Abstract
Background
Remote ischemic preconditioning (RIPC) by repeated brief cycles of limb ischemia/reperfusion attenuates myocardial ischemia/reperfusion injury. We aimed to identify a functional parameter reflecting the RIPC‐induced protection in human. Therefore, we measured mitochondrial function in right atrial tissue and contractile function of isolated right atrial trabeculae before and during hypoxia/reoxygenation from patients undergoing coronary artery bypass grafting with RIPC or placebo, respectively.
Methods and Results
One hundred thirty‐seven patients under isoflurane anesthesia underwent RIPC (3×5 minutes blood pressure cuff inflation on the left upper arm/5 minutes deflation, n=67) or placebo (cuff uninflated, n=70), and right atrial appendages were harvested before ischemic cardioplegic arrest. Myocardial protection by RIPC was assessed from serum troponin I/T concentrations over 72 hours after surgery. Atrial tissue was obtained for isolation of mitochondria (RIPC/placebo: n=10/10). Trabeculae were dissected for contractile function measurements at baseline and after hypoxia/reoxygenation (60 min/30 min) and for western blot analysis after hypoxia/reoxygenation (RIPC/placebo, n=57/60). Associated with cardioprotection by RIPC (26% decrease in the area under the curve of troponin I/T), mitochondrial adenosine diphosphate–stimulated complex I respiration (+10%), adenosine triphosphate production (+46%), and calcium retention capacity (+37%) were greater, whereas reactive oxygen species production (−24%) was less with RIPC than placebo. Contractile function was improved by RIPC (baseline, +7%; reoxygenation, +24%). Expression and phosphorylation of proteins, which have previously been associated with cardioprotection, were not different between RIPC and placebo.
Conclusions
Cardioprotection by RIPC goes along with improved mitochondrial and contractile function of human right atrial tissue.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01406678.
Keywords: cardioprotection, contractile function, mitochondria, troponin
Subject Categories: Translational Studies, Myocardial Infarction
Clinical Perspective
What Is New?
Patients undergoing elective cardiac surgery under volatile anesthesia had cardioprotection by remote ischemic conditioning, as reflected by reduced postoperative troponin release.
This cardioprotection was reflected by better mitochondrial and contractile function in their right atrial appendages even before ischemic cardioplegic arrest.
No cardioprotective molecular signal was identified.
What Are the Clinical Implications?
Mitochondria appear to be an important subcellular effector organelle in protection by remote ischemic conditioning.
Mitochondria are therefore essential targets to better understand confounding influences on cardioprotection in patients and also potential targets for pharmacological recruitment of cardioprotection.
Introduction
Remote ischemic conditioning by brief episodes of ischemia/reperfusion in parenchymal organs or limbs before, during, or following sustained myocardial ischemia with subsequent reperfusion protects the myocardium from irreversible ischemia/reperfusion injury. Protection by remote ischemic conditioning has been confirmed in all species tested so far.1 Remote ischemic conditioning by repetitive limb ischemia/reperfusion also reduces myocardial damage in patients undergoing elective interventional2 or surgical coronary revascularization3, 4, 5, 6 and in those with reperfused acute myocardial infarction.7, 8, 9, 10, 11, 12, 13 Cardioprotection was confirmed by reduced release of cardiac biomarkers2, 3, 4, 5, 6, 10 or by increased salvage in cardiac imaging7, 8, 9, 11 but also resulted in improved short‐5, 8 and more long‐term2, 4, 13, 14, 15 clinical outcome in retrospective analyses. Recently, the first prospective, randomized trial on patients with reperfused acute myocardial infarction confirmed improved clinical outcome as a primary end point of remote ischemic conditioning during follow‐up for 3.6 years.13 Even if there is good evidence for cardioprotection by remote ischemic conditioning in patients, not all studies are positive,16 and the underlying signaling is not well understood. Obviously, remote ischemic conditioning induces a systemic response because it protects also organs other than the heart. Various experimental models suggest that myocardial signaling is similar to that by local ischemic conditioning.17 However, the underlying signal transduction is species specific. Whereas in rodents the activation of the reperfusion injury salvage kinase and the survivor activating factor enhancement pathway was identified as causal for cardioprotection by remote ischemic conditioning, in pigs only the activation of the survivor activating factor enhancement pathway was causally involved, as reflected by the phosphorylation of signal transducer and activator of transcription (STAT)3 in left ventricular myocardial biopsies.18, 19, 20
It is currently impossible to analyze the underlying signaling in the myocardium of patients suffering from acute myocardial infarction. However, in cardiac surgery settings, after ethical approval and with patients’ consent, it is possible to obtain human myocardial specimens. In a previous study, we analyzed left ventricular biopsies from patients undergoing coronary artery bypass grafting (CABG) with and without remote ischemic preconditioning. Among the 22 analyzed proteins of the above pathways, none of the previously identified proteins was actually activated in the patient tissue. However, STAT5 phosphorylation was associated with remote ischemic conditioning,21, 22 highlighting again species‐specific signaling differences. Independently of the involved myocardial signaling, mitochondria are viewed as end effectors of cardioprotective strategies.23, 24
We now aimed to identify functional parameters ex vivo, which reflect the remote ischemic conditioning‐induced protection, to further analyze the underlying signaling in humans. Right atrial appendages were obtained during routine cardiac surgery without risk for the patients, and this tissue permitted the preparation of mitochondria and atrial trabeculae for functional measurements. We obtained right atrial appendages from consecutive patients undergoing CABG with remote ischemic preconditioning (RIPC) or placebo, respectively, and myocardial protection was reflected by reduced postoperative release of the cardiac biomarkers, troponin I (TnI) and troponin T (TnT). Ex vivo, we measured mitochondrial function at baseline and contractile function of isolated right atrial trabeculae before and during hypoxia/reoxygenation. In right atrial trabeculae after hypoxia/reoxygenation, western blot analysis was used to detect changes in expression and phosphorylation of established signaling proteins.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The authors declare that all data that support the presented findings are available within the article. When further details are of interest, these are available from the corresponding author upon reasonable request.
Ethics Statement
The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The local Institutional Review Board (08–3683 and 13–5507) approved the study, and patients gave written informed consent. Patients were enrolled between July 2014 and June 2017 within the framework of a larger, randomized, prospective, double‐blind, placebo‐controlled trial (ClinicalTrials.gov NCT01406678). Eligible patients were adults with double‐ or triple‐vessel coronary artery disease, who were scheduled to undergo primary, isolated, elective CABG surgery under cardiopulmonary bypass or combined valve and CABG surgery. Exclusion criteria were preoperative renal insufficiency (serum creatinine concentration >200 μmol/L), peripheral arterial disease affecting the upper limbs, acute coronary syndrome within the previous 4 weeks, inotropic or mechanical circulatory support before induction of anesthesia, any disorder that could potentially increase preoperative serum TnI or TnT concentrations (eg, percutaneous coronary intervention within the previous 6 weeks), coronary surgery without cardiopulmonary bypass, and emergency, repeat, or concomitant surgery.4
Study Procedure
Anesthesia was induced with sufentanil (1 μg/kg), etomidate (0.3 mg/kg), and rocuronium (0.6 mg/kg) and maintained with isoflurane (0.6–1.0% end‐tidal) and sufentanil, as required. We became aware in our initial studies that propofol interferes with RIPC25, 26 and have since performed all our studies with isoflurane anesthesia. The RIPC protocol consisted of 3 cycles of 5‐minute left upper arm ischemia (by inflation of a blood pressure cuff to 200 mm Hg)/5 min reperfusion (cuff deflated). Data were compared to placebo (cuff left deflated for 30 minutes). CABG was performed using median sternotomy, mild systemic hypothermia (>32°C), and antegrade cold crystalloid Bretschneider (Köhler Chemie GmbH, Bensheim, Germany) cardioplegia, with additional topical cooling and single aortic cross‐clamping for all distal anastomoses.4
Serum Troponin I and T
Venous blood samples were drawn before and at 1, 6, 12, 24, 48, and 72 hours after surgery for measurement of serum TnI or TnT concentrations. The serum TnI concentration was measured using a specific 2‐sided immunoassay with the DimensionR RxL MaxR integrated system (Dimension Flex; Dade Behring GmbH, Marburg, Germany) in the central laboratory of the University Essen Medical School. The detection range of TnI was 0.04 to 40 μg/L, with the upper limit of normal 0.1 μg/L. The serum TnT concentration was measured using an electrochemiluminescence immunoassay with the Cobas e 801 immunoassay analyzer (Roche, Basel, Switzerland) in the laboratory of the Herzchirurgie Essen‐Huttrop. The detection range of TnT was 0.003 to 10 μg/L, with the upper limit of normal 0.1 μg/L. The area under the curve for serum TnI and TnT concentrations was calculated according to the trapezoidal rule. Missing values were replaced by linear inter‐ and extrapolation.4 For comparison, serum TnI and TnT concentrations were normalized to their respective 99th percentile of the reference population (TnI, 0.07 μg/L; TnT, 0.014 μg/L).
