Our results suggest the need to assess dietary quality of all patients with heart failure, especially overweight/obese patients who may otherwise be overlooked.
Some limitations are recognized. First, micronutrient deficiencies were determined from 4‐day food diaries collected at 1 time point. At a group level, we had diet data from food diaries completed during all 12 months, but at an individual level, diaries did not account for potential seasonal variability in individual patients’ diets. Regardless, the consistency of our findings with previous studies lends credence to our results; however, the purpose of this study was to examine the effect of overall diet quality rather than individual micronutrients. Second, our results demonstrate that a focus on single micronutrients would be of limited value because deficiencies do not commonly occur in isolation. The current focus on whole food‐eating patterns rather than targeted nutrients also limits interest in any single nutrient.56 Third, our sample was predominantly male with few minorities and may not reflect other subpopulations of patients with heart failure. Even though race was not a predictor of time to event, the significantly higher number of minority‐race patients in the high‐deficiency group suggests that patients of minority race/ethnicity may be more likely to have diets with micronutrient deficiencies. Consequently, additional research is needed that includes a more diverse sample to provide better understanding of the relationships among patient characteristics, nutritional intake, and outcomes in heart failure.
Several strengths of this study are noted. First, our sample of patients with heart failure is the largest to date to provide comprehensive dietary data. Second, our standardized protocol for collection of food diary data was designed to ensure diaries were as accurate and complete as possible. Recognizing that, even under the best of circumstances, food diaries of some patients will be inaccurate, we eliminated patients with diet dairies that were not clinically plausible from the analysis. Finally, we used the most rigorous method available to define dietary micronutrient deficiencies,24 which increased confidence that patients’ diets were habitually deficient in those micronutrients rather than simply during the 4 days of the food diary.
Conclusion
The results of this study provide additional convincing evidence that diet quality of patients with heart failure plays an important independent role in heart failure outcomes. Our results, combined with previous studies, suggest that patients with comorbid depressive symptoms may be particularly vulnerable to micronutrient deficiencies and shortened event‐free survival. However, the potential relationships among depressive symptoms, diet, and event‐free survival require additional evidence before definitive conclusions can be drawn. Calcium and magnesium intake appear to be nearly universally deficient across multiple countries in Europe, South America, Asia, and the United States. Although diet monotony has been clinically observed and anecdotally noted in our study, it has not been systematically documented in a research study. Attention to contribution of diet monotony to micronutrient deficiency in future research will help identify potential interventions that achieve the goal determining ideal eating patterns to promote optimal health among heart failure patients of all ages.
Sources of Funding
This work was supported by National Institute of Nursing Research NIH RO1NR009280, National Institute of Nursing Research NIH P20NR0106791, American Heart Association, Great Rivers Affiliate Postdoctoral Fellowship, National Center for Research Resources, NIH UL1 RR025008, National Center for Advancing Translational Sciences, NIH UL1TR000117, General Clinical Research Centers NIH: Indiana University M01RR000750, Atlanta Veterans Administration Medical Center, and Clarian Health Partners (Indiana). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding sponsors.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007251 DOI: 10.1161/JAHA.117.007251.)
References
- 1. Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur J Heart Fail. 2013;15:1304–1310. [DOI] [PubMed] [Google Scholar]
- 2. Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. [DOI] [PubMed] [Google Scholar]
- 3. Silver MA. Dietary research in heart failure: beyond the salt shaker. J Am Coll Cardiol. 2003;42:1224–1225. [DOI] [PubMed] [Google Scholar]
- 4. Rich MW, Hauptman PJ. Nutrition in heart failure: more than drugs and devices. J Card Fail. 2015;21:943–944. [DOI] [PubMed] [Google Scholar]
- 5. Gorelik O, Almoznino‐Sarafian D, Feder I, Wachsman O, Alon I, Litvinjuk V, Roshovsky M, Modai D, Cohen N. Dietary intake of various nutrients in older patients with congestive heart failure. Cardiology. 2003;99:177–181. [DOI] [PubMed] [Google Scholar]
- 6. Grossniklaus DA, O'Brien MC, Clark PC, Dunbar SB. Nutrient intake in heart failure patients. J Cardiovasc Nurs. 2008;23:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catapano G, Pedone C, Nunziata E, Zizzo A, Passantino A, Incalzi RA. Nutrient intake and serum cytokine pattern in elderly people with heart failure. Eur J Heart Fail. 2008;10:428–434. [DOI] [PubMed] [Google Scholar]
