Abstract
Background
Cystathionine is an intermediate product in the transsulfuration pathway and formed during the B6‐dependent conversion of methionine to cysteine. Elevated plasma cystathionine has been related to atherosclerosis, which is a major etiological factor for ischemic stroke. However, the role of cystathionine in stroke development is unknown. Therefore, we prospectively assessed the association of circulating levels of cystathionine with risk of total and ischemic stroke.
Methods and Results
Two‐thousand thirty‐six patients (64% men; median age, 62 years) undergoing coronary angiography for suspected stable angina pectoris were included. Stroke cases were identified by linkage to the CVDNOR (Cardiovascular Disease in Norway) project. Hazard ratios with confidence intervals (95% confidence interval) were estimated by using Cox‐regression analyses. During 7.3 years of median follow‐up, 124 (6.1%) incident strokes were ascertained, which comprised 100 cases of ischemic stroke. There was a positive association of plasma cystathionine with risk of total stroke and ischemic stroke. Comparing the fourth versus the first cystathionine quartiles, age‐ and sex‐adjusted hazard ratios (95% confidence interval) were 2.11 (1.19–3.75) and 2.56 (1.31–4.99) for total and ischemic stroke, respectively. Additional adjustment for major stroke risk factors only slightly attenuated the associations, which tended to be stronger in patients without previous or existing atrial fibrillation at baseline (hazard ratio [95% confidence interval], 2.43 [1.27–4.65] and 2.88 [1.39–5.98] for total and ischemic stroke, respectively).
Conclusions
In patients with suspected stable angina pectoris, plasma cystathionine was independently related to increased risk of total stroke and, in particular, ischemic stroke.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00354081.
Keywords: angina pectoris, atrial fibrillation, biomarker, epidemiology, stroke
Subject Categories: Ischemic Stroke, Cerebrovascular Disease/Stroke, Risk Factors, Atrial Fibrillation, Epidemiology
Clinical Perspective
What Is New?
Higher plasma cystathionine levels are associated with increased risk of total stroke and ischemic stroke in patients with suspected stable angina pectoris.
Risk associations tended to be stronger in patients without previous atrial fibrillation at baseline.
What Are the Clinical Implications?
The findings of our study are hypothesis generating and should be confirmed by additional prospective studies with alternative designs.
Introduction
Stroke is a leading cause of mortality and long‐term disability in the world and remains a massive public health burden.1 This highlights a pressing need to identify novel risk associations for stroke and improve our current understanding of its underlying pathophysiology.
Cystathionine is a sulphur‐containing amino acid produced from homocysteine (Hcy) during the conversion of methionine (Met) to cysteine (Cys) by the pyridoxal 5′‐phosphate–dependent enzymes, cystathionine β‐synthase and cystathionine γ‐lyase.2 In rats, high dietary intake of Met has been shown to induce hypercholesterolemia,3 in addition to impairing endothelial morphology and promoting arteriosclerosis.4 Increased flux through cystathionine β‐synthase has also been suggested to worsen outcome of ischemic stroke in experimental studies.5 Notably, elevated circulating cystathionine concentrations were observed in patients with cardiovascular disease (CVD),6 and increased levels were associated with oxidative damage7 and endothelial dysfunction.4, 8 Both these processes play a critical role in the pathogenesis of atherosclerotic disease9 and hence to clinical manifestations such as ischemic stroke.1, 10 Moreover, increased expression of cystathionine metabolizing enzymes have recently been found in human atherosclerotic lesions and are shown to exacerbate angiogenesis, thereby increasing the risk of plaque instability,11 often associated with atherothrombotic events.12 However, the role of plasma cystathionine in relation to stroke risk has not previously been reported.
We conducted a prospective study among patients with suspected stable angina pectoris to examine the association of circulating levels of cystathionine with subsequent risk of total and ischemic stroke.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Cohort
A detailed description of the study population has been published previously.13 In brief, 4164 patients who underwent coronary angiography for suspected stable angina pectoris at Haukeland and Stavanger University Hospitals in Western Norway during 2000–2004 were included. Of these, 2573 (61.8%) were subsequently enrolled in the WENBIT (Western Norway B‐vitamin Intervention Trial; NCT00354081) and received either (1) folic acid, vitamin B12 and vitamin B6 (n=642), (2) folic acid and vitamin B12 (n=642), (3) vitamin B6 (n=643), or (4) placebo (n=646).14 Because B‐vitamin supplementation has been reported to lower the risk of stroke in some studies,15, 16 we excluded patients who received supplementation with B‐vitamins in the WENBIT. In addition, patients with missing baseline data on cystathionine and 1 patient with an extremely high cystathionine level (20 μmol/L) were excluded, leaving 2036 patients eligible for the current analyses (Figure 1).
Figure 1.
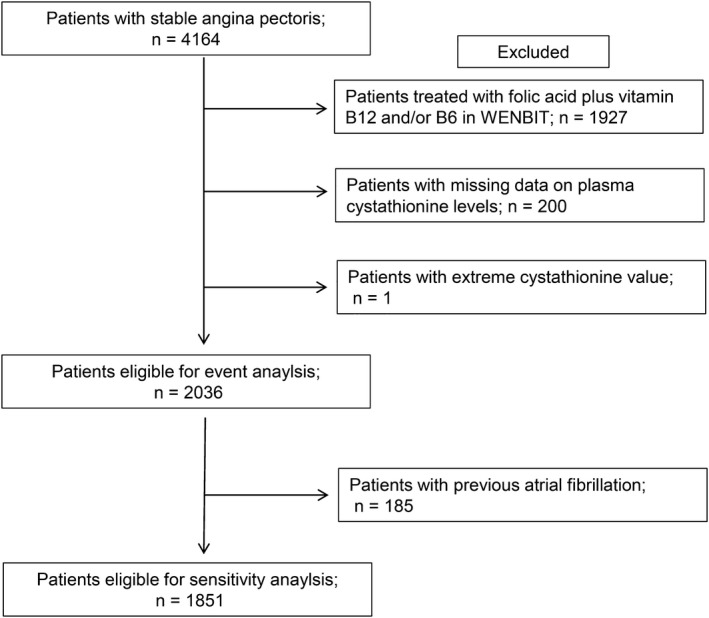
Flow diagram showing patient selection eligible for the study. WENBIT indicates Western Norway B‐Vitamin Intervention Trial.
The study was carried out according to the principles of the Declaration of Helsinki and was approved by the regional ethics committee (approval number 2010/1880) and the Norwegian Data Protection Authority. All study patients signed a consent form.