Right Atrial Appendages
Right atrial appendages (n=137; 424±30 mg; mean±SEM) were obtained at the onset of the cardiopulmonary bypass procedure 10 to 15 minutes after the last RIPC cycle or placebo, respectively, from the cannulation site (right atrium), placed in cardioplegic buffer (mmol/L: 100 NaCl, 10 KCl, 5 MgSO4·7H2O, 1.2 KH2PO4, 50 taurine, 5 MOPS, and 22 glucose) and immediately transported to the laboratory. From these right atrial appendages, mitochondria were isolated for functional measurements (n=20), atrial trabeculae dissected for contractile function measurements (n=74), or proteins extracted for western blot analysis (n=50; Figure 1).
Figure 1.
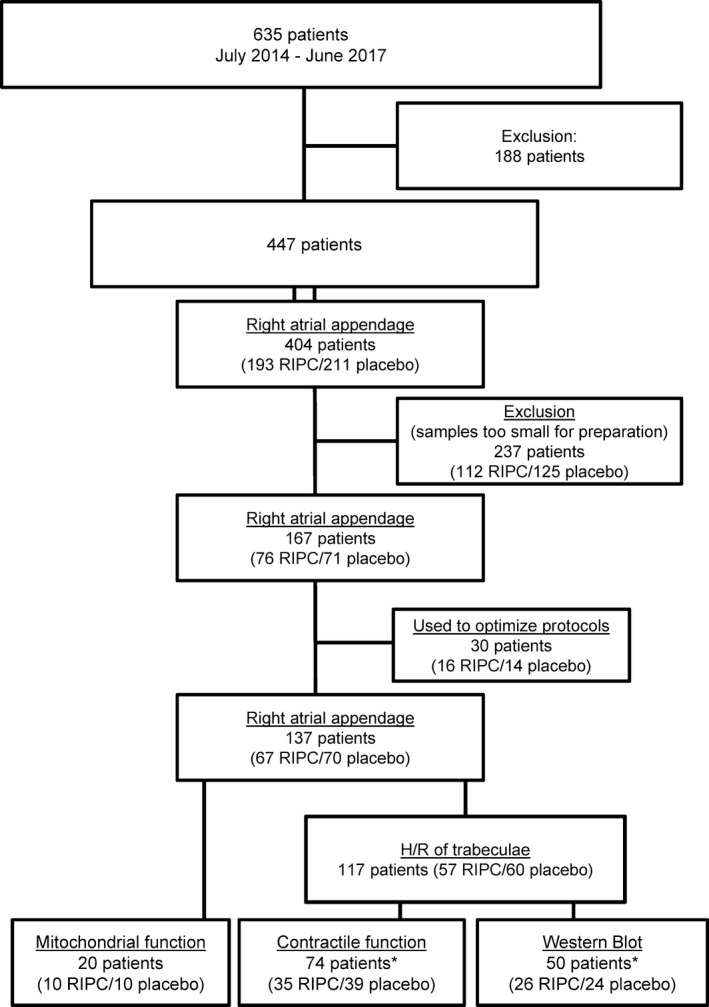
Flow chart. *Atrial specimens from 4 RIPC and 3 placebo patients were analyzed for both contractile function and protein expression and phosphorylation by western Blot. H/R indicates hypoxia/reoxygenation; RIPC, remote ischemic preconditioning.
Mitochondrial Function
Mitochondrial isolation
All procedures were performed on ice or at 4°C. Right atrial appendages of patients with RIPC (n=10) or placebo (n=10), respectively, were placed in ice‐cold isolation buffer (pH 7.4), containing in mmol/L: 250 sucrose, 10 HEPES, and 1 EGTA, with 0.5% (w/v) BSA. Right atrial appendages were minced thoroughly using scissors and then homogenized with a tissue homogenizer (Ultra‐Turrax; IKA, Staufen, Germany) using two 10‐second treatments at a shaft rotation rate of 6500 rpm to release subsarcolemmal mitochondria. Further homogenization with a Teflon pestle in the presence of proteinase type XXIV (2‐IU/g tissue weight) then released interfibrillar mitochondria.27 The homogenate containing subsarcolemmal and interfibrillar mitochondria was centrifuged at 700g for 10 minutes. The supernatant was collected and centrifuged at 14 000g for 10 minutes. The resulting pellet was resuspended in isolation buffer without BSA and centrifuged at 10 000g for 5 minutes. The last procedure was repeated, and the pellet was resuspended in 50 μL of isolation buffer.28 Protein concentration of the resuspended pellet was determined using a protein assay (Lowry method; Bio‐Rad, Hercules, CA) with BSA as the standard (Thermo Scientific, Waltham, MA).
Mitochondrial respiration
Oxygen consumption was measured with a Clark‐type electrode (Strathkelvin, Glasgow, UK) at 37°C during magnetic stirring in incubation buffer, containing in mmol/L: 125 KCl, 10 MOPS, 2 MgCl2, 5 KH2PO4, and 0.2 EGTA, with 5 glutamate and 5 malate as substrates for complex I. The electrode was calibrated using the solubility coefficient of 217 nmol of O2/mL at 37°C. For the measurement of complex I respiration, suspended mitochondria (corresponding to a protein mass of 50 μg) were added to 0.5 mL of incubation buffer. After 2 minutes, ADP (0.4 mmol/L) was added, and ADP‐stimulated respiration was measured over 2 to 3 minutes.
Then, mitochondria were used to either measure complex IV respiration and maximal uncoupled oxygen uptake in the respiration chamber, or incubation buffer containing mitochondria was taken from the respiration chamber to measure ATP or reactive oxygen species (ROS) production, respectively.