- 8. Lemon SC, Olendzki B, Magner R, Li W, Culver AL, Ockene I, Goldberg RJ. The dietary quality of persons with heart failure in NHANES 1999–2006. J Gen Intern Med. 2010;25:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lourenco BH, Vieira LP, Macedo A, Nakasato M, Marucci Mde F, Bocchi EA. Nutritional status and adequacy of energy and nutrient intakes among heart failure patients. Arq Bras Cardiol. 2009;93:541–548. [DOI] [PubMed] [Google Scholar]
- 10. Arcand J, Floras V, Ahmed M, Al‐Hesayen A, Ivanov J, Allard JP, Newton GE. Nutritional inadequacies in patients with stable heart failure. J Am Diet Assoc. 2009;109:1909–1913. [DOI] [PubMed] [Google Scholar]
- 11. Colin‐Ramirez E, Castillo‐Martinez L, Orea‐Tejeda A, Zheng Y, Westerhout CM, Ezekowitz JA. Dietary fatty acids intake and mortality in patients with heart failure. Nutrition. 2014;30:1366–1371. [DOI] [PubMed] [Google Scholar]
- 12. Cohen N, Almoznino‐Sarafian D, Zaidenstein R, Alon I, Gorelik O, Shteinshnaider M, Chachashvily S, Averbukh Z, Golik A, Chen‐Levy Z, Modai D. Serum magnesium aberrations in furosemide (frusemide) treated patients with congestive heart failure: pathophysiological correlates and prognostic evaluation. Heart. 2003;89:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boxer RS, Kenny AM, Cheruvu VK, Vest M, Fiutem JJ, Pina II. Serum 25‐hydroxyvitamin D concentration is associated with functional capacity in older adults with heart failure. Am Heart J. 2010;160:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song EK, Kang SM. Micronutrient deficiency independently predicts adverse health outcomes in patients with heart failure. J Cardiovasc Nurs. 2017;32:47–53. [DOI] [PubMed] [Google Scholar]
- 15. Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17. Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory‐II in clinically depressed outpatients. J Clin Psychol. 1999;55:117–128. [DOI] [PubMed] [Google Scholar]
- 18. Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31:160–168. [DOI] [PubMed] [Google Scholar]
- 19. Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory‐Second Edition in a sample of college students. Depress Anxiety. 2004;19:187–189. [DOI] [PubMed] [Google Scholar]
- 20. Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual. 2nd ed San Diego, CA: Harcourt Brace; 1996. [Google Scholar]
- 21. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Neilson MP, Whellan DJ, Schulman KA, Levy WC, Reed SD. Associations between Seattle Heart Failure Model scores and health utilities: findings from HF‐ACTION. J Card Fail. 2013;19:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlstrom U, Sartipy U, Maggioni A, Swedberg K, O'Conner C, Levy WC. Seattle Heart Failure and Proportional Risk Models predict benefit from implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2017;69:2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute of Medicine . Dietary Reference Intakes. Applications in Dietary Assessment. Washington, DC: National Academic Press; 2000. [Google Scholar]
- 25. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. [DOI] [PubMed] [Google Scholar]
- 26. Moser DK, McKinley S, Dracup K, Chung ML. Gender differences in reasons patients delay in seeking treatment for acute myocardial infarction symptoms. Patient Educ Couns. 2005;56:45–54. [DOI] [PubMed] [Google Scholar]
- 27. McKinley S, Dracup K, Moser DK, Ball C, Yamasaki K, Kim CJ, Barnett M. International comparison of factors associated with delay in presentation for AMI treatment. Eur J Cardiovasc Nurs. 2004;3:225–230. [DOI] [PubMed] [Google Scholar]
- 28. Biddle M, Moser D, Song EK, Heo S, Payne‐Emerson H, Dunbar SB, Pressler S, Lennie TA. Higher dietary lycopene intake is associated with longer cardiac event‐free survival in patients with heart failure. Eur J Cardiovasc Nurs. 2013;12:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dekker RL, Lennie TA, Albert NM, Rayens MK, Chung ML, Wu JR, Song EK, Moser DK. Depressive symptom trajectory predicts 1‐year health‐related quality of life in patients with heart failure. J Card Fail. 2011;17:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee KS, Song EK, Lennie TA, Frazier SK, Chung ML, Heo S, Wu JR, Rayens MK, Riegel B, Moser DK. Symptom clusters in men and women with heart failure and their impact on cardiac event‐free survival. J Cardiovasc Nurs. 2010;25:263–272. [DOI] [PubMed] [Google Scholar]
