Abstract
Background
Trastuzumab is life‐extending therapy for breast cancer patients overexpressing the human epidermal growth factor receptor 2 (HER2+), but has known cardiotoxic risk. We sought to determine if trastuzumab can be administered to patients with reduced baseline cardiac function at no higher cardiotoxicity risk than in those with normal cardiac function at baseline.
Methods and Results
We performed a retrospective study of women treated with trastuzumab for human epidermal growth factor receptor 2 breast cancer at Mayo Clinic Rochester between January 1, 2000 and August 31, 2015 with pre‐ and on‐therapy echocardiograms available for review. A left ventricular ejection fraction (LVEF) <53% was considered abnormal, and a ≥10% decline in LVEF as evidence of cardiotoxicity based on the criteria of the American Society of Echocardiography. A total of 428 women were identified; 408 had a normal cardiac function (LVEF 63.4±5%) and 20 had an impaired cardiac function (LVEF 45.4±7%) before trastuzumab. Seven women (35%) with reduced LVEF at baseline had a ≥10% reduction in LVEF, compared with 179 (43.9%) of those with normal LVEF before trastuzumab initiation (P=NS). Symptomatic heart failure developed more often in patients with reduced versus normal baseline LVEF (25% versus 4.2%, P<0.05). After adjusting for patient age and breast cancer disease stage, survival rates over 5 years from time of diagnosis were found to be lower for patients with reduced baseline LVEF compared with patients with normal baseline LVEF (P<0.001); the adjusted proportion of patients surviving at 5 years for those with low LVEF at baseline was 79% and for those with normal LVEF was 93%.
Conclusions
Women undergoing trastuzumab therapy for breast cancer with impaired baseline cardiac function experience no higher risk of LVEF decline, but more frequently develop symptomatic heart failure. While trastuzumab could be considered, these patients should be co‐managed by a cardiologist.
Keywords: breast cancer, cardiomyopathy, cardiotoxicity, chemotherapy, heart failure, HER2, left ventricular ejection fraction, trastuzumab, treatment
Subject Categories: Heart Failure, Echocardiography, Cardiomyopathy, Women, Treatment
Clinical Perspective
What is New?
This study compared the incidences of left ventricular ejection fraction declines and heart failure with trastuzumab therapy in female breast cancer patients with normal or abnormal cardiac function at baseline, based on a left ventricular ejection fraction cutoff of normal at ≥53% per American Society of Echocardiography Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy.
Women with reduced cardiac function at baseline had no higher risk of any, or a ≥10% decline, in left ventricular ejection fraction with trastuzumab therapy.
The incidence of heart failure, however, was higher in women who underwent trastuzumab therapy with a reduced left ventricular ejection fraction at baseline.
What Are the Clinical Implications?
The current study indicates that breast cancer patients with a reduced cardiac function at baseline could be considered for trastuzumab therapy, but this consideration is to be combined with appropriate counseling regarding the higher cardiac risks involved.
Female breast cancer patients, especially those with a reduced cardiac function at baseline, should be followed and co‐managed closely by a cardiologist or cardio‐oncologist for best possible therapeutic outcomes.
Introduction
Overexpression of the human epidermal growth factor receptor 2 (HER2) protein, amplification of the HER2 gene, or both occurs in ≈20% to 25% of breast cancers, and is associated with a more aggressive disease phenotype.1, 2, 3 A humanized monoclonal antibody against the extracellular domain of HER2, trastuzumab (Herceptin®, Genentech, San Francisco, CA) has been shown to benefit patients with HER2+ breast cancer, alone or in combination with chemotherapy.4, 5 Adverse events typically associated with chemotherapy, such as alopecia, myelosuppression, and nausea and vomiting are not seen with trastuzumab. Cardiotoxicity, either leading to an asymptomatic decrease in left ventricular ejection fraction (LVEF) or heart failure (HF), remains the most important adverse effect.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
As outlined by Ewer and Lippman, trastuzumab‐induced cardiotoxicity (TIC) presents differently from anthracycline‐induced cardiomyopathy.17 For instance, the risk of TIC is not related to the cumulative dose. Also, most importantly, there is at least partial reversibility of cardiac function decline, in most cases, with drug cessation alone. There is no assumed recurrence with subsequent stressors, and re‐challenge is generally tolerated after recovery.18 These dynamics relate to the concept that HER2 inhibition is of functional but not structural consequence.19, 20, 21
Current recommendations for cardiac function monitoring were largely prompted by the unexpected incidence of HF in nearly 30% of patients receiving trastuzumab and anthracycline therapy in a pivotal phase 3 clinical trial.22 The trastuzumab package insert (available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/trasgen020900LB.htm) recommends assessment of LVEF before and at regular intervals during treatment, and to hold trastuzumab for at least 4 weeks if: ≥16% absolute decrease in LVEF from pre‐treatment values, LVEF below institutional limits of normal, and ≥10% absolute decrease in LVEF from pretreatment values. Trastuzumab can be resumed if, within 4 to 8 weeks, the LVEF returns to normal or the absolute decrease is ≤15% from baseline. It is further recommended that trastuzumab be permanently discontinued for persistent (>8 weeks) LVEF declines or trastuzumab suspensions >3 times for cardiomyopathy. Understandably, a reduced cardiac function at baseline may pose a drawback to HER2‐directed therapy in view of its cardiotoxicity risk. The current study was conducted to address the question of cardiac safety of trastuzumab in this group of female breast cancer patients with reduced cardiac function at baseline.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure unless approved by all authors after review and conclusion of a reasonable request.
Patient Population
We performed a single center, retrospective cohort study of patients diagnosed with cancer who received at least one dose of trastuzumab between January 1, 2000 and August 31, 2015 at the Mayo Clinic in Rochester, Minnesota based on pharmacy records. Patients who were male or had another cancer type other than breast cancer were excluded. The remaining female patients were cross‐referenced with the Mayo Clinic Rochester echocardiography database to identify those who had at least one echocardiogram before and one after trastuzumab therapy. This study was approved by the Mayo Clinic Institutional Review Board.
