Introduction
An increasing number of stable patients with evidence of ischemia but no obstructive coronary artery disease (CAD) at coronary angiography, now termed INOCA, are seen. Objective myocardial ischemia or limited coronary flow reserve (CFR) consistent with coronary microvascular dysfunction (CMD) are identified in most of these patients. Although these patients were previously thought to be at low risk for major adverse cardiovascular events (MACE) and were provided only reassurance, newer data document that stable INOCA patients are a heterogeneous population with an elevated MACE risk. Primary prevention cardiovascular risk scores for asymptomatic populations may underestimate risk in these patients, while secondary prevention risk scores developed in patients with established cardiovascular disease may overestimate risk. Medical therapies may be underutilized when no obstructive CAD is documented, and patients are commonly discharged from specialty practice. We review the existing knowledge regarding observed and predicted risk using available risk scores in stable INOCA patients to identify knowledge gaps and plan investigation needed to develop evidence‐based guidelines for this growing patient population.
INOCA—Prevalence
Patients with chest pain, evidence of ischemia but no obstructive CAD at coronary angiography, now termed ischemia with no obstructive CAD or INOCA,1 are increasingly recognized. Although there is likely overlap between INOCA and myocardial infarction (MI) with no obstructive coronary arteries, which appears to be increasingly described, our primary focus is INOCA, the non‐MI syndromes. These stable patients typically have symptoms of chest pain suspected to be angina and/or abnormal stress testing, in the setting of no obstructive CAD at coronary angiography.1, 2 The definition of obstructive CAD varies between different guidelines or studies.3, 4, 5, 6, 7, 8, 9, 10 In general, “normal”‐appearing coronary arteries are defined as 0% luminal stenosis or <20%, and non–obstructive CAD (NOCAD) is defined as luminal stenosis >20% but <50%.3, 8, 9, 10 However, some studies use a threshold of <70% for NOCAD,4 while anatomical scores consider a stenosis ≥50% as significant.5, 6, 11 Traditional understanding of obstructive CAD was 70%12; however, recent European Society of Cardiology and American College of Cardiology/American Heart Association (ACC/AHA) guidelines shifted to include stenosis of 50% to 70% if there is associated inducible ischemia or fractional flow reserve ≤0.08 when considering the physiological significance of stenosis and revascularization management in patients with stable CAD.6, 7
Depending on the study, up to half of patients undergoing coronary angiography have no obstructive CAD,13, 14, 15 with a relatively higher prevalence in women (65% in women versus 32% in men).15 Overall estimates in women and men from the Veterans Administration Cardiovascular Assessment Reporting and Tracking System,16 the National Cardiac Data Registry, and the National Heart, Lung, and Blood Institute–sponsored Women's Ischemia Syndrome Evaluation (WISE)10 databases indicate that there are at least 3 to 4 million American women and men with stable INOCA. Incurred healthcare costs are similar to those for obstructive CAD.
Potential explanations for the apparent increasing prevalence of stable INOCA include more sensitive diagnostics, including advanced cardiac imaging and high‐sensitive troponins, which likely contribute to earlier detection of ischemic heart disease. Furthermore, improved primary prevention risk factor control (reduced smoking, increased aspirin and statin use) has likely contributed to altered atherosclerosis burden with relatively less large‐vessel plaque rupture, potentially leading to less adverse arterial remodeling/obstructive CAD,17 while increasing rates of obesity18 and diabetes mellitus19 may contribute to increasing prevalence of CMD or pathology.20, 21
The ACC/AHA non–ST‐segment–elevation MI guidelines refer to patients with MI and no obstructive CAD as having Cardiac Syndrome X (CSX),22 while the European Society of Cardiology stable CAD guidelines no longer use the term CSX when describing patients with angina and no obstructive CAD12 because testing now allows the diagnosis of CMD or macrovascular dysfunction12 in a majority of these patients. Previously, the term CSX was used to refer to patients with no obstructive CAD but did not require proof of ischemia23, 24 and also included patients with acute coronary syndromes and no obstructive CAD.22, 23, 25 Advanced evaluation can now identify CMD or macrovascular dysfunction by invasive or noninvasive measurements of CFR in a majority of these patients,12, 26 while coronary atherosclerosis and better characterization of plaques can be assessed by intravascular ultrasound, optical coherence tomography, or computed coronary tomography angiography when not evident or appreciated at invasive coronary angiography.27, 28, 29 Since CSX also includes clinical entities other than ischemia, such as pericardial pain, inappropriate pain perception, and psychiatric syndromes,30 the term INOCA was established to improve the identification and management of patients with ischemia and no obstructive CAD.1 In our opinion, the European Society of Cardiology stable CAD guidelines more directly address recent INOCA data and practice implications compared with the ACC/AHA guidelines.
Among both women and men, up to 60% of stable INOCA patients have documented CMD defined as the presence of microvascular endothelial‐dependent and/or nonendothelial‐dependent dysfunction.31, 32, 33 Furthermore, while the CMD was shown to be poorly correlated with traditional risk factors, age was found to be an independent predictor of CMD in both women and men.33 Female sex had a nearly significant association with CMD, with an odds ratio of 1.21 (95% confidence interval, 0.98–1.40) compared with men.33 The high prevalence of endothelial and/or nonendothelial‐dependent CMD26, 31, 33 and their correlation with outcomes26 underscores the role of comprehensive assessment in patients with INOCA. Such functional alterations can be identified at a stage when atherosclerotic lesions are not evident34 and may be useful in designing early effective interventions to prevent the occurrence of subsequent coronary events.
Evidence of an Adverse Prognosis
Evidence from prospective registries indicate that stable INOCA patients are at more elevated risk for future MACE, including death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure or angina than previously thought.
