Abstract
Background
Only 50% of eligible atrial fibrillation (AF) patients receive anticoagulation (AC). Feasibility and effectiveness of electronic medical record (EMR)–based interventions to profile and raise provider AC percentage is poorly understood. The SUPPORT‐AF (Supporting Use of AC Through Provider Profiling of Oral AC Therapy for AF) study aims to improve rates of adherence to AC guidelines by developing and delivering supportive tools based on the EMR to providers treating patients with AF.
Methods and Results
We emailed cardiologists and community‐based primary care providers affiliated with our institution reports of their AC percentage relative to peers. We also sent an electronic medical record–based message to these providers the day before an appointment with an atrial fibrillation patient who was eligible but not receiving AC. The electronic medical record message asked the provider to discuss AC with the patient if he or she deemed it appropriate. To assess feasibility, we tracked provider review of our correspondence. We also tracked the change in AC for intervention providers relative to alternate primary care providers not receiving our intervention. We identified 3786, 1054, and 566 patients cared for by 49 cardiology providers, 90 community‐based primary care providers, and 88 control providers, respectively. At baseline, the percentage of AC was 71.3%, 63.5%, and 58.3% for these 3 respective groups. Intervention providers reviewed our e‐mails and electronic medical record messages 45% and 96% of the time, respectively. For providers responding, patient refusal was the most common reason for patients not being on AC (21%) followed by high bleeding risk (19%). At follow‐up 10 weeks later, change in AC was no different for either cardiology or community‐based primary care providers relative to controls (0.2% lower and 0.01% higher, respectively).
Conclusions
Our intervention profiling AC was feasible, but not sufficient to increase AC in our population.
Keywords: anticoagulation, atrial fibrillation, electronic medical record
Subject Categories: Atrial Fibrillation, Anticoagulants, Quality and Outcomes
Clinical Perspective
What Is New?
Electronic medical record–based messaging along with e‐mails can be used to prompt clinicians to review their anticoagulation prescribing practices for their patients with atrial fibrillation.
What Are the Clinical Implications?
Raising the percentage of anticoagulation use, however, may require more than electronic messaging.
Introduction
Atrial fibrillation (AF) currently affects 5.2 million Americans, with 12 million projected by 2050.1, 2, 3 AF accounts for 15% of all ischemic strokes and one third of all strokes in the elderly.4 These strokes result in permanent disability in 60% of patients and death in up to 20%.5 Anticoagulation (AC) therapy is the cornerstone for stroke prevention in patients with AF. However, only around half of eligible patients receive AC in contemporary US‐based, ambulatory registries.6
Providers and patients struggle with the decision to initiate AC in new cases of AF or resume AC in patients previously on it. The American Heart Association/American College of Cardiology guidelines advise providers about stroke risk, recommending initiation of AC for eligible patients (ie, those with a stroke risk CHA2DS2‐VASc score of 2 or higher).7 Providers often point to concerns about complications from AC in older, frail patients and prohibitive bleeding risks as reasons for not prescribing AC, but data show that physicians often overestimate risks of bleeding and falling and underestimate risks of stroke.6 Although much work has been done to educate providers about the risks of undertreatment, an “AC treatment gap” between eligible and treated AF patients persists, leading to significant morbidity and mortality.8, 9
Recently, the American College of Cardiology began collecting AC data from cardiovascular specialists through the PINNACLE (Practice Innovation and Clinical Excellence) registry. Initial findings from the PINNACLE registry confirm the low prescription percentage found in previous studies and help to identify undertreatment of AF as a national problem.10 Although the PINNACLE registry provides a first step toward identifying the gap in AC prescription, raising percentages will likely require more‐structured interventions that will need to be integrated into individual healthcare system electronic medical record (EMR) and provider workflows. Previous work by 1 of our team members (S.L.) has determined that identifying patients with AF and their stroke risk using the EMR is feasible.11 We built on that work to test whether e‐mailing providers reports with the percentage of AC they prescribed relative to peers was feasible. In addition, we assessed whether sending providers EMR‐based messages to notify them of an upcoming appointment with an eligible AF patient not on AC was also feasible. Finally, we assessed the effectiveness of our intervention by measuring the change in AC percentage by provider over a short time period available to us before the rollout of a new EMR. In contrast to the PINNACLE registry, we include primary care providers (in addition to cardiology providers) in our intervention in order to understand their contribution to AC decision making. Our intervention also stands in contrast to 3 recent publications12, 13, 14 that assess the impact of clinical decision support interventions, but without peer comparison.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The project was approved by the Institutional Review Board at the University of Massachusetts Medical School, and waiver of consent was obtained.
Setting
The UMass‐Memorial Medical Center is the largest not‐for‐profit healthcare system in Central Massachusetts with 1600 physicians and 13 500 employees. Its comprehensive network of care includes a 3‐campus academic medical center, 3 in‐network community hospitals, and several affiliated community hospitals. The UMass‐Memorial Medical Center has a large ambulatory service footprint, with an effective catchment area of nearly 1 million individuals. This nonprofit organization offers a fully integrated healthcare continuum with a multicampus academic medical center, member and affiliated community hospitals, freestanding physician practices, and ambulatory clinics. The UMass‐Memorial Medical Center used the Allscripts ambulatory EMR (Allscripts, Inc, Chicago, IL)15 during the period under study, that captures visit dates, billing codes, problem lists (diagnostic codes), and is used to either document or prescribe medication, including AC.
