Abstract
Background
Mechanistic studies suggest that aldosterone impairs glucose metabolism. We investigated the cross‐sectional associations of aldosterone and plasma renin activity with fasting plasma glucose, insulin resistance (IR), β‐cell function, and longitudinal association with incident diabetes mellitus among adults in MESA (the multiethnic study of atherosclerosis) prospective cohort study.
Methods and Results
Homeostatic model assessment of IR (HOMA2‐IR) and HOMA2‐β were used to estimate IR and β‐cell function, respectively. Incident diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or anti‐diabetic medication use at follow‐up. Linear regression was used to examine cross‐sectional associations of aldosterone with fasting plasma glucose, HOMA2‐IR and HOMA2‐β; Cox regression was used to estimate hazard ratios (HR) for incident diabetes mellitus with multivariable adjustment. There were 116 cases of incident diabetes mellitus over 10.5 years among 1570 adults (44% non‐Hispanic white, 13% Chinese American, 19% Black, 24% Hispanic American, mean age 64±10 years, 51% female). A 100% increase in log‐aldosterone was associated with a 2.6 mg/dL higher fasting plasma glucose, 15% higher HOMA2‐IR and 6% higher HOMA2‐β (P<0.01). A 1‐SD increase in log‐aldosterone was associated with a 44% higher risk of incident diabetes mellitus (P<0.01) with the greatest increase of 142% (P<0.01) observed in Chinese Americans (P for interaction=0.09 versus other ethnicities). Similar cross‐sectional findings for log‐plasma renin activity existed, but log‐plasma renin activity was not associated with incident diabetes mellitus after full adjustment.
Conclusions
Aldosterone is associated with glucose homeostasis and diabetes mellitus risk with graded associations among Chinese Americans and blacks, suggesting that pleiotropic effects of aldosterone may represent a modifiable mechanism in diabetes mellitus pathogenesis with potential racial/ethnic variation.
Keywords: aldosterone, race and ethnicity, renin angiotensin system, type 2 diabetes mellitus
Subject Categories: Diabetes, Type 2; ACE/Angiotension Receptors/Renin Angiotensin System; Race and Ethnicity; Epidemiology
Clinical Perspective
What Is New?
In this contemporary, prospective study of multiethnic adults without type 2 diabetes mellitus, aldosterone was positively associated with fasting plasma glucose, insulin resistance, and risk of incident type 2 diabetes mellitus over 10.5 years.
Type 2 diabetes mellitus risk per log‐aldosterone standard deviation was higher among participants with both unsuppressed renin phenotype and suppressed renin phenotype suggesting physiologically, and autonomously higher aldosterone may increase risk of type 2 diabetes mellitus.
Dose‐dependent associations of aldosterone with incident type 2 diabetes mellitus existed in Chinese Americans and blacks but not in Hispanic Americans or non‐Hispanic whites with the greatest magnitude of association among Chinese Americans.
What Are the Clinical Implications?
Further clarification on the role of aldosterone in the development of diabetes mellitus and potential racial/ethnic differences is necessary to optimize risk stratification.
Elucidation of mechanisms for the racial/ethnic differences in the association of aldosterone with incident type 2 diabetes mellitus is of preeminent importance given the potential to lower risk of type 2 diabetes mellitus through targeted modulation of the renin‐angiotensin‐aldosterone system.
Introduction
Type 2 diabetes mellitus incidence has plateaued among non‐Hispanic whites (NHWs) but continues to rise among US racial/ethnic minorities.1 Thus, novel preventive intervention targets are of paramount importance, one potential target is the renin‐angiotensin‐aldosterone (RAAS). Aldosterone can impair insulin secretion and sensitivity, both of these actions are critical factors in the development of type 2 diabetes mellitus.2, 3, 4 In a German case‐control study, type 2 diabetes mellitus was more prevalent in subjects with primary aldosteronism (a state of high aldosterone, low angiotensin II and low plasma renin activity [PRA], most commonly because of bilateral adrenal gland enlargement or adrenal adenomas) with hypertension than in hypertensive controls.5 Among blacks in the Jackson Heart Study,6 aldosterone was positively associated with insulin resistance, while higher levels of aldosterone across the spectrum were associated with a dose‐dependent higher risk of the development of type 2 diabetes mellitus over 8 years. Recent observations demonstrate that aldosterone can be produced autonomously from aldosterone producing cell clusters within morphologically normal adrenal glands with a large spectrum and high prevalence of subclinical aldosteronism.7, 8, 9 Thus, we examined the association of aldosterone and PRA with glucose metabolism and incident diabetes mellitus across the spectrum of aldosterone levels in a multiethnic cohort to determine if there are racial/ethnic differences.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
MESA (the Multiethnic Study of Atherosclerosis) is a population‐based sample of 6814 men and women from 4 racial/ethnic groups: NHWs (38%), blacks (28%), Chinese Americans (12%), and Hispanic Americans (22%). Participants were aged 45 to 84 years at baseline without evidence of clinical cardiovascular disease. Details of sampling and recruitment are published.10 The study was approved by the Institutional Review Boards of the 6 participating institutions (Columbia University, New York; Johns Hopkins University, Baltimore; Northwestern University, Chicago; University of California, Los Angeles; University of Minnesota, Twin Cities; Wake Forest University, Winston Salem) and written informed consent was obtained from all participants.
At exams 2 and 3 (≈3 and 4.5 years after study baseline, respectively), a random subsample of 1959 MESA participants had PRA and aldosterone levels measured from stored blood as part of an ancillary study investigating calcified atherosclerosis of the renal arteries by computed tomography (Figure S1). Of the participants with aldosterone and PRA measured, we excluded participants with: no or insufficient aldosterone sample (n=40); known diabetes mellitus or missing diabetes mellitus status at the exam of blood draw (n=334); missing data on follow‐up (n=4); and missing data on important covariates (n=11). The final analytic sample included 1570 participants with aldosterone (602 and 968 participants from exams 2 and 3) and 1474 with PRA measurements (569 and 905 participants from exams 2 and 3), respectively. For continuous aldosterone analyses, 22 participants with aldosterone below the detectable range were excluded (n=1548). For this analysis, the baseline visit is the visit at which both aldosterone and PRA were measured.
Standardized questionnaires were used to obtain participant data including: demographics, level of education, alcohol consumption, medical conditions, and current prescription medication usage. Race/ethnicity was assessed by self‐report and categorized as NHW, black, Hispanic American, or Chinese American. Education was carried forward from exam 1 and classified as bachelor's degree versus < bachelor's degree. Current alcohol use was assessed as a binary variable by asking participants if they “presently drink alcoholic beverages.” Angiotensin‐converting enzyme inhibitors (ACE‐I) and angiotensin receptor blockers (ARB) usage was collected at the exam of aldosterone and PRA measurement. The MESA Typical Week Physical Activity Survey, adapted from the Cross‐Cultural Activity Participation Study, was used to assess physical activity.11 We used the intentional exercise variable (sum of walking for exercise, sports/dancing, and conditioning in Metabolic Equivalent of Task‐minutes/week).11
Calibrated devices were used to measure participants’ weight, and height with body mass index calculated as weight (kilograms)/height2 (meters). Waist circumference was measured at the level of the umbilicus. Resting seated blood pressure was measured 3 times using a Dinamap automated oscillometric sphygmomanometer (model Pro 100, Critikon, Tampa, FL); the last 2 measurements were averaged for analysis.
Laboratory Assessment
Covariates
At visits, fasting (12‐hours) blood samples were drawn in the morning after resting in the sitting position for 1 hour. Participants were instructed to take their usual medications before the clinic visit. Samples were processed using a standardized protocol and sent to the MESA central laboratory at the University of Vermont for further processing and storage. Participant samples were aliquoted into ≈65 aliquots per participant, immediately placed on ice, flash frozen, and stored at −80°C after processing.