Documented MACE rates in stable INOCA patients are summarized in Table 1.4, 10, 15, 26, 35, 36, 37, 38, 39, 40, 41 Additionally, many of these patients have an adverse quality of life, functional status, and exercise capacity with relatively frequent visits to healthcare providers for persistent or recurring disabling symptoms.42 Elevated MACE rates are observed both early after the index coronary angiogram (eg within the first year) and at longer‐term follow‐up. In a study of 13 695 subjects, women with nonobstructive CAD demonstrated a 3‐fold higher MACE rate compared with men and 2.55‐fold increase compared with women with normal coronary arteries in the first year.43 Hospitalization for heart failure was the most frequent event, with an observed 10‐fold higher rate during longer‐term follow‐up compared with asymptomatic community‐based women.35 Studies that additionally characterized function or anatomy such as myocardial ischemia, CFR, plaque characterization, or calcium scoring further demonstrate relatively higher MACE rates related to the presence or degree of such abnormalities (Table 1), in both sexes. CMD was shown to be highly prevalent in stable INOCA patients and a CFR <2 was a powerful incremental predictor of MACE in both women and men.26 In symptomatic subjects from the CONFIRM (coronary CT angiography evaluation for clinical outcomes international multicenter) study there was a 2.5‐fold increase in risk of MI and all‐cause mortality related to a higher CT‐Leaman plaque score.44 Similarly, an increased coronary calcium score was related to greater risk of both 5‐year mortality and MACE in symptomatic subjects without significant luminal narrowing.37, 45 The degree of global cardiac magnetic resonance myocardial perfusion imaging was related to outcome in women with INOCA.39
Table 1.
Annuala MACE Rates in INOCA Patients
| Author, Publication Year | Study Population | Test Performed | End Point | Results—Annual Events Ratea (%) | |
|---|---|---|---|---|---|
| No Obstructive CAD—Anatomical Testing | Normal Coronary Arteries | Nonobstructive CAD | |||
| Gulati, 200935 | Chest pain or noninvasive positive tests for ischemia | Coronary angiography | All‐cause death, nonfatal MI, nonfatal stroke, hospitalization for heart failure | 1.5 | 3.1 |
| Ovrehus, 201136 | Stable angina | Coronary computed tomography angiography | Death and MI | 0 | 0.6 |
| Cardiac death, MI, revascularization | 0 | 1 | |||
| Jespersen, 201215 | Chest pain | Coronary angiography | Cardiovascular mortality, hospitalization for MI, heart failure, or stroke | 1.8 | 2.8 |
| Petretta, 201237 | Anginal symptoms and 15%–85% pretest likelihood of CAD | Coronary computed tomography angiography | Cardiac death, nonfatal MI, unstable angina, revascularization | 0 | 3.4 |
| Maddox, 20144 | Chest pain or noninvasive positive tests for ischemia | Coronary angiography | All‐cause death, MI | 1.48 | 2.41 |
| Nielsen, 201741 | Chest pain | Coronary computed tomography angiography | Revascularization MI, and all‐cause death | 0.4 | 0.9 |
| Kenkre, 201740 | Chest pain or noninvasive positive tests for ischemia | Coronary angiography | All‐cause death | 1 | 1.7 |
| Cardiac death | 0.6 | 1.1 | |||
| No Obstructive CAD—Functional Testing | Normal Test | Abnormal Test | |||
|---|---|---|---|---|---|
| Johnson, 200410 | Chest pain or noninvasive positive tests for ischemia | Magnetic resonance spectroscopy | All‐cause death, MI, heart failure, stroke, other vascular events, and hospitalization for unstable angina | 4.4 | 14 |
| Schindler, 200538 | Chest pain | Positron emission tomography | Cardiovascular death, acute coronary syndrome, MI, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting, ischemic stroke, or peripheral revascularization | 0.9 | 5–7 |
| Doyle, 201039 | Chest pain or noninvasive positive tests for ischemia | Cardiac magnetic resonance imaging | All‐cause death, nonfatal MI, or hospitalization for worsening anginal symptoms | 4 | 12 |
| Murthy, 201426 | Chest pain | Positron emission tomography | Cardiac death, nonfatal MI, late revascularization, and hospitalization for heart failure | 2.7 | 6.7 |
CAD indicates coronary artery disease; INOCA, ischemia and no obstructive coronary artery disease; MACE, major adverse cardiovascular events; MI, myocardial infarction.
Annual MACE rate from the reported mean follow‐up events rate divided by the mean years of follow‐up.
Longer‐term follow‐up data from the WISE project confirmed a worse prognosis than previously thought for stable INOCA women where 10‐year all‐cause death and cardiac death rates were 17% and 11%, respectively, in women with nonobstructive CAD, and 10% and 6%, respectively, in women with normal coronary arteries.40 Furthermore, a recent meta‐analysis of 48 studies including patients presenting with stable symptoms undergoing either invasive or noninvasive coronary angiography demonstrated odds ratios of 1.57 to 1.7 for MACE defined as cardiac death, nonfatal MI, hospitalization for unstable angina, or revascularization in patients with NOCAD compared with their counterparts with normal coronary arteries. The odds ratio remained high after excluding revascularization as an outcome event.46
A number of studies now include comparison of patients with normal coronary arteries, nonobstructive CAD, and obstructive CAD (Figure 1).4, 41, 47 Specifically, in the Veterans Administration—Clinical Assessment, Reporting and Tracking System, patients with nonobstructive CAD in 3 coronary arteries had a similar annual risk for MI and death as patients with single‐vessel obstructive CAD.4 Risk was related not only to the degree of luminal stenosis but also to the extent of the angiographic disease, increasing with the number of vessels affected in both nonobstructive and obstructive disease.4, 47
Figure 1.
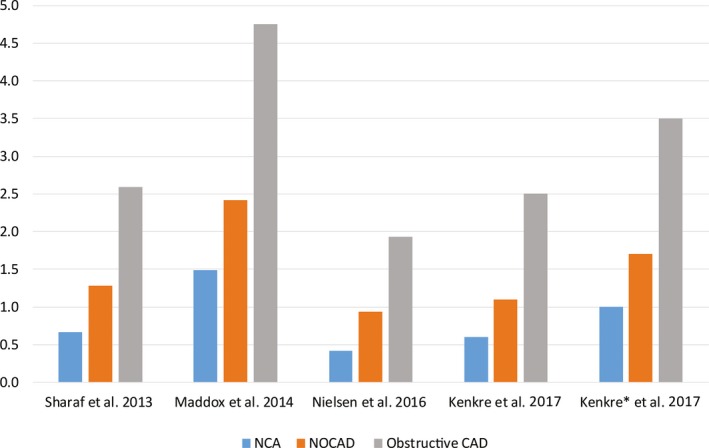
Annual MACE rate stratified by normal coronary arteries, nonobstructive CAD, and obstructive CAD. Annual MACE rates from the reported mean MACE rate divided by the mean years of follow‐up. CAD indicates coronary artery disease; MACE, major adverse cardiovascular events; NCA, normal coronary arteries; NOCAD, nonobstructive coronary artery disease. Outcomes include: Sharaf47: cardiovascular death or nonfatal MI; Maddox4: all‐cause mortality or nonfatal MI; Nielsen41: all‐cause death, MI, late coronary revascularization; Kenkre18: cardiac mortality; Kenkre18*, all‐cause death.