Complex IV respiration was stimulated by adding TMPD (300 μmol/L) and ascorbate (3 mmol/L), which donates electrons to cytochrome oxidase through reduction of cytochrome c. Maximal uncoupled oxygen uptake was measured in the presence of 30 nmol/L of FCCP.28
Mitochondrial ATP production
After measurement of ADP‐stimulated respiration, the incubation buffer containing mitochondria was taken from the respiration chamber and immediately supplemented with ATP assay mix (diluted 1:5; Sigma‐Aldrich, St. Louis, MO). Mitochondrial ATP production was determined immediately and compared with ATP standards using a 96‐well white plate and a Cary Eclipse spectrophotometer (Varian, Mulgrave, Victoria, Australia) at 560 nm emission wavelength.28
ROS production
The Amplex Red Hydrogen Peroxide Assay (Life Technologies, Carlsbad, CA) was used to determine ROS concentration in the extramitochondrial space. Amplex Red reacts in a 1:1 stoichiometry with peroxides under catalysis by HRP and produces highly fluorescent resorufin. The incubation buffer containing mitochondria was removed from the respiration chamber and immediately supplemented with 50 μmol/L of Amplex UltraRed and 2 U/mL of HRP. The supernatant was collected after 120‐minute incubation in the dark. ROS concentration was determined and compared with H2O2 standards using a 96‐well black plate and a Cary Eclipse fluorescence spectrophotometer (Varian) at excitation and emission wavelengths of 540 and 580 nm, respectively.28
Calcium retention capacity
Calcium retention capacity was determined using suspended mitochondria (corresponding to a protein mass of 100 μg) in 1 mL of calcium retention capacity buffer containing in mmol/L: 125 KCl, 10 MOPS, 2 MgCl2, and 5 KH2PO4, with 5 glutamate and 5 malate as substrates in the presence of ADP (0.4 mmol/L) at 37°C. Calcium green‐5N (0.5 μmol/L; Life Technologies) was used to measure extramitochondrial calcium concentration in a spectrophotometer (Cary Eclipse; Varian) at excitation and emission wavelengths of 500 and 530 nm, respectively. Pulses of 10 nmol CaCl2 were added every minute until a rapid increase in calcium green fluorescence indicated mitochondrial permeability transition pore (mPTP) opening.28 Cyclosporine A delays mPTP opening by interaction with cyclophilin D to keep the pore closed.29 Therefore, additional measurements were performed in the presence of cyclosporine A (10 μmol/L).
Hypoxia/Reoxygenation of Right Atrial Trabeculae
Atrial trabeculae (≥3 mm length, ≤1 mm diameter) were immediately dissected from the right atrial appendages of RIPC (n=35) and placebo (n=39) patients and kept at 4°C in cardioplegic buffer.
Measurements of force of contraction were performed in a modified 4‐chamber myograph (Multi Wire Myograph System 610M; DMT A/S, Aarhus, Denmark). For electrical isolation, polyvinyl chloride inserts, covering the bottom and sides of the chambers, respectively, were inserted. Electrical field stimulation was facilitated by inserting platinum wires through the acrylic glass chamber covers, which were then attached to external electrodes. A tube was inserted into a small aperture of the chamber to change the gas supply in the solution.
Right atrial trabeculae were mounted between a fixed and a mobile steel hook, which was attached to a force transducer and suspended in modified, oxygenated (95% O2/5% CO2) Tyrode's solution (mmol/L: 118.5 NaCl, 4.8 KCl, 24.8 NaHCO3, 1.2 KH2PO4, 1.44 MgSO4·7H2O, 1.8 CaCl2·2H2O, 10 glucose, and 10 Na pyruvate) at 37°C.
Right atrial trabeculae were electrically paced with rectangular pulses (5‐ms) field stimulation at 1 Hz and then gradually prestretched to their maximal force of contraction, as previously described.30, 31 Maximal force of contraction was reached when further stretching did not cause greater force of contraction.31 Thereafter, right atrial trabeculae stretch was reduced to 90% of the length with such maximal force, as previously described.32 Force of contraction was continuously recorded and analyzed using LabChart (Powerlab 8/30; A&D Instruments Ltd, Abington, UK). Electrically initiated developed force of contraction (mN) was measured at baseline (95% O2/5% CO2) for 12 minutes. Right atrial trabeculae that did not reach a minimum force of contraction of 4 mN at baseline were excluded. Hypoxia was then induced with glucose‐free Tyrode's hypoxia buffer (mmol/L: 118.5 NaCl, 4.8 KCl, 18.5 NaHCO3, 1.2 KH2PO4, 1.44 MgSO4·7H2O, 1.8 CaCl2·2H2O, and 7 choline chloride) by changing the gas supply (95% N2/5% CO2) and by increasing the stimulation rate (3 Hz) for 60 minutes. Following hypoxia, baseline conditions for reoxygenation were re‐established for 30 minutes. Following reoxygenation, force of contraction was measured in the presence of isoproterenol (10 μmol/L) for 10 minutes. Length and width of relaxed trabeculae were recorded at the end of each experiment, and specimens were then weighed. As described before, the cross‐sectional area of trabeculae was calculated by dividing muscle mass by length times density, assuming a cylindric shape and a density of 1.0 mg/mm3. To ensure that comparisons of developed force of contraction were not affected by variable muscle size, the cross‐sectional area of the right atrial trabeculae was used to calculate the developed contractile stress in mN/mm2.33 Additionally, the developed contractile stress was expressed as a percent of baseline.
For western blot analysis, hypoxia/reoxygenation of right atrial trabeculae from patients with RIPC (n=26) and placebo (n=24), respectively, was performed in the absence of isoproterenol. At the end of reoxygenation, specimens were snap‐frozen in liquid nitrogen and stored at −80°C until use.
Western Blot Analysis
After hypoxia/reoxygenation, right atrial trabeculae (RIPC/placebo, n=26/24) were homogenized in 100 mMol/L of Tris (Sigma‐Aldrich) with 2% SDS (w/v; SERVA Electrophoresis, Heidelberg, Germany), heated at 70°C for 5 minutes, and centrifuged for 10 minutes at 14 000g. Protein concentration of the supernatant was determined using the Lowry method (Bio‐Rad, Munich, Germany). Precasted SDS‐PAGEs (Bio‐Rad) were loaded with 10 μg of total protein per lane. Proteins were separated by electrophoresis and transferred to PVDF membranes (Bio‐Rad). Membranes were stained with Ponceau S solution (SERVA) and documented. After blockade with fat‐free milk (Bio‐Rad), membranes were incubated with antibodies directed against phospho‐protein kinase B (AKT1/2/3)ser473, phospho‐endothelial nitric oxide synthase (eNOS)ser1177, phospho‐eNOSthr495, phospho‐extracellular signal regulated kinase (ERK)1thr202/tyr204/ERK2thr185/tyr187, glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), phospho‐glycogen synthase kinase 3β (GSK3β)ser9, phospho‐p38 mitogen‐activated protein kinase (MAPK)thr180/tyr182, protein kinase C epsilon (PKCε), phospho‐STAT3tyr705, and phospho‐STAT5tyr694 (Table 1) overnight at 4°C.34 After incubation with secondary antibodies, immunoreactive signals were detected by chemiluminescence (Pierce Biotechnology, Rockford, IL) using a charge‐coupled device camera and quantified with LabImage 1‐D software (INTAS, Göttingen, Germany). After detection of the phosphorylated forms of each protein, membranes were stripped and reprobed with antibodies directed against the total form of the respective proteins. Membranes were visually analyzed after Ponceau S staining for equal protein loading. Immunoreactivity of a phosphorylated protein was normalized to that of the respective total form. Immunoreactivity of PKCε was normalized to GAPDH. Furthermore, immunoreactivities of all total proteins were normalized to Ponceau S staining, respectively. For normalization, 2 samples were loaded onto all gels, and the numerical chemiluminescence signals from the phosphorylated and total forms of all analyzed proteins, respectively, were set to be identical on each membrane. Signal intensity of each sample was then normalized by multiplication with the reciprocal value of the sum of the 2 respective signal intensities of the normalization samples.34
Table 1.
