Abstract
Background
Fragmentation of the tunica media is a hallmark of intracranial aneurysm formation, often leading to aneurysmal progression and subsequent rupture. The objective of this study is to determine the plasma level of elastin fragments in the lumen of ruptured versus unruptured human intracranial aneurysms.
Methods and Results
One hundred consecutive patients with/without ruptured saccular intracranial aneurysms undergoing endovascular coiling or stent‐assisted coiling were recruited. Blood samples were collected from the lumen of intracranial aneurysm using a microcatheter. The tip of the microcatheter was placed inside the aneurysm's sac in close proximity to the inner wall of the dome. Plasma levels of elastin fragments were measured using an ELISA‐based method. Mean plasma level of soluble human elastin fragments was significantly greater in ruptured aneurysms when compared with nonruptured aneurysms (102.0±15.5 versus 39.3±9.6 ng/mL; P<0.001). Mean plasma level of soluble human elastin fragments did not have significant correlation with age, sex, size, or aneurysm location.
Conclusions
The present study revealed that a significantly higher concentration of soluble human elastin fragments in the lumen of ruptured intracranial aneurysms when compared with nonruptured ones.
Keywords: biomarker, elastin fragments, ELISA, intracranial aneurysm, rupture
Subject Categories: Cerebral Aneurysm
Clinical Perspective
What Is New?
A significantly higher concentration of soluble elastin fragment was observed in the lumen of ruptured intracranial aneurysms when compared with nonruptured ones.
What Are the Clinical Implications?
The results of our study suggest a pathophysiological scenario where a gradual increase in elastin degradation renders the aneurysm unstable, thus precipitating rupture.
Introduction
Fragmentation of the tunica media is a hallmark of aneurysm formation, progression, and rupture.1 Histological analyses in animals have characterized the wall of intracranial aneurysms, ultimately revealing a lack of an internal elastic lamina and fragmentation of the media. Moreover, analyses of aneurysm wall tissue has shown a larger number of disorganized mural cells (vascular smooth muscle cells, myofibroblasts, and fibroblasts), whereas a few of the walls had entirely lost many of these cells. However, in all intracranial aneurysms, there is fragmentation of the internal elastic membrane. This process is medicated by the uptake of beta‐aminopropionitrile, which suppresses cross‐linking of collagen and elastin.2
Elastin is composed of soluble elastin subunits and is 1 of the major structural matrix proteins in the arterial wall.3 During the aneurysm formation process, an initial tear is formed. Then the dissection tends to expand to the degraded elastin layers, along with an inflammatory infiltrate, a major source of proteolytic enzymes such as elastases and matrix metalloproteinases, which, in turn, dramatically promotes the fragmentation process of the elastin network in the media.4, 5
In this article, we aimed to investigate whether variation exists between plasma level of elastin fragments in the lumen of ruptured and unruptured human intracranial saccular aneurysms.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The study protocol was approved by the University of Iowa Institutional Review Board. Informed consent was obtained for all subjects either by direct signature or consultation of patients’ power of attorney. Consecutive patients undergoing coiling or stent‐assisted coiling for saccular intracranial aneurysms at our institute between July 2014 and December 2015 were recruited. Patients with connective tissue disease, emphysema, chronic obstructive pulmonary disease, myocardial infarction, congestive heart failure, and patients taking steroids or receiving immunosuppressant therapy were excluded from the study.
Blood Sampling
The technique for intraluminal blood sampling has been previously described.6, 7 Briefly, after femoral arterial access was obtained, a sheath was inserted, and a guiding catheter was navigated into the target vessel. Subsequently, a microcatheter was advanced over a micro guide wire and positioned inside the aneurysm's sac. One blood sample (2 mL) was collected from each aneurysm lumen before stent or coil deployment. Blood sampling took ≈3 to 5 minutes for each case. Plasma concentration of soluble elastin fragments (sELAFs) was analyzed using a commercially available ELISA kit (BioSource.com).