Baseline LVEF was defined as the LVEF documented in the last echocardiogram before the first dose of trastuzumab therapy. In agreement with the American Society of Echocardiography (ASE) Consensus report,23 an LVEF of ≥53% was defined as normal, and an LVEF drop of at least 10% to a value of <53% with therapy as reflective of chemotherapy‐related cardiac dysfunction (CRTD). However, as this definition does not apply to patients with a reduced LVEF (<53%) at baseline, a drop in LVEF of ≥10% (to any value) was considered as a significant change in cardiac function. This is in agreement with defining a significant change by more than 2 times the standard deviation for echocardiography‐based measurements of LVEF.24 In agreement with the ASE consensus statement, reversibility of cardiac dysfunction was defined in relation to baseline LVEF: improvement to within 5 percentage points of baseline was considered reversible, improvement by ≥10 percentage points from the nadir but remaining >5 percentage points below baseline was considered partially reversible, and improvement by <10 percentage points from the nadir and remaining >5 percentage points below baseline was considered irreversible.
A chart review was performed for all clinical variables for all patients including demographics, interventions such as medication changes, the impact of these interventions on cardiac function and clinical outcomes including HF and death. External patient health records that had been sent to Mayo Clinic Rochester for clinical purposes were used in addition to internal records from care received at Mayo Clinic Rochester. Patients with incomplete records were excluded. HF was defined by Framingham criteria and the diagnosis of HF was based on chart review by 2 team members; events were adjudicated based on consensus. Premature discontinuation of trastuzumab therapy was defined as discontinuation of trastuzumab before its intended duration, ie, 1 year in the case of stage 1 to 3 disease. Any discontinuation of trastuzumab therapy could be either final (permanent discontinuation) or only for a period of time where therapy is resumed and completed as planned (temporary discontinuation).
Statistical Analysis
The patient cohort was divided into the following 2 groups according to the pre‐trastuzumab treatment LVEF: (1) patients with normal LVEF (LVEF≥53%) at all points before trastuzumab treatment and (2) patients with low LVEF (LVEF<53%) before trastuzumab treatment. Group comparisons were made by Student t test and Mann‐Whitney U‐test for parametric and non‐parametric continuous variables, and the χ2 or Fisher's exact test for categorical variables. 5‐year survival rates were analyzed from the time of the initial diagnosis and adjusted for patient age and breast cancer disease stage using a stratified Cox proportional hazards model. Adjusted Kaplan–Meier curves were produced for each LVEF group and compared by log‐rank test. Outcome associations were analyzed by logistic regression analysis and presented as odds ratio (OR) with 95% confidence interval (CI). All statistical analyses were performed using GraphPad Prism and R (version 3.4.2). A P‐value <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 408 patients with normal LVEF (range 53%–78%) and 20 patients with low LVEF (range 25%–52%) at baseline were analyzed in this study (Figure 1). Clinical features of the patients are shown in Table 1. The 2 patient populations were not significantly different. Mean age at diagnosis was the same for the 2 groups and most women were post‐menopausal. A large proportion of patients in both groups presented with node‐positive disease, only a minority had metastatic disease. The majority of patients in both groups underwent anthracycline and radiation therapy. Patients were administered 240 mg/m2 anthracycline (or less), the standard dose of anthracycline over this time period. Patients with low LVEF were more likely than patients with normal LVEF to have a history of HF and angiotensin converting enzyme (ACE) inhibitor therapy.
Figure 1.
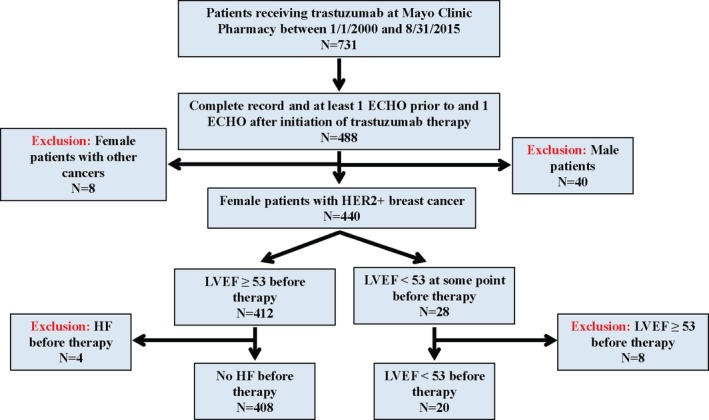
Patient inclusion/exclusion flowchart. ECHO indicates Echocardiogram; HER2+, human epidermal growth factor receptor 2; HF, heart failure; LVEF, left ventricular ejection fraction.
Table 1.