Population
First, we identified patients with AF based on their having an International Classification of Diseases, Tenth Revision (ICD‐10) code of I48.0, I48.1, I48.2, or I48.91 coded in the ambulatory EMR. We then restricted our sample to focus on patients at high stroke risk by identifying those patients with a CHA2DS2‐VASc stroke prediction score of 2 or more based on work by Lip et al.16 This threshold was defined on the basis of the fact that multiple professional societies have defined this risk score as the optimal threshold to initiate AC to prevent ischemic stroke.7, 17 The CHA2DS2‐VASc score assigns 1 point for congestive heart failure, hypertension, age 65 to 74, diabetes mellitus, vascular disease history like myocardial infarction, and female sex and 2 points for age 75+ or previous stroke or transient ischemic attack. Our software calculated each patient's CHA2DS2‐VASc score automatically using active medical problems coded within the EMR. Specifically, we used ICD‐10 codes associated with outpatient clinical encounters (Table S1). Furthermore, to be included in our study, AF patients had to have at least 1 ambulatory visit in the past 12 months with either a cardiology or primary care provider from our medical group. The primary care providers in our sample included healthcare providers from across several disciplines of internal medicine, including family medicine or geriatrics, and providers from multiple training backgrounds, including nurse practitioners and medical doctors.
Provider Assignment
If an AF patient saw a cardiology provider (either nurse practitioner or medical doctor) in the past 12 months, we assigned the patient to him or her. This decision was made on the basis of a prespecified assumption that the patient's PCP would likely defer decision making regarding AC to the treating cardiology provider. For all other AF patients with a medical provider but no cardiology visits, we assigned the patient to the primary care provider.
Determining AC Status
Every AF patient's AC status was automatically coded as “receiving AC,” if the EMR indicated that they had an active prescription for warfarin/coumarin/Coumadin/or any vitamin K antagonist, enoxaparin, dalteparin, dabigatran, rivaroxaban, apixaban, or edoxaban at the time of the report. The same report was run at baseline and after 10 weeks of intervention for the intervention and control medical providers. As a way to validate our identification of AC status, we reviewed 100 charts manually and found that electronic capture of AC status was highly accurate (sensitivity 95% and specificity 100%).
Design of Report With AC Percentage
We designed a graphical report that tracks providers’ change in AC use over time following the Agency for Healthcare Research and Quality's handbook for developing performance measurement tools.18 More specifically, we used Tableau visual analytic software19 to create reports based on live data in the Allscripts EMR to profile AC prescription practices for each provider included in our intervention group (Figure 1A). The content of the report was based on the number of AF patients at high risk for stroke seen within the past 12 months by the healthcare provider (≤10 versus >10). For providers with more than 10 AF patients eligible for AC, in the upper half of our profile page, we displayed a bar graph showing the percentage and number of eligible AF patients currently receiving AC over the total number of AF patients eligible for AC. We provided the report in this fashion to any provider seeing more than 10 eligible patients. We superimposed 2 lines representing benchmarks on the bar graph to enhance impact and help providers assess their prescription rate. The first line showed the percentage of AC prescription for eligible AF patients among “peers,” using the average AC use among eligible AF patients treated by cardiology or primary care providers for cardiology and primary care providers, respectively. We also included a second line at 80%, a target suggested by certain groups as a practice‐level AC prescription goal.20 For providers with 1 to 10 eligible patients, we felt graphing performance would not be as meaningful, and so we reported the percentage of AF patients the provider had treated in the past 12 months without providing a bar graph or peer comparison (Figure 1B). In the lower half of the report, we included the medical record numbers and stroke risk predictors for all AF patients eligible but not currently receiving AC. We also included a list of AC‐eligible AF patients with appointments in the next 31 days to focus providers on an actionable group of patients with whom they might review the use of AC.
Figure 1.
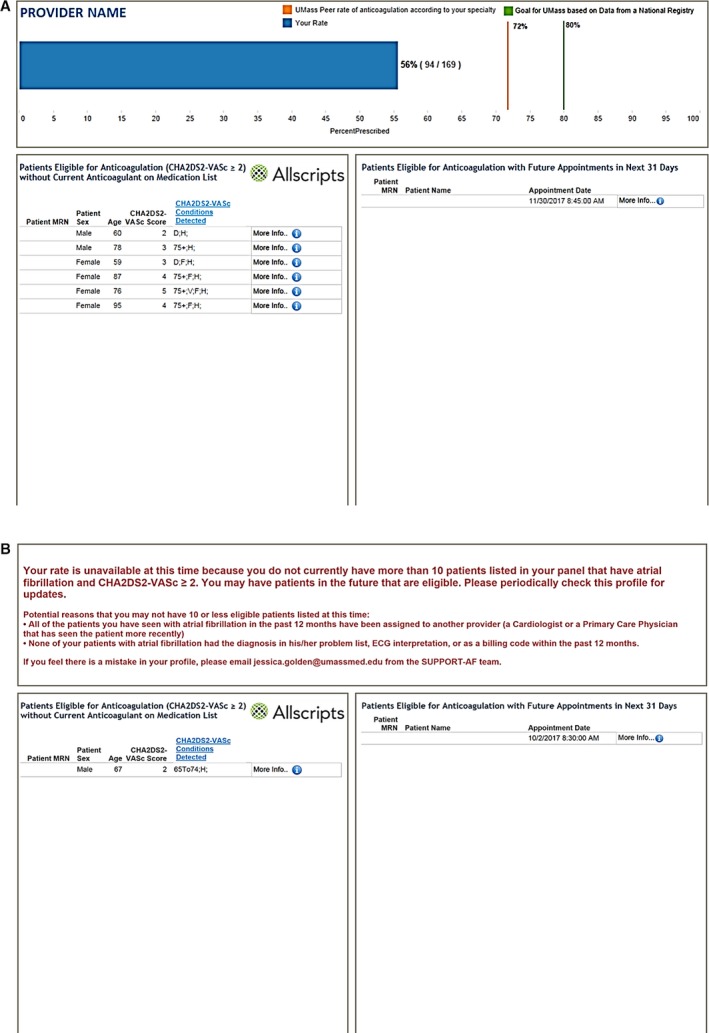
Example of report sent to providers caring for AC‐eligible AF patients with CHA 2 DS 2‐VASc ≥2. A, Sample report for providers with more than 10 eligible patients. MRN indicates medical record number; UMass, University of Massachusetts. B, Sample report for providers with 10 or fewer eligible patients. AC indicates anticoagulation; AF, atrial fibrillation; MRN, medical record number. CHA 2 DS 2‐VASc conditions key: C: Congestive Heart Failure, H: Hypertension, 75+: Age ≥75, D: Diabetes Mellitus, S: Stroke or Transient Ischemic Attack, V: Vascular Disease, 65 to 74: Age 65 to 74, F: Female.