Statistical Analysis
Statistical analysis and graphical display of obtained data were performed using GraphPad software (version 7.03; GraphPad Software Inc, San Diego, CA). Statistical significance of the difference observed between ruptured and nonrupture groups was tested using the independent t test, as well as the Mann–Whitney U test as a nonparametric alternative. Statistical significance between 3 or more groups was tested using 1‐way ANOVA, as well as the Kruskal–Wallis test as nonparametric alternative. Correlation between plasma levels of elastin fragments and age or size of the aneurysm was assessed using the Spearman correlation coefficient (R). All values are reported as mean±SD. In all cases, a P value <0.05 was considered to indicate statistical significance. Sample size was selected to achieve a power of at least 90%. The result from our sample‐size calculation required us to recruit at least 88 patients.
Results
Demographic Characteristics
As summarized in Table, 100 patients were enrolled in this study. Fifty‐three females were included. Mean patient age was 60.9±12.2 years. Forty‐nine aneurysms were ruptured.
Table 1.
Demographic Data for Soluble Human Elastin Fragments ELISA Assay Patients
| Characteristics | Mean (SD) or No. (%) Unless Specified |
|---|---|
| Total no. of patients | 100 |
| Age, y | 60.9±12.2 (range, 24–85) |
| Female | 53 (53) |
| Ruptured aneurysms | 49 (49) |
| Aneurysm size | 7.7±5.8 mm (range, 2.0–43.0) |
| Aneurysm location | |
| ACOM | 36 (36) |
| MCA | 21 (21) |
| ICA | 23 (23) |
| Posterior circulation | 20 (20) |
| Elastin fragments (mean concentration) | 70.0±33.8 ng/mL |
ACOM indicates anterior communicating artery; ICA, internal cerebral artery; MCA, middle cerebral artery.
Plasma Concentration of Elastin Fragments
Mean of plasma concentration of sELAF, across all treated patients, was 70.0±33.8 ng/mL (Table). Results of the independent t test indicated that the mean of plasma concentration of sELAF was significantly higher in ruptured aneurysms than unruptured aneurysms (102.0±15.3 versus 39.3±9.5 ng/mL; P<0.001; Figure 1). There was no association between mean plasma concentrations of sELAF in ruptured aneurysms and age (P=0.06), sex (female, 100.4±16.2 ng/mL; male, 103.6±14.8 ng/mL; P=0.48, independent t test), aneurysm size (P=0.33), and aneurysm location (anterior cerebral artery, 101.1±14.6 ng/mL; middle cerebral artery, 98.9±14.9 ng/mL; intracranial artery, 106.6±19.4 ng/mL; posterior circulation, 102.7±15.1 ng/mL; P=0.74, ANOVA; Figure 2A and 2B). Furthermore, there was no significant difference in mean plasma concentrations of sELAF between anterior and posterior circulation aneurysms. This was consistent for both ruptured (101.8±15.8 versus 102.7±15.1 ng; P=0.86, independent t test) and unruptured aneurysms (39.9±9.6 versus 37.0±9.5 ng; P=0.40, independent t test).
Figure 1.
