Abstract
Background
This study assessed trends in heart failure (HF) hospitalizations and health resource use in patients with adult congenital heart disease (ACHD).
Methods and Results
The Nationwide Inpatient Sample was used to compare ACHD with non‐ACHD HF hospitalization and health resource trends. Health resource use was assessed using total hospital charges, hospital length of stay, and procedural burden. A total of 87 175±2676 ACHD‐related HF hospitalizations occurred between 1998 and 2011. During this time, ACHD HF hospitalizations increased 91% (4620±438–8809±740, P<0.0001) versus a 21% increase in non‐ACHD HF hospitalizations (P=0.003). ACHD HF hospitalization was associated with longer length of stay (ACHD HF versus non‐ACHD HF, 7.2±0.09 versus 6.8±0.02 days; P<0.0001), greater procedural burden, and higher charges ($81 332±$1650 versus $52 050±$379; P<0.0001). ACHD HF hospitalization charges increased 258% during the study period ($26 533±$1816 in 1998 versus $94 887±$8310 in 2011; P=0.0002), more than double that for non‐ACHD HF (P=0.04). Patients with ACHD HF hospitalized in high‐volume ACHD centers versus others were more likely to undergo invasive hemodynamic testing (30.2±0.6% versus 20.7±0.5%; P<0.0001) and to receive cardiac resynchronization/defibrillator devices (4.7±0.3% versus 1.8±0.2%; P<0.0001) and mechanical circulatory support (3.9±0.2% versus 2.4±0.2%; P<0.0001).
Conclusions
ACHD‐related HF hospitalizations have increased dramatically in recent years and are associated with disproportionately higher costs, procedural burden, and health resource use.
Keywords: health services research, heart failure, mortality
Subject Categories: Congenital Heart Disease, Heart Failure, Health Services, Complications
Clinical Perspective
What Is New?
We demonstrate a significant increase in the number of patients with adult congenital heart disease (ACHD) being hospitalized for heart failure (HF) in the United States.
The average cost of ACHD HF hospitalization increased 258% over the past decade, almost double that seen for non‐ACHD HF.
Longer hospital stays and a high burden of HF‐related procedures in patients with ACHD contributed to these costs.
Variation in practice between US centers is demonstrated, with patients admitted to high‐volume ACHD centers being more likely to undergo invasive hemodynamic evaluation, to receive cardiac resynchronization/defibrillator devices, and to undergo heart transplantation.
What Are the Clinical Implications?
The increasing number of patients being hospitalized for ACHD‐related HF presents new challenges for ACHD and HF specialists alike.
Significant variation in the approach taken by high‐ and low‐volume ACHD centers caring for patients with ACHD HF suggests a need for ACHD‐specific HF guidelines, care, and training pathways.
The disproportionate costs associated with caring for patients with ACHD HF highlight the importance of ACHD centers being properly supported when providing specialist care.
Introduction
More than 2.4 million people are living with congenital heart disease (CHD) in the United States.1 For the population with adult CHD (ACHD), heart failure (HF) is now the leading cause of mortality2, 3 and accounts for almost one third of deaths.4 Our nascent understanding of ACHD‐related HF is largely restricted to observational studies and outcomes reported by tertiary ACHD centers,3, 5 an important limitation because many ACHD hospitalizations occur in smaller hospitals with less exposure to ACHD.6 Studies of nationwide ACHD hospital admissions have provided preliminary insights into ACHD HF hospitalization trends,6, 7 but without sufficient detail to inform existing HF services or to anticipate future health resource needs. Little is known about the clinical profile of patients with ACHD HF, including where they are being hospitalized, healthcare costs, and whether treatment varies between high‐ versus low‐volume ACHD centers. This study sought to address these knowledge gaps.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. This study analyzed data from the Nationwide Inpatient Sample (NIS), the largest publicly available all‐payer inpatient care database in the United States. The NIS is a stratified sample of 20% of US academic and nonacademic hospitals that contains data on 28 million hospitalizations each year. Sampling weights are used to produce national estimates. It is not intended that individual patients or hospitals be identified using the NIS.
This study included adults ≥18 years of age at the time of hospitalization between 1998 and 2011 with an International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis code for HF (listed in Table 1). If one of the HF codes was listed in the first position, the admission was considered to be a primary HF hospitalization; otherwise, the admission was considered to be “nonprimary.”8 Two analytic samples were generated: analytic sample 1, for evaluating procedural trends, including heart and heart‐lung transplantation; and analytic sample 2, for evaluating HF hospitalization trends and health resource use (Figure 1). All procedures captured by ICD‐9 codes (invasive hemodynamics, transcatheter, and surgery) were included in analytic sample 1. HF hospitalizations were further categorized into ACHD and non‐ACHD HF; patients with ACHD HF were those with ICD‐9 diagnosis codes for both CHD and HF.7 ICD‐9 codes with lower specificity for ACHD, including atrial septal defect, bicuspid aortic valve, aortic stenosis, and unspecified congenital anomalies, were excluded. Hospitalizations including ICD‐9 codes for pregnancy were also excluded. This study used previously collected deidentified data and was, therefore, exempted from institutional review board approval, and the need for informed consent was waived.
