Abstract
Background
Impact of liver disease on development of atrial fibrillation (AF) is unclear. The purpose of the study was to evaluate prevalence of AF in the setting of liver disease and whether increasing severity of liver disease, using Model for End‐Stage Liver Disease (MELD), is independently associated with increased risk of AF.
Methods and Results
Retrospective data analysis of 1727 patients with liver disease evaluated for liver transplantation between 2006 and 2015 was performed, and patient characteristics were analyzed from billing codes and review of medical records. Multivariable time‐dependent Cox proportional hazards model was performed to determine effect of increasing MELD score on risk of developing AF. Prevalence of AF was 11.2%. Incidence of AF at median follow‐up time of 1.04 years was 8.5%. Both prevalence and incidence of AF increased with increasing MELD scores. Prevalence of AF was 3.7%, 6.4%, 16.7%, and 20.2% corresponding with MELD quartiles 1 to 10, 11 to 20, 21 to 30, and >30, respectively. Compared with patients with MELD quartile 1 to 10, patients with MELD quartile of 11 to 20 had hazard ratio of 2.73 (confidence interval, 1.47–5.07), those in the MELD quartile of 21 to 30 had a hazard ratio of 5.17 (confidence interval, 2.65–10.09), and those with MELD values >30 had hazard ratio of 9.33 (confidence interval, 3.93–22.14) for development of new‐onset AF. Other significant variables associated with new‐onset AF were age, sleep apnea, valvular heart disease, hemodynamic instability, and reduced left ventricular ejection fraction <50% (hazard ratio, of 1.06, 2.17, 3.21, 2.00, and 2.44, respectively).
Conclusions
Prevalence and incidence of AF in patients with liver disease is high. Severity of liver disease, as measured by MELD, is an important predictor of new‐onset AF. This novel finding suggests an interaction between inflammatory and neurohormonal changes in liver disease and pathogenesis of AF.
Keywords: atrial fibrillation, atrial fibrillation arrhythmia, cirrhosis, liver disease, Model for End‐Stage Liver Disease
Subject Categories: Atrial Fibrillation
Clinical Perspective
What Is New?
Prevalence of atrial fibrillation (AF) in patients with liver disease is high.
This large, retrospective study shows a clear association between liver disease and development of AF, independent of other risk factors for AF.
Severity of liver disease, as measured by Model for End‐Stage Liver Disease, is an important predictor of new‐onset AF, with increasing risk of developing this arrhythmia with increased Model for End‐Stage Liver Disease values.
What Are the Clinical Implications?
Severity of liver disease, as measured by Model for End‐Stage Liver Disease, is an important independent predictor of new‐onset AF.
This novel finding suggests an interaction between inflammatory and neurohormonal changes in liver disease and pathogenesis of AF.
Given the prevalence of AF in this population, cardiologists and hepatologists should consider screening for symptoms and signs of AF in patients with liver disease, particularly in those with higher Model for End‐Stage Liver Disease values.
Liver disease can affect circulating inflammatory peptides and lead to autonomic dysfunction, creating a proarrhythmic substrate.1, 2, 3, 4 An elevated risk of atrial fibrillation (AF) among patients with liver disease and structurally normal hearts seems plausible given that inflammation and autonomic dysfunction have been shown to contribute to pathogenesis of AF.5 However, studies on incidence and prevalence of AF in patients with liver disease are few and have shown mixed results.6, 7 In addition, results of these studies have been confounded by the lack of assessment of liver disease severity in the specific population studied. In this study, we hypothesized that the risk of new‐onset AF is driven by severity of liver disease. We therefore hypothesized that increasing Model for End‐Stage Liver Disease (MELD) values is associated with new‐onset AF, after adjusting for other comorbidities and risk factors.
Methods
As per the Journal of American Heart Association (JAHA) implementation of transparency and openness policy, the data, analytical methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon request.
Study Population
Retrospective data analysis of consecutive patients who were diagnosed with liver disease between January 2006 and December 2015 and were evaluated for potential liver transplantation (LT) was performed. The electronic health records database was made available by the University of California, Los Angeles Clinical and Translational Science Institute, supported by the NIH/National Center for Advancing Translational Science University of California Los Angeles Clinical and Translational Science Institute Grant No. UL1TR000124. This study was reviewed and approved by the University of California Institutional Review Board (Los Angeles, CA). Requirement for patient informed consent was waived given the retrospective nature of the study. Baseline patient characteristics were noted at the time of first available MELD score.
MELD Score
Severity of liver disease was assessed by MELD score and calculated using the formula:
MELD values for each patient were calculated and used for survival analysis. MELD scores confounded by use of warfarin and argatroban were treated as unknown values and excluded from analysis.