Features of Patients With Normal and Low LVEF At Baseline
| Normal LVEF (n=408) | Low LVEF (n=20) | P Value | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Post‐menopausal, % (n) | 55.4 (n=225) | 73.4 (n=14) | 0.19 |
| Obesity, % (n) | 39.0 (n=159) | 50.0 (n=10) | 0.32 |
| Smoking, % (n) | 12.5 (n=51) | 20.0 (n=4) | 0.32 |
| Alcohol consumption, % (n) | 68.7 (n=279) | 50.0 (n=10) | 0.09 |
| Chronic kidney disease, % (n) | 0.5 (n=2) | 5.0 (n=1) | 0.02 |
| Hyperlipidemia, % (n) | 21.6 (n=88) | 15.0 (n=3) | 0.48 |
| Diabetes mellitus, % (n) | 7.6 (n=31) | 15.0 (n=3) | 0.23 |
| Hypothyroidism, % (n) | 10.0 (n=41) | 5.0 (n=1) | 0.46 |
| Hypertension, % (n) | 26.5 (n=108) | 40.0 (n=8) | 0.18 |
| Coronary artery disease, % (n) | 14.0 (n=57) | 15.0 (n=3) | 0.90 |
| Heart failure history, % (n)) | 0.0 (n=0) | 35.0 (n=7) | 0.0001 |
| Baseline LVEF, mean (SD) | 63.4 (5.0) | 45.4 (7.0) | 0.019 |
| Cardiovascular medications at baseline | |||
| ACE inhibitors, % (n) | 9.3 (n=38) | 35.0 (n=7) | 0.0003 |
| Angiotensin II receptor blockers, % (n) | 2.2 (n=9) | 0.0 (n=0) | 0.50 |
| β‐blockers, % (n) | 12.3 (n=50) | 15.0 (n=3) | 0.72 |
| Cancer demographics | |||
| Mean (SD) age at cancer diagnosis, y | 52.8 (12.5) | 54.0 (10.4) | 0.62 |
| Age | |||
| <45 y, % (n) | 28.2 (n=115) | 25.0 (n=5) | 0.68 |
| 45 to 60 y, % (n) | 43.9 (n=179) | 55.0 (n=11) | |
| >60 y, % (n) | 27.9 (n=114) | 20.0 (n=4) | |
| Disease stage | |||
| Stage 1 disease, % (n) | 24.2 (n=96) | 30.0 (n=6) | 0.27 |
| Stage 2 disease, % (n) | 39.9 (n=158) | 20.0 (n=4) | |
| Stage 3 disease, % (n) | 25.5 (n=101) | 35.0 (n=7) | |
| Stage 4 disease, % (n) | 10.4 (n=41) | 15.0 (n=3) | |
| Node positive disease at diagnosis, % (n) | 63.0 (n=257) | 75.0 (n=15) | 0.28 |
| Distant metastases at diagnosis, % (n) | 10.8 (n=44) | 5.0 (n=1) | 0.41 |
| Cancer treatment | |||
| Anthracycline use, % (n) | 72.8 (n=297) | 85.0 (n=17) | 0.23 |
| Anthracycline exposure as part of current treatment plan, % (n) | 62.5 (n=255) | 60 (n=12) | 0.82 |
| Mean (SD) duration between anthracycline use as part of current treatment plan and trastuzumab treatment, mo | 0.9 (1.4) | 2.2 (3.0) | 0.0002 |
| Anthracycline exposure unrelated to current treatment (remote), % (n) | 10.3 (n=42) | 25 (n=5) | 0.04 |
| Mean (SD) duration between anthracycline use as part of remote treatment plan and trastuzumab treatment, mo | 91.3 (94.4) | 96.0 (48.0) | 0.83 |
| Chest irradiation, % (n) | 70.3 (n=287) | 80.0 (n=16) | 0.35 |
| Left‐sided chest irradiation, % (n) | 49.8 (n=143) | 50.0 (n=10) | 0.17 |
| Right‐sided chest irradiation, % (n) | 54.7 (n=157) | 55.0 (n=11) | 0.14 |
| Combined chest irradiation and anthracycline, % (n) | 56.4 (n=230) | 75.0 (n=15) | 0.10 |
| Mean (SD) duration of trastuzumab treatment, mo | 16.9 (17.6) | 15.0 (10.7) | 0.83 |
| Mean (SD) duration between anthracycline use and trastuzumab treatment, mo | 13.9 (47.5) | 29.8 (50.2) | 0.01 |
| Cardiac events during therapy | |||
| Mean (SD) time between treatment initiation and HF, mo | 5.9 (6.2) | 15.2 (24.9) | 0.17 |
| Heart failure during therapy, % (n) | 4.2 (n=17) | 25.0 (n=5) | 0.0001 |
| LVEF decline | |||
| No decline, % (n) | 17.9 (n=73) | 50.0 (n=10) | 0.006 |
| <5% decline in LVEF, % (n) | 13.2 (n=54) | 10.0 (n=2) | |
| 5–10% decline in LVEF, % (n) | 25.0 (n=102) | 5.0 (n=1) | |
| ≥10% decline in LVEF, % (n) | 43.9 (n=179) | 35.0 (n=7) | |
| LVEF<53% and 10% decline with therapy, % (n) | 17.6 (n=72) | n/a | ··· |
| Changes in trastuzumab therapy | |||
| Permanent discontinuation of trastuzumab therapy, % (n) | 6.9 (n=28) | 20.0 (n=4) | 0.03 |
| Temporary cessation of trastuzumab therapy, % (n) | 6.6 (n=27) | 35.0 (n=7) | 0.0001 |
| Continuation of trastuzumab therapy after medication changes (%) | 8.1 (n=33) | 35.0 (n=7) | 0.0001 |
ACE indicates angiotensin converting enzyme; LVEF, left ventricular ejection fraction; SD, standard deviation.
Cardiac Function Decline and Heart Failure
The spectrum of the magnitude of LVEF changes on an individual scale is outlined in Figure 2. All cardiac function dynamics and events were recorded from the time of start of trastuzumab exposure. Eighty‐two percent of patients with normal baseline LVEF had a decline in LVEF of any degree with therapy. This rate was 50% in those with low LVEF before trastuzumab therapy (P<0.001 compared with those with normal LVEF before trastuzumab therapy). Notably, a ≥10% decline in LVEF was seen in a similar proportion of patients with normal versus reduced LVEF before trastuzumab therapy (43.9% versus 35% P>0.05) (Table 1). A total of 72 patients (17.6%) with normal LVEF before trastuzumab met the criteria of the ASE Consensus report23 for CRTD (Table 1).
Figure 2.
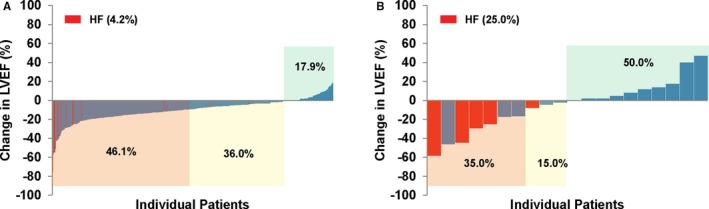
Waterfall plot of maximum percentage point reduction in LVEF from baseline to any point in time during therapy with trastuzumab among patients with normal LVEF before trastuzumab (A) and patients with low LVEF before trastuzumab (B). LVEF indicates left ventricular ejection fraction.