Table 1.
ICD‐9 Diagnosis Categories and Codes
| Variable | ICD‐9 Code Numbers |
|---|---|
| ICD‐9 diagnostic codes | |
| Heart failure | 428.1, 428.21, 428.22, 428.23, 428.31, 428.32, 428.33, 428.41, 428.42, 428.43 |
| Cardiogenic shock | 785.51, 429.4, 997.1, 398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93 |
| Adult congenital heart diseasea | 745, 746, 747.0 to 747.4, 747.9 |
| Single ventricle | 745.3, 746.0, 746.1, 746.7 |
| Transposition of the great arteries | 745.12, 745.10, 745.19 |
| Tetralogy of Fallot | 745.2 |
| Atrioventricular septal defect | 745.60, 745.61, 745.69 |
| Ebstein anomaly | 746.2 |
| ICD‐9 procedural codes | |
| MCS | 37.52, 37.60, 37.62, 37.65, 37.66, 37.68 |
| Intra‐aortic balloon pump | 37.61 |
| Cardiac resynchronization | 00.50, 00.51, 00.52, 00.53, 00.54 |
| Right‐ and left‐sided heart catheterization | 3722, 3723 |
| Central venous catheter placement | 3893 |
| Implantation of cardiac defibrillator | 3794 |
ICD‐9 indicates International Classification of Diseases, Ninth Revision; MCS, mechanical circulatory support, including extracorporeal mechanical assistance.
Exclusions included pregnancy (V22.2), heart transplant (37.51)/heart‐lung transplant (33.6), and adult congenital heart disease subgroups for which ICD‐9 coding is less accurate: atrial septal defect (745.5), congenital anomalies of the cardiovascular system (747) and other connective tissue disease (756.83), and aortic stenosis (excluded congenital stenosis of aortic valve [746.3] and bicuspid aortic valve [746.4]).
Figure 1.
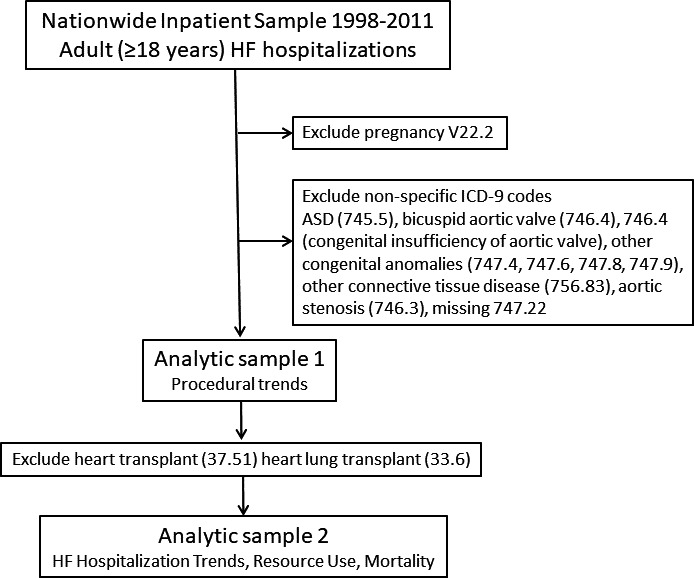
Deriving the analytic samples. ASD indicates atrial septal defect; HF, heart failure; ICD‐9, International Classification of Diseases, Ninth Revision.
Clinical characteristics assessed at admission included age, sex, and comorbidities. A composite comorbidity point score was calculated using the van Walraven modification9 of the Elixhauser comorbidity measure.10 This measure condenses the comorbidity burden into a single numeric score that has been shown to discriminate health outcomes in patients with ACHD.7, 11 Procedures performed during HF hospitalization were assessed using ICD‐9 procedural codes. Table 1 summarizes the ICD‐9 diagnostic and procedural codes used to determine inclusion and exclusion in this study.
HF hospitalization and procedural trends in ACHD are presented as absolute numbers rather than population‐adjusted rates.7
Hospital charges were indexed to healthcare‐specific inflation by adjusting all values to 2011 dollars using the Bureau of Labor Statistics Consumer Price Index. The Consumer Price Index subindex specific to inpatient hospital services was used, with a baseline of December 1996 taken as 100, and yearly Consumer Price Index values were used to generate a conversion factor to 2011 dollars.12 High resource use (HRU) was defined as >90th percentile of all (ACHD and noncongenital combined) HF total charge distribution. Using this threshold, HRU has been observed in patients with ACHD undergoing surgery in pediatric hospitals and linked to increased mortality.13 High‐volume ACHD centers are more likely to care for complex patients and undertake procedures that directly influence health outcomes. To further understand factors influencing health resource use and outcomes, we assessed the impact of being hospitalized in high‐ versus low‐volume centers, with high ACHD volume defined as hospitalizations exceeding the 90th percentile of ACHD admission distribution for all hospitals.