Baseline Characteristics and Assessment of AF
Baseline characteristics were collected from patient medical records, including physician notes, laboratory results, medications, billing codes, ECGs, ambulatory monitors, and echocardiograms. AF diagnosis was obtained from physician notes and billing codes and verified by detailed review of medical records, which included review of all available ECGs and ambulatory monitors. AF prevalence was calculated using the last available time point preceding LT and after the first presentation for clinical evaluation for liver disease or LT. Severe valvular heart disease was assessed from echocardiographic reports and defined as presence of any severe valvular regurgitation or stenosis. Echocardiographic reports were also used to evaluate for moderate or severe left atrial (LA) enlargement. To assess whether severity of liver disease was associated with new‐onset AF, occurrence of new‐onset AF after first available MELD score was assessed. Patients were followed up until occurrence of LT, death, or loss to follow‐up. Patients were then binned into quartiles of MELD categories, which included those with MELD score of 1 to 10 as the lowest quartile, followed by 10 to 20, 20 to 30, and >30. Percentages of patients with missing variables are shown in Table S1.
Statistical Analysis
Continuous variables were assessed as means with SDs or medians with 25% to 75% percentiles, and categorical variables as percentages. Patient characteristics by occurrence of AF were assessed using a 2‐tailed Student t test. Univariable analyses of patient characteristics were performed during the time frame of MELD score availability. Clinically relevant variables, such age and sex, as well as statistically significant variables (P<0.05) on univariable analysis, were included in the multivariable analysis. Multivariable time‐dependent Cox regression analysis was performed to analyze the effect of MELD score on incidence of new‐onset AF, adjusted for age, sex, reduced left ventricular (LV) ejection fraction (LVEF) <50%, LA enlargement, LV hypertrophy, severe valvular heart disease, portal hypertension, hypertension, diabetes mellitus, hemodynamic instability, and sleep apnea. Missing data were imputed using an Expectation‐Maximization algorithm developed by Honaker et al for missing data in time series and 15 multiply imputed data sets generated.8 All estimates were combined using Rubin's rules and P values combined using methods from Meng and Rubin and Marshall et al.9, 10, 11 In addition, the multivariable time‐dependent Cox regression analysis was also performed on available (nonimputed data) to further confirm the results. For survival analysis, only patients with at least 2 MELD scores from distinct dates were considered for analysis. All analyses were performed using the R statistical package (Version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria).12 P≤0.05 was considered statistically significant. The data, analytical methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure.
Results
Patient Characteristics
Between 2006 and 2015, 1727 patients (mean age, 54.4±12.2; 40% female) were diagnosed with liver disease and evaluated for possible LT. Median follow‐up time was 1.04 years (interquartile range, 2 months to 3 years). Baseline characteristics are shown in Table. Mean MELD score in the overall population was 17.5±8.5, and 28% of patients underwent LT at the end of follow‐up. Mean LVEF was 61±8%, sleep apnea was present in 0.5%, severe valvular heart disease in 0.3%, and moderate or severe LA enlargement was 0.3%. LV hypertrophy was present 0.5%, and LV diastolic dysfunction was present in 6.4% of the population. Beta‐blocker use at baseline in the population was 5.7%, and during the follow‐up period, was 11.9 new prescriptions per 100 patient years. Baseline CHA2DS2‐VASc was 0.83 at baseline and baseline HAS‐BLED score was 2.0.
Table 1.
Baseline Characteristics
| Characteristic | N=1727 |
|---|---|
| Age, y | 54.4±12.3 |
| Male (%) | 1034 (60) |
| BMI | 29.0±18.2 |
| White | 668 (38.7) |
| Portal hypertension | 602 (34.9) |
| Etiology of liver disease | |
| Hepatitis C | 489 (28.3) |
| Nonalcoholic steatohepatitis | 290 (16.8) |
| Alcohol abuse | 1083 (62.7) |
| Primary liver cancer | 273 (15.8) |
| Hemodynamic instability | 109 (6.3) |
| Sleep apnea | 9 (0.5) |
| Diabetes mellitus | 197 (11.4) |
| Hypertension | 196 (11.4) |
| Coronary artery disease | 15 (0.9) |
| Severe valvular disease | 6 (0.4) |
| Diastolic dysfunction | 110 (6.4) |
| LV ejection fraction, % | 60.9±7.5 |
| Reduced LV ejection fraction (<50%) | 84 (4.9) |
| LAE (moderate or severe) | 6 (0.3) |
| LA volume, mL | 53.8±24.9 |
| LV hypertrophy | 9 (0.5) |
| MELD Score | 17.5±8.5 |
| 1 to 10 | 390 (22.6) |
| 11 to 20 | 810 (46.9) |
| 21 to 30 | 380 (22.0) |
| >30 | 147 (8.5) |
| Abnormal TSH | 386 (22.4) |
| Abnormal potassium/magnesium | 335 (19.4) |
| Beta‐blocker use | 98 (5.7) |
| Class 1 antiarrhythmic use | 4 (0.2) |
| Amiodarone use | 4 (0.2) |
| Digoxin use | 3 (0.2) |
Values are mean±SD or n (%). BMI indicates body mass index; LA, left atrium; LAE, left atrial enlargement; LV, left ventricle; MELD, Model for End‐Stage Liver Disease; TSH, thyroid‐stimulating hormone.