Serum glucose was measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Insulin was determined using a radioimmunoassay Linco Human Insulin Specific RIA Kit (Linco Research). The homeostasis model assessment (HOMA)‐2 of insulin resistance (HOMA2‐IR) and β‐cell function (HOMA2‐β) were calculated using the updated computer‐based HOMA index of insulin resistance (HOMA2‐IR) and computer‐based HOMA2‐β% (https://www.dtu.ox.ac.uk/homacalculator/).12
Serum creatinine was measured by rate reflectance spectrophotometry using thin‐film adaptation of the creatinine amidinohydrolase method on the VITROS analyzer (Johnson & Johnson Clinical Diagnostics) and calibrated to Cleveland Clinic. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.13 Adiponectin, leptin, and tumor necrosis factor‐α were measured by Human Serum Adipokine Panel A and B LINCOplex Kit (Linco Research, Inc., St. Charles, MO). All assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Exposures—PRA and aldosterone
Aldosterone and PRA were measured after 2 and 3 freeze–thaw cycles, respectively. PRA and aldosterone assays were run in duplicate and averaged. Aldosterone was measured using a competition‐based radioimmunoassay (ALDOCTK‐2; Diasorin, Stillwater, MN). Intra‐assay coefficients of variation ranged from 6.30% to 8.87%. Angiotensin I levels directly correlate with PRA. Therefore, PRA was measured using a radioimmunoassay of generated angiotensin I (GammaCoat PRA 125 I Kit; DiaSorin, Stillwater, MN). The assay range was 0.05 to 5.0 ng/mL per hour. Intra‐assay coefficients of variation ranged from 18.07% to 21.19%. These assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Outcome—incident type 2 diabetes mellitus
Among those without diabetes mellitus at visit 2 or 3, participants newly using glucose lowering medication or with fasting plasma glucose (FPG) ≥7 mmol/L (126 mg/dL) at 1 of the subsequent exams (the last follow‐up visit occurring in 2010–2012) were considered to have incident type 2 diabetes mellitus in accordance with the American Diabetes Association definition.14
Statistical Analysis
Due to the non‐normal distribution of aldosterone, PRA, HOMA2‐IR, and HOMA2‐β, these variables were log transformed before analysis. To explore the potential non‐linear relationships and evaluate for dose‐response relationships, aldosterone, and PRA were divided into tertiles. Descriptive statistics were used to compare the baseline characteristics of all included participants by log‐aldosterone in tertiles and included versus excluded participants using one‐way ANOVA for normally distributed continuous variables, Mann‐Whitney and Kruskal–Wallis test for non‐normally distributed continuous variables and the χ2 test for categorical variables. We used multivariable linear regression to examine the cross‐sectional associations between log‐aldosterone and log‐PRA with FPG, log‐HOMA2‐IR and log‐HOMA2‐β. Sequential multivariable adjustment modeling was performed:
Model 1: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate CKD‐EPI, and systolic blood pressure;
Model 2: Model 1+waist circumference (cm);
Model 3: Model 2+ACE‐I and ARB.
The adjustments for waist circumference were performed because of prior literature demonstrating an association of aldosterone with adiposity,15 which is independently associated with insulin resistance and diabetes mellitus.16 ACE‐inhibitors and ARBs modulate the RAAS system and thus were included in Model 3. Unadjusted type 2 diabetes mellitus incidence rates for log‐aldosterone were calculated using person‐time analysis assuming a Poisson distribution. Participants were censored at the last attended follow‐up exam or development of type 2 diabetes mellitus, whichever came first. Incidence rate ratios were assessed using the Mantel‐Cox method. Cox proportional hazards modeling was used to estimate hazard ratios (HR) associated with the aforementioned classifications after confirming no significant violations of the proportional hazards assumption using Schoenfeld residuals. Sequential multivariable adjustment modeling was performed: Model 1 through Model 3 were the same as the cross‐sectional adjustments, Model 4: Model 2+log‐PRA for log‐aldosterone analyses/log‐aldosterone for log‐PRA analyses. Statistical significance was defined as 2‐sided α<0.05.
To assess the role of adipokines and inflammation in the aldosterone/PRA‐incident type 2 diabetes mellitus associations, we added to Model 2 and 3 sets of covariates assessing adipokines (leptin and adiponectin) and inflammatory markers (high‐sensitivity C‐reactive protein (hsCRP), interleukin‐6 and tumor necrosis factor alpha (TNF‐α) or both. Additionally, we assessed the shape of the association of aldosterone with incident type 2 diabetes mellitus using cubic spline regression with knots at the 25th, 50th, and 75th percentiles of aldosterone to explore potential non‐linear relationships.
Analyses were performed using 3 different exposures with incident diabetes mellitus as the outcome, to provide insight into the physiology of aldosterone/renin in the association with incident diabetes mellitus; first, log‐aldosterone:PRA ratio was set as an exposure as has been performed to test for primary hyperaldosteronism (a state of autonomous aldosterone production).17 Second, participants were classified into 3 categories of PRA of ≤0.50, 0.51 to 0.99, and ≥1.0 μg/L per hour, to characterize “suppressed renin phenotype (a state of autonomous aldosterone production),” “indeterminate renin phenotype,” and “unsuppressed renin phenotype” (a state of potentially appropriate mineralocorticoid receptor activation in the setting of physiologic renin‐dependent secretion of aldosterone). The referent category (≥1.0 μg/L per hour) was compared with the 0.51 to 0.99 and ≤0.50 μg/L per hour categories for the association with incident diabetes mellitus.9 Third, participants were classified into 3 categories of PRA of ≤0.50, 0.51 to 0.99, and ≥1.0 μg/L per hour and the association of log‐aldosterone with incident diabetes mellitus was assessed among participants in the 3 PRA classifications.9
Given that the association of aldosterone with type 2 diabetes mellitus risk may differ by age, sex, waist circumference, and race/ethnicity, we tested for interaction of these factors with log‐aldosterone by inserting an interaction term in the model using unadjusted and Model 1 adjustments. The likelihood ratio test was used to test for significance, defined as P<0.10 for interactions. The interactions for age, sex, and waist circumference were non‐significant. The interaction of Chinese Americans versus the other racial/ethnic groups was significant in the continuous association of log‐aldosterone with incident type 2 diabetes mellitus (P=0.0489 unadjusted and P=0.0885 adjusted) (Table S1). Analyses were performed using Stata 13.1 (Statacorp, College Station, TX).
Role of the Funding Source
The funding sources had no role in the collection, analysis, and interpretation of data; writing of the report; or the decision to submit for publication.
Results
The baseline characteristics at visit of aldosterone/renin measurement for the 1570 participants across tertiles of log aldosterone are presented in Table 1. Compared with those in the lowest tertile, participants in higher tertiles of log aldosterone at baseline had higher diastolic blood pressure, glucose, HOMA2‐IR, HOMA2‐β, PRA, interleukin‐6, TNF‐α, alcohol use, and lower estimated glomerular filtration rate, (all comparisons, P<0.05). Baseline characteristics by race/ethnicity are presented in Table S2. Blacks had the lowest aldosterone and PRA levels, whereas NHWs had the highest aldosterone and PRA levels (P<0.01).
Table 1.