Symptomatic HF developed more often in patients with low than with normal baseline LVEF (25% versus 4.2%, OR 7.67 [95% CI 2.5–23.56], P<0.05). Furthermore, HF developed more often in patients who had an LVEF decline of ≥10% with trastuzumab therapy (8.2% versus 2.6%, OR 3.35 [95% CI 1.28–8.73], P<0.05). Among patients with HF, 72.7% had a drop in LVEF by ≥10% (Figure 3).
Figure 3.
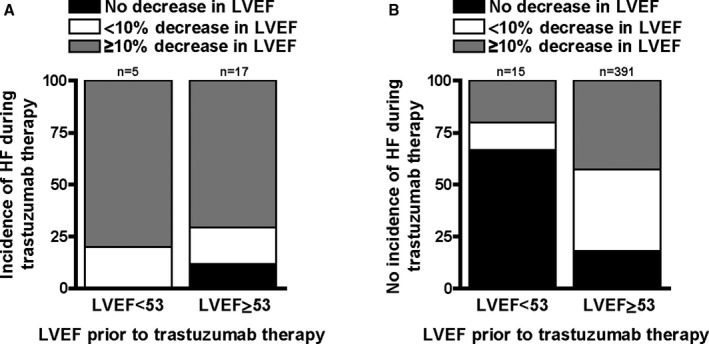
Bar graphs illustrating the distribution of LVEF changes with trastuzumab therapy in patients diagnosed with HF (A) and patients without HF (B), stratified by baseline cardiac function (P=NS for panel A and P=0.0002 for panel B for group comparisons). LVEF indicates left ventricular ejection fraction.
Follow‐up periods were similar for patients with low and normal baseline LVEF (89.7±61.9 and 82.1±61.7 months, respectively, P>0.05). The adjusted proportion of patients surviving 5 years from initial diagnosis was lower in patients with a reduced compared with those with a normal LVEF before trastuzumab therapy (79% versus 93%), and the adjusted survival rates over this 5 year period differed by LVEF status (P<0.001, Figure S1). As outlined in Table S1, there was no significant difference in long‐term non‐cardiac cancer death between low and normal baseline LVEF patients whereas patients with a reduced baseline LVEF tended to have a higher long‐term cardiac mortality (odds ratio 8.89 [95% CI 1.22–59.35], P=0.07). The 2 cardiac deaths in this group of patients occurred late and were both attributable to HF. One patient with a prior history of HF and a reversible <10% decline in LVEF on trastuzumab therapy died 3 years later. The other patient had no prior history of HF but developed an irreversible >10% decline on trastuzumab and died 10 years after trastuzumab therapy. Both patients had undergone left‐sided radiation therapy and the second patient anthracycline therapy as well.
Management of Trastuzumab‐Induced Cardiotoxicity
In patients with a normal LVEF at baseline, 83.8% patients were able to continue trastuzumab therapy without interruption, 6.1% had a temporary hold, and 10% experienced premature permanent discontinuation. The comparable proportions among patients with a normal LVEF at baseline but a reduction in LVEF ≥10% with therapy were 73.2%, 11.2%, and 15.6%, respectively. Among patients with <5% and 5% to 10% decline in LVEF while on trastuzumab therapy, the therapy continuation rates were 94% and 92%, respectively. Two patients (3.7%) in the group with an LVEF decline of <5%, 6 patients (5.9%) in the group with a 5% to 10% LVEF decline, and 28 patients in the group with a ≥10% LVEF decline (15.6%, P<0.05 versus other two groups) received new or intensified cardiovascular drug therapy (50.0% β‐blockers, 26.7% ACE inhibitors or angiotensin receptor blockers [ARBs], and 23.3% both). Table 2 summarizes outcomes with trastuzumab therapy.
Table 2.
Changes in LVEF and Interventions With Trastuzumab Therapy
| Continued Trastuzumab Therapy | Temporary Hold of Trastuzumab Therapy | Discontinued Trastuzumab Therapy | Received CV Therapy | |
|---|---|---|---|---|
| Normal LVEF (n=408) | ||||
| Number (%) | 83.8 (n=342) | 6.1 (n=25) | 10.0 (n=41) | 9.8 (n=40) |
| <5% decline in LVEF (%) | 94.4 (n=51) | ‐ | 5.6 (n=3) | 3.7 (n=2) |
| 5–10% decline in LVEF (%) | 92.2 (n=94) | 2.9 (n=3) | 4.9 (n=5) | 5.9 (n=6) |
| ≥10% decline in LVEF (%) | 73.2 (n=131) | 11.2 (n=20) | 15.6 (n=28) | 15.6 (n=28) |
| Low LVEF (n=20) | ||||
| Number (%) | 55.0 (n=11) | 25.0 (n=5) | 20.0 (n=4) | 35.0 (n=7) |
| <5% decline in LVEF (%) | 100.0 (n=2) | ··· | ··· | ··· |
| 5–10% decline in LVEF (%) | ··· | ··· | 100.0 (n=1) | ··· |
| ≥10% decline in LVEF (%) | ··· | 57.1 (n=4) | 42.9 (n=3) | 71.4 (n=5) |
LVEF indicates left ventricular ejection fraction.
In patients with an impaired LVEF at baseline, only 55% (n=11) were able to continue trastuzumab therapy without interruption, 25% (n=5) had a temporary hold and 20% (n=4) experienced permanent discontinuation of trastuzumab therapy (P<0.01 versus patients with normal baseline LVEF for the three comparisons). The comparable proportions were 0% (n=0), 57.1% (n=4), and 42.9% (n=3) in patients with a reduced LVEF at baseline and further reduction by ≥10% with therapy (P<0.05 versus patients with normal baseline LVEF for all three comparisons). Less than 6% of patients with a <10% LVEF decline received new or intensified cardiovascular therapy during treatment (2.5% received β‐blockers, 2.1% received ACE inhibitors, and 0.8% received both). In comparison, almost 20% of patients in the group with ≥10% LVEF decline received new or intensified cardiovascular therapy during treatment (12.4% received β‐blockers, 7.0% received ACE inhibitors, and 3.2% received both, P<0.05 versus patients without significant LVEF decline receiving similar treatment).