Continuous variables are presented as means±SEM. Discrete variables are presented as numbers (percentages). Intergroup comparisons were performed using 2‐sample t tests for continuous variables and the Pearson χ2 test for categorical variables. Hospitalization, resource use, and procedural trends were examined using the Mann Kendall test, a nonparametric test that assesses the presence and direction of a trend over time.14 To understand the relationships between mortality and hospitalization in a high ACHD volume center, we performed multivariable logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. P<0.05 was required for retention in the final model. All analyses were performed with SAS software, versions 9.3 and 9.4 (SAS Institute Inc, Cary, NC), except the Mann Kendall test, which was performed with R and R package.15
Results
A total of 87 175±2676 ACHD HF hospitalizations occurred between 1998 and 2011; almost all (98%) occurred in adult hospitals. The annual number of ACHD HF hospitalizations increased 91% (4620±438 in 1998 to 8809±740 in 2011; P<0.0001) versus a 21% increase in non‐ACHD HF hospitalizations (3 829 372±170 842 in 1998 to 4 637 214±219 817 in 2011; P=0.003) (Figure 2). HF accounted for almost one third (28.3±0.8%) of ACHD hospitalizations. HF as a primary diagnosis in patients with ACHD increased 47% over the study period (1262±122 in 1998 versus 1864±175 in 2011; P=0.001). By contrast, primary hospitalizations were unchanged for non‐ACHD HF (Figure 3).
Figure 2.
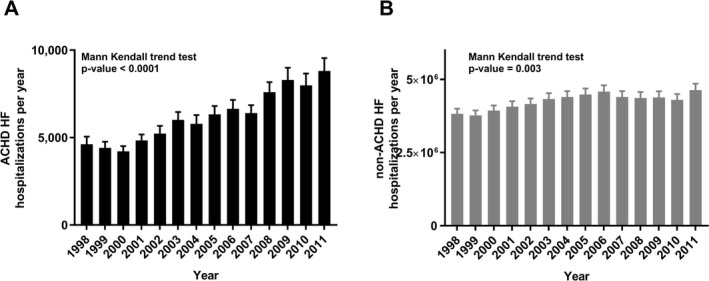
Adult congenital heart disease (ACHD; A) vs non‐ACHD (B) heart failure (HF) annual hospitalization trends, 1998 to 2011.
Figure 3.
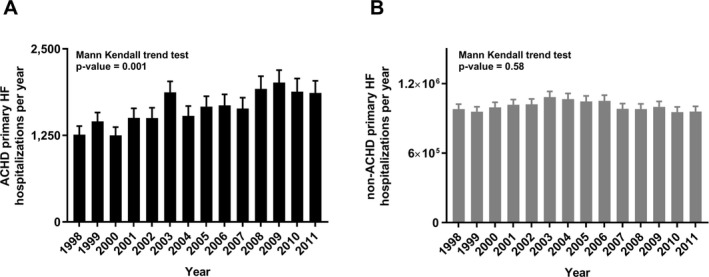
Adult congenital heart disease (ACHD; A) vs non‐ACHD (B) primary heart failure (HF) hospitalizations, 1998 to 2011.
The percentages of ACHD‐related HF hospitalizations by age groups were 13±0.4% of patients aged 18 to 30 years, increasing to 29±0.4% of patients aged 31 to 50 years, 23±0.4% of patients aged 51 to 65 years, and 35±0.5% of patients aged >65 years. HF hospitalizations in patients with non‐ACHD HF peaked after the age of 65 years (Figure 4). The percentage of ACHD‐related HF hospitalizations followed a similar distribution. Patients with tetralogy of Fallot (TOF) accounted for the highest number of ACHD HF hospitalizations (5309±335 HF hospitalizations). However, when considered proportionally by ACHD subgroup, HF was most common in patients with transposition of the great arteries (40±1.6%), Ebstein anomaly (34±1.5%), atrioventricular septal defect (34±1.5%), TOF (33±1.0%), and single‐ventricle physiological feature (28±0.9%) (Figure 5).
Figure 4.
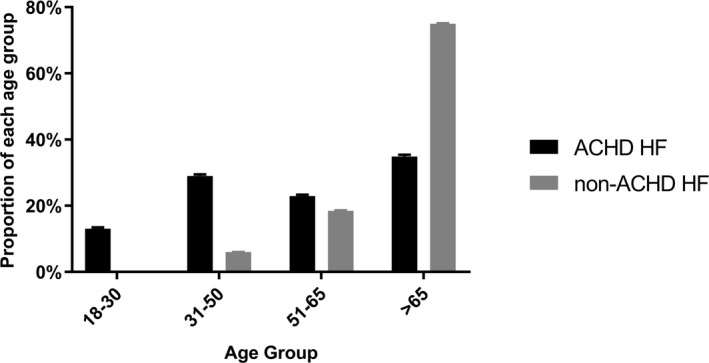
Adult congenital heart disease (ACHD) vs non‐ACHD heart failure (HF) hospitalizations across age groups.
Figure 5.