Antibodies for Western Blot Analysis
| Kinase/Phosphorylation Site | Manufacturer | Order No. | Antibody Dilution | Source | Clonality |
|---|---|---|---|---|---|
| Phospho‐AKT1/2/3ser473 | Cell Signaling | #9271 | 1:500 | Rabbit | Polyclonal |
| AKT1/2/3 | Cell Signaling | #9272 | 1:500 | Rabbit | Polyclonal |
| Phospho‐eNOSser1177 | Cell Signaling | #9571 | 1:500 | Rabbit | Polyclonal |
| eNOS | BD Biosciences | #610296 | 1:500 | Mouse | Polyclonal |
| Phospho‐ERK1thr202/tyr204Phospho‐ERK2thr185/tyr187 | Cell Signaling | #9101 | 1:1000 | Rabbit | Polyclonal |
| ERK1/2 | Cell Signaling | #9102 | 1:1000 | Rabbit | Polyclonal |
| GAPDH | HyTest | #5G4 | 1:500 | Mouse | Monoclonal |
| Phospho‐GSK‐3βser9 | Cell Signaling | #9336 | 1:500 | Rabbit | Polyclonal |
| GSK‐3β | BD Biosciences | #610202 | 1:500 | Mouse | Polyclonal |
| Phospho‐p38 MAPKthr180/tyr182 | Cell Signaling | #9211 | 1:750 | Rabbit | Polyclonal |
| p38 MAPK | Cell Signaling | #9212 | 1:1000 | Rabbit | Polyclonal |
| PKCε | Santa Cruz Biotechnology | #sc‐214 | 1:500 | Rabbit | Polyclonal |
| Phospho‐STAT3tyr705 | Cell Signaling | #9138 | 1:750 | Mouse | Monoclonal |
| STAT3 | Cell Signaling | #12640 | 1:500 | Rabbit | Monoclonal |
| Phospho‐STAT5tyr694 | Cell Signaling | #9359 | 1:250 | Rabbit | Monoclonal |
| STAT5 | Cell Signaling | #9363 | 1:500 | Rabbit | Polyclonal |
AKT indicates protein kinase B; eNOS, endothelial nitric oxide synthase; ERK1/2, extracellular signal‐regulated kinase 1/2; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; GSK3β, glycogen synthase kinase 3β; MAPK, p38 mitogen‐activated protein kinase; PKCε, protein kinase C epsilon; STAT, signal transducer and activator of transcription.
Statistical Analysis
Data are presented as mean±SEM. To identify potential sex‐specific effects, we present data of the entire cohort and in addition separately for males and females, respectively. Data on mitochondrial function and western blot data are presented in box plots. Statistics were performed using SigmaStat software (SigmaStat 2.03; SPSS Inc, Chicago, IL). Patient demographics and intraoperative characteristics were compared between RIPC and placebo using unpaired Student t test (continuous data) and 2‐tailed Fisher's exact test (categorical data). Serum TnI/TnT of patients was analyzed by 2‐way (group, time) ANOVA for repeated measures. The area under the curve for serum TnI/TnT over 72 hours was compared between RIPC and placebo by unpaired Student t test. Differences in developed contractile stress between right atrial trabeculae from patients with RIPC and with placebo were analyzed by 2‐way (group, time) measures ANOVA. Whenever an interactive effect was present, the analysis was followed by Fisher's test of least significant difference. Mitochondrial respiration, ATP production, ROS production and calcium retention capacity, as well as western blot data were compared between RIPC and placebo by unpaired Student t test. Differences were considered significant at the level of P<0.05.
Results
Patient Characteristics
As expected, enrolled patients were 76% men and 24% women. Demographics and intra‐ and postoperative characteristics were not different between patients with RIPC and placebo, respectively (Table 2). These findings are in line with our previous study in patients undergoing elective bypass surgery,4 where RIPC had no impact on intensive care unit and hospital stay, but reduced the release of troponin I. In this previous study, short‐ and long‐term clinical outcome was improved.4, 15 Preoperative serum TnI/TnT concentration did not differ between patients with RIPC and placebo, respectively. Serum TnI/TnT concentration area under the curve over 72 hours was less with RIPC than with placebo, reflecting cardioprotection (234±21 versus 316±31 TnI/TnT 1000× fold of reference 99th percentile × 72 hours; P=0.033; Figure 2). In males, cardioprotection by RIPC was reflected by reduced troponin release (219±23 versus 318±34 TnI/TnT 1000× fold of reference 99th percentile × 72 hours, P=0.021), whereas in females there was only a small reduction of troponin release (275±44 versus 307±76 TnI/TnT 1000× fold of reference 99th percentile × 72 hours; P=0.709).
Table 2.
Demographics and Intra‐ and Postoperative Characteristics
| RIPC (n=67) | Placebo (n=70) | P Value | RIPC (n=49) | Placebo (n=55) | P Value | RIPC (n=18) | Placebo (n=15) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | |||||||
| Demographics | |||||||||
| Age, y | 70±1 | 68±1 | 0.309 | 69±1 | 67±1 | 0.279 | 73±2 | 74±2 | 0.698 |
| Sex, male | 49 (73%) | 55 (79%) | 0.549 | 49 (100%) | 55 (100%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
| Body weight, kg | 84±2 | 87±2 | 0.326 | 88±2 | 91±2 | 0.322 | 73±3 | 71±3 | 0.638 |
| Risk factors and comorbidities | |||||||||
| Diabetes mellitus | 27 (40%) | 18 (26%) | 0.101 | 20 (41%) | 12 (22%) | 0.055 | 7 (39%) | 6 (40%) | 1.000 |
| Hypertension | 59 (88%) | 63 (90%) | 0.789 | 42 (86%) | 50 (91%) | 0.541 | 17 (94%) | 13 (87%) | 0.579 |
| Hyperlipidemia | 26 (39%) | 32 (46%) | 0.460 | 19 (39%) | 22 (40%) | 1.000 | 7 (39%) | 10 (67%) | 0.166 |
| Peripheral arterial disease | 11 (16%) | 10 (14%) | 0.814 | 8 (16%) | 5 (9%) | 0.374 | 3 (17%) | 5 (33%) | 0.418 |
| Chronic obstructive pulmonary disease | 8 (12%) | 11 (16%) | 0.624 | 6 (12%) | 8 (15%) | 0.780 | 2 (11%) | 3 (20%) | 0.639 |