Characteristics of Participants in MESA by Log‐Aldosterone in Tertiles at Baseline
| Baseline Characteristicsa | All | Tertile 1 | Tertile 2 | Tertile 3 | P Valuea |
|---|---|---|---|---|---|
| n=1570 | n=524 | n=522 | n=524 | ||
| Age, y | 64.4 (9.7) | 64.6 (9.8) | 64.8 (9.5) | 63.7 (9.8) | 0.1456 |
| Female, sex (%) | 802 (51) | 269 (51) | 264 (51) | 269 (51) | 0.960 |
| Race/Ethnicity (%) | |||||
| Non‐Hispanic white | 682 (43) | 214 (41) | 217 (42) | 251 (48) | |
| Chinese American | 205 (13) | 61 (12) | 86 (16) | 58 (11) | |
| Black | 311 (20) | 147 (28) | 86 (16) | 78 (15) | |
| Hispanic American | 372 (24) | 102 (19) | 133 (25) | 137 (26) | <0.01 |
| Education ≥ Bachelor's degree (%) | 610 (39) | 194 (37) | 206 (39) | 201 (40) | 0.563 |
| Current alcohol use (%) | 867 (55) | 283 (54) | 272 (52) | 312 (60) | 0.043 |
| Ace‐inhibitor or ARB (%) | 299 (19) | 103 (20) | 107 (21) | 89 (17) | 0.319 |
| Exercise physical activity (MET‐min/week) | 1430 (1844) | 1443 (2095) | 1435 (1680) | 1414 (1731) | 0.9656 |
| Body mass index, kg/m2 | 27.7 (4.9) | 27.6 (5.1) | 27.7 (4.7) | 27.8 (4.9) | 0.9060 |
| Waist circumference, cm | 97.1 (13.5) | 96.6 (13.9) | 96.9 (13.1) | 97.8 (13.7) | 0.3012 |
| Systolic blood pressure, mm Hg | 123 (20) | 123 (20) | 123 (20) | 123 (21) | 0.9971 |
| Diastolic blood pressure, mm Hg | 70 (10) | 69 (10) | 70 (10) | 71 (10) | 0.0031 |
| Fasting plasma glucose (mmol/L, mg/dL) | 5.05 (0.56), 91 (10) | 5.00 (0.50), 90 (9) | 5.05 (0.56), 91 (10) | 5.11 (0.56), 92 (10) | 0.0002 |
| Creatinine (μmol/L, mg/dL) | 83.10 (19.45), 0.94 (0.22) | 82.21 (20.33), 0.93 (0.23) | 82.21 (19.45), 0.93 (0.22) | 83.98 (19.45), 0.95 (0.22) | 0.1740 |
| Estimated glomerular filtration rate (mL/s per m2, mL/min per 1.73 m2) | 1.32 (0.27), 79 (16) | 1.34 (0.28), 80 (17) | 1.32 (0.27), 79 (16) | 1.29 (0.27), 77 (16) | 0.0245 |
| Non‐Parametric Continuous Variables | Median (Interquartile Range) | ||||
|---|---|---|---|---|---|
| Aldosterone (pmol/L, pg/mL)b | 363.12 (260.48, 507.36), 130.9 (93.9, 182.9) | 222.20 (176.43, 257.98), 80.1 (63.6, 93.0) | 359.51 (325.11, 401.40), 129.6 (117.2, 144.7) | 580.88 (505.98, 741.49) 209.4 (182.4, 267.3) | 0.0001 |
| PRA, ng/mL per hc | 0.5 (0.3, 1.1) | 0.4 (0.2, 0.7) | 0.5 (0.3, 0.9) | 0.8 (0.4, 1.5) | 0.0001 |
| Homeostatic model assessment‐2—insulin resistanced | 0.81 (0.57, 1.16) | 0.73 (0.52, 1.02) | 0.83 (0.61, 1.17) | 0.89 (0.61, 1.27)) | 0.0001 |
| Homeostatic model assessment‐2—β‐cell function (%)d | 81.5 (66.5, 102.9) | 77.8 (64.7, 96.6) | 81.7 (66.8, 103.5) | 84.2 (67.5, 107.2) | 0.0018 |
| Interleukin‐6, pg/mLe | 1.8 (1.2, 2.7) | 1.7 (1.1, 2.6) | 1.7 (1.2, 2.6) | 1.9 (1.2, 3.0) | 0.0226 |
| High sensitivity C‐reactive protein, mg/Lf | 1.4 (0.7, 3.2) | 1.4 (0.7, 2.9) | 1.4 (0.7, 3.3) | 1.4 (0.8, 3.3) | 0.7377 |
| Tumor necrosis factor‐α, pg/mLg | 4.5 (3.4, 6.2) | 4.4 (3.4, 6.0) | 4.4 (3.3, 6.3) | 4.7 (3.6, 6.4) | 0.0431 |
| Adiponectin, ng/mL | 17 973 (12 347, 26 678) | 18 743 (12 695, 28 051) | 17 704 (12 442, 26 007) | 17 676 (12 024, 26 348) | 0.1503 |
| Leptin, pg/mLh | 13 006 (5455, 27 881) | 11 799 (4871, 26 668) | 13 020 (5522, 27 991) | 13 802 (5942, 28 089) | 0.3355 |
ARB indicates angiotensin receptor blockers; CI, confidence interval; MESA, the Multiethnic Study of Atherosclerosis; MET, metabolic equivalent of task; PRA, plasma renin activity.
Mean (SD), median (interquartile range) or percentages are listed except where noted, Pvalues calculated using chi‐square (categorical variables), ANOVA (parametric continuous variables) and Kruskal–Wallis test (non‐parametric continuous variables).
n=1548 at baseline in continuous analyses (Tertiles 1–3: n=502, n=522, n=524).
n=1474 participants with plasma renin activity at baseline (Tertiles 1–3: n=484, n=496, n=494).
n=1568 participants with HOMA‐IR and HOMA‐β without diabetes mellitus at baseline (Tertiles 1–3: n=524, n=521, n=523).
n=1541 participants with interleukin‐6 at baseline (Tertiles 1–3: n=513, n=514, n=514).
n=1544 participants with high‐sensitivity C‐reactive protein at baseline (Tertiles 1–3: n=514, n=515, n=515).
n=1564 participants with tumor necrosis factor‐α at baseline (Tertiles 1–3: n=521, n=521, n=522).
n=1563 participants with leptin at baseline (Tertiles 1–3: n=521, n=521, n=521).
The cross‐sectional associations of aldosterone and PRA with FPG, log HOMA2‐IR, and log‐HOMA2‐β, are summarized in Table 2. A 100% increase in aldosterone was associated with a 2.58 mg/dL (0.14 mmol/L) higher FPG (95% confidence interval (CI): 1.69, 3.48), 15% higher HOMA2‐IR (95% CI: 10%, 19%), and a 4% higher HOMA2‐β (95% CI: 1%, 7%) (Model 3). A 100% increase in PRA was associated with a 1.04 mg/dL (0.06 mmol/L) higher FPG (95% CI; 0.61, 1.47), 6% higher HOMA2‐IR (95% CI: 4%, 8%) and a 2% higher HOMA2‐β (95% CI: 0%, 3%) (Model 3). The association of log aldosterone with FPG varied by race/ethnicity (Table S3), a 100% increase in aldosterone was associated with a 3.8 mg/dL (0.21 mmol/L), 3.2 mg/dL (0.18 mmol/L), 2.3 mg/dL (0.13 mmol/L) higher FPG among Chinese Americans, blacks, and NHWs, respectively (all P<0.05) and was non‐significant among Hispanic Americans (Model 3). The association of log PRA with FPG was only significant among NHWs (β‐coefficient 1.31, P<0.01, Model 3) (Table S3).
Table 2.