Design of EMR Message
We sent EMR messages from a user account created for our study team to individual providers 1 day before any scheduled appointment with an AC‐eligible AF patient (Figure 2). The EMR message was linked to the AF patient's medical record, facilitating review of patient charts by providers as well as providing a format for providers to communicate back with our team.
Figure 2.
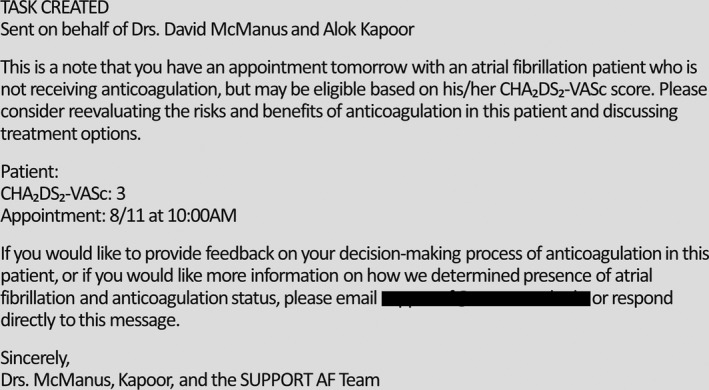
Screenshot of EMR message sent to providers 1 day before an appointment with an AC‐eligible AF patient. AC indicates anticoagulation; AF, atrial fibrillation; EMR, electronic medical record.
Intervention Groups
Based on strong support for our work within UMass‐Memorial Medical Center cardiovascular medicine and community‐based primary care leadership, we targeted providers from these 2 groups to receive our AC intervention. As a first step, the chiefs of the 2 intervention cohorts distributed an e‐mail notifying their respective providers about our project and impending distribution of reports by our group. One week later, in July 2017, we sent e‐mails with the report, first to cardiology providers, and then 2 weeks later to the community‐based primary care group. Subsequently, we sent 2 follow‐up e‐mails at 2‐week intervals from the initial mailing. These were identical to the first e‐mail in structure, but included reports with updated AC information.
Because our institution moved to a new EMR in October 2017, we stopped sending AC‐related practice profile reports and EMR messages in September 2017. Cardiology providers received the intervention over a 10‐week period and community‐based primary care over 8 weeks. This time period (July 1, 2017 to September 30, 2017) also served as our follow‐up period for the purpose of all our analyses.
Control Group
We did not deliver our intervention to an alternate group of providers based at our university campus. The percentage of AC use prescribed by these alternate providers served as controls (reference) to the patients cared for by our intervention providers in order to adjust for temporal trends in AC use. The control group included patients seen by primary care providers from the disciplines of general medicine, family medicine, or geriatrics. Because we initiated the intervention at different time points for cardiology versus community‐based primary care providers, the controls paired with each intervention group differed slightly in order to conform to inclusion criterion which required that each patient saw his or her assigned provider within the past 12 months. Thereby, the inception date for controls to the community‐based primary care providers lagged by 2 weeks compared with the inception date for controls to the cardiology providers.
Outcomes
To assess feasibility of the intervention, we tabulated how often providers in the intervention groups read our e‐mails with report of their AC percentage using Microsoft Outlook–based read receipts. We also tracked how many providers reviewed the EMR message items we sent them. Specifically, if the provider replied to the EMR message or marked the EMR message as complete, in progress, or removed, we assumed that the EMR message had been reviewed. If the provider did not reply and the EMR message remained “active” in the provider's inbox, we counted the EMR message as not reviewed. Finally, we also tallied and thematically coded providers’ reasons for not prescribing AC from responses to our provider EMR messages.
To measure the effectiveness of our intervention on AC use, we calculated the change in percentage of eligible patients on AC for intervention and control groups.
Statistical Analysis
For our primary analyses, we only included patients with no change in provider and who met the 12‐month eligibility criterion at baseline as well as at the end of the follow‐up period. Patients’ follow‐up (postintervention) anticoagulation status was modeled as a function of patients’ baseline (preintervention) anticoagulation status, provider's intervention/control status, and CHA2DS2‐VASc score. We hypothesized that the change in AC percentage would be higher in patients of intervention providers compared with those of control providers. To account for within‐provider clustering, we used generalized estimation equation logistic regression.21 In these analyses, patient AC status was modeled using generalized estimation equation logistic regression as a function of time period (baseline/follow‐up), provider's intervention or control status, and their interaction, again adjusting for CHA2DS2‐VASc score. We hypothesized a statistically significant interaction, such that the follow‐up versus baseline difference in AC prescription percentage would be larger for patients treated by intervention providers than for those treated by control providers. To examine whether any intervention‐control difference varied by provider type, these analyses were conducted separately for cardiology (intervention) versus control providers and for community‐based primary care providers (intervention) versus control providers. In both analyses, control providers were the same; the set of control patients included varied slightly for the 2 analyses because the 12‐month inclusion criterion was applied 2 weeks earlier for analyses including cardiology providers than for analyses including community‐based primary care providers.
In a sensitivity analysis, we included patients with a change in provider or who met the 12‐month inclusion criterion only at baseline or only at follow‐up (ie, the patient only contributed to a provider's percentage at baseline or follow‐up but not both). These analyses again were conducted separately for cardiology providers versus controls and for community‐based primary care providers versus controls. Finally, we re‐examined the effect of our intervention in several subsets. These included examining the effect in the subset of patients who saw their assigned provider during the intervention period, the subset of patients assigned to providers who read varying numbers of our e‐mails containing the report with AC prescription percentage, the subset of patients assigned to a provider who replied to multiple EMR messages we sent, and the subset of patients with CHA2DS2‐VASc score of 2 or 3 and CHA2DS2‐VASc score 4 or 5 (versus CHA2DS2‐VASc score 6+).