Baseline Data
Information on patients’ lifestyle and medical history were obtained through a self‐administered questionnaire and was validated against hospital records, as previously reported.14 Obesity was identified as having body mass index ≥30 kg/m2. Current smokers were defined as those who reported currently smoking, those who quit smoking within <1 month preceding examination, or having serum cotinine levels ≥85 nmol/L at baseline.13 Diabetes mellitus included types 1 and 2 and was classified according to previous diagnosis or by having plasma fasting glucose >7 mmol/L, nonfasting glucose >11.1 mmol/L, or a single measurement of glycated hemoglobin >6.5%.17 Hypertension was defined according to existing diagnosis. Left ventricular ejection fraction was obtained either by echocardiography or ventriculography during cardiac catheterization. Angiographic extent of coronary artery disease (CAD) was graded as 0 to 3 according to the number of significantly stenotic coronary arteries.
Follow‐up and Study End Points
Patients were followed from angiography until the onset of stroke or through December 31, 2009 (end of follow‐up). The primary end point was total stroke, including hospitalization or death attributed to stroke according to the International Statistical Evaluation of Disease, Tenth Revision (ICD‐10; I60–I64 except I63.6), whereas the secondary end point was ischemic stroke (I63 except I63.6). Information on clinical outcomes was obtained from the CVDNOR (Cardiovascular Disease in Norway) project (https://cvdnor.b.uib.no/), recording all CVD discharge diagnosis from the patient administrative systems at Norwegian public hospitals during 1994–2009.18 Fatal stroke events were ascertained by record linkage to the Cause of Death Registry at Statistics Norway (http://www.ssb.no/en).
Biochemical Analyses
Details on the collection of blood samples and biochemical analyses for relevant clinical indices have been described previously.13 Among patients recruited at Haukeland University Hospital, blood samples were drawn 1 to 3 days before coronary angiography and immediately stored at −80°C, whereas such samples from patients at Stavanger University Hospital were drawn after the angiographic procedure and subsequently transported within 48 hours to the core laboratory, until separated and frozen at −80°C. All study‐specific analyses were carried out at the laboratory of Bevital AS (Bergen, Norway; http://www.bevital.no) by laboratory staff blinded to the clinical outcomes of the patients. Plasma cystathionine, Met, Hcy, and Cys concentration were determined with gas chromatography/tandem mass spectrometry.19 Plasma cystathionine had within‐day coefficient of variation of ≤1.8% and between‐day coefficient of variation of ≤3.6%. Serum apolipoprotein A1, apolipoprotein B, and C‐reactive protein (CRP) were measured as previously described.13 Low‐density lipoprotein cholesterol and estimated glomerular filtration rate (eGFR) were calculated using the Friedewald and Chronic Kidney Disease Epidemiology Collaboration formula, respectively.
Statistical Analysis
Baseline continuous variables are reported as median (interquartile range), whereas categorical variables are presented as counts (percentages). Differences in baseline characteristics across plasma cystathionine quartiles were tested with logistic regression for categorical variables and linear median regression for continuous and ordinal data. Associations between cystathionine and related 1‐carbon metabolites were evaluated by Pearson's correlation, adjusted for age and sex.
Kaplan–Meier curves for stroke events were constructed to evaluate survival across plasma cystathionine quartiles and difference tested by the log‐rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident stroke were estimated using Cox regression models and reported according to quartiles of plasma cystathionine, with the lowest quartile as the reference category and per 1‐SD increment in‐log‐transformed plasma cystathionine. Additionally, HR and 95% CI for trends across increasing quartiles were assessed to determine whether their associations with stroke followed a linear pattern. These trend tests were performed by including the quartile‐specific median cystathionine value as a continuous variable in the Cox proportional hazards model and assessing significance with the Wald test. The numeric value of HR greater than 1 was considered a trend toward increased risk (positive trend) across the lowest to highest quartile of plasma cystathionine. Proportionality assumptions were assessed by inspecting log minus log plots and calculating Schoenfeld residuals. Potential nonlinear relationships between cystathionine and risk of incident strokes were visualized by generalized additive regression plots for multivariate models. Patients who did not experience any stroke event during follow‐up were censored. Model 1 included age (years) and sex, and the multivariate model 2 additionally included the categorical variables, obesity, diabetes mellitus, hypertension, smoking, previous acute myocardial infarction, and atrial fibrillation. Because B‐vitamins are crucial in the metabolism of cystathionine20, 21 and systemic B‐vitamin levels have been associated with CVD risk,22, 23 we did not include these variables in the main multivariate model. However, to assess their potential influence on any cystathionine‐stroke relationship, these variables were additionally included in an extended model, as was eGFR, because elevated circulating cystathionine concentrations have been reported in renal patients.21
To investigate effect modifications, survival analyses were explored according to the median values of continuous variable and subgroups of categorical variables, and possible interactions tested by including the interaction products term in Cox model 2. Moreover, the possibility of unmeasured confounding was investigated by performing additional sensitivity analysis and applying E‐value formula to the multivariate model, according to the recent recommendations for observational studies.24
Given the hypothesis‐testing nature of our analyses, we did not adjust for multiple comparisons and a P<0.05 was considered statistically significant.25 Statistical analyses were performed using SPSS (Sample Power, Version 23; SPSS IBM, Armonk, NY) and R software (version 3.1.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
Baseline characteristics according to plasma cystathionine quartiles are presented in Table 1. The study population consisted of 64.0% men, and median (interquartile range) age was 62 (16) years, whereas plasma concentrations of cystathionine were 0.27 (0.20) μmol/L. Cutoffs for serum plasma cystathionine in quartiles were <0.20, 0.20 to 0.27, 0.27 to 0.39, and >3.20 μmol/L in the first, second, third, and fourth quartiles, respectively. SD was 0.48 for plasma cystathionine and 0.61 for log‐transformed plasma cystathionine. Patients in the upper quartiles of plasma cystathionine more often were older, male, and obese and had hypertension, diabetes mellitus, and established CVD. Accordingly, plasma cystathionine showed an inverse association with left ventricular ejection fraction and a strong positive relationship with more‐extensive CAD at angiography, also reflected by more‐frequent use of CVD medications among subjects in the upper cystathionine quartile. Furthermore, we observed positive associations of cystathionine with serum CRP and plasma asymmetric dimethylarginine, whereas there were inverse associations with eGFR.
Table 1.