The Association of Log Aldosterone and Log Plasma Renin Activity With Fasting Plasma Glucose, Insulin Resistance and β‐Cell Function
| Fasting Plasma Glucose—Linear Regression β Coefficientsa | ||||
|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2b | Model 3b | |
| Log‐aldosteronec | ||||
| Continuous | 2.15 (1.22, 3.08) | 2.75 (1.83, 3.68) | 2.54 (1.64, 3.44) | 2.58 (1.69, 3.48) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 1.90 (0.73, 3.08) | 1.94 (0.80, 3.09) | 1.79 (0.68, 2.91) | 1.78 (0.67, 2.89) |
| Tertile 3 | 2.32 (1.15, 3.49) | 2.81 (1.65, 3.97) | 2.64 (1.51, 3.77) | 2.69 (1.56, 3.82) |
| Log‐plasma renin activityd | ||||
| Continuous | 0.90 (0.48, 1.31) | 1.16 (0.75, 1.58) | 1.06 (0.66, 1.47) | 1.04 (0.61, 1.47) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 1.14 (−0.07, 2.36) | 1.59 (0.40, 2.79) | 1.59 (0.42, 2.75) | 1.56 (0.39, 2.72) |
| Tertile 3 | 2.36 (1.15, 3.58) | 3.01 (1.79, 4.24) | 2.75 (1.55, 3.94) | 2.58 (1.35, 3.82) |
| Log‐HOMA2‐IR—Linear Regression β Coefficientsa | ||||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Log‐aldosteronee | ||||
| Continuous | 0.16 (0.11, 0.22) | 0.17 (0.12, 0.22) | 0.15 (0.10, 0.19) | 0.15 (0.10, 0.19) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 0.13 (0.06, 0.20) | 0.14 (0.07, 0.21) | 0.12 (0.06, 0.18) | 0.12 (0.06, 0.18) |
| Tertile 3 | 0.18 (0.12, 0.25) | 0.18 (0.12, 0.25) | 0.16 (0.10, 0.22) | 0.16 (0.11, 0.22) |
| Log‐plasma renin activityf | ||||
| Continuous | 0.05 (0.03, 0.08) | 0.07 (0.05, 0.09) | 0.06 (0.04, 0.08) | 0.06 (0.04, 0.08) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 0.03 (−0.04, 0.10) | 0.06 (−0.01, 0.13) | 0.06 (−0.00, 0.12) | 0.06 (−0.00, 0.12) |
| Tertile 3 | 0.15 (0.08, 0.22) | 0.19 (0.12, 0.26) | 0.17 (0.10, 0.23) | 0.17 (0.10, 0.23) |
| Log‐HOMA2‐β—Linear Regression β Coefficientsa | ||||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Log‐aldosteronee | ||||
| Continuous | 0.06 (0.03, 0.10) | 0.05 (0.02, 0.09) | 0.04 (0.01, 0.07) | 0.04 (0.01, 0.07) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 0.04 (0.00, 0.09) | 0.05 (0.01, 0.09) | 0.04 (0.00, 0.08) | 0.04 (0.00, 0.08) |
| Tertile 3 | 0.07 (0.03, 0.12) | 0.06 (0.02, 0.11) | 0.05 (0.01, 0.09) | 0.05 (0.01, 0.09) |
| Log‐plasma renin activityf | ||||
| Continuous | 0.02 (0.00, 0.03) | 0.02 (0.01, 0.04) | 0.02 (0.00, 0.03) | 0.02 (0.00, 0.03) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | −0.01 (−0.05, 0.04) | 0.01 (−0.04, 0.05) | 0.00 (−0.04, 0.05) | 0.01 (−0.04, 0.05) |
| Tertile 3 | 0.05 (0.00, 0.09) | 0.06 (0.02, 0.11) | 0.05 (0.01, 0.09) | 0.05 (0.01, 0.10) |
Linear regression—a 100% increase in aldosterone or plasma renin activity is associated with an X mg/dL increase in glucose and a 100% increase in aldosterone or plasma renin activity results in a 100 times X percent increase in HOMA‐IR (homeostatic model assessment of insulin resistance) or HOMA‐β (homeostatic model assessment of β‐cell function), where X equals the β‐coefficient.
Models: Model 1: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate and systolic blood pressure; Model 2: Model 1+waist circumference (cm); Model 3: Model 2+angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers.
n=1548 participants in continuous analyses and 1570 participants in categorical analyses.
n=1474 participants in continuous and categorical analyses.
n=1546 participants in continuous analyses and 1568 participants in categorical analyses.
n=1472 participants in continuous and categorical analyses.
Longitudinal Assessments
During a median follow‐up of 10.5 years, 116 participants developed type 2 diabetes mellitus (incidence rate 7.9 per 1000 person‐years). Incident rates for log‐aldosterone were higher in tertiles 2 and 3 (10.7 and 9.3 per 1000 person‐years) compared with tertile 1 (3.6 per 1000 person‐years) (P<0.01) in Table 3. The HRs for the association of log‐aldosterone and log PRA with incident type 2 diabetes mellitus are presented in Table 3. After Model 3 adjustment including waist circumference and ACE‐I/ARBs, a 1‐SD increase in log‐aldosterone was associated with a 44% higher risk of incident type 2 diabetes mellitus (HR 1.44, 95% CI: 1.16, 1.78). Similarly, the second and third tertile of log‐aldosterone were associated with a 237% (HR 3.37, 95% CI: 1.96, 5.80) and 225% (HR 3.25, 95% CI: 1.86, 5.69) higher risk of incident type 2 diabetes mellitus versus tertile 1. These findings were not attenuated after additional adjustment for log‐PRA (Model 4). Additional adjustment for inflammatory markers (hsCRP, interleukin‐6, and TNF‐α) and adipokines (adiponectin and leptin), mildly attenuated the continuous association (HR 1.34, 95% CI: 1.07, 1.66) (Table 4).
Table 3.
The Association of Log‐Aldosterone and Log‐Plasma Renin Activity With Incident Type 2 Diabetes Mellitus
| Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus | |||||||
|---|---|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Incidence of Diabetes Mellitus (Per 1000 Person‐Years, 95% CI) | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4a | |
| Log‐aldosterone | |||||||
| Per 1‐unit SD (continuous) | 1548/116 | 7.9 (6.6, 9.5) | 1.28 (1.05, 1.57) | 1.45 (1.17, 1.79) | 1.43 (1.15, 1.77) | 1.44 (1.16, 1.78) | 1.39 (1.10, 1.76) |
| Tertile 1b | 524/18 | 3.6 (2.2, 5.6) | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 522/52 | 10.7 (8.1, 14.0) | 3.03 (1.77, 5.17) | 3.33 (1.93, 5.72) | 3.37 (1.95, 5.80) | 3.37 (1.96, 5.80) | 3.29 (1.88, 5.76) |
| Tertile 3 | 524/46 | 9.3 (7.0, 12.4) | 2.64 (1.53, 4.55) | 3.24 (1.86, 5.67) | 3.20 (1.83, 5.60) | 3.25 (1.86, 5.69) | 2.99 (1.67, 5.38) |
| Log‐plasma renin activity | |||||||
| Per 1‐unit SD (continuous) | 1474/110 | 7.8 (6.5, 9.5) | 1.18 (0.98, 1.42) | 1.31 (1.08, 1.59) | 1.27 (1.04, 1.54) | 1.21 (0.98, 1.50) | 1.10 (0.89, 1.36) |
| Tertile 1c | 491/28 | 6.1 (4.2, 8.8) | Referent | Referent | Referent | Referent | Referent |
| Tertile 2 | 492/41 | 8.7 (6.4, 11.8) | 1.44 (0.89, 2.33) | 1.63 (1.00, 2.56) | 1.60 (0.98, 2.62) | 1.58 (0.96, 2.58) | 1.42 (0.86, 2.34) |
| Tertile 3 | 491/41 | 8.7 (6.4, 11.9) | 1.46 (0.90, 2.36) | 1.84 (1.12, 3.04) | 1.72 (1.04, 2.84) | 1.56 (0.93, 2.63) | 1.23 (0.72, 2.11) |
Model 1: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate and systolic blood pressure. Model 2: Model 1+waist circumference (cm). Model 3: Model 2+angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers. Model 4 (aldosterone analyses): Model 2+log‐plasma renin activity. Model 4 (plasma renin activity analyses): Model 2+log‐aldosterone.