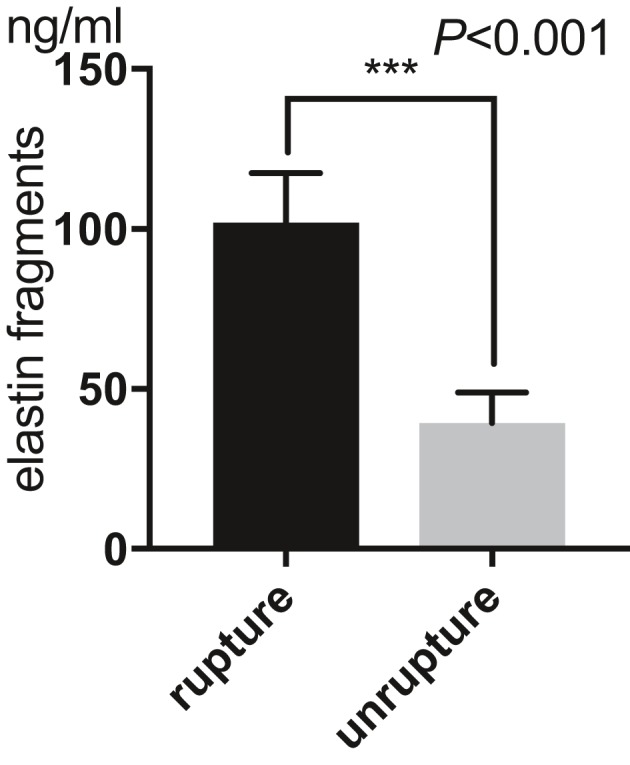
Luminal soluble elastin fragment concentration in ruptured and unruptured intracranial aneurysms. Results of the independent t test indicated that the mean of plasma concentration of elastin fragments was significantly higher in ruptured aneurysms (102.0±15.3 ng/mL) vs unruptured aneurysms (39.3±9.5 ng/mL; P<0.001). ***P<0.001.
Figure 2.
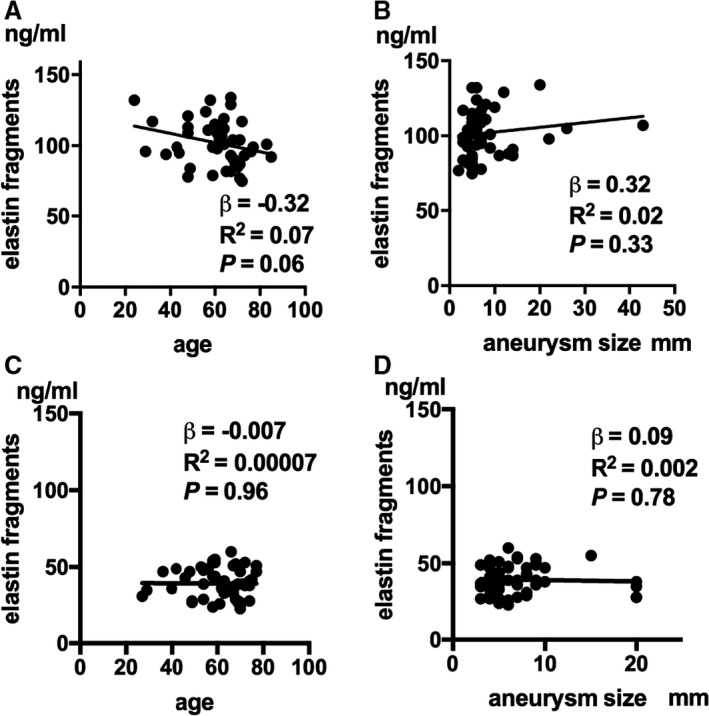
Luminal soluble elastin fragment concentration with respect to age and aneurysm size. A, Relationship between soluble elastin fragment (sELAF) concentration and age in ruptured aneurysms (R 2=0.07; P=0.06). B, Relationship between concentration of sELAF and aneurysm size in ruptured aneurysms (R 2=0.02; P=0.33). C, Relationship between concentration of sELAF and age in unruptured aneurysms (R 2=0.00007; P=0.96). D, Relation between concentration level of sELAF and aneurysm size in unruptured aneurysms (R 2=0.002; P=0.78). R indicates correlation coefficient; β, slope of regression line.
In regard to the unruptured sample, there was no association between mean plasma concentration of sELAF and age (P=0.96), sex (female, 59.5±12.4 ng/mL; male, 62.8±11.3 ng/mL; P=0.35, Mann–Whitney U test), aneurysm size (P=0.78), and location (anterior cerebral artery, 40.6±8.4 ng/mL; middle cerebral artery, 41.1±9.1 ng/mL; intracranial artery, 38.0±11.9 ng/mL; posterior circulation, 37.0±9.5 ng/mL; P=0.69, ANOVA; Figure 2C and 2D).