Including pre‐existing AF at the time of evaluation, overall prevalence of AF among this population was 11.2% (confidence interval [CI], 9.8–12.7). AF prevalence by final MELD score (Figure 1) was 3.7% in the lowest MELD quartile and 20.2% in highest quartile. Freedom from AF (Figure 2) decreased over time with 8.5% of patients experiencing new‐onset AF over the median follow‐up period. Incidence of new‐onset AF by MELD categories (1–10, 11–20, and 21–30) over a 12‐month period is shown in Figure 3. There was a trend of increasing incidence of AF as MELD severity increased (P=0.056). Of note, patients in the highest MELD group (>30) had a very short median follow‐up time (<1 month) attributed to either death or LT.
Figure 1.
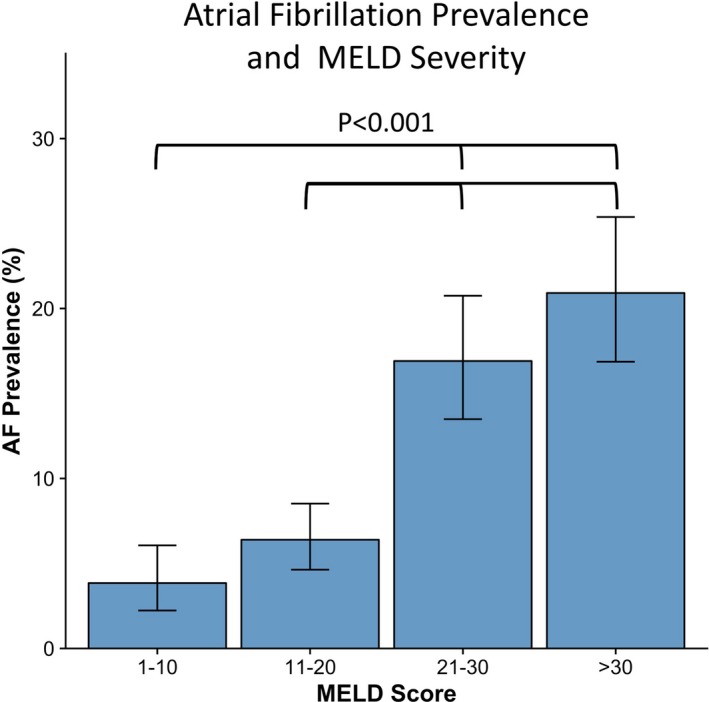
Atrial fibrillation prevalence and MELD severity. Prevalence of AF is significantly higher in patients with higher MELD values. 95% confidence intervals are represented by error bars and significant comparisons between MELD groups are noted. AF indicates atrial fibrillation; MELD, Model for End‐Stage Liver Disease.
Figure 2.
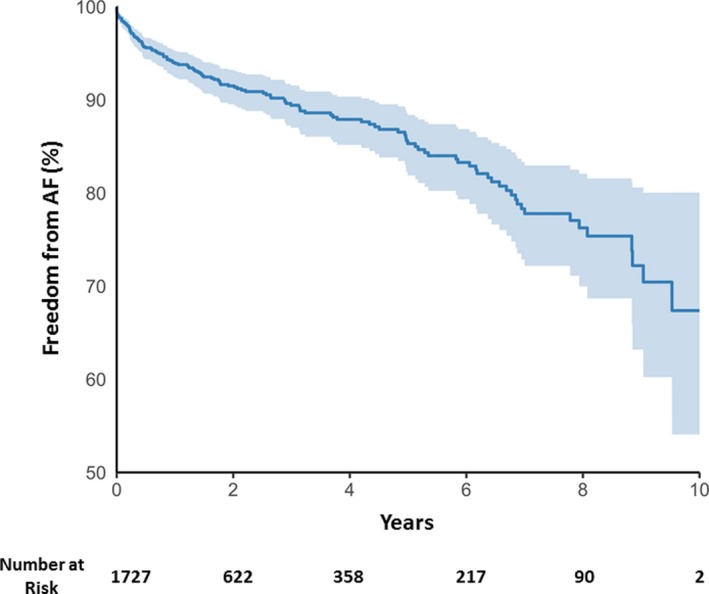
Freedom from AF over time in the entire population Kaplan–Meier curve for freedom from AF over time in the entire population is shown. AF indicates atrial fibrillation.
Figure 3.