Baseline Characteristics According to Quartiles of Plasma Cystathionine
| Quartiles of Plasma Cystathionine | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P trend a | P trend b | |
| Age, y | 58 (14) | 61 (15) | 64 (16) | 67 (14) | <0.001 | ··· |
| Male sex, n (%) | 303 (59.5) | 321 (63.1) | 322 (63.3) | 356 (69.9) | 0.001 | ··· |
| Coronary risk factor, n (%) | ||||||
| Obesity | 77 (15.2) | 100 (19.7) | 107 (21.0) | 119 (23.4) | 0.001 | <0.001 |
| Hypertension | 189 (37.1) | 230 (45.2) | 253 (49.7) | 276 (54.2) | <0.001 | <0.001 |
| Diabetes mellitus | 211 (41.9) | 210 (41.7) | 222 (43.6) | 267 (52.5) | 0.002 | 0.003 |
| Current smoking | 191 (37.6) | 160 (31.9) | 150 (29.6) | 134 (26.5) | <0.001 | 0.16 |
| eGFR, mL/min/1.73 m2 | 95 (16) | 90 (20) | 87 (23) | 78 (29) | <0.001 | <0.001 |
| Serum CRP, mg/L | 1.58 (2.50) | 1.80 (3.0) | 1.89 (2.97) | 2.12 (3.44) | <0.001 | <0.001 |
| Plasma ADMA, μmol/L | 0.53 (0.15) | 0.54 (0.13) | 0.58 (0.16) | 0.59 (0.17) | <0.001 | <0.001 |
| Plasma 1‐carbon metabolites, μmol/L | ||||||
| Met | 25.6 (7.2) | 27.4 (8.1) | 29.9 (11.3) | 31.4 (12.7) | <0.001 | <0.001 |
| tHcy | 9.27 (3.13) | 10.5 (3.69) | 10.9 (4.27) | 12.4 (5.32) | <0.001 | <0.001 |
| Cys | 283 (45.2) | 294 (49.2) | 298 (50.1) | 306 (53) | <0.001 | <0.001 |
| Plasma B‐vitamin status | ||||||
| Folate, nmol/L | 11.9 (9.8) | 9.81 (7.27) | 9.75 (6.51) | 9.89 (7.01) | <0.001 | <0.001 |
| Cobalamin (B12), pmol/L | 390 (213) | 393 (193) | 391 (210) | 391 (231) | 0.75 | 0.44 |
| PLP, nmol/L | 45.6 (43.6) | 42.3 (32.4) | 41.9 (31.2) | 40.2 (31.9) | <0.001 | <0.001 |
| Serum lipids | ||||||
| Triglycerides, mmol/L | 1.28 (0.86) | 1.45 (1.02) | 1.52 (1.18) | 1.60 (1.11) | <0.001 | <0.001 |
| ApoB, g/L | 0.91 (0. 31) | 0.89 (0.35) | 0.87 (0.30) | 0.86 (0.31) | 0.002 | 0.02 |
| Apo A1, g/L | 1.36 (0. 39) | 1.34 (0.36) | 1.35 (0.36) | 1.28 (0.31) | <0.001 | <0.001 |
| LDL‐C | 3.1 (1.38) | 3.0 (1.45) | 2.9 (1.40) | 2.8 (1.28) | <0.001 | <0.001 |
| Previous CVD, n (%) | ||||||
| AMI | 166 (32.6) | 166 (32.6) | 189 (37.1) | 228 (44.8) | <0.001 | 0.02 |
| PAD | 38 (7.5) | 38 (7.5) | 49 (9.6) | 64 (12.6) | 0.002 | 0.17 |
| AF | 35 (6.9) | 42 (8.3) | 48 (9.4) | 60 (11.8) | 0.005 | 0.34 |
| Cerebrovascular diseasec | 26 (5.1) | 35 (6.9) | 33 (6.5) | 54 (10.6) | 0.002 | 0.18 |
| Extent of CAD, n (%) | <0.001 | 0.09 | ||||
| No significant stenosis | 225 (44.2) | 210 (41.3) | 192 (37.7) | 141 (27.7) | ||
| 1‐vessel disease | 102 (20.0) | 83 (16.3) | 97 (19.1) | 94 (18.5) | ||
| 2‐vessel disease | 84 (16.5) | 102 (20.0) | 99 (19.4) | 92 (18.1) | ||
| 3‐vessel disease | 98 (19.3) | 114 (22.4) | 121 (23.8) | 182 (35.8) | ||
| LVEF (%) | 70 (10) | 70 (10) | 66 (10) | 65 (11) | <0.001 | <0.001 |
| Medications before angiography, n (%) | ||||||
| Aspirin | 393 (77.2) | 405 (79.6) | 395 (77.6) | 386 (75.8) | 0.46 | 0.25 |
| Warfarin | 18 (3.5) | 24 (4.7) | 25 (4.9) | 47 (9.2) | <0.001 | 0.01 |
| Statins | 318 (62.5) | 339 (66.6) | 348 (68.4) | 363 (71.3) | 0.002 | 0.06 |
| β‐blocker | 333 (65.4) | 354 (69.5) | 376 (73.9) | 388 (76.2) | <0.001 | 0.002 |
| ACEIs | 74 (14.5) | 84 (16.5) | 102 (20.0) | 146 (28.7) | <0.001 | <0.001 |
| Medications after angiography, n (%) | ||||||
| Aspirin | 362 (71.1) | 382 (75.0) | 381 (74.9) | 398 (78.2) | 0.02 | 0.81 |
| Warfarin | 17 (3.3) | 26 (5.1) | 23 (4.5) | 43 (8.4) | 0.001 | 0.02 |
| Statins | 349 (68.6) | 371 (72.9) | 375 (73.7) | 397 (78.0) | 0.001 | 0.22 |
| β‐blocker | 315 (61.9) | 330 (64.8) | 352 (69.2) | 382 (75.0) | <0.001 | 0.004 |
| ACEIs | 78 (15.3) | 85 (16.7) | 106 (20.8) | 144 (28.3) | <0.001 | <0.001 |
Continuous variables are presented as medians (interquartile range), and categorical variables are reported as counts (%). ACEIs indicates angiotensin‐converting‐enzyme inhibitors; ADMA, asymmetric dimethylarginine; AF, atrial fibrillation; AMI, acute myocardial infarction; apoA1, apolipoprotein A1; apoB, apolipoprotein B; BMI, body mass index; CAD, coronary artery disease; CRP, C‐reactive protein; CVD, cardiovascular disease; Cys, cysteine; eGFR, estimated glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; Met, methionine; PAD, peripheral artery disease; PLP, pyridoxal 5′‐phosphate; tHcy, total homocysteine.
Unadjusted.
Adjusted for age and sex.
Composite of stroke, transient ischemic attack, or carotid stenosis.
As anticipated, plasma cystathionine was positively associated with the 1‐carbon metabolites, plasma Met, total Hcy, and total Cyst (r=0.24, 0.11, and 0.12, respectively; P<0.001) and negatively to serum folate and plasma pyridoxal 5′‐phosphate, whereas no relationship was observed with serum cobalamin. Patients in the higher cystathionine quartiles also had higher levels of serum triglycerides and lower serum apolipoprotein A1, apolipoprotein B, and low‐density lipoprotein cholesterol.
Plasma Cystathionine and Risk of Stroke
During a median (interquartile range) follow‐up of 7.3 (2.4) years, an incident stroke event occurred in 124 patients (6.1%), which comprised 100 (4.9%) cases of ischemic stroke. As illustrated in Figure 2, there was higher incidences of stroke throughout higher plasma cystathionine quartiles (P<0.001 for log‐rank test).
Figure 2.