| Renal disease, creatinine >200 μmol/L | 7 (10%) | 4 (6%) | 0.359 | 5 (10%) | 3 (5%) | 0.470 | 2 (11%) | 1 (7%) | 1.000 |
| Cardiac status | |||||||||
| Angina CCS III to IV | 9 (13%) | 10 (14%) | 1.000 | 4 (8%) | 6 (11%) | 1.000 | 5 (28%) | 4 (27%) | 1.000 |
| Previous myocardial infarction | 16 (24%) | 11 (16%) | 0.285 | 13 (27%) | 11 (20%) | 0.285 | 3 (17%) | 0 (0%) | 0.233 |
| Left ventricular ejection fraction, % | 52±1 | 53±1 | 0.673 | 51±1 | 52±1 | 0.398 | 56±2 | 54±2 | 0.606 |
| Medication | |||||||||
| Aspirin | 61 (91%) | 63 (90%) | 1.000 | 47 (96%) | 50 (91%) | 0.442 | 14 (96%) | 13 (87%) | 0.664 |
| Clopidogrel | 5 (7%) | 6 (9%) | 1.000 | 4 (8%) | 5 (9%) | 1.000 | 1 (6%) | 1 (7%) | 1.000 |
| β‐blockers | 57 (85%) | 56 (80%) | 0.503 | 43 (88%) | 42 (76%) | 0.203 | 14 (78%) | 14 (93%) | 0.345 |
| Statins | 45 (67%) | 47 (67%) | 1.000 | 33 (67%) | 39 (71%) | 0.832 | 12 (67%) | 8 (53%) | 0.492 |
| ACE inhibitors or ARBs | 45 (67%) | 45 (64%) | 0.665 | 36 (73%) | 37 (67%) | 0.526 | 9 (50%) | 8 (53%) | 1.000 |
| Risk scores | |||||||||
| Additive EuroSCORE | 5±1 | 4±0 | 0.349 | 5±1 | 4±1 | 0.418 | 5±1 | 5±1 | 0.874 |
| Logistic EuroSCORE, % | 6±1 | 5±1 | 0.656 | 7±1 | 5±1 | 0.515 | 5±1 | 6±2 | 0.547 |
| EuroSCORE II, % | 3±0 | 2±0 | 0.169 | 3±1 | 2±0 | 0.172 | 3±1 | 4±1 | 0.311 |
| Intraoperative characteristics | |||||||||
| Time from end of RIPC/placebo to ischemic cardioplegic arrest, min | 74±14 | 73±10 | 0.930 | 79±14 | 76±12 | 0.862 | 67±21 | 68±13 | 0.973 |
| Time from end of RIPC/placebo to reperfusion, min | 146±12 | 135±7 | 0.436 | 151±14 | 135±8 | 0.328 | 119±5 | 140±13 | 0.238 |
| Aortic cross‐clamp duration, min | 62±3 | 57±3 | 0.425 | 63±4 | 56±3 | 0.213 | 61±7 | 59±6 | 0.899 |
| Cardioplegia, mL | 1569±46 | 1500±28 | 0.195 | 1609±58 | 1497±33 | 0.085 | 1453±58 | 1511±54 | 0.478 |
| Reperfusion time, min | 29±2 | 28±1 | 0.806 | 29±2 | 28±1 | 0.587 | 28±3 | 30±3 | 0.654 |
| Number of bypass grafts | 3±0 | 3±0 | 0.587 | 3±0 | 3±0 | 0.317 | 3±0 | 3±0 | 0.512 |
| Transit time graft flow, mL/min | 73±8 | 81±8 | 0.464 | 81±8 | 80±9 | 0.750 | 58±15 | 64±10 | 0.587 |
| Postoperative characteristics | |||||||||
| ICU/IMC stay, days | 3±1 | 2±0 | 0.069 | 3±1 | 2±0 | 0.150 | 3±1 | 2±0 | 0.220 |
| Hospital stay, days | 9±1 | 10±1 | 0.667 | 9±1 | 9±1 | 0.959 | 9±1 | 11±0 | 0.438 |
| In‐hospital mortality | 1 (1%) | 1 (1%) | 1.000 | 1 (2%) | 1 (2%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
Data are mean±SEM or number (%). Demographics and intra‐ and postoperative characteristics were compared using unpaired Student t test (continuous data) and 2‐tailed Fisher's exact test (categorical data). Reperfusion time: time from release of aortic cross‐clamp to end of cardiopulmonary bypass. ACE indicates angiotensin‐converting enzyme; ARBs, angiotensin‐II receptor blockers; CCS, Canadian Cardiovascular Society score; EuroSCORE, European system for cardiac operative risk evaluation; ICU, intensive care unit; IMC, intermediate care unit; RIPC, remote ischemic preconditioning.
Figure 2.
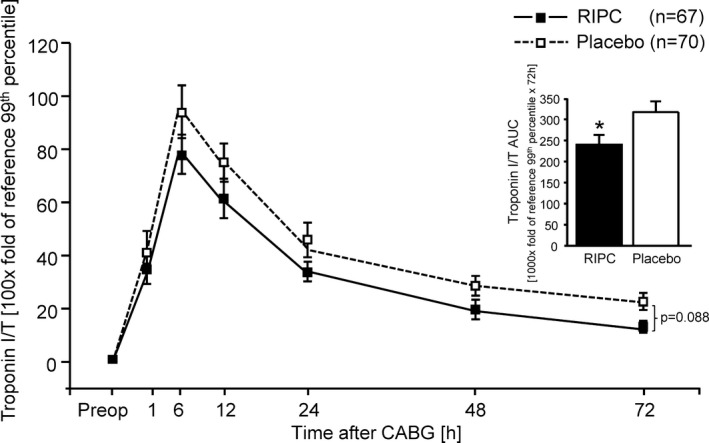
Serum troponin I/T concentrations in peripheral venous blood at baseline before (preop) and over 72 hours after coronary artery bypass graft (CABG) surgery in patients with remote ischemic preconditioning (RIPC; black symbols/bars) or with placebo (white symbols/bars). Decreased serum troponin I/T concentrations confirmed protection by RIPC. Insert: area under the curve (AUC) for serum troponin I/T concentrations over 72 hours. Data are mean±SEM. Serum troponin I/T of patients was analyzed by 2‐way (group, time) ANOVA for repeated measures followed by Fisher's least significant difference test. The AUC for the serum troponin I/T over 72 hours was compared between RIPC and placebo by unpaired Student t test. *P<0.05, RIPC vs placebo.
Mitochondrial Function
Baseline respiration was not different between mitochondria isolated from patients with RIPC and from placebo patients (Figure 3A). ADP‐stimulated complex I respiration was greater with RIPC than with placebo (Figure 3B). Mitochondrial complex IV respiration and maximal oxygen uptake of uncoupled mitochondria were not different between groups, reflecting equal loading of viable mitochondria (Figure 3C and 3D). Mitochondrial ATP production was greater with RIPC than with placebo (Figure 3E). Mitochondrial ROS production was less with RIPC than with placebo (Figure 3F). Calcium retention capacity of mitochondria was better with RIPC than with placebo, with and without cyclosporine A, respectively (Figure 3G and 3H).
Figure 3.
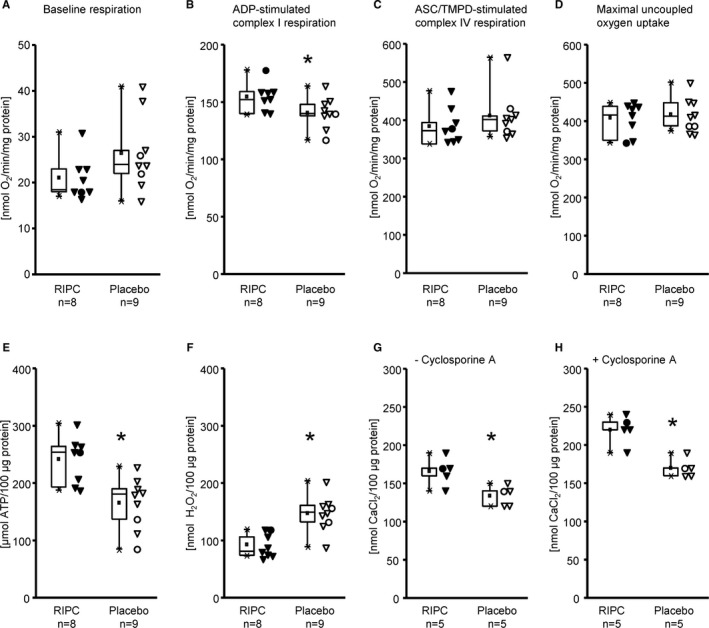
Mitochondrial function of isolated mitochondria from right atrial appendages of patients with remote ischemic preconditioning (RIPC; black symbols) and with placebo (white symbols); triangles for males and circles for females. Baseline respiration (A), ADP‐stimulated complex I respiration (B), ASC/TMPD‐stimulated complex IV respiration (C), maximal uncoupled oxygen uptake with FCCP (D), ATP production (E), reactive oxygen species production (F), and calcium retention capacity, without (G) and with cyclosporine A (H). Data are presented as box plot showing minimum and maximum (crosses), interquartile range from 25% to 75% (box), mean (square), and median (line) and as individual data points, respectively; differences between RIPC and placebo were analyzed by unpaired Student t test. *P<0.05, RIPC vs placebo.