Primary Prevention Risk Scores
Current guidelines endorse use of primary prevention risk scores in asymptomatic patients. Related to the asymptomatic populations used to develop primary prevention scores, the Framingham Risk Score (FRS) appears to underestimate risk in women,48 while the Reynolds Risk Score may perform better in selected populations.49 Among asymptomatic subjects enrolled in the MESA (Multi‐Ethnic Study of Atherosclerosis), the current guideline Atherosclerotic Cardiovascular Disease score and 3 older FRS‐based risk scores overestimate MACE by 37% to 154% in men and 8% to 67% in women, while the Reynolds Risk Score underestimated risk in women by almost one quarter.50 Whether knowledge of the enrolled MESA subjects’ coronary artery calcium score led to activities to reduce risk is not clear.51 The Atherosclerotic Cardiovascular Disease score accurately predicted risk in the Reasons for geographic and racial differences in stroke, a contemporary US dataset that includes representative ethnicity and socioeconomic status.52 A recent study in stable INOCA patients undergoing Coronary Reactivity Testing (CRT) demonstrated that a majority were classified as intermediate risk by FRS, which did not accurately predict MACE, while the addition of coronary macro‐ and microvascular endothelial dysfunction to the FRS correctly reclassified 23%, with a net reclassification index of 0.23.53 Coronary endothelial dysfunction, both micro‐ and macrovascular, added to the FRS in INOCA improved discrimination and risk stratification, further emphasizing the crucial role of functional assessment.
Obstructive CAD Likelihood Scores
In symptomatic patients, clinical likelihood scores (eg Diamond/Forrester, Morise, and CAD Consortium Pretest Probability score) assess the likelihood of obstructive CAD. Several analyses now indicate that these scores overestimate the likelihood of obstructive CAD in contemporary symptomatic patients undergoing noninvasive computed coronary tomography angiography. Although designed for predicting likelihood of obstructive CAD, the Diamond/Forrester and CAD Consortium Pretest Probability score were also tested for prediction of MACE in the PARTNERS Registry, and demonstrated that the CAD Consortium score had the highest discriminatory ability (area under the curve 0.687; 95% confidence interval, 0.646–0.728) for MACE.54 Similarly, the Morise pretest clinical score that includes 9 variables to estimate the likelihood of obstructive CAD effectively stratified WISE subjects according to the combined end point of cardiac death/MI during a mean follow‐up of 3.4 years, with separation between the low‐risk group and the others (P=0.012).55 The intermediate‐ and high‐risk groups were separable for as long as 1.5 years, but thereafter, became less clearly separable.55 Other obstructive CAD prediction scores developed in stable or acute chest pain patients have not been tested for MACE prediction.
The newly developed PROMISE (Prospective Multicenter Imaging Study of Chest Pain) minimal risk tool was designed to identify “low‐risk” patients in whom deferred noninvasive testing (noninvasive coronary angiography or functional stress testing) may be considered.56 Subjects with minimal risk had a low‐risk profile (0.5% risk of cardiovascular death and MI at a median 25 months). While this could be of use for risk stratification in INOCA, prior studies suggest that the majority of INOCA patients are classified in the “intermediate‐risk” class.36
Among patients with an intermediate pretest risk for obstructive CAD, with a normal ECG and who can exercise, guidelines recommend that exercise ECG stress testing be considered as the first test. In women evaluated for signs and symptoms of ischemia undergoing clinically ordered coronary angiography in the WISE project, a pretest clinical score and an exercise test score designed for use in women with suspected CAD performed better than the commonly used Duke score in stratifying women with a low prevalence of obstructive CAD.41, 55
Nevertheless, treadmill ECG stress testing, stress echocardiography, and single photon emission computed tomography stress all have a limited sensitivity and specificity for detection of ischemia in INOCA patients.57 This is not surprising given the lack of a large regional territory of ischemia, as in the obstructive CAD populations used to validate these techniques. Among subjects from the National Cardiovascular Data Registry's Cath Percutaneous Coronary Intervention (CathPCI) undergoing invasive coronary angiography for stable chest pain, while low‐ or intermediate‐risk findings on noninvasive testing were associated with no obstructive CAD, the ability to predict MACE was not tested.58 Notably, >50% of MACE occurred in subjects with normal stress testing in the PROMISE,59 emphasizing the lower sensitivity and specificity to detect ischemia in less than obstructive CAD. An anatomical computed coronary tomography angiography approach offered better prognostic information once NOCAD was visualized.59 Furthermore, mechanisms leading to acute coronary syndromes are not solely depending on degree of luminal stenosis. An ischemic event is the consequence of a complex interaction among plaque characteristics, endothelial dysfunction, coronary blood flow hemodynamics, hemostasis factors, and metabolic, inflammatory, neurohormonal, and environmental factors60 that are not addressed by commonly used tests.
Secondary Prevention Risk Scores
The Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (Syntax) II is a coronary angiographic‐based score used to optimize outcomes relative to revascularization in obstructive CAD.12 Other secondary prevention scores are relevant in early61, 62 or longer‐term63 risk stratification after a vascular event. Scores using obstructive CAD variables are not applicable to INOCA patients. Recently, the Gensini score, which includes lesser‐than‐obstructive CAD (epicardial luminal diameter stenosis <50%), was found to be useful for prognosis in men and women referred for invasive coronary angiography with no obstructive CAD.64 Previously, in women, a WISE coronary angiographic score that assigned points according to severity of stenosis, adjusted for the presence of collaterals and weighted by lesion location, predicted MACE in stable INOCA patients. Specifically, MACE risk was positively associated with increased coronary atherosclerosis scores in the absence of obstructive CAD.47
Additional scores developed for secondary prevention in patients with established cardiovascular disease (CVD) do not include obstructive CAD as a variable, but were developed in populations dominated by obstructive CAD,63, 65, 66, 67, 68, 69, 70, 71 and therefore are of unknown appropriateness for stable INOCA patients.