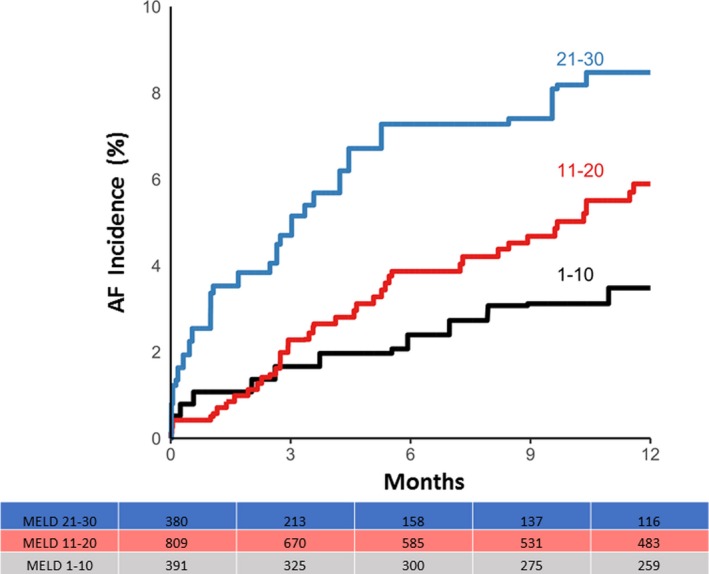
AF incidence and liver disease severity. Kaplan–Meier curves for AF incidence over time by different MELD quartiles are shown. AF incidence increases with increasing severity of liver disease. AF indicates atrial fibrillation; MELD, Model for End‐Stage Liver Disease.
Predictors of New‐Onset AF
On univariable analysis (Table S2), variables commonly associated with AF, including age, severe valvular heart disease, hemodynamic instability, LVEF, LA enlargement, and sleep apnea, were significantly associated with new‐onset AF. Therefore, these variables, in addition to categories of MELD score, were included in the final multivariable analysis, which included: age, diabetes mellitus, hemodynamic instability, hypertension, LA enlargement, LV hypertrophy, MELD score groups (1–10, 11–20, 21–30, and >30), portal hypertension, LVEF, sleep apnea, and valvular heart disease. Patient characteristics by occurrence of new‐onset AF are shown in Table S3.
Using multivariable time‐dependent Cox proportional hazard regression analysis (Figure 4), increasing severity of MELD was independently associated with new‐onset AF. Specifically, as compared with patients in the lowest quartile (MELD 1–10), those in the MELD group of 11 to 20 had a hazard ratio (HR) of 2.73 (CI, 1.47–5.07), those with MELD score of 21 to 30 had an HR of 5.41 (CI, 2.77–10.55), and those in the highest quartile (MELD >30) had an HR of 9.33 (CI, 3.93–22.14) for new‐onset AF. Other variables that were significantly associated with new‐onset AF were age (HR=1.06 [CI, 1.04–1.08]), sleep apnea (HR=2.2 [CI, 1.02–4.61]), severe valvular heart disease (HR=3.21 [CI, 1.68–6.11]), hemodynamic instability (HR=2.00 [CI, 1.24–3.25]), and reduced LVEF (HR=2.44 [CI, 1.44–4.14]). Based on the Cox proportional hazard model, a predicted freedom from AF curve was constructed for the quartiles of MELD scores and is also shown in Figure 4. Similar results were obtained with the Cox proportional hazard model using available (nonimputed) data (Table S4).
Figure 4.
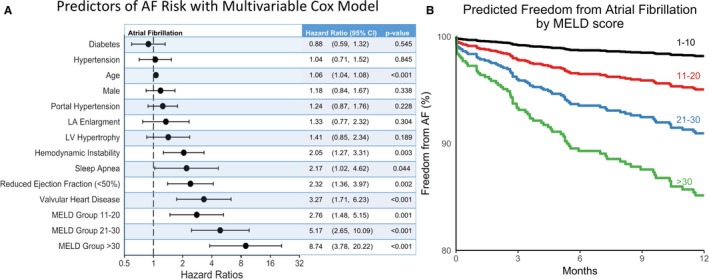
Multivariable assessment of factors associated with new‐onset AF. A, Multivariable Cox proportional hazard model was used to assess the effect of increasing MELD quartiles on new‐onset AF. On multivariable analysis, each increase in MELD quartile was significantly and independently associated with new‐onset AF after adjusting for other variables. For hazard ratios of MELD quartiles, MELD score of 1 to 10 was used as reference. B, Predicted 1‐year freedom from AF curves by the different MELD quartiles using the results from the multivariable time‐dependent Cox model. All variables other than MELD were held at their median values. AF indicates atrial fibrillation; CI, confidence interval; Diabetes, diabetes mellitus; EF, ejection fraction; LA, left atrial; LV, left ventricular; MELD, Model for End‐Stage Liver Disease.
With regard to stroke, rate of stroke was 1.6 events per 100 years of follow‐up in patients with AF (ischemic stroke rate of 1.3% and hemorrhagic stroke rate of 0.6%). CHA2DS2‐VASc score in these patients was 1.1±1.0, likely explaining the low incidence of stroke in this patient population with otherwise low comorbidities. Risk of stroke (both ischemic and hemorrhagic) did significantly increase with increasing MELD score (P<0.001; Tables S5 and S6).
Discussion
Major Findings
In this large, retrospective study, incidence and prevalence of AF in liver disease was high and severity of liver disease, as measured by MELD, was found to be a novel risk factor for new‐onset AF, after adjusting for other traditional risk factors.