- 31. Lennie TA, Song EK, Wu JR, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three gram sodium intake is associated with longer event‐free survival only in patients with advanced heart failure. J Card Fail. 2011;17:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song EK, Lee Y, Moser DK, Dekker RL, Kang SM, Lennie TA. The link of unintentional weight loss to cardiac event‐free survival in patients with heart failure. J Cardiovasc Nurs. 2014;29:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song EK, Lennie TA, Moser DK. Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18:1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event‐free survival in patients with mild heart failure. Eur J Cardiovasc Nurs. 2014;13:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song EK, Moser DK, Frazier SK, Heo S, Chung ML, Lennie TA. Depressive symptoms affect the relationship of N‐terminal pro B‐type natriuretic peptide to cardiac event‐free survival in patients with heart failure. J Card Fail. 2010;16:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song EK, Moser DK, Dekker RL, Lennie TA. The impact of body mass index on the link between depressive symptoms and health outcome in patients with heart failure. J Cardiovasc Nurs. 2015;30:529–536. [DOI] [PubMed] [Google Scholar]
- 37. Song EK, Moser DK, Rayens MK, Lennie TA. Symptom clusters predict event‐free survival in patients with heart failure. J Cardiovasc Nurs. 2010;25:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song EK, Son YJ, Lennie TA. Trait anger, hostility, serum homocysteine, and recurrent cardiac events after percutaneous coronary interventions. Am J Crit Care. 2009;18:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moser DK, Stevenson WG, Woo MA, Stevenson LW. Timing of sudden death in patients with heart failure. J Am Coll Cardiol. 1994;24:963–967. [DOI] [PubMed] [Google Scholar]
- 40. Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self‐reported, medication adherence independently predicts event‐free survival in patients with heart failure. J Card Fail. 2008;14:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu JR, Moser DK, Rayens MK, De Jong MJ, Chung ML, Riegel B, Lennie TA. Rurality and event‐free survival in patients with heart failure. Heart Lung. 2010;39:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song EK, Moser DK, Kang SM, Lennie TA. Association of depressive symptoms and micronutrient deficiency with cardiac event‐free survival in patients with heart failure. J Card Fail. 2015;21:945–951. [DOI] [PubMed] [Google Scholar]
- 43. Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, Robinson S, Cooper LS. Randomized, controlled trial to improve self‐care in patients with heart failure living in rural areas. Circulation. 2014;130:256–264. [DOI] [PubMed] [Google Scholar]
- 44. National Heart Lung and Blood Institute . The NHLBI practical guide: identification, evaluation, and treatment of overweight and obesity in adults. 2000. NIH Publication No. 00‐4084 2000.
- 45. Frediani JK, Reilly CM, Higgins M, Clark PC, Gary RA, Dunbar SB. Quality and adequacy of dietary intake in a southern urban heart failure population. J Cardiovasc Nurs. 2013;28:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanches Machado d'Almeida K, Dalira Schweigert Perry I, Clausell N, Correa Souza G. Adequacy of energy and nutrient intake in patients with heart failure. Nutr Hosp. 2015;31:500–507. [DOI] [PubMed] [Google Scholar]
- 47. McKeag NA, McKinley MC, Harbinson MT, McGinty A, Neville CE, Woodside JV, McKeown PP. Dietary micronutrient intake and micronutrient status in patients with chronic stable heart failure: an observational study. J Cardiovasc Nurs. 2017;32:148–155. [DOI] [PubMed] [Google Scholar]
- 48. Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, Pastoris O. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol. 2003;42:1218–1223. [DOI] [PubMed] [Google Scholar]
- 49. Vadiveloo M, Dixon LB, Mijanovich T, Elbel B, Parekh N. Development and evaluation of the US Healthy Food Diversity index. Br J Nutr. 2014;112:1562–1574. [DOI] [PubMed] [Google Scholar]
- 50. Pelchat ML, Schaefer S. Dietary monotony and food cravings in young and elderly adults. Physiol Behav. 2000;68:353–359. [DOI] [PubMed] [Google Scholar]
- 51. Lennie TA, Moser DK, Heo S, Chung ML, Zambroski CH. Factors influencing food intake in patients with heart failure: a comparison with healthy elders. J Cardiovasc Nurs. 2006;21:123–129. [DOI] [PubMed] [Google Scholar]
- 52. Jefferson K, Ahmed M, Choleva M, Mak S, Allard JP, Newton GE, Arcand J. Effect of a sodium‐restricted diet on intake of other nutrients in heart failure: implications for research and clinical practice. J Card Fail. 2015;21:959–962. [DOI] [PubMed] [Google Scholar]
- 53. Shetty PM, Hauptman PJ, Landfried LK, Patel K, Weiss EP. Micronutrient deficiencies in patients with heart failure: relationships with body mass index and age. J Card Fail. 2015;21:968–972. [DOI] [PubMed] [Google Scholar]
- 54. Minamisawa M, Miura T, Motoki H, Ueki Y, Nishimura H, Shimizu K, Shoin W, Harada M, Mochidome T, Senda K, Yoshie K, Oguchi Y, Hashizume N, Abe N, Saigusa T, Ebisawa S, Izawa A, Koyama J, Ikeda U, Kuwahara K. Geriatric Nutritional Risk Index predicts cardiovascular events in patients at risk for heart failure. Circ J. 2018;82:1614–1622. [DOI] [PubMed] [Google Scholar]
- 55. Adejumo OL, Koelling TM, Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant. 2015;34:1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. United States Department of Agriculture . Dietary guidelines for Americans 2015–2020. 2015.