In continuous and categorical analyses, n=1453 with 110 type 2 diabetes mellitus cases and n=1474 with 110 type 2 diabetes mellitus cases, respectively.
n=1548 for continuous analyses and 1570 for categorical analyses. The rate‐ratio for incidence of type 2 diabetes mellitus per tertile using Mantel‐Cox comparison was 1.46 (95% CI: 1.17, 1.82) for log‐aldosterone.
The rate‐ratio for incidence of type 2 diabetes mellitus per tertile using Mantel‐Cox comparison was 1.19 (95% CI: 0.95, 1.50) for log‐plasma renin activity.
Table 4.
The Association of Log‐Aldosterone and Log‐Plasma Renin Activity With Incident Diabetes Mellitus Adjusted for Additional Potential Mediators
| Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Diabetes Mellitus | ||
|---|---|---|
| Log‐aldosterone SD | ||
|
Model 1a
n=1548 |
Model 1 | 1.44 (1.16, 1.78) |
|
Model 2 (adipokines) n=1542 |
Model 1+log‐leptin and log‐adiponectin | 1.38 (1.12, 1.72) |
|
Model 3 (inflammatory markers) n=1493 |
Model 1+log‐hsCRP, log‐IL‐6, and log‐TNF‐α | 1.38 (1.10, 1.71) |
|
Model 4 (adipokines+inflammatory markers) n=1488 |
Model 1+log‐leptin, log‐adiponectin, log‐hsCRP, log‐IL‐6, and log‐TNF‐α | 1.34 (1.07, 1.66) |
| Log‐plasma renin activity SD (n=1447) | ||
|
Model 1a
n=1474 |
Model 1 | 1.21 (0.98, 1.50) |
|
Model 2 (adipokines) n=1467 |
Model 1+log‐leptin and log‐adiponectin | 1.18 (0.96, 1.45) |
|
Model 3 (inflammatory markers) n=1423 |
Model 1+log‐hsCRP, log‐IL‐6, and log‐TNF‐α | 1.14 (0.92, 1.41) |
|
Model 4 (adipokines+inflammatory markers) n=1417 |
Model 1+log‐leptin, log‐adiponectin, log‐hsCRP, log‐IL‐6, and log‐TNF‐α | 1.12 (0.91, 1.38) |
hsCRP indicates high‐sensitivity C‐reactive protein; IL‐6, interleukin 6; TNF‐α, tumor necrosis factor‐α.
Model 1 adjustments: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate, systolic blood pressure, waist circumference (cm), angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers.
In regard to log‐PRA (Table 3), after Model 2 adjustments including waist circumference, a 1‐SD increase in log‐PRA was associated with a 27% higher risk of incident type 2 diabetes mellitus (HR 1.27, 95% CI: 1.04, 1.54). After Model 2 adjustments, participants in tertile 3 versus tertile 1 had a 72% higher risk of incident type 2 diabetes mellitus (HR 1.72, 95% CI: 1.04, 2.84). These findings were attenuated and non‐significant after adjustment for ACE‐I/ARB medications and categorical analyses were non‐significant after adjustment for ACE‐I/ARB medications (Model 3).
Formal race/ethnicity interaction testing was significant for Chinese Americans versus the other racial/ethnic groups (Table S1). Thus, we proceeded with race/ethnicity stratified analyses (Table 5), after Model 3 adjustment including waist circumference and ACE‐I/ARBs, a 1‐SD increase in log‐aldosterone was associated with a HR 2.42 (95% CI: 1.31, 4.46), 1.49 (95% CI: 0.94, 2.36), 1.42 (95% CI: 0.99, 2.03) and 1.01 (95% CI: 0.66, 1.55) for Chinese Americans, blacks, NHWs, and Hispanic Americans, respectively. In categorical analyses, there was a significant graded association among Chinese Americans (P=0.011) and blacks (P=0.008). Tertile 3 compared with 1 was associated with a HR 12.59 (95% CI: 1.50, 105.55) among Chinese Americans, HR 3.72 (95% CI: 1.40, 9.91) among blacks and HR 2.93 (95% CI: 1.03, 8.33) among NHWs (Model 3). These racial/ethnic findings are concordant with spline data presented in Figure, revealing dose‐dependent linear associations among Chinese Americans and blacks and non‐linear associations among NHWs and Hispanic Americans.
Table 5.
The Association of Log‐Aldosterone With Incident Type 2 Diabetes Mellitus by Race/Ethnicity
| Log‐Aldosterone | Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitusa | |||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Non‐Hispanic white (per 1‐SD increase)b | 1.28 (0.92, 1.79) | 1.53 (1.07, 2.21) | 1.39 (0.97, 1.99) | 1.42 (0.99, 2.03) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 4.59 (1.74, 12.13) | 5.18 (1.95, 13.79) | 4.80 (1.79, 12.91) | 4.87 (1.81, 13.09) |
| Tertile 3 | 2.48 (0.89, 6.88) | 3.32 (1.16, 9.45) | 2.84 (1.00, 8.07) | 2.93 (1.03, 8.33) |
| P for trend | P=0.174 | P=0.039 | P=0.097 | P=0.081 |
| Chinese American (per 1‐SD increase)c | 2.21 (1.25, 3.88) | 2.44 (1.36, 4.41) | 2.55 (1.34, 4.84) | 2.42 (1.31, 4.46) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 7.13 (0.90, 56.28) | 8.71 (1.05, 72.23) | 7.96 (0.95, 66.60) | 8.84 (1.04, 74.77) |
| Tertile 3 | 10.96 (1.39, 86.56) | 12.56 (1.52, 103.81) | 11.17 (1.33, 93.92) | 12.59 (1.50, 105.55) |
| P for trend | P=0.009 | P=0.009 | P=0.016 | P=0.011 |
| Black (per 1‐SD increase)d | 1.33 (0.88, 2.02) | 1.42 (0.91 2.22) | 1.42 (0.91, 2.22) | 1.49 (0.94, 2.36) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 2.35 (0.90, 6.18) | 2.27 (0.83, 6.17) | 2.26 (0.83, 6.15) | 2.27 (0.81, 6.31) |
| Tertile 3 | 3.01 (1.17, 7.76) | 3.49 (1.31, 9.27) | 3.49 (1.31, 9.29) | 3.72 (1.40, 9.91) |
| P for trend | P=0.020 | P=0.011 | P=0.011 | P=0.008 |
| Hispanic American (per 1‐SD increase)e | 1.05 (0.69, 1.59) | 0.99 (0.64, 1.52) | 1.01 (0.65, 1.54) | 1.01 (0.66, 1.55) |
| Tertile 1 | Referent | Referent | Referent | Referent |
| Tertile 2 | 1.79 (0.62, 5.16) | 1.78 (0.61, 5.17) | 1.87 (0.64, 5.47) | 1.89 (0.65, 5.50) |
| Tertile 3 | 1.85 (0.65, 5.25) | 1.67 (0.57, 4.85) | 1.73 (0.59, 5.01) | 1.72 (0.60, 4.99) |
| P for trend | P=0.278 | P=0.401 | P=0.370 | P=0.372 |
Models: Model 1: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate and systolic blood pressure. Model 2: Model 1+waist circumference (cm). Model 3: Model 2+angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers.
n=674 with 41 cases in continuous analyses and n=682 with 41 cases in tertile analyses.
n=204 with 19 cases in continuous analyses and n=205 with 19 cases in tertile analyses.
n=301 with 28 cases in continuous analyses and n=311 with 28 cases in tertile analyses.
n=369 with 28 cases in continuous analyses and n=372 with 28 cases in tertile analyses.