End‐Stage Liver Disease and AF
Although many variables play a role in the pathogenesis of AF, liver disease has not been traditionally viewed as 1 of these risk factors. The few studies that have evaluated prevalence and risk of developing AF in patients with liver disease have shown mixed results. Mwalitsa et al evaluated 335 patients with cirrhosis and found that, over 24 months of follow‐up, there was no association between cirrhosis or MELD score and new‐onset AF. It is possible that the small sample size of this study and the method used for verifying AF could have underestimated the incidence of AF in this population.7 In another study of 1302 cirrhotic patients, incidence of AF was found to be very low at 0.15%, and this study suggested that liver disease may be protective against AF. However, in this study, disease severity was not assessed and intermittent ECGs were used for verification of AF diagnosis, leading to underdiagnosis of asymptomatic episodes.13 On the other hand, Lee et al. focused on new‐onset AF and found a 46% relatively higher risk of AF in cirrhotic patients compared with noncirrhotic patients.6 Given the small number and controversial results of these previous studies, we hypothesized that severity of liver disease, as assessed by MELD score, would be independently associated with new‐onset AF. We found that in our population of 1727 patients, prevalence of AF was high (11.2%) and increasing liver disease severity was associated with an increasing HR for new‐onset AF, even after adjusting for other AF risk factors. Importantly, prevalence of AF in our study was higher than previously reported studies. This may be attributed to the fact that all patients in this study were followed by a cardiologist as part of their disease workup, which may have increased the detection of AF. In this study, we focused on analyzing the effect of liver disease on developing new‐onset AF preceding transplantation. Perioperative AF, which can be associated with inflammation, complications at the time of surgery, and blood loss, has been previously shown to be associated with worse clinical outcomes acutely. Predictors of perioperative AF included MELD score and previous history of AF.14
Clinical Implications
Patients with liver disease have been traditionally viewed as having low cardiac comorbidities, with a focus placed on treatment of associated renal dysfunction and coagulopathy. A previous study had even suggested that liver disease may play a protective role against the development of AF.13 Our study suggests that patients with cirrhosis are at increased risk of AF, with increasing risk of developing AF as their MELD score rises. Severity of liver disease was independently associated with increased risk of AF after adjusting for other traditional cardiac and noncardiac risk factors, such as sleep apnea. We noted an AF prevalence of 20.2% in patients with the highest MELD category with a new AF incidence of 8.5% at 12 months of follow‐up in the overall population. Mean age of our population was only 54.4±12 years. Based on a study by Wilke et al looking at 8.3 million people in Germany, prevalence of AF has been reported to be between 1% and 2% in men (ages 50–55 and 55–59 years, respectively) and 0.4% to 0.8% in women (ages 50–55 and 55–59 years, respectively) in the general population.15 Incidence of AF in the same study was 0.3% and 0.5% in men (ages 50–55 and 55–59 years, respectively) and 0.12% to 0.26% in women (ages 50–55 and 55–59 years, respectively).15 Prevalence and incidence of AF were much higher in our patient population with liver disease, despite the fact that many of the previously reported historical controls had presence of cardiac risk factors for AF, such as hypertension and heart failure.15 High prevalence of AF, particularly as liver disease progresses, suggests that both cardiologists and hepatologists should be aware of the emerging risk of AF, inquire about arrhythmia symptoms, and consider periodic evaluations with ECGs and ambulatory monitors.
Higher prevalence of AF as well as increasing incidence of AF with increasing severity of liver disease bring to the forefront the question of benefit versus risk of anticoagulation. The benefit of anticoagulation for ischemic stroke prevention in patients with liver disease and AF remains unknown. Few studies have evaluated the benefit of anticoagulation systematically or in a randomized fashion. Kuo et al evaluated liver disease patients with CHA2DS2‐VASc score ≥2 in the setting of AF and showed a reduction in ischemic stroke events in patients treated with warfarin compared with those with and without antiplatelet therapy, notably without increased intracranial hemorrhage.16 However, an increased risk of hemorrhagic stroke and intracranial hemorrhage has been reported in patients with cirrhosis as compared with those without,17, 18 and in 1 study was reported to be higher than ischemic stroke risk.19 A tailored strategy has been proposed and includes anticoagulation in the setting of less‐severe liver disease, while refraining from anticoagulation in more severe cirrhotic patients with AF.20 The results of our study, demonstrating a high prevalence of AF and increasing incidence of AF in the setting of more‐advanced liver disease, highlight the need for better designed studies in this specific population to define the benefit and risk of anticoagulation, particularly with the advent of novel oral anticoagulants. It is possible that patients at high risk of bleeding and stroke may also benefit from LA appendage closure devices, a question that could not be assessed in our study, given the low overall CHA2DS2‐VASc score and low incidence of comorbidities in this population.
It has been suggested that AF is associated with worsened outcomes in the perioperative period and immediate postoperative period in patients undergoing LT.21, 22 Given the findings of this study, AF is likely a marker of illness severity rather than a major cause of poor outcomes.