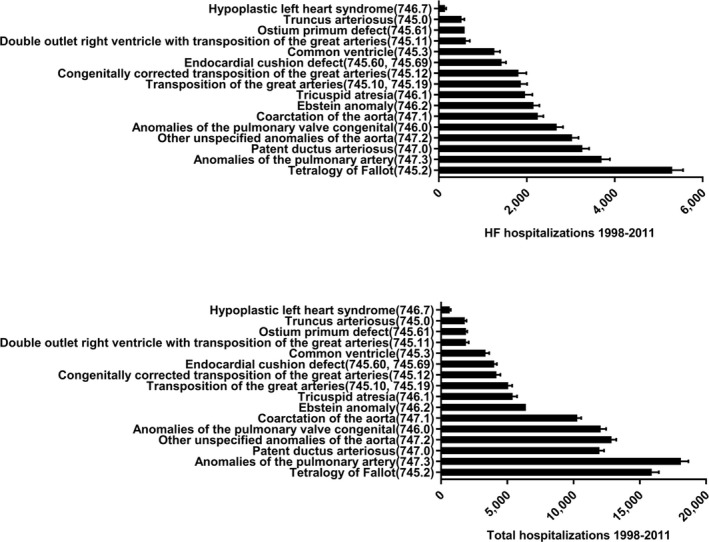
Adult congenital heart disease heart failure (HF) hospitalizations by International Classification of Diseases, Ninth Revision (ICD‐9) code and anatomic subgroup.
The most common and relevant comorbidities in patients hospitalized for ACHD‐related HF are listed in Table 2. Although the comorbidity index was lower for patients with ACHD HF versus those with non‐ACHD HF (1.74±0.015 versus 2.45±0.005; P<0.001) comorbidities were not uncommon, including coronary artery disease in 21±0.4%, hypertension in 29±0.04%, diabetes mellitus in 19±0.01%, and chronic kidney disease in 11±0.03%.
Table 2.
Common Comorbidities in Patients Hospitalized With ACHD‐Related HF
| Comorbiditiesa | Patients With ACHD HF, % |
|---|---|
| Hypertension | 28±0.4 |
| Fluid or electrolyte disturbance | 24±0.4 |
| Chronic lung disease | 22±0.4 |
| Coronary artery disease | 21±0.4 |
| Diabetes mellitus | 19±0.1 |
| Chronic kidney disease | 11±0.3 |
| Iron‐deficiency anemia | 10±0.4 |
| Hypothyroidism | 10±0.2 |
| Coagulopathy | 8±0.2 |
| Obesity | 8±0.2 |
ACHD indicates adult congenital heart disease; HF, heart failure.
International Classification of Diseases, Ninth Revision (ICD‐9), codes for these comorbidities are available in Table S1.
Compared with noncongenital HF, ACHD‐related HF hospitalization led to longer length of stay (7.2±0.09 versus 6.8±0.02 days; P<0.0001) and higher charges ($81 332±$1650 versus $52 050±$379; P<0.0001). Total hospital charges for ACHD‐related HF increased 258% ($26 533±$1816 in 1998 versus $94 887±$8310 in 2011; P=0.0002), more than double the 172% increase in non‐ACHD HF charges ($19 455±$465 in 1998 versus $52 994±$1712 in 2011; P=0.04) (Figure 6). The 90th percentile for total charges defining HRU for all patients with HF was $115 427. Criteria for HRU were met in 21% of ACHD HF hospitalizations versus 10% of noncongenital HF (P<0.001).
Figure 6.
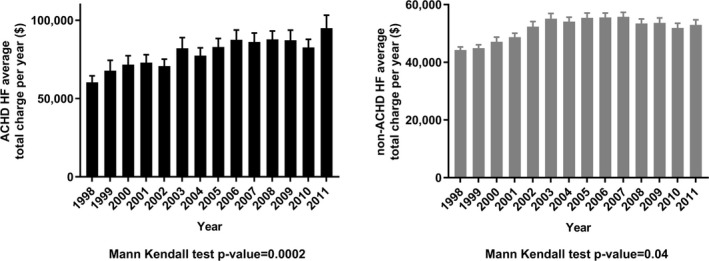
Total hospital charges for adult congenital heart disease (ACHD; A) vs non‐ACHD (B) heart failure (HF) hospitalizations, 1998 to 2011.
HF‐related procedures were much more common in ACHD HF versus noncongenital HF hospitalizations (Table 3). A significant increase in ACHD HF–related procedures occurred during the study period, including those seen for right‐ and left‐sided heart catheterization (851±106 in 1998 versus 1693±178 in 2011; P<0.0001), central line placement (204±40 in 1998 versus 925±109 in 2011; P<0.0001), and intra‐aortic balloon pump placement (109±25 in 1998 versus 352±52 in 2011; P<0.001). Significant increases were also seen for cardiac resynchronization therapy (9±6 in 2002 versus 140±28 in 2011; P=0.001), cardiac defibrillator implants (64±20 versus 170±39 years; P<0.001), mechanical circulatory support (7±6 versus 55±26 years; P<0.0001), and heart transplantation (10±7 in 1999 versus 59±24 in 2011; P<0.001) (Figure 7).
Table 3.