Irrespective of baseline LVEF, complete reversibility of cardiac dysfunction (defined as improvement to within 5 percentage points of baseline) was seen in all patients with a <10% LVEF decline during trastuzumab therapy. In contrast, this was the case in only 64.7% of patients (n=65) with a normal baseline LVEF and a ≥10% decline in LVEF, and another 22.9% (n=41) had only partial reversibility. Among patients with a low baseline LVEF and ≥10% LVEF decline on therapy, reversibility was seen in 71.4% of patients, and the remainder (28.6%) had an irreversible decline in LVEF with trastuzumab therapy. As detailed in Figure 4, initiation or intensification of cardiovascular medications with or without transient or permanent cessation of trastuzumab therapy was associated with a smaller fraction of patients with irreversible decline in LVEF to <20%.
Figure 4.
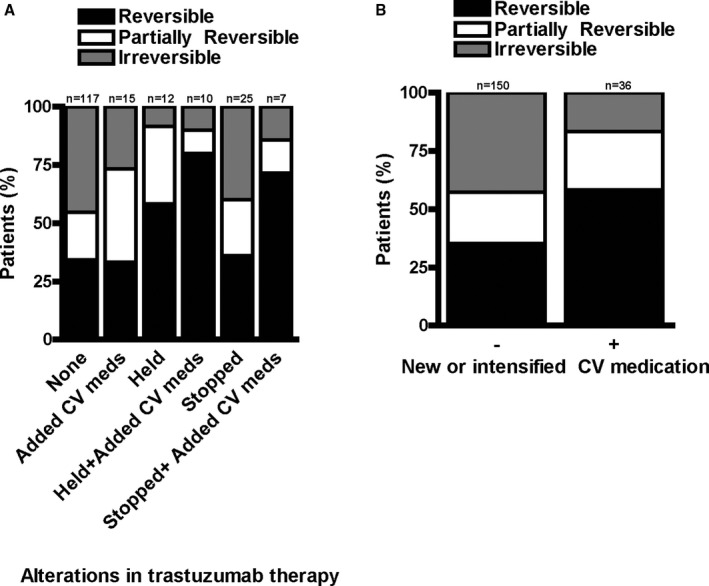
Reversibility of trastuzumab‐induced decreases in LVEF, (A) related to the trastuzumab treatment regimen: no change in trastuzumab therapy (none), cessation of trastuzumab (held), or permanent stop of trastuzumab (stopped) with and without the addition of cardiovascular medication (added CV meds), and (B) by cardiovascular medications alone (P=0.03 for panel A and P=0.01 for (B) for group comparisons). CV indicates cardiovascular; LVEF, left ventricular ejection fraction.
Predictors for Trastuzumab‐Induced Cardiotoxicity
Comparing patients who experienced a ≥10% LVEF decline with those who had none or <10% LVEF decline (Table 3), the only identifiable predictor of cardiotoxicity was baseline LVEF, which was higher in patients with a significant LVEF decline on therapy. History of HF, CAD, tobacco use, alcohol consumption, obesity, and presence of distant metastases were not associated with a higher risk of significant LVEF decline. Likewise, cardiovascular medications at baseline were not associated with a lower risk of decreases in LVEF on trastuzumab therapy.
Table 3.
Predictors of Significant LVEF Decline With Trastuzumab Therapy
| None or <10% LVEF Decline (n=242) | ≥10% LVEF Decline (n=186) | P Value | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Post‐menopausal, % (n) | 58.3 (n=140) | 53.5 (n=99) | 0.34 |
| Obesity, % (n) | 39.7 (n=96) | 39.2 (n=73) | 0.93 |
| Smoking, % (n) | 12.8 (n=31) | 12.9 (n=24) | 0.98 |
| Alcohol consumption, % (n) | 68.9 (n=166) | 66.5 (n=123) | 0.59 |
| Chronic kidney disease, % (n) | 1.2 (n=3) | 0.0 (n=0) | 0.13 |
| Hyperlipidemia, % (n) | 23.6 (n=57) | 18.3 (n=34) | 0.19 |
| Diabetes mellitus, % (n) | 8.7 (n=21) | 7.0 (n=13) | 0.52 |
| Hypothyroidism, % (n) | 12.0 (n=29) | 7.0 (n=13) | 0.09 |
| Hypertension, (%) | 28.1 (n=68) | 25.8 (n=48) | 0.60 |
| Coronary artery disease, % (n) | 15.3 (n=37) | 12.4 (n=23) | 0.39 |
| Heart failure history, (%) | 2.1 (n=5) | 1.1 (n=2) | 0.42 |
| Baseline LVEF, mean (SD) | 61.4 (6.1) | 64.1 (6.1) | 0.0001 |
| Cardiovascular medications at baseline | |||
| ACE inhibitors, % (n) | 12.4 (30) | 9.1 (17) | 0.28 |
| Angiotensin II receptor blockers, % (n) | 4.5 (11) | 2.7 (5) | 0.31 |
| β‐blockers, % (n) | 12.8 (30) | 12.9 (24) | 0.87 |
| Cancer demographics | |||
| Mean age at cancer diagnosis, years (SD) | 53.5 (12.9) | 52.1 (11.8) | 0.37 |
| Age | |||
| Aged <45 y, % (n) | 28.1 (n=68) | 28.0 (n=52) | 0.45 |
| Aged 45 to 60 y, % (n) | 42.1 (n=102) | 47.3 (n=88) | |
| Aged >60 y, % (n) | 29.8 (n=72) | 24.7 (n=46) | |
| Node positive disease at diagnosis, % (n) | 59.9 (n=145) | 67.7 (n=126) | 0.10 |
| Distant metastases at diagnosis, % (n) | 11.6 (n=28) | 9.1 (n=17) | 0.42 |
| Cancer treatment | |||
| Anthracycline use, % (n) | 71.5 (n=173) | 75.8 (n=141) | 0.32 |
| Chest irradiation, % (n) | 70.7 (n=171) | 71.0 (n=132) | 0.94 |
| Left‐sided chest irradiation, % (n) | 35.5 (n=86) | 34.9 (n=65) | 0.90 |
| Right‐sided chest irradiation, % (n) | 40.1 (n=97) | 37.1 (n=69) | 0.53 |
| Combined chest irradiation and anthracycline, % (n) | 56.6 (n=137) | 58.1 (n=108) | 0.76 |
| Mean (SD) duration of trastuzumab treatment, mo | 15.5 (13.0) | 18.5 (22.0) | 0.56 |
| Mean (SD) duration between anthracycline use and trastuzumab treatment, mo | 18.0 (49.7) | 10.7 (45.0) | 0.21 |
| 5‐year survival, % | 89.9 | 82.5 | 0.07 |
| LVEF decline | |||
| Cardiac events during therapy | |||
| No LVEF decline with trastuzumab therapy, % (n) | 34.3 (n=83) | 0.0 (n=0) | |
| <5% decline in LVEF, % (n) | 23.1 (n=56) | n/a | |
| 5–10% decline in LVEF, % (n) | 42.6 (n=103) | n/a | |
| ≥10% decline in LVEF, % (n) | n/a | 100.0 (n=186) | |
| LVEF<53% and 10% decline with therapy, % (n) | n/a | 42.5 (n=79) | ··· |
| Heart failure during therapy, % (n) | 2.5 (n=6) | 8.6 (n=16) | 0.0047 |
ACE indicates angiotensin converting enzyme; LVEF, left ventricular ejection fraction; SD, standard deviation.