Contractile Function of Right Atrial Trabeculae
Baseline contractile function and recovery of contractile function during reoxygenation (20 and 30 minutes) were better in right atrial trabeculae from patients with RIPC than from those with placebo, also in the presence of isoproterenol (Figure 4A). When normalized to baseline, recovery of contractile function during reoxygenation was also better with RIPC than placebo at 10‐minute reoxygenation (Figure 4B). This improved contractile function was also evident in right atrial trabeculae from males (Figure 4C and 4D), but not in those from females (Figure 4E and 4F).
Figure 4.
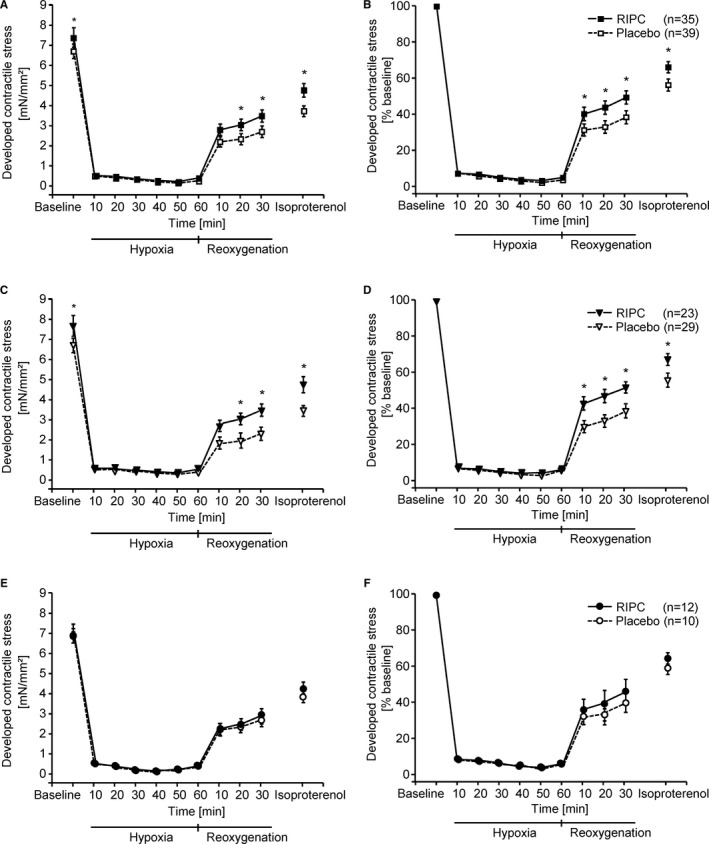
Developed contractile stress of right atrial trabeculae from all patients (A and B), separately from males (C and D) and from females (E and F) with remote ischemic preconditioning (RIPC; black symbols) and with placebo (white symbols) during baseline, hypoxia, reoxygenation, and exposure to isoproterenol, without (A, C, E) and with (B, D, F) normalization to baseline, respectively. Data are presented as mean±SEM. Differences between RIPC and placebo were analyzed by 2‐way (group, time) repeated‐measures ANOVA followed by Fisher's least significant difference test. *P<0.05, RIPC vs placebo.
Western Blot Analysis
Protein expression and/or protein phosphorylation of AKT1/2/3, eNOS, ERK1/2, GSK3β, p38 MAPK, PKCƐ, STAT3, and STAT5 were not different in right atrial trabeculae after hypoxia/reoxygenation between RIPC and placebo, respectively (Figure 5A through 5H for all original western blots and protein phosphorylation/expression and Table 3 for protein expression).
Figure 5.
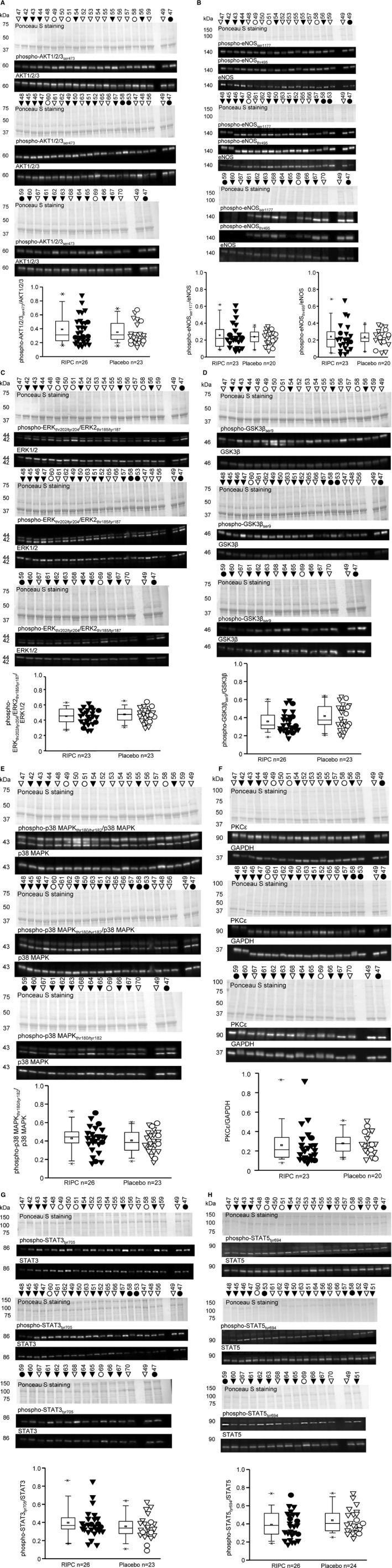
Western blot analysis of phosphorylation/expression of protein kinase B (AKT1/2/3) (A), endothelial nitric oxide synthase (eNOS) (B), extracellular signal‐regulated kinase 1/2 (ERK1/2) (C), glycogen synthase kinase 3β (GSK3β) (D), p38 mitogen‐activated protein kinase (MAPK) (E), protein kinase C epsilon (PKCε) (F), signal transducer and activator of transcription (STAT)3 (G), and STAT5 (H) in right atrial trabeculae after hypoxia and reoxygenation from patients with remote ischemic preconditioning (RIPC; black symbols) or with placebo (white symbols); triangles for males and circles for females. Ponceau S staining and blots of phosphorylated (p‐) and total proteins. Signal intensities from different western blots were normalized by the mean value of samples 47 and 49, respectively, which were available on all gels and set as identical. Data are presented as box plot showing minimum and maximum (crosses), interquartile range from 25% to 75% (box), mean (square), and median (line) and as individual data points, respectively; differences between RIPC and placebo were analyzed by unpaired Student t test.
Table 3.
Protein Expressions of AKT1/2/3, eNOS, ERK1/2, GSK3β, p38 MAPK, PKCε, STAT3, and STAT5 Normalized to Ponceau S Staining
| RIPC | Placebo | P Value | |
|---|---|---|---|
| AKT1/2/3 | 0.94±0.05 | 1.02±0.06 | 0.356 |
| eNOS | 1.73±0.20 | 1.91±0.17 | 0.488 |
| ERK1/2 | 0.97±0.04 | 0.95±0.03 | 0.563 |
| GSK‐3β | 0.98±0.07 | 0.99±0.05 | 0.895 |
| p38 MAPK | 0.77±0.04 | 0.77±0.04 | 0.916 |
| PKCε | 1.07±0.10 | 1.06±0.07 | 0.971 |
| STAT3 | 0.85±0.05 | 0.98±0.07 | 0.124 |
| STAT5 | 0.99±0.05 | 0.98±0.07 | 0.843 |
Data are mean±SEM. Protein expressions were compared using unpaired Student t test. eNOS indicates endothelial nitric oxide synthase; ERK1/2, extracellular signal‐regulated kinase 1/2; GSK3β, glycogen synthase kinase 3β; MAPK, p38 mitogen‐activated protein kinase; AKT, protein kinase B; PKCε, protein kinase C epsilon; RIPC, remote ischemic preconditioning; STAT, signal transducer and activator of transcription.