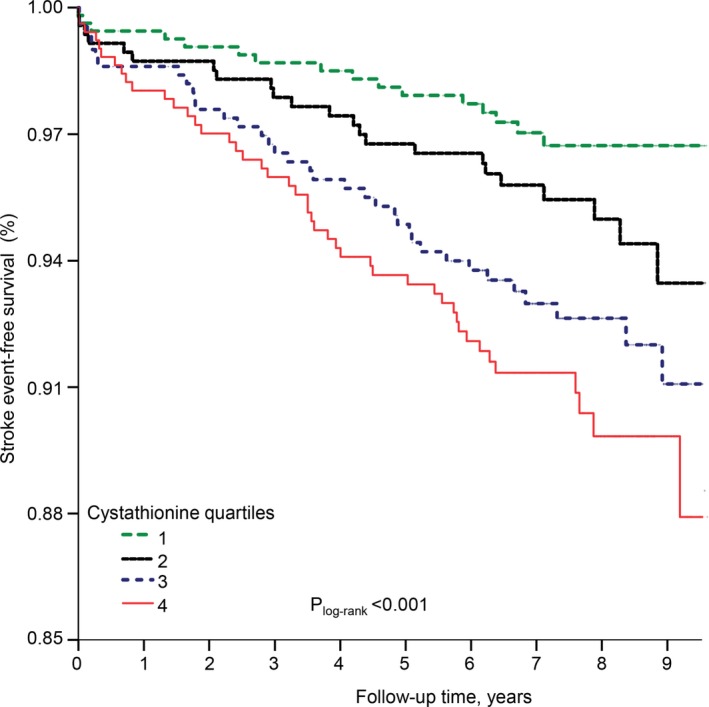
Stroke‐event–free survival. Kaplan–Meier plot showing crude stroke‐free survival according to the quartiles of plasma cystathionine (designated as quartiles 1, 2, 3, and 4). Difference between quartiles was compared by the log‐rank test.
Accordingly, we found an approximately linear positive trend between plasma cystathionine and subsequent risk of stroke (Figure 3). In an unadjusted model, those in the highest plasma cystathionine quartile compared with the lowest had increased risk of total stroke (HR, 3.12; 95% CI, 1.78–5.44). This association was particularly strong for ischemic strokes (HR, 3.69; 95% CI, 1.92–7.08). Corresponding HRs (95% CIs) were 2.11 (1.19–3.75) and 2.56 (1.31–4.99) in age‐ and sex‐adjusted analyses and essentially similar in the multivariate models (Table 2).
Figure 3.
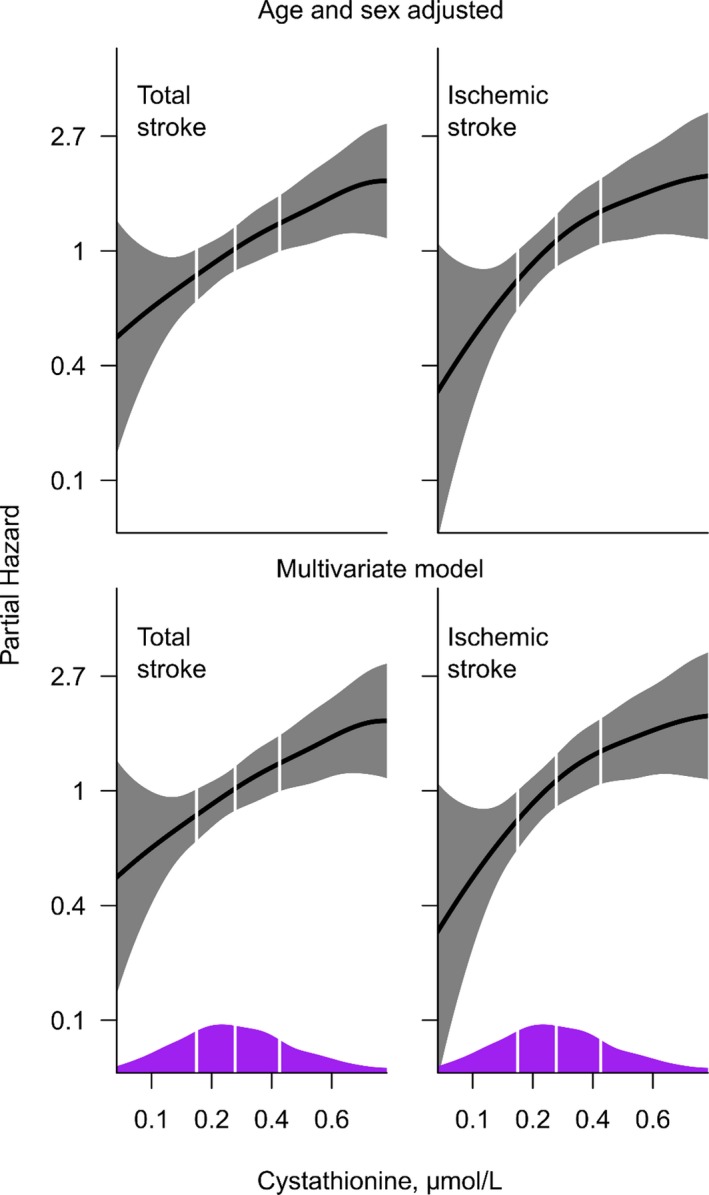
Association of log‐transformed plasma cystathionine with stroke and ischemic stroke. Multivariable models include age, sex, obesity, diabetes mellitus (yes/no), hypertension (yes/no), smoking (yes/no), previous acute myocardial infarction (yes/no), and atrial fibrillation (yes/no). Kernel density plots are superimposed along the x‐axis, to display distribution of plasma cystathionine. Vertical lines depict the 25th, 50th, and 75th percentile of the population.
Table 2.
Risk Association Between Plasma Cystathionine and Stroke
| Plasma Cystathionine | |||||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Age and Sex Adjusted | Multivariatea | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Total Stroke | |||||||
| Events | 124 | ||||||
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.53 (0.82–2.87) | 0.18 | 1.33 (0.70–2.49) | 0.37 | 1.30 (0.69–2.45) | 0.42 | |
| Q3 | 2.47 (1.39–4.38) | 0.002 | 1.87 (1.04–3.33) | 0.04 | 1.78 (0.99–3.19) | 0.06 | |
| Q4 | 3.12 (1.78–5.44) | <0.001 | 2.11 (1.19–3.75) | 0.01 | 2.01 (1.14–3.61) | 0.02 | |
| Trend | 1.46 (1.23–1.72) | <0.001 | 1.28 (1.08–1.52) | 0.004 | 1.26 (1.06–1.50) | 0.01 | |
| Per 1‐SDb | 1.41 (1.22–1.63) | <0.001 | 1.26 (1.07–1.47) | 0.005 | 1.23 (1.05–1.44) | 0.01 | |
| Ischemic Stroke | |||||||
| Events | 100 | ||||||
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.82 (0.88–3.75) | 0.11 | 1.57 (0.76–3.25) | 0.22 | 1.53 (0.74–3.16) | 0.26 | |
| Q3 | 2.98 (1.52–5.82) | 0.001 | 2.28 (1.16–4.48) | 0.02 | 2.16 (1.09–4.26) | 0.03 | |
| Q4 | 3.69 (1.92–7.08) | <0.001 | 2.56 (1.31–4.99) | 0.01 | 2.39 (1.23–4.72) | 0.01 | |
| Trend | 1.51 (1.25–1.82) | <0.001 | 1.34 (1.11–1.62) | 0.003 | 1.32 (1.09–1.59) | 0.005 | |
| Per 1‐SDb | 1.46 (1.25–1.71) | <0.001 | 1.31 (1.11–1.56) | 0.002 | 1.29 (1.09–1.54) | 0.004 | |
CI indicates confidence interval; HR, hazard ratio; Q1, first quartile; Q4, fourth quartile.