Potential Mechanisms Behind Development of AF in Cirrhosis
Liver disease can directly or indirectly influence the pathogenesis of AF. The autonomic nervous system is known to play a significant role in development of AF.5 Abnormal autonomic neurotransmission, with increased parasympathetic and sympathetic activation, is associated with AF.23 Vagal afferent and efferent fibers also innervate the portal vein, hepatic arteries, and biliary ducts in addition to liver parenchyma.24 Specifically, autonomic dysfunction has been observed in the setting of portal hypertension1, 2, 3 and nonalcoholic fatty liver disease,25 and denervation of liver parenchyma has been observed in cirrhosis.26 Cardiac metaiodobenzylguanidine studies have shown an increased washout rate consistent with abnormal myocardial norepinephrine reuptake in patients with cirrhosis. In addition, patients with cirrhosis have been shown to have significantly decreased heart rate variability and baroreflex sensitivity,27 with increased prevalence of abnormal heart rate variability with increasing severity of liver disease.28 Finally, improvements in autonomic indices are observed post‐LT.29
Cirrhosis can also lead to increased levels of neuropeptides that have been shown to play a role in development of AF. Vasoactive intestinal peptide is increased in the setting of cirrhosis, possibly attributed to decreased turnover by the liver.30, 31 Vasoactive intestinal peptide has also been implicated in the onset of vagally mediated AF.32, 33 Whereas low and moderate levels of vagal nerves stimulation have been shown to be protective in preventing AF, vasoactive intestinal peptide is coreleased with acetylcholine during high‐level vagal nerve stimulation, shortening action potential duration, increasing atrial action potential duration spatial heterogeneity, and causing intra‐atrial conduction block.33, 34 Another potential mechanism behind the elevated risk of AF in the setting of cirrhosis is the elevated levels of galectin‐3 observed in patients with liver disease.35, 36 Galectin‐3 is implicated in fibrosis of both the liver and heart, and associated with increased incidence of AF.37, 38 Other potential mediators that share the common pathway of fibrosis and could play a role include transforming growth factor beta39 and connective tissue growth factor.40 Also, many inflammatory cytokines, such as interleukin‐6, interleukin‐8, and tumor necrosis factor‐alpha,41 and oxidative radicals, such as superoxides and peroxynitrite,42 are elevated in the setting of significant liver disease and also play a role in development of AF.43, 44, 45 Finally, many circulatory cytokines and neuropeptides that play a role in AF are upregulated by the autonomic dysfunction that occurs in liver disease.46
Limitations
This study is a single‐center retrospective study and represents outcomes at a specialized center for evaluation of patients with liver disease. To best capture AF prevalence, patients with cirrhosis undergoing evaluation for LT were selected, because this group at our center undergoes extensive and rigorous cardiac evaluation regardless of cardiac history. Focusing efforts on this population, however, may create a bias toward patients who do not have a significant burden of comorbidities that would preclude consideration for transplantation. However, using a population with less comorbidity allows for a more accurate assessment of the impact of liver disease on development of AF, the primary goal of this study. Using International Classification of Diseases, Ninth Revision (ICD‐9) billing code data to identify comorbidities associated with AF, such as sleep apnea, has limitations. However, in this study, many of the risk factors previously reported to be associated with AF continued to be statistically significant in our multivariable model. Of note, in this study, manual chart review was performed to ensure that all AF diagnoses had also been documented on physician notes, ECGs, and ambulatory monitors in addition to ICD‐9 codes. Finally, although we performed a review of all available clinical data to verify the diagnosis of AF, it is possible that patients with asymptomatic AF were overlooked, and therefore, despite the higher prevalence of AF observed in this study, it may yet represent a conservative estimate as to the true prevalence of AF in this population. Finally, we noted a higher prevalence and incidence of AF in patients with liver disease as compared with previous studies that reported the prevalence and incidence of AF by age in the general population. However, we did not have access to data of similar age‐/sex‐matched controls without liver disease with similar CHA2DS2‐VASc scores to show a lower incidence of AF in a similar population without liver disease.
Conclusions
Prevalence of AF in patients with liver disease is notable. Incidence of AF increases with increasing severity of liver disease, which is independently associated with new‐onset AF, even after adjusting for other common risk factors. This finding may provide additional mechanistic insight into the pathogenesis of AF, which shares the underlying link of autonomic dysfunction and inflammation, known to play a role in development of AF. Given the prevalence of AF in this population, cardiologists and hepatologists should consider screening for symptoms and signs of AF in patients with liver disease, particularly in patients with higher MELD values. The results also highlight the need for future well‐designed studies to evaluate risks and benefits of anticoagulation in the setting of liver disease.
Sources of Funding
This work was supported by the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124 and NIH1DP21 DP2 HL132356‐01 to Vaseghi.
Disclosures
None.