Percentages of Non‐ACHD HF vs ACHD HF Hospitalizations Associated With an HF‐Related Procedure
| Procedure | Hospitalizations, % | P Value | |
|---|---|---|---|
| Non‐ACHD HF | ACHD HF | ||
| Intra‐aortic balloon pump | 0.84±0.01 | 3.07±0.14 | <0.0001 |
| Right‐ and left‐sided heart catheterization | 7.86±0.07 | 19.02±0.36 | <0.0001 |
| Central venous catheter placement | 7.20±0.05 | 7.97±0.24 | <0.001 |
| Implantation of cardiac defibrillator | 0.80±0.01 | 1.89±0.11 | <0.0001 |
| Cardiac resynchronization | 0.66±0.02 | 1.46±0.10 | <0.0001 |
| Mechanical circulatory support | 0.05±0.01 | 0.32±0.05 | <0.0001 |
| Heart transplant | 0.02±0.01 | 0.43±0.07 | <0.0001 |
Data are given as hospitalizations±SEM. ACHD indicates adult congenital heart disease; HF, heart failure.
Figure 7.
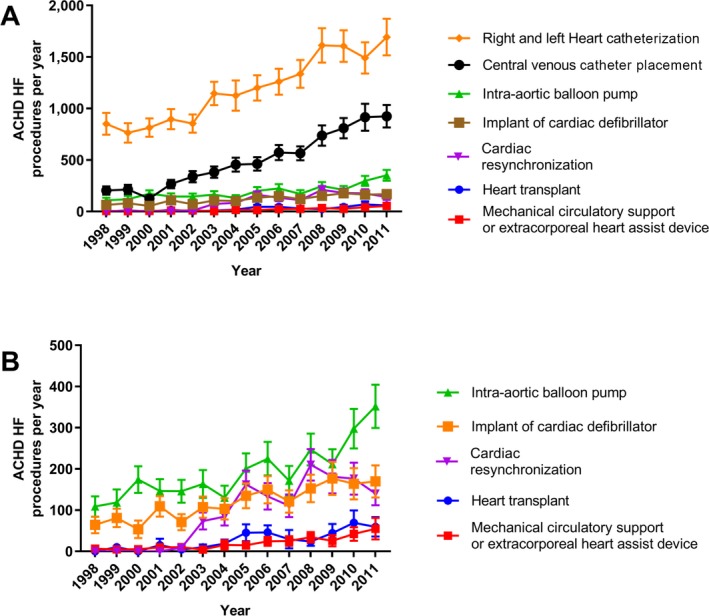
A and B, Procedural trends in adult congenital heart disease (ACHD) heart failure (HF) hospitalizations. Please note that in B, the trend for central, right‐ and left‐sided heart catheterizations has been removed to enable scale adjustment and review of heart transplant and mechanical circulatory support.
ACHD volume was used as a surrogate for ACHD expertise. High‐volume ACHD centers were (by definition) 10% of all hospitals; however, they admitted 53±0.09% of patients with ACHD HF. Compared with admission to lower‐volume centers, admission to a high‐volume center was associated with higher rates of invasive hemodynamic measurements: cardiac catheterization and central line placement (30.2±0.6% versus 20.7±0.5%; P<0.0001), cardiac resynchronization/cardiac defibrillator device therapy (4.7±0.3% versus 1.8±0.2%; P<0.0001), and advanced mechanical circulatory support (3.9±0.2% versus 2.4±0.2%; P<0.0001) (Table 4). Total charges for ACHD HF hospitalizations were substantially higher in high‐ versus low‐volume centers ($74 286±$2397 versus $42 206±$1046; P<0.0001). Almost one third (28±0.9%) of patients with ACHD HF hospitalized in high‐volume centers met criteria for HRU.
Table 4.
Patient and Procedural Profile for ACHD High‐Volume Centers vs Other Adult Centers
| Variable | ACHD HF Hospitalization Center | P Value | |
|---|---|---|---|
| High ACHD Volume | Other Adult Centers | ||
| Age, y | 52±0.4 | 59±0.3 | <0.0001 |
| Female sex, % | 49±0.6 | 54±0.6 | <0.0001 |
| Total charge, $ | 100 692±2692 | 59 596±1418 | <0.0001 |
| Meeting HRU, % | 27.7±0.9 | 13.4±0.5 | <0.0001 |
| Comorbidity index | 1.67±0.02 | 1.83±0.02 | <0.0001 |
| Payer category, % | |||
| Medicare | 16.0±0.6 | 14.7±0.5 | <0.0001 |
| Medicaid | 43.4±0.7 | 58.4±0.7 | <0.0001 |
| Othera | 40.5±0.7 | 26.9±0.6 | <0.0001 |
| Invasive hemodynamics, %b | 30.2±0.6 | 20.7±0.5 | <0.0001 |
| CRT or ICD, % | 4.7±0.3 | 1.8±0.2 | <0.0001 |
| Advanced HF therapies, %c | 3.9±0.2 | 2.4±0.2 | <0.0001 |
ACHD indicates adult congenital heart disease; CRT, cardiac resynchronization therapy; HF, heart failure; HRU, high resource use; ICD, implantable cardiac defibrillator.
Private insurance, self‐pay, no charge, other.
Central, left and right heart catheterization.
Heart transplant and mechanical circulatory support.