Adjusted for age, sex, obesity, diabetes mellitus, hypertension, smoking, previous acute myocardial infarction, and atrial fibrillation.
Log‐transformed.
Adjusting for previous CVD, extent of CAD, CRP, low‐density lipoprotein cholesterol, or medications at baseline had no significant effect on the risk associations between plasma cystathionine and either end point (data not shown).
Met, Hcy, and Cys are important metabolites of 1‐carbon cycle and are closely associated with cystathionine metabolism.2, 7 Hence, we also studied the potential influence by adjusting for these parameters on the cystathionine‐stroke risk associations. In the multivariate model, neither plasma Met nor Cys were significantly associated with stroke risk, whereas plasma total Hcy was only borderline positively related to the risk before controlling for cystathionine (Table 3). However, additional adjustment for these 1‐carbon metabolites or plasma markers of B‐vitamins status in the multivariate model did not appreciably alter the associations (Table 4), whereas including eGFR slightly attenuated the risk estimates (HR [95% CI] were 1.88 [1.04–3.41] for total stroke and 2.19 [1.09–4.41] for ischemic stroke, when comparing cystathionine quartiles 4–1).
Table 3.
Hazard Ratios for Incident Stroke According to Plasma Levels of Methionine, Homocysteine, and Cysteine
| Total Stroke | Ischemic Stroke | |||||||
|---|---|---|---|---|---|---|---|---|
| Q4 vs Q1 | P Value | Per 1‐SDa | P Value | Q4 vs Q1 | P Value | Per 1‐SDa | P Value | |
| Plasma Met | ||||||||
| Unadjusted | 0.92 (0.54–1.57) | 0.77 | 0.88 (0.74–1.06) | 0.19 | 0.93 (0.51–1.67) | 0.81 | 0.89 (0.73–1.09) | 0.89 |
| Model 1b | 1.04 (0.61–1.78) | 0.88 | 0.92 (0.76–1.09) | 0.35 | 1.05 (0.58–1.89) | 0.88 | 0.93 (0.76–1.13) | 0.44 |
| Model 2c | 1.02 (0.60–1.75) | 0.93 | 0.91 (0.76–1.08) | 0.29 | 1.01 (0.55–1.83) | 0.98 | 0.91 (0.75–1.11) | 0.37 |
| Model 3d | 0.91 (0.51–1.62) | 0.75 | 0.87 (0.73–1.06) | 0.18 | 0.90 (0.48–1.75) | 0.79 | 0.88 (0.71–1.08) | 0.23 |
| Plasma tHcy | ||||||||
| Unadjusted | 2.63 (1.55–4.44) | <0.001 | 1.40 (1.21–1.61) | <0.001 | 2.74 (1.55–4.83) | <0.001 | 1.44 (1.23–1.69) | <0.001 |
| Model 1b | 1.76 (1.01–3.04) | 0.05 | 1.27 (1.08–1.50) | 0.003 | 1.87 (1.03–3.38) | 0.04 | 1.32 (1.11–1.59) | 0.002 |
| Model 2c | 1.49 (0.85–2.60) | 0.16 | 1.21 (1.02–1.45) | 0.04 | 1.57 (0.86–2.87) | 0.14 | 1.24 (1.03–1.54) | 0.03 |
| Model 3d | 1.27 (0.72–2.28) | 0.39 | 1.14 (0.95–1.39) | 0.12 | 1.30 (0.71–2.43) | 0.40 | 1.16 (0.97–1.44) | 0.10 |
| Plasma tCys | ||||||||
| Unadjusted | 3.38 (1.91–5.97) | <0.001 | 1.49 (1.26–1.76) | <0.001 | 2.88 (1.51–5.47) | <0.001 | 1.37 (1.14–1.65) | 0.001 |
| Model 1b | 1.80 (0.99–3.29) | 0.54 | 1.16 (0.96–1.41) | 0.10 | 1.51 (0.77–2.97) | 0.23 | 1.06 (0.87–1.31) | 0.56 |
| Model 2c | 1.75 (0.95–3.21) | 0.07 | 1.13 (0.93–1.36) | 0.20 | 1.44 (0.7–2.85) | 0.29 | 1.02 (0.83–1.26) | 0.84 |
| Model 3d | 1.63 (0.89–3.01) | 0.12 | 1.10 (0.91–1.33) | 0.31 | 1.31 (0.66–2.61) | 0.44 | 0.98 (0.81–1.22) | 0.96 |
Met indicates methionine; Q1, first quartile; Q4, fourth quartile; tCyst, total cysteine; tHcy, total homocysteine.
Log‐transformed.
Adjusted for age and sex.
Adjusted for variables in model 1 plus obesity, diabetes mellitus, hypertension, smoking, previous myocardial infarction, and atrial fibrillation.
Adjusted for variables in model 2 plus plasma cystathionine.
Table 4.
Risk Association Between Plasma Cystathionine and Incident Stroke, When Separately Adjusted for Related 1‐Carbon Metabolites and Markers of B‐Vitamin Status
| Total Stroke | Ischemic Stroke | |||||||
|---|---|---|---|---|---|---|---|---|
| Q4 vs Q1 | P Value | Per 1‐SDa | P Value | Q4 vs Q1 | P Value | Per 1‐SDa | P Value | |
| Plasma cystathionine | ||||||||
| Model 2+Metb | 2.10 (1.16–3.85) | 0.01 | 1.23 (1.05–1.45) | 0.01 | 2.50 (1.26–5.08) | 0.01 | 1.30 (1.09–1.55) | 0.004 |
| Model 2+tHcyb | 1.92 (1.05–3.57) | 0.04 | 1.20 (1.03–1.43) | 0.04 | 2.26 (1.10–4.63) | 0.03 | 1.24 (1.03–1.50) | 0.03 |
| Model 2+tCysb | 1.96 (1.08–3.49) | 0.03 | 1.22 (1.04–1.42) | 0.03 | 2.34 (1.18–4.62) | 0.01 | 1.28 (1.07–1.53) | 0.01 |
| Model 2+folateb | 2.09 (1.17–3.76) | 0.01 | 1.24 (1.05–1.45) | 0.01 | 2.42 (1.23–4.81) | 0.01 | 1.29 (1.09–1.54) | 0.004 |
| Model 2+PLPb | 1.95 (1.06–3.59) | 0.03 | 1.21 (1.03–1.44) | 0.03 | 2.27 (1.13–4.64) | 0.02 | 1.26 (1.06–1.53) | 0.01 |
| Model 2+Cobb | 2.05 (1.04–4.06) | 0.04 | 1.26 (1.05–1.52) | 0.02 | 2.52 (1.12–5.66) | 0.02 | 1.33 (1.09–1.64) | 0.01 |
Cob indicates cobalamin (B12); Met, methionine; PLP, pyridoxal 5′‐phosphate; Q1, first quartile; Q4, fourth quartile; tCyst, total cysteine; tHcy, total homocysteine.