Figure 1.
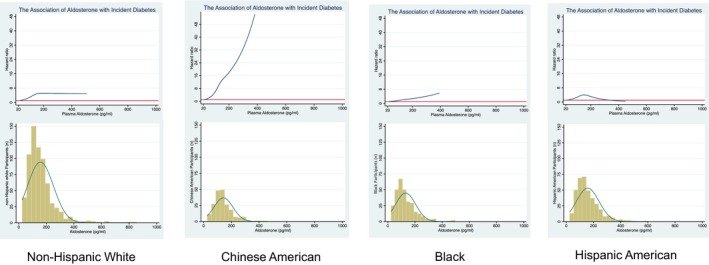
Race/ethnicity‐specific associations of aldosterone with incident diabetes mellitus. The cubic spline regressions estimate the hazard ratio of incident diabetes mellitus, according to concentrations of aldosterone (picograms per milliliter, pg/mL) examined as a continuous variable up to the 99th percentile with 3 knots placed at the 25th, 50th, and 75th percentiles. Splines are adjusted for age, sex, study site, education, current alcohol use, physical activity, estimated glomerular filtration rate, systolic blood pressure, and waist circumference. Below each spline is the histogram of the distribution of aldosterone concentration among participants with detectable aldosterone (n=1548).
The association of log‐aldosterone by renin phenotypes, renin phenotypes alone and log‐aldosterone:renin ratio with incident type 2 diabetes mellitus are presented in Table 6. Participants with PRA ≥1.0 μg/L per hour had a 66% higher risk of incident type 2 diabetes mellitus per 1‐SD higher log‐aldosterone (HR 1.66, 95% CI: 1.08, 2.23) and participants with PRA ≤0.50 μg/L per hour had a 79% higher risk of incident type 2 diabetes mellitus (HR 1.79, 95% CI: 1.21, 2.64) per 1‐SD higher log‐aldosterone in models adjusted for age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate, systolic blood pressure, waist circumference, and ACE‐I/ARBs. These findings remained significant after exclusion of participants taking ACE‐I/ARBs. The association PRA of ≥1.0 μg/L per hour compared with ≤0.51 to 0.99 and 0.50 μg/L per hour with incident type 2 diabetes mellitus was non‐significant. Additionally, the association of log‐aldosterone/PRA ratio with incident diabetes mellitus was non‐significant.
Table 6.
The Association of Log‐Aldosterone by Renin Phenotypes, Renin Phenotypes Alone and Log‐Aldosterone:Renin Ratio With Incident Type 2 Diabetes Mellitus
| (A) Log‐Aldosterone Per 1‐Unit SD (Continuous, n=1453)b | Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus | ||||
|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Median Aldosterone (pg/mL, IQR) | Unadjusted | Model 1a | Model 2a | |
| “Unsuppressed Renin Phenotype” PRA ≥1.0 μg/L per h | 386/34 | 169 (118, 234) | 1.13 (0.78, 1.63) | 1.31 (0.88, 1.94) | 1.66 (1.08, 2.53) |
| “Indeterminate Renin Phenotype” PRA 0.51 to 0.99 μg/L per h | 389/28 | 130 (99, 182) | 1.05 (0.69, 1.60) | 1.16 (0.74, 1.83) | 1.18 (0.74, 1.88) |
| “Suppressed Renin Phenotype” PRA ≤0.50 μg/L per h | 678/48 | 116 (85, 156) | 1.57 (1.10, 2.25) | 1.74 (1.19, 2.54) | 1.79 (1.21, 2.64) |
| (B) Log‐Aldosterone Per 1‐Unit SD (Continuous, n=1179)b |
Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus Excluding Participants Taking ACE‐Inhibitors and Angiotensin Receptor Blockers |
||||
|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Median Aldosterone (pg/mL, IQR) | Unadjusted | Model 1a | Model 2a | |
| “Unsuppressed Renin Phenotype” PRA ≥1.0 μg/L per h | 246/14 | 188 (140, 267) | 1.74 (0.95, 3.16) | 2.23 (1.12, 4.43) | ··· |
| “Indeterminate Renin Phenotype” PRA 0.51 to 0.99 μg/L per h | 338/24 | 136 (101, 187) | 0.99 (0.63, 1.43) | 1.12 (0.68, 1.84) | ··· |
| “Suppressed Renin Phenotype” PRA ≤0.50 μg/L per h | 595/42 | 114 (84, 152) | 1.65 (1.12, 2.42) | 2.00 (1.31, 3.06) | ··· |
| (C) Plasma Renin Activity (n=1453)c | Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus | ||||
|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Median Aldosterone (pg/mL, IQR) | Unadjusted | Model 1a | Model 2a | |
| “Unsuppressed Renin Phenotype” ≥1.0 μg/L per h | 386/34 | 169 (118, 234) | Referent | Referent | Referent |
| “Indeterminate Renin Phenotype” 0.51 to 0.99 μg/L per h | 389/28 | 130 (99, 182) | 0.80 (0.48, 1.32) | 0.79 (0.48, 1.31) | 0.87 (0.52, 1.47) |
| “Suppressed Renin Phenotype” ≤0.50 μg/L per h | 678/48 | 116 (85, 156) | 0.80 (0.52, 1.24) | 0.71 (0.45, 1.12) | 0.79 (0.49, 1.28) |
| (D) Plasma Renin Activity (n=1179)c |
Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus Excluding Participants Taking ACE‐Inhibitors and Angiotensin Receptor Blockers |
||||
|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Median Aldosterone (pg/mL, IQR) | Unadjusted | Model 1a | Model 2a | |
| “Unsuppressed Renin Phenotype” ≥1.0 μg/L per h | 246/14 | 188 (140, 267) | Referent | Referent | ··· |
| “Indeterminate Renin Phenotype” 0.51 to 0.99 μg/L per h | 338/24 | 136 (101, 187) | 1.24 (0.64, 2.40) | 1.13 (0.58, 2.20) | ··· |
| “Suppressed Renin Phenotype” ≤0.50 μg/L per h | 595/42 | 114 (84, 152) | 1.26 (0.69, 2.31) | 1.04 (0.56, 1.96) | ··· |
| (E) Log‐Aldosterone:Plasma Renin Activity Ratio Per 1‐Unit SD (Continuous)d | Cox Proportional Hazards Model—Hazards Ratio (95% CI) for Incident Type 2 Diabetes Mellitus | ||||
|---|---|---|---|---|---|
| Total No. Participants/Diabetes Mellitus Cases | Median Aldosterone (pg/mL, IQR) | Unadjusted | Model 1a | Model 2a | |
| All participants | 1453/110 | 131 (95, 183) | 0.93 (0.77, 1.12) | 0.91 (0.75, 1.10) | 0.97 (0.79, 1.19) |
| Excluding participants taking ACE‐inhibitors and angiotensin receptor blockers | 1179/80 | 133 (95, 186) | 1.10 (0.85, 1.41) | 1.07 (0.82, 1.40) | ··· |
ACE indicates angiotensin‐converting enzyme inhibitors; CI, confidence interval; IQR, interquartile range.
Model 1: age, education, sex, study site, race, alcohol, physical activity, estimated glomerular filtration rate, systolic blood pressure and waist circumference (cm). Model 2+ACE‐inhibitors and angiotensin receptor blockers.