The Long‐Term Intervention with Pravastatin in Ischemic Disease (LIPID)63 score identifies higher‐risk subjects on statin therapy, developed again in an obstructive CAD population. A score from the Guangdong Coronary Artery Disease Cohort (GCADC) study65 had good predictive value for mortality among secondary prevention patients, and the European trial on reduction of cardiac events with perindopril in stable coronary artery disease (EUROPA) score model predicted CVD mortality but not nonfatal outcomes or combined end points.71 Again, these studies addressed mostly obstructive CAD patients.
The Second Manifestation of Arterial Disease (SMART),69 Thrombolysis In Myocardial Infarction Risk Score for Secondary Prevention (TIMI‐TRS2oP),70 and A Coronary Disease Trial Investigating Outcome with Nifedipine (ACTION) risk66 scores, all showed substantial variability in risk among patients with stable CVD, but more importantly that aggressive guideline treatment in high‐risk patients decreased their risk.69 Similarly, the PREDICT CVD (New Zealand Primary Care Cohort Study) score developed for patients with previous CVD recognizes patient‐specific risks of future events and how they may be reduced through therapeutic and behavioral strategies.68
The “Cardiovascular Disease Research Using Linked Bespoke Studies and Electronic Health Records” (CALIBER) score67 used real‐world commonly available data that contributed to important prognostic information in unselected patients with a wide phenotype of stable ischemic heart disease. The CALIBER models had good calibration and discrimination in internal and external validation with C‐index 0.811 (0.735) for all‐cause mortality and 0.778 (0.718) for nonfatal MI or coronary death in established stable ischemic heart disease.67
Primary and Secondary Prevention Risk Versus Observed INOCA Risk
Comparison of primary, secondary prevention risk versus observed risk in an example of stable INOCA patient is presented in Figure 2.40, 47, 56, 63, 65, 66, 67, 68, 69, 70, 71 The primary prevention scores, developed in asymptomatic populations, predicted that risks vary between 1% and ≈5% and underestimate the observed INOCA risk. The related primary prevention risk guidelines for these low‐risk scores would include therapeutic lifestyle change and not statin therapy. Among secondary risk scores, developed in symptomatic but mainly obstructive CAD patients, the predicted risk varied widely, either over‐ or underestimating the observed INOCA risk.
Figure 2.
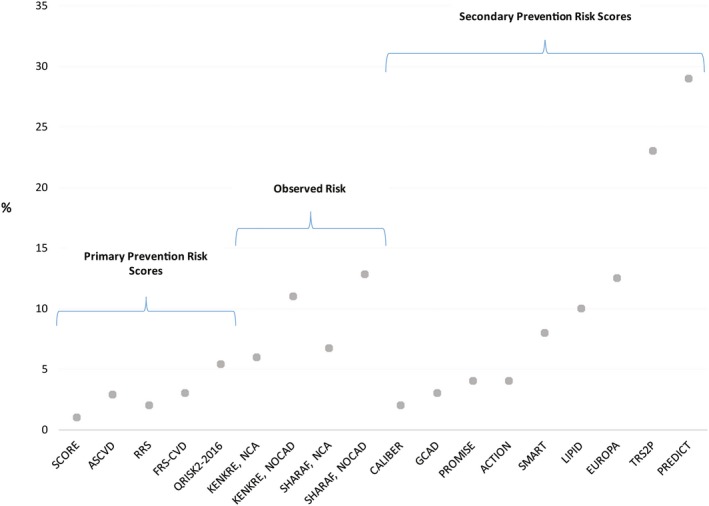
Predicted primary and secondary prevention scores risk vs observed 10‐year risk in an example INOCA patient. Model variables used: female, 55 years, hypertension, systolic blood pressure 139 mm Hg on treatment, heart rate 80 bpm, total cholesterol 200 mg/dL (5.17 mmol/L); low‐density lipoprotein 80 mg/dL (2.068 mmol/L), high‐density lipoprotein 60 mg/dL (1.55 mmol/L), high‐sensitivity C‐reactive protein (hs‐CRP) 2 mg/dL, creatinine 0.9 mg/dL (79 μmol/L), white blood cell count 10 K3/mL, hemoglobin 12 g/dL, no family history, height 5′ 67″ (170 cm), weight 158 pounds (72 kg), body mass index 24.9, low‐risk country, chest pain related to physical/mental stress, glomerular filtration rate 60 mL/min per 1.73 m2. Predicted 10‐year Risk: Primary Prevention Risk Scores: ASCVD—risk of cardiovascular death, nonfatal MI, nonfatal stroke; SCORE—risk of fatal cardiovascular disease; Reynolds (RRS)—risk of myocardial infarction, ischemic stroke, coronary revascularization and cardiovascular death; QRISK2—risk of MI or Stroke; FRS CVD—risk of CHD or coronary insufficiency death, MI, or angina; Secondary Prevention Risk Scores: CALIBER—myocardial infarction, cardiovascular death; GCAD—cardiovascular death; PROMISE—myocardial infarction, cardiovascular death; ACTION—myocardial infarction, stroke, all‐cause death; SMART—myocardial infarction, stroke, vascular death; LIPID—myocardial infarction, cardiovascular death; EUROPA—cardiovascular death; TRS2P—myocardial infarction, stroke, cardiovascular death; PREDICT—myocardial infarction, stroke, cardiovascular death. The 10‐year risk was calculated from the reported risk divided by the numbers of follow‐up years and then projected to 10 years. Observed 10‐year Risk: Sharaf—cardiovascular death or MI (median follow‐up of 9.3 years); Kenkre—cardiac mortality (median follow‐up 9.5 years). ACTION indicates A Coronary disease Trial Investigating Outcome with Nifedipine; ASCVD, Atherosclerotic Cardiovascular Disease; CAD, coronary artery disease; CALIBER, Cardiovascular disease research using Linked Bespoke studies and Electronic Health Records; EUROPA, European trial On reduction of cardiac events with Perindopril in stable coronary Artery disease; FRS‐CVD, Framingham Risk Score Cardiovascular Disease; GCAD, Guangdong Coronary Artery Disease Cohort; INOCA, ischemia and no obstructive coronary artery disease; LIPID, Long‐Term Intervention with Pravastatin in Ischemic Disease; NCA, normal coronary arteries; NOCAD, nonobstructive coronary artery disease; PREDICT, Patients with Renal Impairment and Diabetes undergoing Computed Tomography; PROMISE, Prospective Multicenter Imaging Study of Chest Pain; QRISK2, QRESEARCH cardiovascular disease risk score; RRS, Reynolds Risk Score; SMART, Second Manifestation of Arterial Disease; SCORE, Systematic Coronary Risk Evaluation; TRS2P, Thrombolysis In Myocardial Infarction Risk Score for Secondary Prevention.