Log‐transformed.
Adjusted for age, sex, obesity, diabetes mellitus, hypertension, smoking, previous myocardial infarction, and atrial fibrillation.
Subgroup Analyses
Figure 4 and Table 5 depict the risk associations between plasma cystathionine and stroke according to several traditional risk factors and plasma B‐vitamin status, respectively. Notably, we observed a stronger risk estimate of plasma cystathionine among patients without than those with previous atrial fibrillation at baseline (P interaction=0.03). The risk relationship did not differ significantly according to other subgroup parameters.
Figure 4.
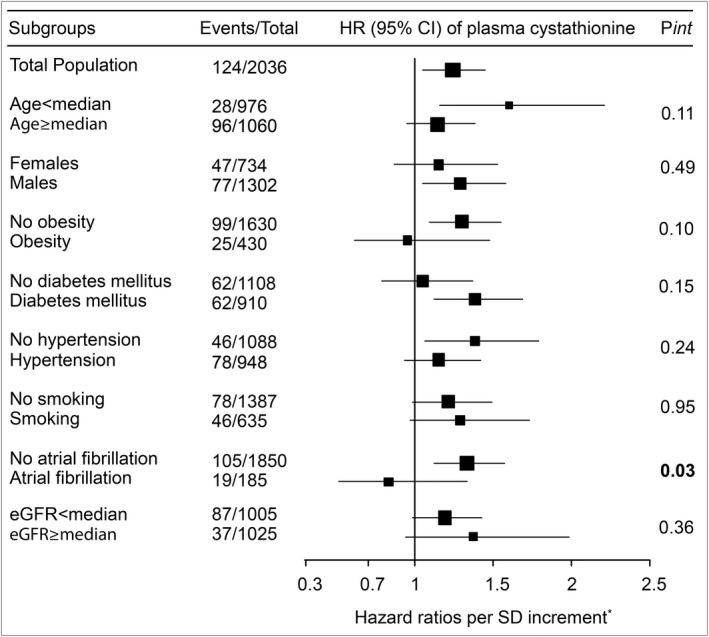
Forest plot depicting hazard ratios between plasma cystathionine and total stroke according to several traditional risk factors. Box areas illustrate sample sizes, and horizontal lines depict 95% confidence intervals (CIs). eGFR indicates estimated glomerular filtration rate; HR, hazard ratio. *Adjusted for age, sex, obesity, diabetes mellitus, hypertension, smoking, previous myocardial infarction, and atrial fibrillation.
Table 5.
Association Between Plasma Cystathionine Per SD (Log‐Transformed) and Total Stroke According to Plasma Markers of B‐Vitamin Status
| Subgroups | Events/Total | HR (95% CI)a | P Value | P int |
|---|---|---|---|---|
| Folate | ||||
| <Median | 55/1015 | 1.20 (0.92–1.55) | 0.027 | 0.56 |
| ≥Median | 69/1015 | 1.21 (0.96–1.51) | 0.09 | |
| PLP | ||||
| <Median | 80/1011 | 1.26 (1.03–1.55) | 0.02 | 0.58 |
| ≥Median | 44/1014 | 1.13 (0.85–1.49) | 0.40 | |
| Cob | ||||
| <Median | 46/800 | 1.39 (1.05–1.84) | 0.02 | 0.44 |
| ≥Median | 41/800 | 1.15 (0.86–1.51) | 0.34 | |
CI indicates confidence interval; Cob, cobalamin (B12); HR, hazard ratio; PLP, pyridoxal 5′‐phosphate.
Adjusted for age, sex, obesity, diabetes mellitus, hypertension, smoking, previous myocardial infarction, and atrial fibrillation.
Sensitivity Analysis
To elucidate the influence of previous atrial fibrillation further, we performed additional sensitivity analysis after excluding patients with previous atrial fibrillation (Figure 1). Notably, we obtained numerically stronger associations of cystathionine with risk in the remaining 1851 patients without atrial fibrillation. In the multivariate model, HR (95% CI) were 2.43 (1.27–4.65; P<0.005) for total stroke and 2.88 (1.39–5.98; P<0.004) for ischemic stroke, when comparing cystathionine quartiles 4 to 1.
In addition, application of E‐value formula to the multivariate model, also including eGFR, revealed high sensitivity of the observed association between cystathionine and total stroke, with an E‐value of 3.19 and 1.28 each, for the estimate and lower reported CI, respectively. Corresponding E‐values were 3.80 and 1.40 for the ischemic stroke event analysis.
Discussion
Principal Findings
In a large cohort of patients undergoing elective coronary angiography for stable angina pectoris, elevated concentrations of plasma cystathionine were strongly associated with increased risk of total stroke and ischemic stroke during follow‐up. The risk relationship was independent of several established stroke risk factor and potential confounders, and tended to be stronger in patients without previous atrial fibrillation.
Strengths and Limitations
The major strengths of this study include the large sample size, detailed clinical information on patient characteristics, and its prospective design. Although the influence of residual confounding cannot be ruled out in an observational study, the risk estimates were consistent also after the adjustment for major stroke risk factors. Notably, the high E‐value validates the strength and robustness of the observed findings to the presence of an unmeasured cofounder.24
There are several additional aspects of the present study that merit consideration. First, because of lack of data, we were unable to perform separate analyses on the subtypes of stroke. Second, the current study is based on the measurement of blood parameters at only 1 time point, thus preventing assessment of the possible variation of cystathionine during follow‐up. However, the within‐subject reproducibility has been shown to be fair to good for plasma cystathionine,26 which allows 1‐exposure assessment of plasma cystathionine status, with reduced risk of regression dilution bias. Third, we performed multiple subgroup comparisons, and it is possible that the significant interaction between cystathionine and previous atrial fibrillation on stroke occurrence is attributed to chance (type I error). This is, however, not immediately supported by our observation of stronger risk estimates after excluding patients with atrial fibrillation at baseline. Fourth, the accuracy of self‐reported information on use of medications, disease conditions, and other health issues pose a concern, despite validation of the questionnaires. Fifth, blood samples from Stavanger University hospital were transported to Bevital AS, thus exposing the samples to room temperature for ≈3 days. This is unlikely to have introduced a bias, however, given that plasma cystathionine concentrations are reported to be fairly stable during short‐term storage under such conditions.27 Furthermore, the potential of analytical bias was reduced by using 1 central laboratory for analyzing the plasma samples. Sixth, the long follow‐up time and prospective design minimizes the potential risk of reverse causation. Yet, we cannot exclude that patients with established CVD at baseline may also have had lifestyle modifications or other interventions performed, which could somehow influence plasma cystathionine status. Seventh, information on incident stroke end points were ascertained from the patient administrative database only, which indicates that cases with asymptomatic stroke without hospitalization might not have been reported. However, we do not suspect that such misclassification differs according to levels of plasma cystathionine. Furthermore, the possibility of detection bias is unlikely, because each patient's unique national identification number was linked to health registries with almost 100% coverage of all endpoints. Eighth, we studied patients treated with various medications for CVD, and thus our results may not be applicable to the general healthy population. Finally, our results are hypothesis generating and should be confirmed by additional prospective studies with alternative designs.