Hazard ratio for incident type 2 diabetes mellitus with log‐aldosterone standard deviations as a continuous exposure, by renin phenotype.
Hazard ratio for incident type 2 diabetes mellitus by renin phenotype.
Hazard ratio for incident type 2 diabetes mellitus with log‐aldosterone/PRA standard deviations as a continuous exposure.
Discussion
In this contemporary, prospective study of multiethnic adults without type 2 diabetes mellitus, higher aldosterone and PRA were associated with higher FPG and insulin resistance at baseline, along with higher risk of incident type 2 diabetes mellitus over 10.5 years. The associations of aldosterone with abnormalities of glucose homeostasis were independent of known type 2 diabetes mellitus risk factors including age, socioeconomic status, race/ethnicity, physical activity, waist circumference, adipokines (leptin and adiponectin) and inflammation (hsCRP, interleukin‐6, TNF‐α). Additionally, PRA did not modify the association, suggesting that the effects of aldosterone are independent of not only known type 2 diabetes mellitus risk factors but also PRA. On the contrary, the association of PRA with incident type 2 diabetes mellitus was attenuated and became non‐significant after adjustment for ACE‐I and ARBs or aldosterone. Type 2 diabetes mellitus risk was higher per log‐aldosterone SD among participants with both unsuppressed renin phenotype and suppressed renin phenotype indicating that individuals with physiologically and autonomously higher aldosterone are both at higher risk of type 2 diabetes mellitus. There were differences in the magnitude of the associations of aldosterone with type 2 diabetes mellitus across racial/ethnic groups with Chinese Americans having the strongest associations. In the tertile and spline analyses, there were dose‐dependent associations of aldosterone with incident type 2 diabetes mellitus in Chinese Americans and blacks but not in Hispanic Americans or NHWs, suggesting potential underlying differences in physiology.
Mechanisms
Preclinical studies suggest that aldosterone induced mineralocorticoid activation increases insulin resistance and impairs insulin secretion.2 Aldosterone inhibits insulin signaling and insulin‐stimulated glucose uptake via glut‐4 translocation in adipocytes, skeletal muscle, and vascular smooth muscle cells, as well as a reduction in adiponectin and peroxisome proliferator‐activated receptor‐gamma.3, 4 Aldosterone impairs β‐cell function and insulin secretion, in preclinical models through blunted glucose‐stimulated insulin secretion, moreover, in aldosterone synthase deficient mice glucose‐stimulated insulin secretion is markedly increased.18 Consistent with these findings, we found higher insulin resistance and lower compensatory β‐cell function with higher levels of aldosterone, suggesting the inability of the β‐cell to increase compensatory insulin secretion in the face of higher insulin resistance may be critical in the pathophysiology of type 2 diabetes mellitus.
In our study, Chinese Americans and blacks had graded associations of aldosterone with incident type 2 diabetes mellitus and greater magnitude of associations for Chinese Americans. There are many plausible mechanisms including salt‐sensitivity, production of aldosterone, receptor binding, and post‐receptor signal transduction in response to aldosterone. In the Dahl Salt‐Sensitive rat, aldosterone is suppressed acutely in response to salt loads, but aldosterone becomes paradoxically elevated over time.19 Inappropriate RAAS activation, combined with the maladaptive inability to lower aldosterone levels in response to high salt intake constitute a major issue in all ethnicities, but may be especially relevant in populations with higher levels of salt‐sensitivity including individuals of Chinese and African ancestry.20, 21 We have previously shown a dose‐response relationship between aldosterone and incident type 2 diabetes mellitus among blacks.6 In MESA, blacks had the lowest serum aldosterone compared with the other racial/ethnic groups, but importantly, the level may be less critical than the post‐receptor signaling cascade evoked by aldosterone. Concordant with this hypothesis, a study of blacks and NHWs found lower aldosterone in blacks during childhood and adulthood, but positive associations of plasma aldosterone with blood pressure only among blacks.22 Furthermore, administration of 9‐α fludrocortisone (synthetic steroid with mineralocorticoid receptor activity) increased blood pressure in blacks but not NHWs.22
Aldosterone is synthesized by aldosterone synthase (CYP11B2). The most studied polymorphism of CYP11B2 is T‐344C in the promoter region and the polymorphism is associated with higher aldosterone levels.23, 24 In a large population of individuals of Chinese and Japanese ancestry, CYP11B2 polymorphisms (Arg‐173 and T‐344C) were associated with higher FPG levels, higher 2‐hour post load glucose, impaired fasting glucose and type 2 diabetes mellitus,25 which is consistent with our findings of a high risk of type 2 diabetes mellitus in Chinese Americans with higher levels of aldosterone. Additionally, a prospective Japanese cohort study with 10 years of follow‐up demonstrated that plasma aldosterone levels predict the development of insulin resistance,26 suggesting our findings might extend to individuals with ancestry from the continent of Asia. Thus, genetic differences in production or response to aldosterone may be responsible for racial/ethnic differences. Our finding that aldosterone's association with glucose metabolism is independent of PRA is consistent with observations made in the pathological state of primary aldosteronism, which is associated with higher levels of insulin resistance.3 The treatment of primary aldosteronism with adrenalectomy or mineralocorticoid receptor antagonism improves insulin sensitivity and insulin secretion.27, 28 The largest pharmacologic diabetes mellitus prevention trials examining RAAS antagonism as a primary end point, the DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) study and the NAVIGATOR (Nateglinide and Valsartan in Impaired Glucose Tolerance Outcome Research) study, both showed modest reductions in risk of diabetes mellitus with an ACE‐I or ARB, which were significant in NAVIGATOR (14% risk reduction; P<0.0001).29, 30 These trials were majority NHW and the graded associations of aldosterone with incident diabetes mellitus among blacks and Chinese Americans but not among NHWs, suggests a potential for greater risk reduction with modulation of the RAAS system among blacks and Chinese Americans. Consistent with this hypothesis, incident diabetes mellitus was analyzed as a secondary end point in the AASK trial (African American Study of Kidney Disease and Hypertension Trial) and in the Candesartan Antihypertensive Survival Evaluation in Japan (CASE‐J).29, 31, 32 Among black participants in AASK ramipril (ACE‐I) compared with metoprolol or amlodipine was associated with a 36% reduction (95% CI: 0.45–0.90) in incident diabetes mellitus.29 Among Japanese participants in CASE‐J, candesartan versus placebo was associated with a 36% relative risk reduction (hazard ratio: 0.64; 95% CI: 0.43–0.97; P=0.033) with similar blood pressure reductions in both groups. When considered in comparison to the 9% and 14% diabetes mellitus risk reduction in DREAM and NAVIGATOR, these findings provide additional support for the possibility of racial/ethnic differences in the glycemic response to modulation of the RAAS system. Notably, although these are secondary analyses, the diabetes mellitus risk reduction of 36% in both the AASK and CASE‐J trials are greater than the 31% diabetes mellitus risk reduction with metformin in the Diabetes Prevention Program, a medication currently recommended by national guidelines for prevention/delay of type 2 diabetes mellitus.33, 34
Strengths and Limitations
Our findings should be considered in light of potential limitations. First, aldosterone and PRA were measured at 1 point in time, not allowing us to assess changes in aldosterone and PRA with glucose metabolism. Second, we did not have a measure of 24‐hour urinary sodium in the cohort and thus were unable to assess the impact of dietary salt intake. Third, sample sizes varied for the race/ethnic groups with power implications for detecting significant racial/ethnic interactions in stratified analyses. Fourth, we were unable to distinguish between type 1 and type 2 diabetes mellitus, but incident type 1 diabetes mellitus is extremely uncommon in older adults, so we assume a predominance of type 2 diabetes mellitus. Lastly, we may have underestimated the relationship of aldosterone with incident type 2 diabetes mellitus, as individuals with 2‐hour post load glucose impairment or hemoglobin A1c ≥6.5% (48 mmol/mol) may have remained undetected. The strengths of our study include a racial/ethnically diverse cohort with long‐term follow‐up. We also have a comprehensive, laboratory‐ and medication‐based documentation of diabetes mellitus, in addition to data on estimates of insulin resistance and β‐cell function.