Cardiovascular Treatment Rates in INOCA Patients
Registry data demonstrate that half or less of stable INOCA patients are treated with cardiovascular medication effective for ischemic heart disease, such as angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, β‐blocker, calcium channel blocker, or statin therapies (Table 2).15, 41, 47, 72, 73, 74, 75, 76, 77 Furthermore, the intensity of treatment, specifically for the maximally tolerated angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker and potent statin categories, is unknown in these registries. These findings suggest that the presence of normal coronary arteries or NOCAD at coronary angiography may be associated with diagnostic and therapeutic uncertainty, resulting in patients being less often treated with either primary prevention or secondary prevention guidelines therapy. This practice seems unchanged despite knowledge about adverse outcome in this population. The current ACC/AHA guidelines for patients with stable CAD echoes the ACC/AHA recommendations for patients with unstable angina/non–ST‐segment–elevation MI for subgroups of patients with no obstructive CAD, which was defined as CSX.22, 78 While there have been studies evaluating therapy in patients with CSX,79, 80, 81, 82 these studies are limited in their characterization of coronary vasomotor function. Indeed, this emerging INOCA patient population remains underdiagnosed and undertreated, likely perseverating this observed therapeutic equipoise. The observed elevated MACE rate endorses this as a knowledge gap. Even mild degrees of atherosclerosis or abnormal coronary vasoreactivity are related to increased health risk.4, 26, 83, 84 Furthermore, the majority of MIs result from rupture of nonobstructive plaque, highlighting the importance of optimizing therapy in these patients.85, 86 To date, limited data exist about the effectiveness of therapy in stable patients with no CAD and high prevalence of CMD. Nevertheless, prior work in obstructive CAD has demonstrated that atherosclerotic progression can be slowed and MACE reduced with optimal medical therapy,69 while surrogate outcome trials in CMD patients indicate improvement in endothelial function, CFR, and angina with optimal medical therapy, as well.87 Patients with INOCA deserve to receive optimal treatment as per current guidelines while awaiting future dedicated trials.
Table 2.
Cardiovascular Treatment Rates in INOCA Patients
| Author, Publication Year (n) | Hypertension/Angina Therapy (%) | Statin Therapy (%) |
|---|---|---|
| Maddox, 201072 (n=237 167) | 51 | 47 |
| Johnston, 201173 (n=5386) | 21–56 | 51 |
| Shaw, 201174 (n=824) | 10–20 | 32 |
| Jespersen, 201215 (n=5183) | 44 | 50 |
| Sedlak, 201275 (n=1864) | 34 | 32 |
| Sharaf, 201347 (n=567) | 2–39 | 10a/31b |
| Chow, 201576 (n=10 418) | N/A | 33.3 |
| Nielsen, 201741 (n=14 205) | 11.8–32.3 | 25–39.2 |
| Galway, 201777 (n=2642) | 18–46 | 34–59 |
Hypertension/Angina therapy includes: angiotensin‐converting enzyme inhibitor, angiotensin II receptor blocker, β‐blocker, and calcium channel blocker medication. INOCA indicates ischemia and no obstructive coronary artery disease; N/A, not applicable.
Normal coronary arteries.
Nonobstructive coronary artery disease.
Implications and Conclusions
An increasing number of stable INOCA patients and observed elevated MACE rate calls attention to several important knowledge gaps (Table 3). Existing primary and secondary prevention risk assessment tools do not appear to predict MACE risk in INOCA patients; investigation is needed to specifically address tools to accurately assess risk in these patients. Furthermore, there appears to be diagnostic and therapeutic uncertainty in INOCA patients with potentially inappropriately low rates of cardiovascular therapy given the documented atherosclerotic and ischemia burden. Evidence‐based primary or secondary treatment guidelines do not specifically address this population, which is indicative of the absence of cardiovascular outcome trials in INOCA subjects. This important knowledge gap must be addressed to get ahead of this emerging issue. Clinical trials designed to test the impact of optimal medical therapy in INOCA patients are needed.
Table 3.
Knowledge Gaps in Stable INOCA
| CVD Primary Prevention Guidelines | Stable CAD Guidelines | Secondary CVD Prevention Guidelines | Knowledge Gaps | ||
|---|---|---|---|---|---|
| Detection | N/A | Likelihood of CAD score | Limited to the presence of obstructive CAD | Limited to established coronary or other atherosclerotic vascular disease | Evidence regarding the utility, benefits, and risks of invasive and noninvasive detection strategies in INOCA patients is needed to develop evidence‐based detection guidelines |
| Stress testing | Limited to the presence of obstructive CAD | ||||
| CCTA | Limited to anatomical coronary plaque/stenosis and obstructive CAD flow | ||||
| Coronary angiography | Limited to anatomical stenosis and obstructive CAD flow; no evidence‐based guidelines for less than obstructive CAD | ||||
| Risk assessment | Limited to asymptomatic patients | Limited to stable known or suspected obstructive CAD | Risks scores limited to prior MI and established CAD | Risk scores developed in INOCA populations to develop evidence‐based risk assessment guidelines are needed | |
| Treatment | Limited to asymptomatic patients | Echoes treatment recommendations for specific subgroups of patients from UA/NSTEMI guidelines. Emphasis on the lack of dedicated treatment trials for INOCA | Limited to established coronary or other atherosclerotic vascular disease | MACE trials to inform evidence‐based guidelines for treatment strategies are needed | |
CAD indicates coronary artery disease; CCTA, computed coronary tomography angiography; CVD, cardiovascular disease; INOCA, ischemia and no obstructive coronary artery disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; N/A, not applicable; NSTEMI, non–ST‐segment–elevation myocardial infarction; UA, unstable angina.