Cystathionine and CVD in Other Epidemiological Studies
Data on the relationship between cystathionine status and CVD are scant. In a small study among 14 healthy participants, plasma cystathionine was related to impaired vascular function following Met loading.8 Another study reported high plasma cystathionine levels among patients with CVD compared with those without CVD.6 To the best of our knowledge, this is the first large‐scale investigation to demonstrate a significant relationship between plasma cystathionine and subsequent risk of both total stroke and ischemic stroke. However, the magnitude of risk association was stronger for ischemic, as compared with total stroke, and particularly stronger in patients without a history of atrial fibrillation, suggesting that elevated cystathionine is associated with the atherosclerotic processes leading to stroke events.
Possible Mechanisms
Plasma cystathionine, lipids, and CAD
Elevation of plasma cystathionine has been related to an unfavorable lipid profile.6 Dyslipidemia is a less‐well‐known risk factor for stroke28; however, it is pivotal in atherosclerosis and thereby may be linked to stroke.9 Interestingly, although higher plasma cystathionine was related to an overall adverse CVD risk profile, including extent of CAD and previous CVD, we somewhat unexpectedly observed an inverse association between plasma cystathionine and low‐density lipoprotein cholesterol. However, this may be attributed to statin therapy, which was received by a majority of the patients. Nevertheless, adjusting for these parameters did not attenuate the risk estimates, implying that factors other than those traditionally predisposing to atherosclerosis are likely to play a role in the relationship between cystathionine and stroke development.
Plasma cystathionine and inflammation
Previous studies have reported increased cystathionine concentrations in patients with asthma, a condition known to be related to systemic inflammation.29 Accordingly, in our study, plasma cystathionine concentration was directly associated with CRP, a marker of inflammation.30 Accumulating data indicate that inflammation may play a key role in the pathophysiology of atherothrombosis.30 However, adjusting for CRP did not influence the relationship between cystathionine and stroke risk, indicating that the associations are not mediated solely by CRP‐related inflammation.
Plasma cystathionine, endothelial dysfunction, and oxidative stress
Both experimental and clinical data have shown that increased systemic cystathionine levels are related to alterations in endothelial function.4, 8 Endothelial dysfunction, characterized by reduced nitric oxide–mediated vasodilatation of arteries, is a crucial step in the early formation of atherosclerotic lesions9 and is associated with plaque progression and subsequent ischemic stroke events.10 It is therefore interesting that plasma cystathionine demonstrated a positive association with asymmetric dimethylarginine in our study, given that asymmetric dimethylarginine may induce vascular dysfunction through suppression of endothelial nitric oxide synthase.31 In addition, systemic cystathionine elevation has previously been related to impaired downstream catabolism to cysteine, rather than increased production per se.7, 32 Reduced cystathionine‐transsulfuration may impair glutathione production and thereby lead to oxidative stress.7 Oxidative stress can, in turn, further impair endothelial morphology by inactivating nitric oxide,9 in addition to mediating several other biological processes such as lipid peroxidation and induction of vascular lesions.1 Notably, a previous study from a subsample of the current cohort showed that higher plasma cystathionine concentration are related to low levels of reduced glutathione,33 indicating impaired antioxidant defence.7
Plasma status of cystathionine
Elevated cystathionine may simply be a marker of high Met intake, which enhances flux through cystathionine β‐synthase.2, 4 This could explain a strong association between plasma cystathionine and Cys at baseline. However, a prospective study in 26 556 male Finnish smokers found no significant association between Met intake and risk of stroke.34 Accordingly, we observed no significant relationship between either plasma Met or Cys with stroke risk, making them unlikely as confounders. Likewise, cystathionine remained a significant predictor of stroke risk even after the adjustment for Hcy. Although we cannot exclude the influence of this metabolic precursor on our results, it is, however, unlikely that the observed associations of cystathionine with stroke in the current study are confounded by Hcy status. Alternatively, high plasma cystathionine may reflect suboptimal nutritional status.20, 21 In this regard, previous studies showed that low serum concentrations of B‐vitamins, in particular vitamin B6, were strongly associated with high cystathionine levels.21, 29 However, any influence by poor nutrition status seems unlikely, because plasma levels of B‐vitamins neither attenuated the association between plasma cystathionine and stroke events nor introduced any significant effect modification. Conceivably, renal mechanism may be considered given that elevated circulating cystathionine has been observed in patients with renal disease, possibly attributed to the combination of reduced urinary excretion and increased production.21 This could explain our observation of negative correlation of cystathionine with eGFR. Notably, renal dysfunction is a major risk factor for ischemic stroke.35 However, including eGFR had a minor influence on our estimates, suggesting other mechanisms than renal impairment being responsible for the current findings.
Conclusions
In conclusion, our observational study suggests that elevated plasma cystathionine is associated with increased risk of total stroke and ischemic stroke in patients with stable angina pectoris, particularly in those without a history of atrial fibrillation. Our results motivate future studies to elucidate the role of cystathionine in stroke development.
Author Contributions
Nygård and Dhar designed research; Dhar analyzed the data, interpreted findings and wrote the manuscript; Dhar, Svingen, Ueland, Tell, and Nygård conducted research; Dhar and Lysne performed statistical analysis; Dhar, Svingen, Ueland, Lysne, Svenningsson, Tell, and Nygård critically revised the manuscript. All authors read and approved the final version of the manuscript.
Sources of Funding
This work has been funded by the KG Jebsen Centre for Diabetes Research, University of Bergen, the Department of Heart Disease, Haukeland University Hospital, Norway, the Western Norway Regional Health Authority, and the Foundation to Promote Research into Functional Vitamin B12 Deficiency, Norway.
Disclosures
None.
Acknowledgments
We thank all the coworkers and laboratory personnel at Haukeland University Hospital, Bergen; Stavanger University Hospital, Stavanger; and Bevital A/S, Bergen, Norway. We are also grateful to Tomislav Dimoski at the Norwegian Knowledge Centre for the Health Services, Oslo, Norway, for his contribution by developing the software necessary for obtaining admission data from Norwegian hospitals and conducting data collection and quality assurance of data in this project.