Discussion
Human right atrial appendages provide a unique opportunity to investigate functional effects of RIPC on human myocardium and its underlying signaling in patients. In the present study, cardioprotection by RIPC was confirmed by reduced postoperative release of troponin in patients undergoing CABG surgery under isoflurane anesthesia. When analyzing this cohort separately for males and females, there was a significant troponin reduction by RIPC only in males. Lack of apparent protection by RIPC in females may also relate to the small sample size, given that in larger cohorts there was no interaction of sex with protection by RIPC in a meta‐analysis of trials in patients with elective percutaneous coronary intervention,35 in a retrospective analysis of the CONDI (Effect of RIC on Clinical Outcomes in STEMI Patients Undergoing pPCI) trial in patients with primary percutaneous coronary intervention,36 and in a retrospective analysis of our previous study in patients undergoing elective bypass surgery.37 Cardioprotection was associated with improved function of mitochondria, confirming the notion that mitochondria are a target of RIPC's protection. Contractile function of human atrial trabeculae was improved at baseline and after hypoxia/reoxygenation by RIPC. However, we were unable to identify the activation of 1 of the established cardioprotective signaling proteins in such atrial tissue with improved mitochondrial and contractile function.
Previous studies have already suggested an association of RIPC with improved mitochondrial function. In mitochondria taken from isolated perfused rat hearts after RIPC in vivo, there was a preserved mitochondrial respiration after ischemia/reperfusion.38 In mitochondria taken from atrial tissue of patients undergoing cardiac surgery with RIPC, there was also a preserved respiration when the atrial tissue was obtained after aortic cross‐clamping, but not when obtained before aortic cross‐clamping.39, 40 However, these studies have failed to confirm cardioprotection by RIPC and thus could not establish a relation between improved mitochondrial function and protection, as would be determined by triphenyl tetrazolium chloride staining in rats38 or biomarker (creatine kinase and its isoenzyme, MB, and troponin) release in patients.39, 40
In contrast, in the present study in myocardial tissue taken from patients with documented cardioprotection by RIPC, mitochondrial function (ie, ADP‐stimulated complex I respiration, ATP production, mPTP opening, and ROS formation) was improved already before myocardial ischemia/reperfusion. We can only speculate on whether such improved mitochondrial function at baseline reflects, and is relevant for, cardioprotection that becomes manifest during subsequent ischemia/reperfusion. It is certainly conceivable that improved cellular energy balance and reduced oxidative stress before myocardial ischemia result in reduced cell death during ischemia/reperfusion. Mitochondrial function is also essential for a proper cardiomyocyte contraction and, as such, may also underlie the improved contractile function of atrial trabeculae at baseline in patients with RIPC. Both improved mitochondrial function and improved contractile function at baseline may reflect a “memory” of the RIPC maneuver in the atrial tissue.
The beneficial effect of RIPC on contractile function of atrial trabeculae persisted after hypoxia/reoxygenation. In previous studies, local pre‐ and postconditioning ex vivo has improved contractile function of atrial trabeculae during reoxygenation after sustained hypoxia.41, 42, 43, 44 Contractile function of atrial trabeculae was also improved during reoxygenation when plasma dialysate, taken from human volunteers after RIPC, was added before sustained hypoxia.45 The improved contractile function of atrial trabeculae in response to humoral transfer of cardioprotection was related to a posttranslational modification of proteins by O‐linked beta‐N‐acetylglucosamine.45 In rat cardiomyocytes, such increased concentration of O‐linked beta‐N‐acetylglucosamines inhibited mPTP opening and reduced ROS formation.46 Improved mitochondrial and contractile function of human atrial tissue in association with protection by RIPC open the possibility to further analyze cardioprotective signaling in human tissue with the potential to establish a causal relation between signaling and protection.
Study Limitations
Data obtained from right atrial trabeculae cannot simply be extrapolated to left ventricular tissue. In pigs, baseline force of contraction was lower in trabeculae from the right atrium than from the right ventricle.47 Also, iloprost increased contractile function in trabeculae taken from the right atrium, but not from the right ventricle.47
Transport time of right atrial appendages to the laboratory and the time to final ex vivo sample processing may have influenced the present results, and we cannot exclude partial hypoxia during these times.
To our disappointment, we were unable to identify and confirm the activation of previously reported potentially cardioprotective key signaling proteins (ie, AKT1/2/3, ERK1/2, eNOS, GSK3β, p38 MAPK, PKCƐ, STAT3, and STAT5). In our previous study in human left ventricular myocardium, we have demonstrated an association of increased STAT5 phosphorylation with cardioprotection by RIPC.21 This association of increased STAT5 phosphorylation with RIPC was recently confirmed in myocardial tissue from the right ventricular outflow tract of children undergoing cardiac surgery under cardiopulmonary bypass along with increased phosphorylation of AKT, eNOS, and STAT3.48 In the present study, however, we analyzed expression and phosphorylation of potential signaling proteins in human atrial trabeculae at only 1 time point (ie, after in vitro hypoxia/reoxygenation). Thus, we cannot exclude that we may have missed changes in protein expression and phosphorylation at different time points between baseline and reoxygenation. The size of the right atria was too small and precluded a parallel preparation of several atrial trabeculae that would have permitted extraction of proteins from 1 atrium with different lysis buffers. We here used a Tris/SDS buffer to achieve a high protein yield of all cellular components, but may have missed changes in protein expression or phosphorylation of those predominantly expressed in specific cellular components (ie, nucleus, mitochondria, or cytosol).49
Conclusions
Both mitochondrial and contractile function of atrial myocardium reflect cardioprotection by RIPC in patients undergoing cardiovascular surgery. Such right atrial tissue may be suitable for the further analysis of the signaling that underlies RIPC in humans.
Sources of Funding
The present study was supported by the German Research Foundation (SFB 1116 B08).
Disclosures
None.
Acknowledgments
The technical assistance of Astrid Büchert, Jelena Löblein, and Marion Pesch is acknowledged.
(J Am Heart Assoc. 2018;7:e009540 DOI: 10.1161/JAHA.118.009540.)