We performed all analyses in SAS software (version 9.4; SAS Institute Inc, Cary, NC).22
Results
In July 2017 at study onset, we identified 3786 patients with AF receiving care from cardiology providers and 562 treated by controls. Two weeks later, at the time of study onset for community‐based primary care providers, we identified 1054 AF patients cared for by participating community‐based primary care providers as well as 566 treated by controls. The number of AF patients with CHA2DS2‐VASc score of 2, 3, 4, and 5+ was relatively evenly distributed across provider groups (Table 1). Providers receiving our intervention cared for AF patients with slightly higher CHA2DS2‐VASc scores. The proportion of AF patients treated with AC varied across provider types, with cardiologists more frequently prescribing AC for their eligible patients than primary care physicians in both intervention and control groups. Cardiologists also saw a considerably higher volume of AC‐eligible AF patients, with nearly 70% of providers seeing over 100 such patients, in contrast to 0% among primary care providers in both intervention and control groups (Table 1). Race and sex did not vary significantly across the provider groups. However, primary care providers receiving the AC intervention did, on average, see a higher proportion of patients over the age of 75 years.
Table 1.
Comparison of Key Characteristics Recorded at Beginning of Intervention for Patients From 3 Provider Groups
| Characteristic | Cardiology Providers | Community‐Based Primary Care Providers | Controlsa |
|---|---|---|---|
| Frequency (% Out of 3786 Total) | Frequency (% Out of 1054 Total) | Frequency (% Out of 566 Total) | |
| Age, y | |||
| 75+ | 2105 (55.6) | 666 (63.2) | 327 (57.8) |
| 65 to 74 | 1262 (33.3) | 312 (29.6) | 161 (28.5) |
| <65 | 418 (11.0) | 76 (7.2) | 78 (13.8) |
| Female sex | 1672 (44.2) | 490 (46.5) | 281 (50.0) |
| Median area‐level annual income | |||
| ≤100% poverty level | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| 100% to 400% poverty level | 2446 (65.0) | 698 (66.4) | 337 (60.8) |
| Nonwhite race | 243 (6.4) | 47 (4.5) | 36 (6.4) |
| Hispanic ethnicity | 78 (2.1) | 16 (1.5) | 17 (3.0) |
| Non‐English‐language preference | 231 (6.1) | 23 (2.2) | 21 (3.7) |
| Insurance | |||
| Commercial | 430 (11.4) | 104 (9.9) | 69 (12.2) |
| Medicare | 3113 (82.2) | 909 (86.2) | 457 (80.7) |
| Medicaid | 97 (2.6) | 21 (2.0) | 22 (3.9) |
| Other/MA state health insurance exchange | 109 (2.9) | 20 (1.9) | 16 (2.8) |
| Uninsured/self‐pay | 37 (1.0) | 0 (0.0) | 2 (0.4) |
| Individual CHA2DS2‐VASc comorbidities | |||
| CHF | 1066 (28.2) | 202 (19.2) | 99 (17.5) |
| Hypertension | 3046 (80.5) | 870 (82.5) | 442 (78.1) |
| Diabetes mellitus | 972 (25.7) | 310 (29.4) | 182 (32.2) |
| Stroke/TIA | 367 (9.7) | 98 (9.3) | 58 (10.3) |
| Vascular disease | 1366 (36.1) | 295 (28.0) | 123 (21.7) |
| CHA2DS2‐VASc score | |||
| 2 | 741 (19.6) | 200 (19.0) | 127 (22.4) |
| 3 | 953 (25.2) | 248 (23.5) | 158 (27.9) |
| 4 | 1036 (27.4) | 310 (29.4) | 146 (25.8) |
| 5 | 643 (17.0) | 185 (17.6) | 85 (15.0) |
| 6 | 294 (7.8) | 85 (8.1) | 33 (5.8) |
| 7 | 93 (2.5) | 20 (1.9) | 13 (2.3) |
| 8 | 21 (0.6) | 6 (0.6) | 4 (0.7) |
| 9 | 5 (0.1) | 0 (0.0) | 0 (0.0) |
| Anticoagulant use | |||
| Warfarin | 1879 (49.6) | 432 (41.0) | 224 (39.6) |
| Direct oral anticoagulant | 836 (22.1) | 242 (23.0) | 109 (19.3) |
| None | 1071 (28.3) | 380 (36.1) | 233 (41.2) |
| Timing of visit with providerb | |||
| Early | 407 (10.8) | 144 (13.7) | 95 (16.8) |
| Middle | 423 (11.2) | 163 (15.5) | 85 (15.0) |
| Late | 404 (10.7) | 163 (15.5) | 68 (12.0) |
| None | 2552 (67.4) | 584 (55.4) | 318 (56.2) |
| Anticoagulation‐eligible panel size of patient's provider | |||
| 1 to 10 | 51 (1.3) | 249 (23.6) | 230 (40.6) |
| 11 to 50 | 558 (14.7) | 689 (65.4) | 336 (59.4) |
| 51 to 100 | 643 (17.0) | 116 (11.0) | 0 (0.0) |
| >100 | 2534 (66.9) | 0 (0.0) | 0 (0.0) |
CHF indicates congestive heart failure; TIA, transient ischemic attack.
In the table, we report only the frequencies for patients serving as controls for community‐based primary care providers. Controls for cardiology providers were mostly the same patients, but because we rolled out the intervention 2 weeks earlier for cardiology providers, frequencies shifted slightly from 562 to 566 patients based on the requirement to have last appointment within 12 months.
Early visit occurred between days 0 and 32 for cardiology and 0 and 29 for community‐based primary care providers and controls; middle occurred between days 32 and 57 for cardiology and 29 and 49 for community‐based primary care providers and controls; finally, late visits occurred between days 57 and 79 for cardiology and 49 and 65 for community‐based primary care providers and controls.