Sources of Funding
This work was supported by contracts from the National Heart, Lung, and Blood Institute nos. N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, T32HL116273, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1‐RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars‐Sinai Medical Center, Los Angeles, CA, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars‐Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, DC, The Linda Joy Pollin Women's Heart Health Program, and the Erika J. Glazer Women's Heart Research Initiative, Cedars‐Sinai Medical Center, Los Angeles, CA.
Disclosures
None.
J Am Heart Assoc. 2018;7:e008868 DOI: 10.1161/JAHA.118.008868.
References
- 1. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pepine CJ, Ferdinand KC, Shaw LJ, Light‐McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson HB, Pedersen F, Engstrom T, Helqvist S, Jensen MK, Jorgensen E, Kelbaek H, Rader S, Saunamaki K, Bates E, Grande P, Holmvang L, Clemmensen P. Long‐term survival and causes of death in patients with ST‐elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J. 2018;39:102–110. [DOI] [PubMed] [Google Scholar]
- 4. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR Jr, Mack M, Feldman T, Morice MC, Stahle E, Onuma Y, Morel MA, Garcia‐Garcia HM, van Es GA, Dawkins KD, Mohr FW, Serruys PW. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Nucl Cardiol. 2017;24:1759–1792. [DOI] [PubMed] [Google Scholar]
- 7. Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Zamorano JL. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517–592. [DOI] [PubMed] [Google Scholar]
- 8. Dehmer GJ, Weaver D, Roe MT, Milford‐Beland S, Fitzgerald S, Hermann A, Messenger J, Moussa I, Garratt K, Rumsfeld J, Brindis RG. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60:2017–2031. [DOI] [PubMed] [Google Scholar]
- 9. Douglas PS, Patel MR, Bailey SR, Dai D, Kaltenbach L, Brindis RG, Messenger J, Peterson ED. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58:801–809. [DOI] [PubMed] [Google Scholar]
- 10. Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM; National Institutes of Health‐National Heart L, Blood I . Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health‐National Heart, Lung, and Blood Institute‐Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–2999. [DOI] [PubMed] [Google Scholar]
- 11. Syntax score calculator version 2.0. 2009. Available at: http://www.syntaxscore.com/calculator/syntaxscore/frameset.htm. Accessed August 17, 2017.
- 12. Task Force M , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; Guidelines ESCCfP , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document R , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 13. Farrehi PM, Bernstein SJ, Rasak M, Dabbous SA, Stomel RJ, Eagle KA, Rubenfire M. Frequency of negative coronary arteriographic findings in patients with chest pain is related to community practice patterns. Am J Manag Care. 2002;8:643–648. [PubMed] [Google Scholar]
- 14. Bradley SM, Maddox TM, Stanislawski MA, O'Donnell CI, Grunwald GK, Tsai TT, Ho PM, Peterson ED, Rumsfeld JS. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (Veterans Affairs Clinical Assessment Reporting and Tracking). J Am Coll Cardiol. 2014;63:417–426. [DOI] [PubMed] [Google Scholar]
- 15. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 16. Davis MB, Maddox TM, Langner P, Plomondon ME, Rumsfeld JS, Duvernoy CS. Characteristics and outcomes of women veterans undergoing cardiac catheterization in the Veterans Affairs Healthcare System: insights from the VA CART Program. Circ Cardiovasc Qual Outcomes. 2015;8:S39–S47. [DOI] [PubMed] [Google Scholar]
- 17. Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–2987. [DOI] [PubMed] [Google Scholar]
- 18. IAftSoO . International association for the study of obesity. Available at: http://www.iaso.org. Accessed August 17, 2017.
- 19. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet. 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 20. Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, Montisci R, Famoso G, Tellatin S, Foletto M, Giovagnoni A, Iliceto S, Vettor R. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24:447–453. [DOI] [PubMed] [Google Scholar]
- 21. Ahmari SA, Bunch TJ, Modesto K, Stussy V, Dichak A, Seward JB, Pellikka PA, Chandrasekaran K. Impact of individual and cumulative coronary risk factors on coronary flow reserve assessed by dobutamine stress echocardiography. Am J Cardiol. 2008;101:1694–1699. [DOI] [PubMed] [Google Scholar]
- 22. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 23. Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–376. [DOI] [PubMed] [Google Scholar]
- 24. Mukerji V, Beitman BD, Alpert MA. Chest pain and angiographically normal coronary arteries. Implications for treatment. Tex Heart Inst J. 1993;20:170–179. [PMC free article] [PubMed] [Google Scholar]
- 25. Karamitsos TD, Arnold JR, Pegg TJ, Francis JM, Birks J, Jerosch‐Herold M, Neubauer S, Selvanayagam JB. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3‐T cardiovascular magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5:194–200. [DOI] [PubMed] [Google Scholar]
- 26. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute‐Sponsored Women's Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bogale N, Lempereur M, Sheikh I, Wood D, Saw J, Fung A. Optical coherence tomography (OCT) evaluation of intermediate coronary lesions in patients with NSTEMI. Cardiovasc Revasc Med. 2016;17:113–118. [DOI] [PubMed] [Google Scholar]
- 29. Conte E, Annoni A, Pontone G, Mushtaq S, Guglielmo M, Baggiano A, Volpato V, Agalbato C, Bonomi A, Veglia F, Formenti A, Fiorentini C, Bartorelli AL, Pepi M, Andreini D. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non‐obstructive coronary artery disease: a long‐term follow‐up study. Eur Heart J Cardiovasc Imaging. 2016;18:1170–1178. [DOI] [PubMed] [Google Scholar]
- 30. Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73:1133–1140. [DOI] [PubMed] [Google Scholar]
- 32. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 33. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 34. Manfrini O, Cenko E, Verna E, Salerno Uriarte JA, Bugiardini R. Endothelial dysfunction versus early atherosclerosis: a study with high resolution imaging. Int J Cardiol. 2013;168:1714–1716. [DOI] [PubMed] [Google Scholar]