References
- 1. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon DM. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the crisp stent trial long‐term follow‐up. Circ Cardiovasc Interv. 2013;6:246–251. [DOI] [PubMed] [Google Scholar]
- 3. Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–579. [DOI] [PubMed] [Google Scholar]
- 4. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single‐centre randomised, double‐blind, controlled trial. Lancet. 2013;382:597–604. [DOI] [PubMed] [Google Scholar]
- 5. Candilio L, Malik A, Ariti C, Barnard M, Di SC, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;10:185–192. [DOI] [PubMed] [Google Scholar]
- 6. Nouraei SM, Baradari AG, Jazayeri A. Does remote ischaemic preconditioning protect kidney and cardiomyocytes after coronary revascularization? A double blind controlled clinical trial. Med Arch. 2016;70:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bøtker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 8. Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST‐elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 9. White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188. [DOI] [PubMed] [Google Scholar]
- 10. Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size in stemi patients treated by thrombolysis. J Am Coll Cardiol. 2015;65:2764–2765. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Zhao L, Hong D, Gao J. Remote ischaemic preconditioning reduces myocardial ischaemic reperfusion injury in patients with ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. Acta Cardiol. 2016;71:596–603. [DOI] [PubMed] [Google Scholar]
- 12. Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol. 2018;113:15. [DOI] [PubMed] [Google Scholar]
- 13. Gaspar A, Lourenco AP, Pereira MA, Azevedo P, Roncon‐Albuquerque R Jr, Marques J, Leite‐Moreira AF. Randomized controlled trial of remote ischaemic conditioning in ST‐elevation myocardial infarction as adjuvant to primary angioplasty (RIC‐STEMI). Basic Res Cardiol. 2018;113:14. [DOI] [PubMed] [Google Scholar]
- 14. Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Bøtker HE. Improved long‐term clinical outcomes in patients with ST‐elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. [DOI] [PubMed] [Google Scholar]
- 15. Kleinbongard P, Peters J, Jakob H, Heusch G, Thielmann M. Persistent survival benefit from remote ischemic preconditioning in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2018;71:251–262. [DOI] [PubMed] [Google Scholar]
- 16. Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre‐, post‐, and remote conditioning. Circ Res. 2016;119:676–695. [DOI] [PubMed] [Google Scholar]
- 17. Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017;469:159–181. [DOI] [PubMed] [Google Scholar]
- 18. Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across‐species transfer of protection by remote ischemic preconditioning with species‐specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117:279–288. [DOI] [PubMed] [Google Scholar]
- 19. Skyschally A, Kleinbongard P, Lieder HR, Gedik N, Stoian L, Amanakis G, Elbers E, Heusch G. Humoral transfer and intra‐myocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am J Physiol Heart Circ Physiol. 2018;315:H159–H172. [DOI] [PubMed] [Google Scholar]
- 20. Kleinbongard P, Amanakis G, Skyschally A, Heusch G. Reflection of cardioprotection by remote ischemic perconditioning in attenuated ST‐segment elevation during ongoing coronary occlusion in pigs: evidence for cardioprotection from ischemic injury. Circ Res. 2018;122:1102–1108. [DOI] [PubMed] [Google Scholar]
- 21. Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res. 2012;110:111–115. [DOI] [PubMed] [Google Scholar]
- 22. Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, Kleinbongard P. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One. 2014;9:e96567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre‐, post‐, and remote conditioning. Circ Res. 2015;116:674–699. [DOI] [PubMed] [Google Scholar]
- 24. Heusch G. Cardioprotection is alive but remains enigmatic: the nitric oxide‐protein kinases‐mitochondria signaling axis. Circulation. 2017;136:2356–2358. [DOI] [PubMed] [Google Scholar]
- 25. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischaemic preconditioning during coronary artery bypass grafting with isoflurane but not with propofol anesthesia—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. [DOI] [PubMed] [Google Scholar]
- 26. Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376–382. [DOI] [PubMed] [Google Scholar]
- 27. Holmuhamedov EL, Oberlin A, Short K, Terzic A, Jahangir A. Cardiac subsarcolemmal and interfibrillar mitochondria display distinct responsiveness to protection by diazoxide. PLoS One. 2012;7:e44667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gedik N, Maciel L, Schulte C, Skyschally A, Heusch G, Kleinbongard P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch Med Sci. 2017;13:448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin d. J Biol Chem. 2005;280:18558–18561. [DOI] [PubMed] [Google Scholar]
- 30. Lampert FM, Matt P, Grapow M, Lefkovits I, Zerkowski HR, Grussenmeyer T. “Turnover proteome” of human atrial trabeculae. J Proteome Res. 2007;6:4458–4468. [DOI] [PubMed] [Google Scholar]
- 31. Mruck S, Henneken H, Dragu A, Stuttgen B, Tenderich G, Korfer R, Holubarsch C, Kuwert T. Force‐generating preparation from human atria as a model for studying myocardial uptake of radiopharmaceuticals. J Nucl Med. 2000;41:1587–1593. [PubMed] [Google Scholar]
- 32. Deja MA, Golba KS, Malinowski M, Widenka K, Biernat J, Szurlej D, Wos S. Diazoxide provides maximal KATP channels independent protection if present throughout hypoxia. Ann Thorac Surg. 2006;81:1408–1416. [DOI] [PubMed] [Google Scholar]
- 33. Morris SD, Yellon DM. Angiotensin‐converting enzyme inhibitiors potentiate preconditioning trough bradykinin B2 receptor activation in human heart. J Am Coll Cardiol. 1997;29:1599–1606. [DOI] [PubMed] [Google Scholar]
- 34. Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. Stat3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol. 2018;113:3. [DOI] [PubMed] [Google Scholar]
- 35. D'Ascenzo F, Moretti C, Omede P, Cerrato E, Cavallero E, Er F, Presutti DG, Colombo F, Crimi G, Conrotto F, Dinicolantonio JJ, Chen S, Prasad A, Biondi ZG, Gaita F. Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: a meta‐analysis of randomised clinical trials. EuroIntervention. 2014;9:1463–1471. [DOI] [PubMed] [Google Scholar]
- 36. Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sorensen HT, Botker HE; CONDI Investigators . Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open. 2015;5:e006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kleinbongard P, Neuhauser M, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology. 2016;133:128–133. [DOI] [PubMed] [Google Scholar]
- 38. Ferko M, Kancirova I, Jasova M, Carnicka S, Murarikova M, Waczulikova I, Sumbalova Z, Kucharska J, Ulicna O, Ravingerova T, Ziegelhoffer A. Remote ischemic preconditioning of the heart: protective responses in functional and biophysical properties of cardiac mitochondria. Physiol Res. 2014;63(suppl 4):S469–S478. [DOI] [PubMed] [Google Scholar]
- 39. Slagsvold KH, Moreira JB, Rognmo O, Hoydal M, Bye A, Wisloff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and activates pro‐survival protein kinase AKT in the left ventricle during cardiac surgery: a randomized trial. Int J Cardiol. 2014;177:409–417. [DOI] [PubMed] [Google Scholar]
- 40. Slagsvold KH, Rognmo O, Hoydal M, Wisloff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microrna expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851–859. [DOI] [PubMed] [Google Scholar]
- 41. Walker DM, Walker JM, Pugsley WB, Pattinson CW, Yellon DM. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol. 1995;27:1349–1357. [DOI] [PubMed] [Google Scholar]
- 42. Sivaraman V, Mudalgiri NR, Di SC, Kolvekar S, Hayward M, Yap J, Keogh B, Hausenloy DJ, Yellon DM. Postconditioning protects human atrial muscle through the activation of the risk pathway. Basic Res Cardiol. 2007;102:453–459. [DOI] [PubMed] [Google Scholar]
- 43. Mudalagiri NR, Mocanu MM, Di SC, Kolvekar S, Hayward M, Yap J, Keogh B, Yellon DM. Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol‐3 kinase and ERK1/2 activation. Br J Pharmacol. 2008;153:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Bøtker HE. Impact of O‐GLcNAc on cardioprotection by remote ischaemic preconditioning in non‐diabetic and diabetic patients. Cardiovasc Res. 2013;97:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O‐GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holmboe S, Andersen A, Jensen RV, Kimose HH, Ilkjaer LB, Shen L, Clapp LH, Nielsen‐Kudsk JE. Prostacyclins have no direct inotropic effect on isolated atrial strips from the normal and pressure‐overloaded human right heart. Pulm Circ. 2017;7:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu Q, Wang T, Chen S, Zhou Q, Li H, Hu N, Feng Y, Dong N, Yao S, Xia Z. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. Eur Heart J. 2018;39:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gedik N, Krüger M, Thielmann M, Kottenberg E, Skyschally A, Frey UH, Cario E, Peters J, Jakob H, Heusch G, Kleinbongard P. Proteomics/phosphoproteomics of left ventricular biopsies from patients with surgical coronary revascularization and pigs with coronary occlusion/reperfusion: remote ischemic preconditioning. Sci Rep. 2017;7:7629. [DOI] [PMC free article] [PubMed] [Google Scholar]