Discussion
The current analysis indicates that decreases in LVEF during trastuzumab therapy were not more likely among patients with reduced baseline cardiac function. Symptoms of HF, however, occurred more frequently during trastuzumab therapy in patients with reduced baseline LVEF, and long‐term (post‐trastuzumab therapy) cardiac mortality also tended to be higher in this group of patients. The rate of irreversible LVEF decline was <20% with institution or intensification of cardiovascular therapy (± temporary or permanent cessation of trastuzumab therapy) and all cases of LVEF declines <10% were reversible.
The introduction of HER2‐directed therapy has revolutionized the treatment and survival expectations for women with HER2+ breast cancer,25 even for those with metastatic disease.26 Our data suggest that such lifesaving therapy should not be withheld out of concern of a higher cardiotoxicity risk in most women with a reduced cardiac function. Intriguingly, there is paucity of data on this important topic, primarily because patients with baseline reduction of cardiac function were excluded from the enrollment in prospective clinical trials or the analysis of retrospective cohort studies. One exception is the NSABPB‐31 trial, which found that patients with an LVEF in the range of 50% to 54% were at a significantly higher risk for HF, and developed a 5‐year cardiac risk score based on age and baseline LVEF.27 Not over 5 years but over the duration of trastuzumab therapy, the current study made similar observations, ie, patients with a reduced cardiac function at baseline were at a higher risk of developing HF. Paradoxically, average baseline LVEF was higher for patients with a ≥10% LVEF decline in the current study. The absolute difference in LVEF between these two groups was small (only 2.7%) and might be explained by the distribution of patients with reduced baseline LVEF. Only one third of these patients had an LVEF decline ≥10%, leaving the majority of low LVEF patients in the <10% LVEF decline group. No other variable was identified in the current study that would reliably predict or exclude the risk of TIC. This is in distinction from a unique TIC risk prediction model, which was developed based on the SEER database set. It did not include patients with reduced cardiac function at baseline and identified coronary artery disease, atrial fibrillation, hypertension, diabetes mellitus, chronic kidney disease, aged >74 years, and administration of other chemotherapy as risk factors.28 Other retrospective cohort studies, not limited to a Medicare population but including patients of all ages, were unable to identify any predictors for TIC other than >240 mg/m2 doxorubicin exposure.9, 29 However, this has also not been consistently seen as a risk factor. Thus, in clinical practice it might not be easy to define cardiotoxicity risk and tailor cardiac function surveillance accordingly.
In most recent clinical trials, the proportion of patients developing symptomatic HF during trastuzumab has been relatively low (0.6% to 3.8%).27, 30, 31, 32, 33 In fact, the HERA (HERceptin Adjuvant) trial showed an HF incidence of only 0.8% and decreases in LVEF of ≥10% from baseline to an LVEF of <50% in only 7.2% of patients.34 In contrast, real world cohort studies noted a decline in LVEF by ≥10% points in 15% to 40% of patients.9, 29 The current study is in agreement with these previous reports. Among patients with a normal baseline LVEF, the rate of an LVEF decline ≥10% was 43.9% and 17.6% met ASE criteria for cancer therapeutics related cardiac dysfunction (CTRCD). Importantly, in patients with a reduced LVEF by ASE criteria, the incidence of an LVEF decline exceeding 10% was similar at 35%. Approximately 3 in 4 HF events were associated with a ≥10% drop in LVEF, and patients with an LVEF drop ≥10% had a 3.35 times higher risk of HF. These findings confirm that a reduced cardiac reserve in patients with lower baseline LVEF predisposes to HF presentations.