- 35. Gulati M, Cooper‐DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ovrehus KA, Botker HE, Jensen JM, Munkholm H, Johnsen SP, Norgaard BL. Influence of coronary computed tomographic angiography on patient treatment and prognosis in patients with suspected stable angina pectoris. Am J Cardiol. 2011;107:1473–1479. [DOI] [PubMed] [Google Scholar]
- 37. Petretta M, Daniele S, Acampa W, Imbriaco M, Pellegrino T, Messalli G, Xhoxhi E, Del Prete G, Nappi C, Accardo D, Angeloni F, Bonaduce D, Cuocolo A. Prognostic value of coronary artery calcium score and coronary CT angiography in patients with intermediate risk of coronary artery disease. Int J Cardiovasc Imaging. 2012;28:1547–1556. [DOI] [PubMed] [Google Scholar]
- 38. Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, Brink I, Zhang XL, Kreissl M, Magosaki N, Just H, Solzbach U. Positron emission tomography‐measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45:1505–1512. [DOI] [PubMed] [Google Scholar]
- 39. Doyle M, Weinberg N, Pohost GM, Bairey Merz CN, Shaw LJ, Sopko G, Fuisz A, Rogers WJ, Walsh EG, Johnson BD, Sharaf BL, Pepine CJ, Mankad S, Reis SE, Vido DA, Rayarao G, Bittner V, Tauxe L, Olson MB, Kelsey SF, Biederman RW. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI‐Sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging. 2010;3:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kenkre TS, Malhotra P, Johnson BD, Handberg EM, Thompson DV, Marroquin OC, Rogers WJ, Pepine CJ, Bairey Merz CN, Kelsey SF. Ten‐year mortality in the WISE study (Women's Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes. 2017;10:e003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nielsen LH, Botker HE, Sorensen HT, Schmidt M, Pedersen L, Sand NP, Jensen JM, Steffensen FH, Tilsted HH, Bottcher M, Diederichsen A, Lambrechtsen J, Kristensen LD, Ovrehus KA, Mickley H, Munkholm H, Gotzsche O, Husain M, Knudsen LL, Norgaard BL. Prognostic assessment of stable coronary artery disease as determined by coronary computed tomography angiography: a Danish multicentre cohort study. Eur Heart J. 2017;38:413–421. [DOI] [PubMed] [Google Scholar]
- 42. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health—National Heart, Lung, and Blood Institute–Sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 43. Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. [DOI] [PubMed] [Google Scholar]
- 44. Andreini D, Pontone G, Mushtaq S, Gransar H, Conte E, Bartorelli AL, Pepi M, Opolski MP, Ó Hartaigh B, Berman DS, Budoff MJ, Achenbach S, Al‐Mallah M, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Cury R, Delago A, Hadamitzky M, Hausleiter J, Feuchtner G, Kim YJ, Kaufmann PA, Leipsic J, Lin FY, Maffei E, Raff G, Shaw LJ, Villines TC, Dunning A, Marques H, Rubinshtein R, Hindoyan N, Gomez M, Min JK. Long‐term prognostic impact of CT‐Leaman score in patients with non‐obstructive CAD: results from the coronary CT angiography evaluation for clinical outcomes international multicenter (CONFIRM) study. Int J Cardiol. 2016;231:18–25. [DOI] [PubMed] [Google Scholar]
- 45. Cho I, Ó Hartaigh B, Gransar H, Valenti V, Lin FY, Achenbach S, Berman DS, Budoff MJ, Callister TQ, Al‐Mallah MH, Cademartiri F, Chinnaiyan K, Chow BJW, Dunning AM, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Leipsic J, Shaw LJ, Kaufmann PA, Cury RC, Feuchtner G, Kim YJ, Maffei E, Raff G, Pontone G, Andreini D, Chang HJ, Min JK. Prognostic implications of coronary artery calcium in the absence of coronary artery luminal narrowing. Atherosclerosis. 2017;262:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang FY, Huang BT, Lv WY, Liu W, Peng Y, Xia TL, Wang PJ, Zuo ZL, Liu RS, Zhang C, Gui YY, Liao YB, Chen M, Zhu Y. The prognosis of patients with nonobstructive coronary artery disease versus normal arteries determined by invasive coronary angiography or computed tomography coronary angiography: a systematic review. Medicine (Baltimore). 2016;95:e3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ, Bairey Merz CN. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wenger NK. What do the 2011 American Heart Association guidelines tell us about prevention of cardiovascular disease in women? Clin Cardiol. 2011;34:520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, Rossouw JE, Wassertheil‐Smoller S, Ridker PM. Comparison of the Framingham and Reynolds Risk scores for global cardiovascular risk prediction in the multiethnic Women's Health Initiative. Circulation. 2012;125:1748–1756, S1741‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pletcher MJ, Pignone M, Earnshaw S, McDade C, Phillips KA, Auer R, Zablotska L, Greenland P. Using the coronary artery calcium score to guide statin therapy: a cost‐effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colantonio LD, Richman JS, Carson AP, Lloyd‐Jones DM, Howard G, Deng L, Howard VJ, Safford MM, Muntner P, Goff DC Jr. Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. J Am Heart Assoc. 2017;6:e005676 DOI: 10.1161/JAHA.117.005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reriani M, Sara JD, Flammer AJ, Gulati R, Li J, Rihal C, Lennon R, Lerman LO, Lerman A. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis. 2016;27:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bittencourt MS, Hulten E, Polonsky TS, Hoffman U, Nasir K, Abbara S, Di Carli M, Blankstein R. European Society of Cardiology‐recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond and Forrester score: the PARTNERS registry. Circulation. 2016;134:201–211. [DOI] [PubMed] [Google Scholar]
- 55. Morise AP, Olson MB, Merz CN, Mankad S, Rogers WJ, Pepine CJ, Reis SE, Sharaf BL, Sopko G, Smith K, Pohost GM, Shaw L. Validation of the accuracy of pretest and exercise test scores in women with a low prevalence of coronary disease: the NHLBI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Am Heart J. 2004;147:1085–1092. [DOI] [PubMed] [Google Scholar]
- 56. Fordyce CB, Douglas PS, Roberts RS, Hoffmann U, Al‐Khalidi HR, Patel MR, Granger CB, Kostis J, Mark DB, Lee KL, Udelson JE. Identification of patients with stable chest pain deriving minimal value from noninvasive testing: the PROMISE minimal‐risk tool, a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2017;2:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED, Roe MT. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846–852.e842. [DOI] [PubMed] [Google Scholar]