We also compared patients receiving AC and those not receiving AC for the same baseline characteristics. Patients were comparable in age, income, race, and insurance. There were more patients with CHA2DS2‐VASc score ≥2 in the group receiving AC. Patients off AC were more than twice as likely to be under the care of a provider whose AC‐eligible panel size was 1 to 10 (Table 2).
Table 2.
Comparison of Key Characteristics Recorded at Beginning of Intervention for Patients on AC vs Patients Not on AC
| Characteristic | On AC | Off AC |
|---|---|---|
| Frequency (% Out of 3740 Total) | Frequency (% Out of 1666 Total) | |
| Age, y | ||
| 75+ | 2131 (57.0) | 967 (58.0) |
| 65 to 74 | 1228 (32.8) | 507 (30.4) |
| <65 | 381 (10.2) | 192 (11.5) |
| Female sex | 1636 (43.7) | 807 (48.4) |
| Median area‐level annual income | ||
| ≤100% poverty level | 1 (0.0) | 1 (0.1) |
| 100% to 400% poverty level | 2412 (64.9) | 1069 (64.6) |
| Nonwhite race | 236 (6.3) | 90 (5.4) |
| Hispanic ethnicity | 82 (2.2) | 29 (1.7) |
| Non‐English‐language preference | 208 (5.6) | 67 (4.0) |
| Insurance | ||
| Commercial | 399 (10.7) | 204 (12.2) |
| Medicare | 3110 (83.2) | 1369 (82.2) |
| Medicaid | 96 (2.6) | 44 (2.6) |
| Other/MA state health insurance exchange | 107 (2.9) | 38 (2.3) |
| Uninsured/self‐pay | 28 (0.7) | 11 (0.7) |
| Individual CHA2DS2‐VASc comorbidities | ||
| CHF | 1017 (27.2) | 350 (21.0) |
| Hypertension | 3040 (81.3) | 1318 (79.1) |
| Diabetes mellitus | 1058 (28.3) | 406 (24.4) |
| Stroke/TIA | 431 (11.5) | 92 (5.5) |
| Vascular disease | 1251 (33.4) | 533 (32.0) |
| CHA2DS2‐VASc score | ||
| 2 | 668 (17.9) | 400 (24.0) |
| 3 | 939 (25.1) | 420 (25.2) |
| 4 | 1058 (28.3) | 434 (26.1) |
| 5 | 638 (17.1) | 275 (16.5) |
| 6 | 311 (8.3) | 101 (6.1) |
| 7 | 99 (2.6) | 27 (1.6) |
| 8 | 23 (0.6) | 8 (0.5) |
| 9 | 4 (0.1) | 1 (0.1) |
| Anticoagulant use | ||
| Warfarin | 2535 (67.8) | N/A |
| Direct oral anticoagulant | 1187 (37.7) | N/A |
| Other | 18 (0.5) | N/A |
| None | N/A | 1666 (100.0) |
| Timing of visit with providera | ||
| Early | 476 (12.7) | 170 (10.2) |
| Middle | 496 (13.3) | 175 (10.5) |
| Late | 497 (13.3) | 138 (8.3) |
| None | 2271 (60.7) | 1183 (71.0) |
| AC‐eligible panel size of patient's provider | ||
| 1 to 10 | 526 (14.1) | 524 (31.5) |
| 11 to 50 | 1146 (30.6) | 581 (34.9) |
| 51 to 100 | 394 (10.5) | 338 (20.3) |
| >100 | 1674 (44.8) | 223 (13.4) |
AC indicates anticoagulation; AF, atrial fibrillation; CHF, congestive heart failure; N/A, not applicable; TIA, transient ischemic attack.
Early visit occurred between days 0 and 32 for cardiology and 0 and 29 for community‐based primary care providers and controls; middle occurred between days 32 and 57 for cardiology and 29 and 49 for community‐based primary care providers and controls; finally, late visits occurred between days 57 and 79 for cardiology and 49 and 65 for community‐based primary care providers and controls.
In terms of feasibility of the e‐mail component of our intervention, we found that most patients received care from a provider who read at least 1 of our e‐mails. More specifically, among patients receiving care from cardiology providers, 676 (17.9%) received care from providers who read 1 e‐mail and 1391 (36.7%) received care from providers who read more than 1 of our e‐mails. We observed similar trends among patients receiving care from community‐based primary care providers (Table 3).
Table 3.
Frequency of Patients Receiving Care by Providers Reading Variable Number of E‐mails
| Provider Group | No E‐mail Read (%) | One E‐mail Read (%) | More Than 1 E‐mail Read (%) | Total |
|---|---|---|---|---|
| Cardiology | 1719 (45.4) | 676 (17.9) | 1391 (36.7) | 3786 |
| Community‐based primary care providers | 449 (42.6) | 204 (19.4) | 401 (38.0) | 1054 |
In terms of feasibility of the EMR message component of our intervention, we found that providers reviewed our EMR messages 96% of the time. We sent a total of 432 EMR messages to 88 providers (31 cardiology providers and 57 community‐based primary care providers) for upcoming appointments with 362 unique patients over the course of the intervention. Out of these 432 messages, we received 163 responses (or 37.5% response rate) from 64 unique providers (19 cardiology and 45 community based). The 64 providers who responded to our EMR‐based messages cared for 51.7% of all eligible AF patients not on AC. Overall, 77.3% of the responses included reasons for not anticoagulating these patients (Table 4). Of the 163 responses, healthcare providers cited AF patient refusal to start/resume AC as the most common reason for a patient not being on AC (21%), followed by the provider's perception that the patient was at a prohibitively high risk for bleeding (19%) and deferral to primary cardiologist or other physician (15%). Cardiologists and community‐based primary care providers both noted that patients refused AC because they were worried about bleeding risks or other adverse events, or knew someone who died from complications of AC. Patient history of gastric bleeding, nose bleeding, intracerebral bleeding, rectal bleeding, hematuria, and anemia were among the reasons providers cited as explanations for withholding AC. Of the 111 community‐based primary care providers’ responses to the EMR messages, 24.3% deferred to a cardiology specialist. These responses referred to notes in which the patient's cardiologist deemed AC was unnecessary or inappropriate, requested our research group to contact the patient's cardiologist instead, or informed us that the patient would discuss treatment options with his or her cardiologist at a future appointment.