Despite patients with ACHD HF being younger and having a lower comorbidity index, in‐hospital mortality did not differ between patients with ACHD HF and those with non‐ACHD HF (6.5±0.2% versus 6.62±0.02%; P=0.54). Compared with patients with ACHD HF who did not undergo these procedures, higher mortality risk was observed in patients with ACHD HF undergoing invasive hemodynamic assessment (OR, 1.5; 95% CI, 1.3–1.8; P<0.0001) and advanced HF therapies (mechanical circulatory support and heart transplantation) (OR, 9.1; 95% CI, 7.4–11.2; P<0.0001). Mortality was not increased in high ACHD volume centers (OR, 1.0; 95% CI, 0.9–1.1; P=0.43), even though they were more likely to undertake high‐risk interventions. Mortality risk was lower in ACHD HF hospitalizations associated with cardiac resynchronization therapy/implantable cardiac defibrillator (OR, 0.2; 95% CI, 0.1–0.3; P<0.0001) (Table 5).
Table 5.
Multivariable Logistic Regression Exploring the Association Between Clinical Variables and Mortality After Hospitalization for ACHD‐Related HF
| Clinical Variable | OR | 95% CI | P Value |
|---|---|---|---|
| ACHD high‐volume hospitalization | 1.0 | 0.87–1.12 | 0.82 |
| Invasive hemodynamicsa | 1.5 | 1.32–1.75 | <0.0001 |
| Heart transplant or MCS | 9.1 | 7.45–11.18 | <0.0001 |
| CRT or ICD | 0.2 | 0.08–0.34 | <0.0001 |
| Elixhauser comorbidity index | 1.0 | 0.93–1.03 | 0.3602 |
ACHD indicates adult congenital heart disease; CI, confidence interval; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardiac defibrillator; MCS, mechanical circulatory support, including extracorporeal mechanical assistance; OR, odds ratio.
Invasive hemodynamics indicates cardiac catheterization and central venous catheter.
Discussion
It is widely accepted that ACHD HF is “different” from other forms of HF. Yet it remains unclear what these differences mean for health resource use in the contemporary era. Evaluating >85 000 hospital admissions, we report a notable increase in ACHD HF hospitalizations over the past decade, including a growing number of primary likely incident HF hospitalizations in young adults with complex CHD. Longer hospital stays and a steady increase and a steady rise in HF‐related procedures contributed to a substantial increase in total charges per ACHD HF hospitalization, disproportionately higher than non‐ACHD HF. Important differences in the assessment and treatment of ACHD HF are demonstrated, with patients admitted to high‐volume ACHD centers being more likely to undergo cardiac catheterization or invasive hemodynamics, to be implanted with cardiac resynchronization/cardiac defibrillator devices, and to proceed with advanced HF treatments. Despite high‐risk interventions being more common in high‐ versus low‐volume centers, mortality was equivalent between the two.
HF is now a major focus in ACHD specialist care worldwide, accounting for up to one third of all ACHD hospitalizations.6, 16 The 91% increase in ACHD HF hospitalizations observed in this study reflects not only an expansion of the overall population with ACHD but an increasing burden of late‐onset HF often occurring years after surgical repair. Primary ACHD HF hospitalizations increased 47% during the study period. In contrast, primary HF hospitalizations in patients without ACHD were unchanged. Fewer primary HF hospitalizations in patients without ACHD over the past decade has been attributed to improved guidelines‐based treatment and earlier intervention in acquired forms of heart disease.17, 18 Conversely, the increasing numbers of patients with ACHD being hospitalized with incident HF suggest existing HF guidelines may be less applicable to the emerging population of patients with ACHD HF in whom proven HF treatments are lacking.
Comparing the proportion of HF hospitalizations across 4 age groups, ACHD HF hospitalizations start in early adulthood, with 13%, 29%, 23%, and 35% of patients hospitalized between ages 18 and 30, 31 and 50, 51 and 65, and >65 years, respectively. This compares with non‐ACHD HF, which predominantly affected adults aged ≥65 years. The high prevalence of HF in different ACHD subtypes observed herein and in prior studies, including up to 50% of patients with TOF and 30% of patients who underwent Mustard/Senningrepair,19, 20 highlights the fact that these patients are not “cured” but instead remain at high risk for HF. Compared with early studies of subclinical HF reported in often asymptomatic patients with ACHD with elevated serum biomarkers,19 we are now witnessing an increasing number of patients with ACHD with clinically overt HF requiring in‐hospital care. In this study, HF accounted for 40% of all hospitalizations in patients with transposition of the great arteries. The proportions of HF hospitalizations in patients with Ebstein anomaly (34%), atrioventricular septal defect (34%), TOF (33%), and single‐ventricle physiological feature (28%) were similarly high. Reflecting the complex interplay between acquired and congenital disease, we observed a relatively high prevalence of comorbidities, including hypertension in 29%, coronary artery disease in 20%, and chronic kidney disease in 11%.