- 59. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, Fordyce CB, Pellikka PA, Tardif JC, Budoff M, Nahhas G, Chow B, Kosinski AS, Lee KL, Douglas PS. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (prospective multicenter imaging study for evaluation of chest pain). Circulation. 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arbab‐Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. 2016;68:2467–2478. [DOI] [PubMed] [Google Scholar]
- 61. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA Jr, Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 63. Marschner IC, Colquhoun D, Simes RJ, Glasziou P, Harris P, Singh BB, Friedlander D, White H, Thompson P, Tonkin A. Long‐term risk stratification for survivors of acute coronary syndromes. Results from the long‐term intervention with pravastatin in ischemic disease (LIPID) study. LIPID Study Investigators. J Am Coll Cardiol. 2001;38:56–63. [DOI] [PubMed] [Google Scholar]
- 64. Sinning C, Zengin E, Waldeyer C, Seiffert M, Schnabel RB, Lubos E, Zeller T, Bickel C, Blankenberg S, Clemmensen PM, Westermann D. SYNTAX score‐0 patients: risk stratification in nonobstructive coronary artery disease. Clin Res Cardiol. 2016;105:901–911. [DOI] [PubMed] [Google Scholar]
- 65. Chen Q, Ding D, Zhang Y, Yang YN, Li Q, Chen XC, Hu G, Ling WH. Prediction of the risk of mortality using risk score in patients with coronary heart disease. Oncotarget. 2016;7:81680–81690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clayton TC, Lubsen J, Pocock SJ, Voko Z, Kirwan BA, Fox KA, Poole‐Wilson PA. Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ. 2005;331:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rapsomaniki E, Shah A, Perel P, Denaxas S, George J, Nicholas O, Udumyan R, Feder GS, Hingorani AD, Timmis A, Smeeth L, Hemingway H. Prognostic models for stable coronary artery disease based on electronic health record cohort of 102 023 patients. Eur Heart J. 2014;35:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Poppe KK, Doughty RN, Wells S, Gentles D, Hemingway H, Jackson R, Kerr AJ. Developing and validating a cardiovascular risk score for patients in the community with prior cardiovascular disease. Heart. 2017;103:891–892. [DOI] [PubMed] [Google Scholar]
- 69. Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, Amarenco P, LaRosa JC, Cramer MJ, Westerink J, Kappelle LJ, de Borst GJ, Visseren FL. Distribution of estimated 10‐year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134:1419–1429. [DOI] [PubMed] [Google Scholar]
- 70. Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, He P, Lewis BS, Merlini PA, Murphy SA, Sabatine MS, Scirica BM, Morrow DA. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134:304–313. [DOI] [PubMed] [Google Scholar]
- 71. Battes L, Barendse R, Steyerberg EW, Simoons ML, Deckers JW, Nieboer D, Bertrand M, Ferrari R, Remme WJ, Fox K, Takkenberg JJ, Boersma E, Kardys I. Development and validation of a cardiovascular risk assessment model in patients with established coronary artery disease. Am J Cardiol. 2013;112:27–33. [DOI] [PubMed] [Google Scholar]
- 72. Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–641. [DOI] [PubMed] [Google Scholar]
- 73. Johnston N, Schenck‐Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J. 2011;32:1331–1336. [DOI] [PubMed] [Google Scholar]
- 74. Shaw LJ, Mieres JH, Hendel RH, Boden WE, Gulati M, Veledar E, Hachamovitch R, Arrighi JA, Merz CN, Gibbons RJ, Wenger NK, Heller GV. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the what is the optimal method for ischemia evaluation in women (WOMEN) trial. Circulation. 2011;124:1239–1249. [DOI] [PubMed] [Google Scholar]
- 75. Sedlak TIM, Lee M, Gao M, Humphries K, Bairey Merz CN. Clinical outcomes in patients < 65 years with no obstructive coronary disease on coronary angiogram. J Am Coll Cardiol. 2012;59:E1486–E1486. [Google Scholar]
- 76. Chow BJ, Small G, Yam Y, Chen L, McPherson R, Achenbach S, Al‐Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann PA, Kim YJ, Leipsic J, LaBounty T, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry) registry. Arterioscler Thromb Vasc Biol. 2015;35:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Galway S, Adatia F, Grubisic M, Lee M, Daniele P, Humphries KH, Sedlak TL. Sex differences in cardiac medication use post‐catheterization in patients undergoing coronary angiography for stable angina with nonobstructive coronary artery disease. J Womens Health (Larchmt). 2017;26:976–983. [DOI] [PubMed] [Google Scholar]
- 78. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 79. Botker HE, Sonne HS, Schmitz O, Nielsen TT. Effects of doxazosin on exercise‐induced angina pectoris, ST‐segment depression, and insulin sensitivity in patients with syndrome X. Am J Cardiol. 1998;82:1352–1356. [DOI] [PubMed] [Google Scholar]
- 80. Rosen SD, Lorenzoni R, Kaski JC, Foale RA, Camici PG. Effect of alpha1‐adrenoceptor blockade on coronary vasodilator reserve in cardiac syndrome X. J Cardiovasc Pharmacol. 1999;34:554–560. [DOI] [PubMed] [Google Scholar]
- 81. Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide‐5‐mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–856, a858. [DOI] [PubMed] [Google Scholar]
- 82. Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63:286–290. [DOI] [PubMed] [Google Scholar]
- 83. Alzuhairi KS, Sogaard P, Ravkilde J, Azimi A, Maeng M, Jensen LO, Torp‐Pedersen C. Long‐term prognosis of patients with non‐ST‐segment elevation myocardial infarction according to coronary arteries atherosclerosis extent on coronary angiography: a historical cohort study. BMC Cardiovasc Disord. 2017;17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aldrovandi A, Maffei E, Seitun S, Martini C, Berti E, Grilli R, Messalli G, Weustink AC, Mollet NR, Nieman K, Ardissino D, de Feyter PJ, Krestin GP, Cademartiri F. Major adverse cardiac events and the severity of coronary atherosclerosis assessed by computed tomography coronary angiography in an outpatient population with suspected or known coronary artery disease. J Thorac Imaging. 2012;27:23–28. [DOI] [PubMed] [Google Scholar]
- 85. Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69:729–732. [DOI] [PubMed] [Google Scholar]
- 86. Hackett D, Davies G, Maseri A. Pre‐existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988;9:1317–1323. [DOI] [PubMed] [Google Scholar]
- 87. Chen C, Wei J, AlBadri A, Zarrini P, Bairey Merz CN. Coronary microvascular dysfunction‐ epidemiology, pathogenesis, prognosis, diagnosis, risk factors and therapy. Circ J. 2016;81:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