Table 4.
Distribution of Primary Reasons for Not Prescribing AC Among Providers Responding to EMR Messages Sent as Part of Prescriber Profiling Intervention (N=126a)
| Reasona | Overall Frequency (%) N=102 | Cardiology N=38 | Community‐Based Primary Care N=64 |
|---|---|---|---|
| No AF | 5 (4.9) | 1 (2.6) | 4 (6.3) |
| Allergy with no alternative | 1 (1.0) | 0 (0) | 1 (1.6) |
| Hospice | 2 (2.0) | 0 (0) | 2 (3.1) |
| Watchman/closure of appendage | 3 (2.9) | 3 (7.9) | 0 (0) |
| Active bleeding | 1 (1.0) | 0 (0) | 1 (1.6) |
| Intralobar hemorrhageb | 1 (1.0) | 1 (2.6) | 0 (0) |
| Other intracranial hemorrhage | 3 (2.9) | 0 (0) | 3 (4.7) |
| Patient not a good candidatec | 2 (2.0) | 0 (0) | 2 (3.1) |
| Transient condition | 17 (16.7) | 6 (15.8) | 11 (17.2) |
| Nonintracranial site of bleeding | 23 (22.5) | 9 (23.7) | 14 (21.9) |
| Patient already on dual antiplatelet agent; risk too high | 1 (1.0) | 1 (2.6) | 0 (0) |
| Fall risk | 8 (7.8) | 2 (5.3) | 6 (9.4) |
| Patient postablation | 5 (4.9) | 2 (5.3) | 3 (4.7) |
| On aspirin | 5 (4.9) | 0 (0) | 5 (7.8) |
| Patient refusal | 25 (24.5) | 13 (34.2) | 12 (18.8) |
AC indicates anticoagulation; AF, atrial fibrillation; EMR, electronic medical record.
We received 163 responses to our EMR messages, out of which 126 included reasons for not prescribing AC.
Hemorrhage into frontal, parietal, occipital, or temporal lobes; distinctive from deep brain hematoma involving midbrain, also subarachnoid hemorrhage.
Patient nonadherent, has intellectual disability or poor cognitive status and no one to supervise medication, poor AC control, or poor experience with warfarin in the past.
At baseline, patients receiving care from cardiology providers received AC at the highest percentage (71.3%) followed by community‐based primary care providers (63.5%) followed by control providers (58.3%). At follow‐up, there was only a small change in the crude percentage of AC prescription across all groups. Based on generalized estimation equation logistic regression modeling, the adjusted change in difference was not significant for either intervention group and was virtually unchanged from the unadjusted analysis. More specifically, the difference between cardiology and control group in adjusted AC percentage was 12.4% at baseline and 12.3% at follow‐up, a change in difference of −0.2% (P=0.91). The intracluster coefficient for this analysis was 0.014. The difference between community‐based primary care providers and controls was 5.1% at baseline and 5.1% at the time of follow‐up for a change in difference of 0.01% (P=0.98). The intracluster coefficient for this analysis was 0.032 (Table 5).
Table 5.
Comparison of Change in the Difference in Adjusted Percentage on Anticoagulation for Patients Cared for by Cardiology and Community‐Based Primary Care Providers Versus Controls
| Provider Group | At Baseline (Preintervention) | At Follow‐up (Postintervention) | Change in the Difference at Follow‐up vs Baseline % | ||
|---|---|---|---|---|---|
| Adjusted Percentage on Anticoagulation | Difference in Adjusted Percentage on Anticoagulation From Controls | Adjusted Percentage on Anticoagulation | Difference in Adjusted Percentage on Anticoagulation From Controls | ||
| Cardiology (n=3786) | 71.3 | 12.4 | 71.5 | 12.3 | −0.2 (P=0.91) |
| Community (n=1054) | 63.5 | 5.1 | 63.7 | 5.1 | 0.01 (P=0.98) |
| Controls (n=566)a | 58.9 | Reference | 59.2 | Reference | Reference |
In the table, we report only the frequencies for patients serving as controls for community‐based primary care providers. Controls for cardiology providers were mostly the same patients, but because we rolled out the intervention 2 weeks earlier for cardiology providers, frequencies shifted slightly from 562 to 566 patients based on the requirement to have last appointment within 12 months.
In the sensitivity analysis in which we included patients with a change in provider (ie, who met the 12‐month inclusion criterion only at baseline or only at follow‐up), there was similarly no significant change in AC percentage for intervention groups relative to controls. More specifically, relative to control providers, the change in AC percentage was 0.2% higher for cardiology providers (P=0.82) and 0.8% lower for community‐based primary care providers (P=0.61).
Finally, there was no significant effect of our intervention in the various subsets that we examined. Specifically, compared with the −0.2% change in difference in adjusted AC percentage from the main analysis of cardiology providers and 0.01% in community‐based primary care providers, in the subset of patients seeing their assigned provider over the course of follow‐up, the change in difference value was 0.6% for patients of cardiology providers and −0.06 for patients of community‐based primary care providers. Furthermore, there was no significant effect in the cohort of patients seeing their provider in the first 28 to 32 days of follow‐up—change in difference value of −0.8% for cardiology providers and −0.2% for the community‐based primary care providers. The change in difference value was −1.0% in the patients cared for by the subset of cardiology providers not reading any of our e‐mails to 0.9% for patients cared for by a provider who read more than 1 of our e‐mails. The same values in patients of community‐based primary care providers were 0.4% to −0.3%. The change in difference in adjusted AC percentage was 0.55% for the subset of patients with a cardiology provider who replied to multiple EMR messages (as opposed to a single message or no message). This value was −0.06% for the subset of community‐based primary care providers who replied to multiple EMR messages compared with controls. Finally, the range for change in difference in adjusted AC percentage was −0.6% for patients of cardiology providers with CHA2DS2‐VASc of 2 or 3 and −0.7 for patients of cardiology providers with CHA2DS2‐VASc of 6 or more. These values were −0.1% to 0.3% for patients of community‐based primary care providers with CHA2DS2‐VASc 2 or 3 and 6 or more, respectively.