HF‐related procedures and longer length of stay contribute to the disproportionate healthcare costs associated with ACHD HF hospitalization, as demonstrated in this study. Current guidelines21 recommend referral of patients with ACHD HF to a center with combined expertise in ACHD and HF. We demonstrate, for the first time, that implementing this guideline is associated with considerable expense, with hospital charges for the average ACHD HF hospitalization totaling $81 332 (US dollars), 64% higher than the average cost of a non‐ACHD HF admission. Along with a 258% increase in the average cost of ACHD HF hospitalization over the past decade (almost double that observed for non‐ACHD HF), these findings highlight the importance of ACHD centers receiving adequate financial support so that they can continue to provide care to patients with ACHD HF who are increasing in both number and complexity.
Further emphasizing this challenge, ACHD HF hospitalization in a high‐volume center was especially costly, with almost one third of these admissions meeting criteria for HRU (defined as >90th percentile of all HF total charge distributions; in this study, >$115 427). High‐volume centers were more likely to undertake invasive hemodynamics, to implant cardiac devices, and to proceed with advanced HF therapies. Invasive hemodynamic assessment is a prerequisite for patients with ACHD‐related HF, especially those being considered for advanced HF therapies. Although it is tempting to conclude that greater use of HF procedures and treatments in high‐volume centers reflects greater adherence to guidelines‐based care in high‐volume centers, these differences may simply reflect ACHD HF guidelines being enacted and high‐volume centers serving as regional hubs/centers of expertise. Alternatively, the higher rate of procedures in high‐volume centers may reflect other patient differences not captured in the NIS database. Further research is needed to understand the pathways that patients with ACHD HF are taking before receiving expert care, along with the financial implications to stakeholders engaged in a “hub and spoke” model of ACHD HF care.
In‐hospital ACHD HF mortality was 6.5% and was no different from that seen in patients with non‐ACHD HF, despite patients with ACHD being significantly younger and having fewer comorbidities.
Death after HF hospitalization is common, with the immediate postdischarge period being the highest‐risk period.22 Observing 274 patients with ACHD after HF hospitalization, the Dutch CONCOR (Congenital Corvitia) registry reported 1‐ and 3‐year mortality rates of 24% and 35%, respectively. Outcomes in tertiary ACHD centers in Canada and the United Kingdom demonstrate comparable mortality, with HF accounting for 21% and 42% of deaths, respectively.3, 23 Therefore, the in‐hospital mortality reported in this study likely underestimates mortality reported after ACHD HF hospitalization.
This study has several limitations. The NIS is vulnerable to classification error, and analyses are based on hospital admissions rather than individual patients; multiple hospitalizations for each person in a given year cannot be accounted for. Using the NIS, total charges reflect what a hospital billed for services and not what costs a hospital incurred or received in payment. Current Procedural Terminology codes, rather than ICD‐9 codes, are used for procedural billing during a hospitalization. Therefore, a significant amount of data may not be captured from procedure ICD‐9 codes alone. Inherent patient differences not captured in the NIS data set further limit our ability to fully explore factors that are important for ACHD HF hospitalization trends, referral patterns, and outcomes. Even with these limitations, we still believe that our findings are important and provide internally consistent information about ACHD‐related HF hospitalizations. Similar methods have been widely used and accepted for studies of acquired HF.8, 24 We25 and others26 have reported the constraints of administrative data sets for detection and categorization of ACHD. Even so, ICD‐9 codes have been used to investigate CHD‐related births and mortality in the United States.27, 28 TOF accounted for the largest number of HF hospitalizations in this study, consistent with prior studies reporting HF as the most common cause of hospitalization in this group.29 Patients with TOF may also be coded as having anomalies of the pulmonary artery and pulmonary valve, and these groups accounted for a large number of ACHD HF hospitalizations. As such, the numbers of HF hospitalizations reported for TOF and other ACHD subgroups are best viewed as estimates, and further research is needed to understand the true burden of HF in the population with ACHD. Because many patients with ACHD with HF are not hospitalized, the true prevalence and incidence of HF has proven elusive, even in research using a national ACHD database linked to hospital and mortality registries.30 Although it may be appealing to attribute lower ACHD HF in‐hospital mortality to greater use of cardiac resynchronization therapy and defibrillators, we are unable to study cause and effect in this study. Lower mortality may also reflect a subgroup of patients being electively admitted for cardiac device implantation, although hospitalizations <24 hours were excluded to reduce the impact of these admissions.
Conclusions
Young adult survivors with complex CHD are an emerging HF population, with a notable increase in ACHD‐related HF hospitalizations over the past decade. The healthcare costs associated with these hospitalizations are substantial and growing. The combination of lifelong cardiac disease, incident HF in early adulthood, and interplay between congenital and acquired comorbidities differentiates patients with ACHD HF from “traditional” patients with HF around whom HF services and resources are currently centered. The unique clinical profile of patients with ACHD, increasing costs of in‐hospital care, and variability in HF treatment strategy between high‐ and low‐volume ACHD centers suggest a need for ACHD‐specific HF treatment guidelines and care pathways.
Sources of Funding
This study was supported by intramural research funds provided by Oregon Health Science University and the Knight Cardiovascular Research Institute.
Disclosures
None.
Supporting information
Table S1. ICD‐9 codes for ACHD HF patient comorbidities
(J Am Heart Assoc. 2018;7:e008775 DOI: 10.1161/JAHA.118.008775.)