One of the most unique and defining aspects of TIC is reversibility, as emphasized by Ewer and Lippman.17 Indeed, the current study confirms reversibility of cardiac dysfunction in the majority of patients. Baseline cardiac function and the extent of LVEF decrease on therapy were seemingly modifying factors. All declines in LVEF <10% were reversible, which is an important observation in support of surveillance and early detection of and reaction to cardiac function changes. If LVEF drops exceeded 10%, the rate of reversibility number was reduced to 91% in the normal baseline LVEF group and to 71.4% in the low baseline LVEF group. These reversibility numbers are more favorable than those outlined in a prior study by Cardinale et al, which indicated that as many as 40% of patients with TIC might experience an irreversible decline in LVEF.8 However, irreversibility rates this high were noted even in the current study in patients who did not receive new or intensified cardiovascular medications. Importantly, we noted that only a minority of patients received new or intensified β‐blocker and/or renin‐angiotensin system inhibitors. This held true even in those with a reduced cardiac function at baseline. Collectively, these data support previous reports on opportunities for practice improvements.35
Reversibility of trastuzumab‐induced cardiotoxicity was defined by the extent of LVEF change that occurred from the nadir in LVEF value to the last value recorded by the end of trastuzumab treatment. Extending the follow‐up period to beyond completion of trastuzumab therapy (on average 28 months in those with low LVEF before trastuzumab therapy) only made a difference in the classification of 2 patients (1 from reversible to irreversible and another from completely reversible to partially reversible). Both patients were treated with anthracyclines. Thus, a longer follow‐up period, at least in this study, did not result in increased reversibility numbers of trastuzumab cardiotoxicity. Still, future studies will need to further define long‐term outcomes as well as the interaction with anthracyclines as done in a recent study by Narayan et al.36
Another important observation was that >70% of patients continued with trastuzumab, even if the LVEF decline was ≥10% (unless baseline function was impaired, in which case no patient continued with therapy). In this latter group with impairment in baseline cardiac function, 42% had permanent discontinuation of therapy. Overall, only 20% of patients with a reduced baseline cardiac function had their trastuzumab therapy permanently discontinued, just twice the rate than in patients with a normal LVEF. These data would indicate that, indeed, most patients are able to complete trastuzumab therapy, even with a reduced baseline LVEF. However, patients with reduced cardiac function before trastuzumab therapy tended to have a higher cardiac mortality long‐term. These data should be interpreted with caution as it is based on only 2 patients in a group of 20 patients with reduced baseline LVEF. Importantly, no cardiac death was encountered during trastuzumab therapy and both of these deaths occurred years later. While one of the 2 patients had irreversible cardiotoxicity with trastuzumab therapy, change in LVEF with trastuzumab was not found to be predictive of death from cardiac‐related causes in the overall cohort, suggesting that cardiac death does not necessarily correlate with the LVEF decline on trastuzumab therapy. Generally, patients with cardiac compromise are followed more closely, which may have affected the outcome observed in this retrospective study. Prospective clinical studies such as the ongoing SAFE‐HEaRT study (Cardiac Safety Study in Patients With HER2+ Breast Cancer, ClinicalTrials.gov Identifier: NCT01904903) are warranted to better define the safety of trastuzumab in patients with compromised cardiac function.
A few limitations apply to the current study, the first being the small study size. One has to consider though that only 20% to 25% of all breast cancers are HER2+. Furthermore, many patients seen at our tertiary referral center elect to receive therapy elsewhere. Moreover, there might be inter‐individual differences in clinical practice that could have influenced the results.28 This study was not designed to evaluate practice patterns and decision‐making regarding use of trastuzumab in patients with reduced cardiac function. A selection bias in this regard can therefore not be excluded. That said, the cohort examined in this study had a high percentage of prior anthracycline (85%), left‐sided radiation use (50%), as well as cardiovascular risk factors (hypertension in 40%, diabetes mellitus and coronary artery disease in 15% each). Patients were prescribed the standard dose of anthracycline over this time period (240 mg/m2). Three (out of 17) patients in the low LVEF group had a significant lag (years) between last dose of anthracycline and trastuzumab treatment initiation, thereby increasing the mean duration between anthracycline use and trastuzumab treatment. Over 1 in 3 patients in the cohort of patients with LVEF<53% before trastuzumab therapy had a prior history of symptomatic HF. Thus, the cohort represented here is not an exclusive selection of other low risk patients. The definition of reduced cardiac function in this study (<53%) was based on the ASE consensus document for cancer patients receiving chemotherapy23 and not <50% as commonly used.37, 38 However, the mean LVEF in the group with reduced baseline cardiac function was 45%, ie, below either cutoff. Finally, while a prior study in a general cancer cohort found an increase in mortality for patients undergoing chemotherapy with an LVEF <35%, but not for those with an LVEF 35% to 50%,39 the lowest LVEF level permissible for trastuzumab therapy is yet to be defined.
The current study indicates that trastuzumab therapy could be considered in breast cancer patients with a reduced cardiac function at baseline. However, they should be informed about the higher cardiac risks. Our data attest that these patients should be closely followed and co‐managed by a cardiologist or cardio‐oncologist to receive the best possible therapeutic outcome.
Sources of Funding
Herrmann was supported by a K08 grant (HL116952 from the National Heart Lung and Blood Institute) and a grant from Miami Heart Research Institute/Florida Heart Research Foundation. Ruddy was supported by a training grant under the Clinical and Translational Science Awards (CTSA) Grant Program (UL1 TR000135 and KL2TR000136‐09 from the National Center for Advancing Translational Sciences). Nowsheen was supported by the Laura J. Siegel Breast Cancer Fellowship Award from the Foundation for Women's Wellness.
Disclosures
None.
Supporting information
Table S1. Long‐Term Causes of Death
Figure S1. Kaplan–Meier 5‐year survival plot from the time of breast cancer diagnosis for patients with normal and reduced left ventricular ejection fraction (LVEF) at baseline, adjusted for age and stage of breast cancer (P<0.001 for group comparison by log rank). The number of patients is reduced in the normal LVEF by the 10 patients, for which stage of disease information was not available.
(J Am Heart Assoc. 2018;7:e008637 DOI: 10.1161/JAHA.118.008637.)