Discussion
We demonstrated that it is feasible to generate and distribute reports of AC prescription percentages and EMR‐based AC messages to primary care and cardiology providers responsible for treating AF patients within our health system. Involvement of our health system's informatics and leadership teams was essential to our ability to generate automated processes for AF patient identification and stroke risk profiling. Providers were most likely to review and respond to EMR messages as compared with e‐mails. Patient refusal, high bleeding risk, and deference to a cardiology specialist were the most common reasons provided by providers for not treating their AF patients with AC. We did not detect an increase in AC use among intervention providers relative to controls.
Several other provider‐directed intervention studies have addressed AC prescription among AF patients. Three recent trials12, 13, 14 found mixed results for clinical decision support, although the trial12 which obligated intervention providers to explain why his or her patient was not on AC achieved a 1.6% increase in rate of AC use compared with control providers. In 1 cluster‐randomized, multisite trial conducted in the United States,23 there was no added benefit of site visits and direct engagement with providers compared with the distribution of paper‐based reports of AC prescription percentages. Of note, the study follow‐up for that trial was 2 years, much longer than the 8 to 10 weeks available to us for follow‐up. In another UK‐based cluster trial24, 25 looking at direct engagement with educational meetings and visits there was a 10% increase in the proportion of patients with guideline‐concordant AC and aspirin use among those with AF, although like our study, the result was not statistically significant. More recently, investigators conducting the IMPACT‐AF (Integrated Management Program Advancing Community Treatment of Atrial Fibrillation) cluster‐randomized, multinational study25 found that education of patients and providers in 5 middle‐income countries with regular monitoring and feedback increased AC use among intervention patients by 9.1% more than controls. Individual countries were able to customize the educational content and mode of delivery, including use of digital and social media. Our intervention leveraged tools available through the EMR and external software that were not available to, or used by, the previous investigative groups. By contrast, we did not directly engage providers (ie we did not meet with them in person), which had mixed results in the studies cited above. Responses to EMR messages we sent suggest that providers could benefit from discussion with trusted advisors regarding controversial areas and important topics, including shared decision‐making practices, issues of frailty and fall risk in relation to AC benefit, initiation of AC for AF that developed during a transient illness or surgery, and safe practices around AC resumption after a bleeding event. Increasing AC use may therefore require a combination of technology‐based approaches and direct engagement with providers. Finally, we did not directly interact with AF patients. Given the success of IMPACT‐AF, which interacted directly with patients, and our own findings that a number of providers indicated patient refusal as a reason for not starting/resuming AC, other researchers developing interventions in this area may decide to include a component of direct interaction with patients.
Understanding the lack of impact of our findings can benefit from discussion of strategies suggested by experts in a published article for improving effectiveness when delivering practice‐based feedback.26 As recommended by the authors of this article, we obtained endorsement of clinical chiefs supervising providers whose practice we profiled. At the same time, we did not widely solicit feedback from individual providers about the priority of our intervention relative to other quality improvement efforts/other priorities. We provided multiple instances of feedback including 3 e‐mails and separately EMR messages on each occurrence of a patient not on AC visiting his or her provider. We also provided feedback both quantitatively and visually. As mentioned above, we did not directly engage providers, which likely limited our impact.
There are other limitations to the findings we report. We had a short follow‐up period (8–10 weeks), and it is possible that if we had longer to follow AC prescribing, we would have had seen a larger impact. Approximately 40% of patients saw their assigned provider over the duration of the study. We anticipate that with 6 months of follow‐up, nearly every patient would have seen his provider given typical follow‐up for a patient with AF on AC. The considerably higher average AC rates among cardiology and community‐based intervention providers at baseline compared with control providers also limited our ability to detect a significant change in AC prescription. Perhaps, targeting providers with lower AC use would have enabled us to observe a more‐substantial increase in AC rates with our intervention. Unfortunately, our health system's transition to another EMR system limited our ability to identify AF patients, profile their stroke risk, and deliver scripted messages in an uninterrupted fashion. To address this limitation, we looked at a subset of patients who were seen over the course of the intervention and compared the effect with that of the entire sample (most of whom were not seen during the course of the intervention). The subset analysis, however, did not suggest a more‐positive effect. Other limitations include potential for residual confounding from the older age seen in community‐based primary care providers and also from the lower rate of AC use at baseline in the control population. Our inability to identify the discrete effectiveness of each component of the intervention and absence of information about bleed or fall risk in our AC profiling process are other limitations. The subset analysis mentioned above does provide important information about the effectiveness of the EMR messages sent before office visits with AF patients, but it is difficult to disentangle EMR message effects from the e‐mails with reports of AC prescription percentages. We also did not provide clinicians receiving reports with information about bleeding or fall risk/history for their patients. We plan to provide this in our future work.
In summary, we confirmed the feasibility of profiling AC prescription practices for AF patients among ambulatory healthcare providers using a common EMR. Although we did not find a significant effect for our 2‐component intervention, responses from providers, our process evaluation, and our analysis methodology allow us and others to determine the comparative effectiveness of future interventions focused on improving AC prescription practice. Based on our findings, we suspect that profiling and EMR messaging alone is not sufficient to overcome the AC prescription gap among eligible AF patients. Future work should examine the role of direct engagement with providers, focusing on the reasons for not prescribing AC that providers reported to us in the current work.
Sources of Funding
This study was funded by a grant from Bristol‐Myers Squibb under the request ID 28358345.