Supporting information
Table S1. Percentage of Patients With Missing Variables
Table S2. Univariable Analysis
Table S3. Patient Characteristics With and Without Atrial Fibrillation
Table S4. Multivariable Cox Proportional Hazard Ratios Obtained Using Nonimputed Data to Evaluate Factors Associated With New‐Onset AF
Table S5. Ischemic Stroke Risk by MELD Category
Table S6. Hemorrhagic Stroke Risk by MELD Category
(J Am Heart Assoc. 2018;7:e008703 DOI: 10.1161/JAHA.118.008703.)
References
- 1. Dumcke CW, Moller S. Autonomic dysfunction in cirrhosis and portal hypertension. Scand J Clin Lab Invest. 2008;68:437–447. [DOI] [PubMed] [Google Scholar]
- 2. Rangari M, Sinha S, Kapoor D, Mohan JC, Sarin SK. Prevalence of autonomic dysfunction in cirrhotic and noncirrhotic portal hypertension. Am J Gastroenterol. 2002;97:707–713. [DOI] [PubMed] [Google Scholar]
- 3. Voigt MD, Trey G, Levitt NS, Raine R, Lombard CJ, Robson SC, Gordon G, Kirsch RE. Autonomic neuropathy in extra‐hepatic portal vein thrombosis: evidence for impaired autonomic reflex arc. J Hepatol. 1997;26:634–641. [DOI] [PubMed] [Google Scholar]
- 4. Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064–1070. [DOI] [PubMed] [Google Scholar]
- 5. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee H, Choi EK, Rhee TM, Lee SR, Lim WH, Kang SH, Han KD, Cha MJ, Oh S. Cirrhosis is a risk factor for atrial fibrillation: a nationwide, population‐based study. Liver Int. 2017;37:1660–1667. [DOI] [PubMed] [Google Scholar]
- 7. Mwalitsa JP, Maimone S, Filomia R, Alibrandi A, Saitta C, Caccamo G, Cacciola I, Spinella R, Oliva G, Lembo T, Vadala D, Gambino G, Raimondo G, Squadrito G. Atrial fibrillation in patients with cirrhosis. Liver Int. 2016;36:395–400. [DOI] [PubMed] [Google Scholar]
- 8. AMELIA II: a program for missing data [computer program]. 2009.
- 9. Meng XL, Rubin DB. Performing likelihood ratio tests with multiply‐imputed data sets. Biometrika. 1992;79:103–111. [Google Scholar]
- 10. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 12. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 13. Zamirian M, Sarmadi T, Aghasadeghi K, Kazemi MB. Liver cirrhosis prevents atrial fibrillation: a reality or just an illusion? J Cardiovasc Dis Res. 2012;3:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia VW, Worapot A, Huang S, Dhillon A, Gudzenko V, Backon A, Agopian VG, Aksoy O, Vorobiof G, Busuttil RW, Steadman RH. Postoperative atrial fibrillation in liver transplantation. Am J Transplant. 2015;15:687–694. [DOI] [PubMed] [Google Scholar]
- 15. Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. [DOI] [PubMed] [Google Scholar]
- 16. Kuo L, Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Liao JN, Chung FP, Chen TJ, Lip GYH, Chen SA. Liver cirrhosis in patients with atrial fibrillation: would oral anticoagulation have a net clinical benefit for stroke prevention? J Am Heart Assoc. 2017;6:e005307 DOI: 10.1161/JAHA.116.005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parikh NS, Navi BB, Schneider Y, Jesudian A, Kamel H. Association between cirrhosis and stroke in a nationally representative cohort. JAMA Neurol. 2017;74:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parikh NS, Navi BB, Kumar S, Kamel H. Association between liver disease and intracranial hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu HY, Lin CS, Yeh CC, Hu CJ, Shih CC, Cherng YG, Chen TL, Liao CC. Cirrhosis patients’ stroke risks and adverse outcomes: two nationwide studies. Atherosclerosis. 2017;263:29–35. [DOI] [PubMed] [Google Scholar]
- 20. Lee SJ, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B. The safety and efficacy of vitamin K antagonist in patients with atrial fibrillation and liver cirrhosis. Int J Cardiol. 2015;180:185–191. [DOI] [PubMed] [Google Scholar]
- 21. Bargehr J, Trejo‐Gutierrez JF, Patel T, Rosser B, Aranda‐Michel J, Yataco ML, Taner CB. Preexisting atrial fibrillation and cardiac complications after liver transplantation. Liver Transpl. 2015;21:314–320. [DOI] [PubMed] [Google Scholar]
- 22. Vannucci A, Rathor R, Vachharajani N, Chapman W, Kangrga I. Atrial fibrillation in patients undergoing liver transplantation‐a single‐center experience. Transplant Proc. 2014;46:1432–1437. [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. [DOI] [PubMed] [Google Scholar]
- 24. Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:808–820. [DOI] [PubMed] [Google Scholar]