Perspectives
In conclusion, our study suggests that higher levels of aldosterone are associated with insulin resistance and incident type 2 diabetes mellitus among multiethnic individuals, suggesting pleiotropic effects of aldosterone may be relevant to the pathogenesis of type 2 diabetes mellitus. The greatest magnitude of association of aldosterone with incident type 2 diabetes mellitus was in Chinese Americans and dose‐dependent responses were seen in Chinese Americans and blacks. Further research to elucidate mechanisms for the racial/ethnic differences in the association of aldosterone with incident type 2 diabetes mellitus is of preeminent importance given the potential to lower risk of type 2 diabetes mellitus through targeted modulation of the RAAS system.
Sources of Funding
This research was supported by contracts N01‐HC‐95159 through N01‐HC‐95165 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute, as well as an NIDDK grant (R01 DK080015).
Disclosures
None.
Supporting information
Table S1. P for Interaction by Race
Table S2. Characteristics of Participants in MESA by Race/Ethnicity
Table S3. The Association of Log Aldosterone and Log Plasma Renin Activity With Fasting Plasma Glucose by Race/Ethnicity
Figure S1. Multiethnic Study of Atherosclerosis (MESA) calcified renal artery atherosclerosis ancillary study consort flow diagram.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2018;7:e009890 DOI: 10.1161/JAHA.118.009890.)
References
- 1. Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218. [DOI] [PubMed] [Google Scholar]
- 2. Luther JM, Brown NJ. The renin‐angiotensin‐aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity‐related changes in expression of adiponectin, peroxisome proliferator‐activated receptor‐, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reincke M, Meisinger C, Holle R, Quinkler M, Hahner S, Beuschlein F, Bidlingmaier M, Seissler J, Endres S. Is primary aldosteronism associated with diabetes mellitus? Results of the German Conn's Registry. Horm Metab Res. 2010;42:435–439. [DOI] [PubMed] [Google Scholar]
- 6. Joseph JJ, Echouffo‐Tcheugui JB, Kalyani RR, Yeh H‐C, Bertoni AG, Effoe VS, Casanova R, Sims M, Correa A, Wu W‐C, Wand GS, Golden SH. Aldosterone, renin, and diabetes mellitus in African Americans: the Jackson Heart Study. J Clin Endocrinol Metab. 2016;101:1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu C‐J, Sanjanwala AR, Edwards MA, Gomez‐Sanchez CE, Nanba K, Rainey WE. Aldosterone‐stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112:E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age‐related autonomous aldosteronism clinical perspective. Circulation. 2017;136:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JM, Robinson‐Cohen C, Luque‐Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bild DE. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 11. Joseph JJ, Echouffo‐Tcheugui JB, Golden SH, Chen H, Jenny NS, Carnethon MR, Jacobs D Jr, Burke GL, Vaidya D, Ouyang P, Bertoni AG. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: the Multi‐Ethnic Study of Atherosclerosis (MESA). BMJ Open Diabetes Res Care. 2016;4:e000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. [DOI] [PubMed] [Google Scholar]
- 16. Joseph JJ, Golden SH. Type 2 diabetes and cardiovascular disease: what next? Curr Opin Endocrinol Diabetes Obes. 2014;21:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morizane S, Mitani F, Ozawa K, Ito K, Matsuhashi T, Katsumata Y, Ito H, Yan X, Shinmura K, Nishiyama A, Honma S, Suzuki T, Funder JW, Fukuda K, Sano M. Biphasic time course of the changes in aldosterone biosynthesis under high‐salt conditions in Dahl salt‐sensitive rats. Arterioscler Thromb Vasc Biol. 2012;32:1194–1203. [DOI] [PubMed] [Google Scholar]
- 20. Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension. 1996;28:854–858. [DOI] [PubMed] [Google Scholar]
- 21. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, DiMeglio LA, Jung J, Pratt JH. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahimi Z, Moradi M, Nasri H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J Res Med Sci. 2014;19:1090. [PMC free article] [PubMed] [Google Scholar]
- 24. Russo P, Siani A, Venezia A, Iacone R, Russo O, Barba G, D'Elia L, Cappuccio FP, Strazzullo P. Interaction between the C(‐344)T polymorphism of CYP11B2 and age in the regulation of blood pressure and plasma aldosterone levels: cross‐sectional and longitudinal findings of the Olivetti Prospective Heart Study. J Hypertens. 2002;20:1785–1792. [DOI] [PubMed] [Google Scholar]
- 25. Ranade K, Wu KD, Risch N, Olivier M, Pei D, Hsiao CF, Chuang LM, Ho LT, Jorgenson E, Pesich R, Chen YD, Dzau V, Lin A, Olshen RA, Curb D, Cox DR, Botstein D. Genetic variation in aldosterone synthase predicts plasma glucose levels. Proc Natl Acad Sci USA. 2001;98:13219–13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumagai E, Adachi H, Jacobs DR, Hirai Y, Enomoto M, Fukami A, Otsuka M, Kumagae S‐I, Nanjo Y, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Kasahara A, Tsukagawa E, Ohbu‐Murayama K, Imaizumi T. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–1048. [DOI] [PubMed] [Google Scholar]
- 27. Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA. Insulin sensitivity in patients with primary aldosteronism: a follow‐up study. J Clin Endocrinol Metab. 2006;91:3457–3463. [DOI] [PubMed] [Google Scholar]
- 28. Fischer E, Adolf C, Pallauf A, Then C, Bidlingmaier M, Beuschlein F, Seissler J , Reincke M. Aldosterone excess impairs first phase insulin secretion in primary aldosteronism. J Clin Endocrinol Metab. 2013;98:2513–2520. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Mallah M, Khawaja O, Sinno M, Alzohaili O, Samra ABA. Do angiotensin converting enzyme inhibitors or angiotensin receptor blockers prevent diabetes mellitus? A meta‐analysis. Cardiol J. 2010;17:448–456. [PubMed] [Google Scholar]
- 30. NAVIGATOR Study Group , McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. [DOI] [PubMed] [Google Scholar]
- 31. Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, Randall O, Rostand S, Sherer S, Toto RD, Wright JT, Wang X, Greene T, Appel LJ, Lewis J. Cardiovascular outcomes in the African American Study of kidney disease and hypertension (AASK) Trial. Am J Kidney Dis. 2006;48:739–751. [DOI] [PubMed] [Google Scholar]
- 32. Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M, Fukushima Y, Kikuchi M, Fujita T. High‐salt diet enhances insulin signaling and induces insulin resistance in Dahl salt‐sensitive rats. Hypertension. 2002;40:83–89. [DOI] [PubMed] [Google Scholar]
- 33. The Diabetes Prevention Program . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Diabetes Association . Standards of medical care in diabetes—2017. Diabetes Care. 2017;40 (suppl_1):S1–S142.27979885 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. P for Interaction by Race
Table S2. Characteristics of Participants in MESA by Race/Ethnicity
Table S3. The Association of Log Aldosterone and Log Plasma Renin Activity With Fasting Plasma Glucose by Race/Ethnicity
Figure S1. Multiethnic Study of Atherosclerosis (MESA) calcified renal artery atherosclerosis ancillary study consort flow diagram.