(J Am Heart Assoc. 2018;7:e008824 DOI: 10.1161/JAHA.118.008824.)
References
- 1. Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. [DOI] [PubMed] [Google Scholar]
- 2. Guttormsen AB, Solheim E, Refsum H. Variation in plasma cystathionine and its relation to changes in plasma concentrations of homocysteine and methionine in healthy subjects during a 24‐h observation period. Am J Clin Nutr. 2004;79:76–79. [DOI] [PubMed] [Google Scholar]
- 3. Hirche F, Schroder A, Knoth B, Stangl GI, Eder K. Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br J Nutr. 2006;95:879–888. [DOI] [PubMed] [Google Scholar]
- 4. Matthias D, Becker CH, Riezler R, Kindling PH. Homocysteine induced arteriosclerosis‐like alterations of the aorta in normotensive and hypertensive rats following application of high doses of methionine. Atherosclerosis. 1996;122:201–216. [DOI] [PubMed] [Google Scholar]
- 5. Chan SJ, Chai C, Lim TW, Yamamoto M, Lo EH, Lai MK, Wong PT. Cystathionine β‐synthase inhibition is a potential therapeutic approach to treatment of ischemic injury. ASN Neuro. 2015;7:1759091415578711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elshorbagy AK, Valdivia‐Garcia M, Graham IM, Palma Reis R, Sales Luis A, Smith AD, Refsum H. The association of fasting plasma sulfur‐containing compounds with BMI, serum lipids and apolipoproteins. Nutr Metab Cardiovasc Dis. 2012;22:1031–1038. [DOI] [PubMed] [Google Scholar]
- 7. Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M. Cystathionine gamma‐Lyase‐deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers JC, Ueland PM, Wright M, Dore CJ, Refsum H, Kooner JS. Investigation of relationship between reduced, oxidized and protein‐bound homocysteine and vascular endothelial function in healthy human subjects. Circ Res. 2001;89:187–192. [DOI] [PubMed] [Google Scholar]
- 9. Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis—a multifactorial process. Exp Clin Cardiol. 2002;7:40–53. [PMC free article] [PubMed] [Google Scholar]
- 10. Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction, vascular disease and stroke: the ARTICO study. Cerebrovasc Dis. 2009;27:25–37. [DOI] [PubMed] [Google Scholar]
- 11. Sigala F, Efentakis P, Karageorgiadi D, Filis K, Zampas P, Iliodromitis EK, Zografos G, Papapetropoulos A, Andreadou I. Reciprocal regulation of eNOS, H2S and CO‐synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017;12:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–1867. [DOI] [PubMed] [Google Scholar]
- 13. Svingen GF, Ueland PM, Pedersen EK, Schartum‐Hansen H, Seifert R, Ebbing M, Løland KH, Tell GS, Nygård O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–2048. [DOI] [PubMed] [Google Scholar]
- 14. Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygard O. Mortality and cardiovascular events in patients treated with homocysteine‐lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 15. Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators . Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. [DOI] [PubMed] [Google Scholar]
- 16. Spence JD. Homocysteine‐lowering therapy: a role in stroke prevention? Lancet Neurol. 2007;6:830–838. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulo G, Igland J, Vollset SE, Nygård O, Øyen N, Tell GS. Cardiovascular disease and diabetes mellitus in Norway during 1994–2009 CVDNOR—a nationwide research project. Nor Epidemiol. 2013;23:101–107. [Google Scholar]
- 19. Midttun O, McCann A, Aarseth O, Krokeide M, Kvalheim G, Meyer K, Ueland PM. Combined measurement of 6 fat‐soluble vitamins and 26 water‐soluble functional vitamin markers and amino acids in 50 μL of serum or plasma by high‐throughput mass spectrometry. Anal Chem. 2016;88:10427–10436. [DOI] [PubMed] [Google Scholar]
- 20. Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood. 1993;81:3404–3413. [PubMed] [Google Scholar]
- 21. Herrmann W, Schorr H, Geisel J, Riegel W. Homocysteine, cystathionine, methylmalonic acid and B‐vitamins in patients with renal disease. Clin Chem Lab Med. 2001;39:739–746. [DOI] [PubMed] [Google Scholar]
- 22. Robinson K, Arheart K, Refsum H, Brattström L, Boers G, Ueland P, Rubba P, Palma‐Reis R, Meleady R, Daly L, Witteman J, Graham I. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. Circulation. 1998;97:437–443. [DOI] [PubMed] [Google Scholar]
- 23. Weikert C, Dierkes J, Hoffmann K, Berger K, Drogan D, Klipstein‐Grobusch K, Spranger J, Mohlig M, Luley C, Boeing H. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke. 2007;38:2912–2918. [DOI] [PubMed] [Google Scholar]
- 24. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 25. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 26. Midttun O, Townsend MK, Nygard O, Tworoger SS, Brennan P, Johansson M, Ueland PM. Most blood biomarkers related to vitamin status, one‐carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within‐person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr. 2014;144:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hustad S, Eussen S, Midttun O, Ulvik A, van de Kant PM, Morkrid L, Gislefoss R, Ueland PM. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one‐carbon metabolism. Clin Chem. 2012;58:402–410. [DOI] [PubMed] [Google Scholar]
- 28. Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six‐year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320:904–910. [DOI] [PubMed] [Google Scholar]
- 29. Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, Vermaak WJ. The effect of a subnormal vitamin B‐6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly PJ, Murphy S, Coveney S, Purroy F, Lemmens R, Tsivgoulis G, Price C. Anti‐inflammatory approaches to ischaemic stroke prevention. J Neurol Neurosurg Psychiatry. 2018;89:211–218. [DOI] [PubMed] [Google Scholar]
- 31. Alpoim PN, Sousa LP, Mota AP, Rios DR, Dusse LM. Asymmetric dimethylarginine (ADMA) in cardiovascular and renal disease. Clin Chim Acta. 2015;440:36–39. [DOI] [PubMed] [Google Scholar]
- 32. Look MP, Riezler R, Reichel C, Brensing KA, Rockstroh JK, Stabler SP, Spengler U, Berthold HK, Sauerbruch T. Is the increase in serum cystathionine levels in patients with liver cirrhosis a consequence of impaired homocysteine transsulfuration at the level of gamma‐cystathionase? Scand J Gastroenterol. 2000;35:866–872. [DOI] [PubMed] [Google Scholar]
- 33. DeRatt BN, Ralat MA, Lysne V, Tayyari F, Dhar I, Edison AS, Garrett TJ, Midttun Ø, Ueland PM, Nygård OK, Gregory JF. Metabolomic evaluation of the consequences of plasma cystathionine elevation in adults with stable angina pectoris. J Nutr. 2017;147:1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am J Epidemiol. 2008;167:954–961. [DOI] [PubMed] [Google Scholar]
- 35. Koren‐Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67:224–228. [DOI] [PubMed] [Google Scholar]