- 25. Liu YC, Hung CS, Wu YW, Lee YC, Lin YH, Lin C, Lo MT, Chan CC, Ma HP, Ho YL, Chen CH. Influence of non‐alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS One. 2013;8:e61803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JA, Ahmed Q, Hines JE, Burt AD. Disappearance of hepatic parenchymal nerves in human liver cirrhosis. Gut. 1992;33:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moller S, Mortensen C, Bendtsen F, Jensen LT, Gotze JP, Madsen JL. Cardiac sympathetic imaging with mIBG in cirrhosis and portal hypertension: relation to autonomic and cardiac function. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1228–G1235. [DOI] [PubMed] [Google Scholar]
- 28. Dillon JF, Plevris JN, Nolan J, Ewing DJ, Neilson JM, Bouchier IA, Hayes PC. Autonomic function in cirrhosis assessed by cardiovascular reflex tests and 24‐hour heart rate variability. Am J Gastroenterol. 1994;89:1544–1547. [PubMed] [Google Scholar]
- 29. Di Stefano C, Milazzo V, Milan A, Veglio F, Maule S. The role of autonomic dysfunction in cirrhotic patients before and after liver transplantation. Review of the literature. Liver Int. 2016;36:1081–1089. [DOI] [PubMed] [Google Scholar]
- 30. Henriksen JH, Staun‐Olsen P, Borg Mogensen N, Fahrenkrug J. Circulating endogenous vasoactive intestinal polypeptide (VIP) in patients with uraemia and liver cirrhosis. Eur J Clin Invest. 1986;16:211–216. [DOI] [PubMed] [Google Scholar]
- 31. Hunt S, Vaamonde CA, Rattassi T, Berian G, Said SI, Papper S. Circulating levels of vasoactive intestinal polypeptide in liver disease. Arch Intern Med. 1979;139:994–996. [PubMed] [Google Scholar]
- 32. Liu Y, Scherlag BJ, Fan Y, Varma V, Male S, Chaudhry MA, Huang C, Po SS. Inducibility of atrial fibrillation after GP ablations and “autonomic blockade”: evidence for the pathophysiological role of the nonadrenergic and noncholinergic neurotransmitters. J Cardiovasc Electrophysiol. 2013;24:188–195. [DOI] [PubMed] [Google Scholar]
- 33. Xi Y, James Chao ZY, Yan W, Abbasi S, Yin X, Mathuria N, Patel M, Fan C, Sun J, Wu G, Wang S, Elayda M, Gao L, Wehrens XH, Lin SF, Cheng J. Neuronally released vasoactive intestinal polypeptide alters atrial electrophysiological properties and may promote atrial fibrillation. Heart Rhythm. 2015;12:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xi Y, Wu G, Ai T, Cheng N, Kalisnik JM, Sun J, Abbasi S, Yang D, Fan C, Yuan X, Wang S, Elayda M, Gregoric ID, Kantharia BK, Lin SF, Cheng J. Ionic mechanisms underlying the effects of vasoactive intestinal polypeptide on canine atrial myocardium. Circ Arrhythm Electrophysiol. 2013;6:976–983. [DOI] [PubMed] [Google Scholar]
- 35. Gudowska M, Gruszewska E, Cylwik B, Panasiuk A, Rogalska M, Flisiak R, Szmitkowski M, Chrostek L. Galectin‐3 concentration in liver diseases. Ann Clin Lab Sci. 2015;45:669–673. [PubMed] [Google Scholar]
- 36. Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin‐3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. [DOI] [PubMed] [Google Scholar]
- 37. Lippi G, Cervellin G, Sanchis‐Gomar F. Galectin‐3 in atrial fibrillation: simple bystander, player or both? Clin Biochem. 2015;48:818–822. [DOI] [PubMed] [Google Scholar]
- 38. Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, McManus DD, Lubitz SA, Larson MG, Benjamin EJ. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014;167:729–734.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu F, Liu C, Zhou D, Zhang L. TGF‐beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–1079. [DOI] [PubMed] [Google Scholar]
- 41. Martinez‐Esparza M, Tristan‐Manzano M, Ruiz‐Alcaraz AJ, Garcia‐Penarrubia P. Inflammatory status in human hepatic cirrhosis. World J Gastroenterol. 2015;21:11522–11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vairappan B. Endothelial dysfunction in cirrhosis: role of inflammation and oxidative stress. World J Hepatol. 2015;7:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol. 2014;29:20–27. [DOI] [PubMed] [Google Scholar]
- 44. Sovari AA. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract. 2016;2016:9656078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circulation. 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang WA, Shivkumar K, Vaseghi M. Device‐based autonomic modulation in arrhythmia patients: the role of vagal nerve stimulation. Curr Treat Options Cardiovasc Med. 2015;17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of Patients With Missing Variables
Table S2. Univariable Analysis
Table S3. Patient Characteristics With and Without Atrial Fibrillation
Table S4. Multivariable Cox Proportional Hazard Ratios Obtained Using Nonimputed Data to Evaluate Factors Associated With New‐Onset AF
Table S5. Ischemic Stroke Risk by MELD Category
Table S6. Hemorrhagic Stroke Risk by MELD Category