Discussion
In this study, we sought to evaluate the level of sELAF present within the lumen of human intracranial aneurysms. Our analysis indicated that a significantly higher plasma concentration of sELAF exists in lumen of ruptured aneurysms when compared with unruptured aneurysms.
A fluid‐solid‐growth model of saccular aneurysm evolution was developed by Watton et al in 2011.8 The model showed that aneurysms develop after localized degradation of elastin results in a perturbation in the arterial geometry. This creates an altered hemodynamic environment. The collagen fabric adapts, and the artery achieves a new homeostatic configuration. Subsequent degradation of elastin is explicitly linked to low wall shear stress in a confined region of the arterial domain. This leads to the development of a saccular aneurysm. If the collagen fabric was able to adapt, the aneurysm stabilizes in size. Aneurysms are thus subjects to periods with and without enlargement. Although the model failed to pinpoint what causes the progressive slow growth of aneurysms, the researchers suggested that it is not directly linked to wall shear stress and that the causes of progression may be multifactorial.
We postulate that an inciting event would lead to a failure of elastin, precipitating aneurysm growth or rupture. When Nabaei and Fatouraee included the degradation of elastin as a function of vascular wall effective stress in their model,9 thus taking into account the shear‐dependent nature of degradation and the mural‐cell–mediated destructive activities, they found that the model was more consistent with other computational and clinical studies. Furthermore, the evolving microstructural properties of the wall during the evolution process have been better predicted by this new model.
Further highlighting the role of elastin in aneurysmal rupture, a large retrospective series in China reported that 2 single‐nucleotide polymorphisms in elastin genes were associated with intracranial aneurysm formation and rupture.10 Similarly, a study in the Dutch population concluded that variants and haplotypes within the elastin gene were associated with risk of sporadic subarachnoid hemorrhage.11 Finally, Onda et al found a strong association between the intron20/intron23 variant (maps to chromosome 7q11) and intracranial aneurysms in Japan.12 However, this haplotype failed to correlate with subarachnoid hemorrhage in the central European study that examined 30 familial and 175 sporadic subarachnoid hemorrhage cases. This supports increased genetic heterogeneity among the populations examined.13 More important, these studies again highlight the role of elastin in aneurysm formation, progression, and rupture.14
The result of our study indicated that plasma levels of sELAF in unruptured intracranial aneurysms were also presented. This was expected, given that elastin degradation is an inciting event in the development and progression of aneurysms. Shinohara et al showed that sELAF was higher in serum of patients with acute aortic dissections compared with healthy patients and patients with acute myocardial infarction at the same age. Using a threshold for positivity (mean of healthy matched aged patients, ±3 SDs), they discovered that patients with acute aortic dissection with either an open or a partially open pseudo‐lumen were found be 88.9% positive for sELAF, whereas those with its early closure were 0% positive.4
Although we cannot determine whether the soluble elastin fragments are elevated because of the rupture or whether there has been an elevation in soluble fragments before the rupture, we tend to favor the pathophysiological scenario where a gradual increase in elastin degradation renders the aneurysm unstable, thus precipitating rupture. To accurately test this hypothesis, future studies should attempt to follow patients with unruptured aneurysms (that do not require treatment) to evaluate whether subsequent growth is associated with elevations in sELAF concentrations. Obtaining blood samples for measurements of sELAF in unruptured aneurysms using a microcatheter is invasive and not exempt of potential devastating complications. Consequently, we hope that future work explores sELAF consequently in peripheral venous blood samples. Further investigation may ultimately yield a certain threshold concentration of sELAF, which, when above, would confer a higher risk of rupture.
Limitations
We did not compare plasma concentration of sELAF in either the aneurysm wall tissue or venous blood. Moreover, it is unclear whether the higher levels of elastin reported in ruptured aneurysms may correspond to the generalized inflammatory process occurring after rupture. These limitations are subjects of future work.