References
- 1. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population‐based study. J Am Coll Cardiol. 2007;50:1263–1271. [DOI] [PubMed] [Google Scholar]
- 3. Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–1116. [DOI] [PubMed] [Google Scholar]
- 4. Zomer AC, Uiterwaal CS, van der Velde ET, Tijssen JG, Mariman EC, Verheugt CL, Vaartjes I, Pieper PG, Meijboom FJ, Grobbee DE, Mulder BJ. Mortality in adult congenital heart disease: are national registries reliable for cause of death? Int J Cardiol. 2011;152:212–217. [DOI] [PubMed] [Google Scholar]
- 5. Connolly HM, Ammash, NM , Warnes C. One thousand patients with adult congenital heart disease: causes of death and lessons learned. J Am Coll Cardiol. 1997;29A:385A [Google Scholar]
- 6. Agarwal S, Sud K, Menon V. Nationwide hospitalization trends in adult congenital heart disease across 2003–2012. J Am Heart Assoc. 2016;5:e002330 DOI: 10.1161/JAHA.115.002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. [DOI] [PubMed] [Google Scholar]
- 8. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure‐associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. [DOI] [PubMed] [Google Scholar]
- 10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 11. Maxwell BG, Wong JK, Kin C, Lobato RL. Perioperative outcomes of major noncardiac surgery in adults with congenital heart disease. Anesthesiology. 2013;119:762–769. [DOI] [PubMed] [Google Scholar]
- 12. Statistics BoL . Consumer price index detailed reports 1998–2011. 2011.
- 13. Kim YY, Gauvreau K, Bacha EA, Landzberg MJ, Benavidez OJ. Resource use among adult congenital heart surgery admissions in pediatric hospitals: risk factors for high resource utilization and association with inpatient death. Circ Cardiovasc Qual Outcomes. 2011;4:634–639. [DOI] [PubMed] [Google Scholar]
- 14. Hirsch R, Slack JR, Smith RA. Technique of trend analysis for monthly water quality data. Water Resour Res. 1982;18:107–121. [Google Scholar]
- 15. Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 16. Rodriguez FH III, Moodie DS, Parekh DR, Franklin WJ, Morales DL, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of heart failure‐related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis. 2013;8:513–519. [DOI] [PubMed] [Google Scholar]
- 17. Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, Redfield MM, Rodeheffer RJ, Yawn BP, Roger VL. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157:1101–1107. [DOI] [PubMed] [Google Scholar]
- 18. Shafazand M, Rosengren A, Lappas G, Swedberg K, Schaufelberger M. Decreasing trends in the incidence of heart failure after acute myocardial infarction from 1993–2004: a study of 175,216 patients with a first acute myocardial infarction in Sweden. Eur J Heart Fail. 2011;13:135–141. [DOI] [PubMed] [Google Scholar]
- 19. Norozi K, Buchhorn R, Bartmus D, Alpers V, Arnhold JO, Schoof S, Zoege M, Binder L, Geyer S, Wessel A. Elevated brain natriuretic peptide and reduced exercise capacity in adult patients operated on for tetralogy of fallot is due to biventricular dysfunction as determined by the myocardial performance index. Am J Cardiol. 2006;97:1377–1382. [DOI] [PubMed] [Google Scholar]
- 20. Norozi K, Wessel A, Alpers V, Arnhold JO, Geyer S, Zoege M, Buchhorn R. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97:1238–1243. [DOI] [PubMed] [Google Scholar]
- 21. Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL; American Heart Association Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Cardiovascular Radiology and Imaging . Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2016;133:770–801. [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA; Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. [DOI] [PubMed] [Google Scholar]
- 23. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Normand SL, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for medicare fee‐for‐service beneficiaries: progress and continuing challenges. Circulation. 2010;121:1322–1328. [DOI] [PubMed] [Google Scholar]
- 25. Broberg C, Sklenar J, Burchill L, Daniels C, Marelli A, Gurvitz M. Feasibility of using electronic medical record data for tracking quality indicators in adults with congenital heart disease. Congenit Heart Dis. 2015;10:E268–E277. [DOI] [PubMed] [Google Scholar]
- 26. Riehle‐Colarusso TJ, Bergersen L, Broberg CS, Cassell CH, Gray DT, Grosse SD, Jacobs JP, Jacobs ML, Kirby RS, Kochilas L, Krishnaswamy A, Marelli A, Pasquali SK, Wood T, Oster ME; Congenital Heart Public Health Consortium . Databases for congenital heart defect public health studies across the lifespan. J Am Heart Assoc. 2016;5:e004148 DOI: 10.1161/jaha.116.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. [DOI] [PubMed] [Google Scholar]
- 28. Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. [DOI] [PubMed] [Google Scholar]
- 29. Stefanescu Schmidt AC, DeFaria Yeh D, Tabtabai S, Kennedy KF, Yeh RW, Bhatt AB. National trends in hospitalizations of adults with tetralogy of fallot. Am J Cardiol. 2016;118:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zomer AC, Vaartjes I, van der Velde ET, de Jong HM, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur LH, Grobbee DE, Mulder BJ. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol. 2013;168:2487–2493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9 codes for ACHD HF patient comorbidities