References
- 1. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. [DOI] [PubMed] [Google Scholar]
- 2. Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. [DOI] [PubMed] [Google Scholar]
- 3. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the Her‐2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 4. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang C‐S, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Sütő T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 5. Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti‐her2 monoclonal antibody in women who have her2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639. [DOI] [PubMed] [Google Scholar]
- 6. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. [DOI] [PubMed] [Google Scholar]
- 7. Guglin M, Cutro R, Mishkin JD. Trastuzumab‐induced cardiomyopathy. J Card Fail. 2008;14:437–444. [DOI] [PubMed] [Google Scholar]
- 8. Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. [DOI] [PubMed] [Google Scholar]
- 9. Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, Sarti S, Cecconetto L, Pietri E, Ferrario C, Fedeli A, Faedi M, Nanni O, Frassineti GL, Amadori D, Rocca A. Trastuzumab‐induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99:634–639. [DOI] [PubMed] [Google Scholar]
- 10. Sengupta PP, Northfelt DW, Gentile F, Zamorano JL, Khandheria BK. Trastuzumab‐induced cardiotoxicity: heart failure at the crossroads. Mayo Clin Proc. 2008;83:197–203. [DOI] [PubMed] [Google Scholar]
- 11. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 12. Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart‐Gebhart MJ, Bell R; Herceptin Adjuvant (HERA) Trial Study Team . Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2‐positive early breast cancer: a 4‐year follow‐up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. [DOI] [PubMed] [Google Scholar]
- 13. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart‐Gebhart MJ; HERA study team . 2‐year follow‐up of trastuzumab after adjuvant chemotherapy in her2‐positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. [DOI] [PubMed] [Google Scholar]
- 14. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin Adjuvant Trial Study Team . Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 15. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med. 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 16. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:547–558. [DOI] [PubMed] [Google Scholar]
- 17. Ewer MS, Lippman SM. Type II chemotherapy‐related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. [DOI] [PubMed] [Google Scholar]
- 18. Keefe DL. Trastuzumab‐associated cardiotoxicity. Cancer. 2002;95:1592–1600. [DOI] [PubMed] [Google Scholar]
- 19. Herrmann J, Lerman A. An update on cardio‐oncology. Trends Cardiovasc Med. 2014;24:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio‐oncology. Mayo Clin Proc. 2014;89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575. [DOI] [PubMed] [Google Scholar]
- 22. Slamon DJ, Leyland‐Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 23. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wood PW, Choy JB, Nanda NC, Becher H. Left ventricular ejection fraction and volumes: It depends on the imaging method. Echocardiography. 2014;31:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez EA, Romond EH, Suman VJ, Jeong J‐H, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2‐positive breast cancer: planned joint analysis of overall survival from NSABP B‐31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swain SM, Baselga J, Kim S‐B, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero J‐M, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortés J. Pertuzumab, trastuzumab, and docetaxel in HER2‐positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romond EH, Jeong J‐H, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA. Seven‐year follow‐up assessment of cardiac function in NSABP B‐31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node‐positive, human epidermal growth factor receptor 2‐positive breast cancer. J Clin Oncol. 2012;30:3792–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472 DOI: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naumann D, Rusius V, Margiotta C, Nevill A, Carmichael A, Rea D, Sintler M. Factors predicting trastuzumab‐related cardiotoxicity in a real‐world population of women with her2+ breast cancer. Anticancer Res. 2013;33:1717–1720. [PubMed] [Google Scholar]
- 30. Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K, Rugo H, Miller K, Ellis M, Shapira I. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node‐negative, ErbB2‐positive breast cancer. JAMA Oncol. 2016;2:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Advani PP, Ballman KV, Dockter TJ, Colon‐Otero G, Perez EA. Long‐term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cameron D, Piccart‐Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L, Baselga J, Al‐Sakaff N, Lauer S, McFadden E, Leyland‐Jones B, Bell R, Dowsett M, Jackisch C. 11 years’ follow‐up of trastuzumab after adjuvant chemotherapy in her2‐positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slamon DJ EW, Robert NJ, Giermek J, Martin M, Jasiowka M, Mackey JR, Chan A, Liu M‐C, Pinter T, Valero V, Falkson C, Fornander T, Shiftan TA, Bensfia S, Hitier S, Xu N, Bée‐Munteanu V, Drevot P, Press MF, Crown J; On Behalf of the BCIRG‐006 Investigators. UCLA, Los Angeles, CA; GBG, Munchen, Germany; USO, The Woodlands, TX; Maria Sklodowska‐Curie Centre, Warsaw, Poland; GEICAM, Madrid, Spain; Maria Sklodowska‐Curie Memorial Cancer Institute, Krakow, Poland; Cross Cancer Institute, Edmonton, Canada; Breast Cancer Research Centre ‐ WA & Curtin University, Perth, Australia; Sun Yat‐Sen Cancer Center, Taipei, Taiwan; Petz Aladar Megyei Oktato Korhaz Onkoradiologica, Gyor, Hungary; The University of Texas MD Anderson Cancer Centre, Houston, TX; University of Alabama, Birmingham, AL; Karolinska University Hospital, Stockholm, Sweden; Sharp Memorial Hospital, San Diego, CA; Sanofi, Cambridge; Sanofi, Chilly‐Mazarin, France; Genentech, South San Francisco, CA; TRIO, Paris, France; USC/Norris Comprehensive Cancer Center, Los Angeles, CA; ICORG, Dublin, Ireland . Ten year follow‐up of BCIRG‐006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. San Antonio Breast Cancer Symposium. Abstract S5‐04. Presented December 11, 2015. 2015.
- 34. de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor‐Tarh D, Metzger‐Filho O, Steinseifer J, Untch M, Smith IE, Gianni L, Baselga J, Jackisch C, Cameron DA, Bell R, Leyland‐Jones B, Dowsett M, Gelber RD, Piccart‐Gebhart MJ, Suter TM. Trastuzumab‐associated cardiac events at 8 years of median follow‐up in the Herceptin Adjuvant Trial (BIG 1‐01). J Clin Oncol. 2014;32:2159–2165. [DOI] [PubMed] [Google Scholar]
- 35. Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies. J Am Coll Cardiol. 2010;56:1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narayan HK, Finkelman BS, French B, Plappert T, Hyman D, Smith AM, Margulies KB, Ky B. Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow‐up. Circulation. 2017:135:1397–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 38. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 39. Said R, Banchs J, Wheler J, Hess KR, Falchook G, Fu S, Naing A, Hong D, Piha‐Paul S, Ye Y, Yeh E, Wolff RA, Tsimberidou AM. The prognostic significance of left ventricular ejection fraction in patients with advanced cancer treated in phase I clinical trials. Ann Oncol. 2014;25:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Long‐Term Causes of Death
Figure S1. Kaplan–Meier 5‐year survival plot from the time of breast cancer diagnosis for patients with normal and reduced left ventricular ejection fraction (LVEF) at baseline, adjusted for age and stage of breast cancer (P<0.001 for group comparison by log rank). The number of patients is reduced in the normal LVEF by the 10 patients, for which stage of disease information was not available.