Conclusions
The present study revealed a significantly higher concentration of sELAF in the lumen of ruptured intracranial aneurysms when compared with nonruptured aneurysms.
Sources of Funding
This work was partially supported by funding from Nakatani Foundation to Nakagawa.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e010051 DOI: 10.1161/JAHA.118.010051.)
This article was handled independently by Eric Smith, MD, MPH, as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Ro A, Kageyama N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: adventitial rupture, dilated lesion, intimal tear, and medial defect. J Neurosurg. 2013;119:221–227. [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto N, Handa H, Nagata I, Hazama F. Saccular cerebral aneurysms in rats. Am J Pathol. 1983;110:397–399. [PMC free article] [PubMed] [Google Scholar]
- 3. Powell J, Vine N, Crossman M. On the accumulation of D‐aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis. 1992;97:201–208. [DOI] [PubMed] [Google Scholar]
- 4. Shinohara T, Suzuki K, Okada M, Shiigai M, Shimizu M, Maehara T, Ohsuzu F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol. 2003;2003:10. [DOI] [PubMed] [Google Scholar]
- 5. Murray C, Edwards J. Spontaneous laceration of ascending aorta. Circulation. 1973;47:848–858. [DOI] [PubMed] [Google Scholar]
- 6. Chalouhi N, Points L, Pierce G, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013;44:2594–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chalouhi N, Jabbour P, Zanaty M, Starke RM, Torner J, Nakagawa D, Hasan DM. Sex differential in 15‐hydroxyprostaglandin dehydrogenase levels in the lumen of human intracranial aneurysms. J Am Heart Assoc. 2017;6:e006639 DOI: 10.1161/jaha.117.006639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watton P, Selimovic A, Raberger N, Huang P, Holzapfel G, Ventikos Y. Modelling evolution and the evolving mechanical environment of saccular cerebral aneurysms. Biomech Model Mechanobiol. 2011;10:109–132. [DOI] [PubMed] [Google Scholar]
- 9. Nabaei M, Fatouraee N. Microstructural modelling of cerebral aneurysm evolution through effective stress mediated destructive remodelling. J Theor Biol. 2014;354:60–71. [DOI] [PubMed] [Google Scholar]
- 10. Yang S, Wang T, You C, Liu W, Zhao K, Sun H, Mao B, Li X, Xiao A, Mao X, Zhang H. Association of polymorphisms in the elastin gene with sporadic ruptured intracranial aneurysms and unruptured intracranial aneurysms in Chinese patients. Int J Neurosci. 2013;123:454–458. [DOI] [PubMed] [Google Scholar]
- 11. Ruigrok Y, Seitz U, Wolterink S, Rinkel G, Wijmenga C, Urbán Z. Association of polymorphisms and haplotypes in the elastin gene in Dutch patients with sporadic aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:2064–2068. [DOI] [PubMed] [Google Scholar]
- 12. Onda H, Kasuya H, Yoneyama T, Takakura K, Hori T, Takeda J, Nakajima T, Inoue I. Genomewide‐linkage and haplotype‐association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet. 2001;69:804–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hofer A, Hermans M, Kubassek N, Sitzer M, Funke H, Stögbauer F, Ivaskevicius V, Oldenburg J, Burtscher J, Knopp U, Schoch B, Wanke I, Hübner F, Deinsberger W, Meyer B, Boecher‐Schwarz H, Poewe W, Raabe A, Steinmetz H, Auburger G. Elastin polymorphism haplotype and intracranial aneurysms are not associated in central Europe. Stroke. 2003;34:1207–1211. [DOI] [PubMed] [Google Scholar]
- 14. Yamada S, Utsunomiya M, Inoue K, Nozaki K, Miyamoto S, Hashimoto N, Takenaka K, Yoshinaga T, Koizumi A. Absence of linkage of familial intracranial aneurysms to 7q11 in highly aggregated Japanese families. Stroke. 2003;34:892–900. [DOI] [PubMed] [Google Scholar]