Disclosures
Dr Kapoor receives sponsored research support from Bristol‐Myers Squibb for the currently submitted project and has received similar support in the past from Pfizer. Dr McManus receives sponsored research support from Bristol‐Myers Squibb, Pfizer, Biotronik, and Philips Healthcare and has consulted for Bristol‐Myers Squibb, FlexCon, Samsung, Philips, and Pfizer. Dr McManus has equity in Mobile Sense Technologies, LLC. Dr Lubitz receives sponsored research support from Bristol‐Myers Squibb, Bayer HealthCare, Biotronik, and Boehringer Ingelheim and has consulted for St. Jude Medical/Abbott and Quest Diagnostics. All other authors have declared that they do not have any potential conflicts of interest.
Supporting information
Table S1. ICD‐10 Diagnosis Codes for Atrial Fibrillation and CHA2DS2‐VASc Predictors
Acknowledgments
This study was supported by the Center for Data Driven Discovery and HealthCare (D³Health) at the University of Massachusetts Medical School. The data and conclusions derived from this study are solely the responsibility of the authors and do not necessarily represent the official views of D³Health or the medical school.
(J Am Heart Assoc. 2018;7:e009946 DOI: 10.1161/JAHA.118.009946.)
References
- 1. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21‐year community‐based study. J Am Coll Cardiol. 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 4. Heart and Stroke Foundation of Canada . “Atrial fibrillation”. 2016. Available at: http://www.heartandstroke.ca/heart/conditions/atrial-fibrillation. Accessed June 19, 2016.
- 5. Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, Silver FL, Kapral MK. Potentially preventable strokes in high‐risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. [DOI] [PubMed] [Google Scholar]
- 6. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 8. Marcucci M, Nobili A, Tettamanti M, Iorio A, Pasina L, Djade CD, Franchi C, Marengoni A, Salerno F, Corrao S, Violi F, Mannucci PM. Joint use of cardio‐embolic and bleeding risk scores in elderly patients with atrial fibrillation. Eur J Intern Med. 2013;24:800–806. [DOI] [PubMed] [Google Scholar]
- 9. Steinberg BA, Kim S, Thomas L, Fonarow GC, Hylek E, Ansell J, Go AS, Chang P, Kowey P, Gersh BJ, Mahaffey KW, Singer DE, Piccini JP, Peterson ED. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) registry. Circulation. 2014;129:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu JC, Chan PS, Tang F, Maddox TM, Marcus GM. Oral anticoagulant prescription in patients with atrial fibrillation and a low risk of thromboembolism: insights from the NCDR PINNACLE Registry. JAMA Intern Med. 2015;175:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khurshid S, Keaney J, Ellinor PT, Lubitz SA. A simple and portable algorithm for identifying atrial fibrillation in the electronic medical record. Am J Cardiol. 2016;117:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlsson LO, Nilsson S, Bang M, Nilsson L, Charitakis E, Janzon M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: a cluster‐randomized trial in a Swedish primary care setting (the CDS‐AF study). PLoS Med. 2018;15:e1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holt TA, Dalton A, Marshall T, Fay M, Qureshi N, Kirkpatrick S, Hislop J, Lasserson D, Kearley K, Mollison J, Yu LM, Hobbs FD, Fitzmaurice D. Automated software system to promote anticoagulation and reduce stroke risk: cluster‐randomized controlled trial. Stroke. 2017;48:787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arts DL, Abu‐Hanna A, Medlock SK, van Weert HC. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: a cluster randomized controlled trial. PLoS One. 2017;12:e0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allscripts [computer program]. Chicago, IL: Allscripts Healthcare Solutions, Inc.; 1986. [Google Scholar]
- 16. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 17. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Alexandru Popescu B, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Rev Esp Cardiol (Engl Ed). 2017;70:50. [DOI] [PubMed] [Google Scholar]
- 18. Module 7. Measuring and benchmarking clinical performance. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ) Content last reviewed May 2013. Available at: http://www.ahrq.gov/professionals/prevention-chronic-care/improve/system/pfhandbook/mod7.html. Accessed April 26, 2018. [Google Scholar]
- 19. Tableau [computer program]. Seattle, WA: Tableau Software; 2003. [Google Scholar]
- 20. Cullen MW, Kim S, Piccini JP Sr, Ansell JE, Fonarow GC, Hylek EM, Singer DE, Mahaffey KW, Kowey PR, Thomas L, Go AS, Lopes RD, Chang P, Peterson ED, Gersh BJ. Risks and benefits of anticoagulation in atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) registry. Circ Cardiovasc Qual Outcomes. 2013;6:461–469. [DOI] [PubMed] [Google Scholar]
- 21. Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. New York, NY: Springer‐Verlag; 2005. [Google Scholar]
- 22. SAS [computer program]. Cary, NC: SAS Institute; 2013. [Google Scholar]
- 23. Ornstein S, Jenkins RG, Nietert PJ, Feifer C, Roylance LF, Nemeth L, Corley S, Dickerson L, Bradford WD, Litvin C. A multimethod quality improvement intervention to improve preventive cardiovascular care: a cluster randomized trial. Ann Intern Med. 2004;141:523–532. [DOI] [PubMed] [Google Scholar]
- 24. Wright J, Bibby J, Eastham J, Harrison S, McGeorge M, Patterson C, Price N, Russell D, Russell I, Small N, Walsh M, Young J. Multifaceted implementation of stroke prevention guidelines in primary care: cluster‐randomised evaluation of clinical and cost effectiveness. Qual Saf Health Care. 2007;16:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ezekowitz MD, Kent AP. The impact of IMPACT‐AF. Lancet. 2017;390:1717–1718. [DOI] [PubMed] [Google Scholar]
- 26. Brehaut JC, Colquhoun HL, Eva KW, Carroll K, Sales A, Michie S, Ivers N, Grimshaw JM. Practice feedback interventions: 15 suggestions for optimizing effectiveness. Ann Intern Med. 2016;164:435–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐10 Diagnosis Codes for Atrial Fibrillation and CHA2DS2‐VASc Predictors


