Abstract
Background
Long‐term data on durability of currently available transcatheter heart valves are sparse. We sought to assess the incidence of long‐term (8‐year) structural valve dysfunction and bioprosthetic valve failure in a cohort of patients with transcatheter aortic valve replacement (TAVR) who reached at least 5‐year follow‐up.
Methods and Results
Consecutive patients with at least 5‐year follow‐up available undergoing TAVR from June 4, 2007 to March 30, 2012 were included. Structural valve dysfunction and bioprosthetic valve failure were defined according to newly standardized European Association of Percutaneous Cardiovascular Interventions/European Society of Cardiology/European Association for Cardio‐Thoracic Surgery criteria and reported as cumulative incidence function to account for the competing risk of death. A total of 288 consecutive patients with a mean age of 80.7±5.3 years and with a mean Society of Thoracic Surgery mortality score of 8.1±5.1% were analyzed. Survival rate at 8 years was 29.8%. Mean pressure gradients decreased from 53.3±15.9 mm Hg (pre‐TAVR) to 10.5±4.5 mm Hg (in‐hospital post‐TAVR) (P<0.001). There was a small, not significant, increase in the transaortic gradient throughout follow‐up. Bioprosthetic valve failure was observed in a total of 11 patients (8‐year cumulative incidence function: 4.51%; 95% confidence interval, 1.95%–8.76%). Severe and moderate structural valve dysfunctions were reported in 7 patients (8‐year cumulative incidence function: 2.39%; 95% confidence interval, 0.77%–5.71%) and 13 patients (8‐year cumulative incidence function: 5.87%; 95% confidence interval, 3.06%–9.96%), respectively. Aortic valve reintervention (redo TAVR) was successfully performed in 2 patients (0.7%) presenting with symptomatic severe restenosis and intraprosthetic regurgitation subsequent to endocarditis.
Conclusions
In an aged population of patients with symptomatic severe aortic stenosis treated with first‐generation bioprostheses, TAVR was associated with a survival rate of 30% but low rates of bioprosthetic valve failure and structural valve dysfunction at 8 years.
Keywords: aortic stenosis, durability, prosthetic heart valve, transcatheter aortic valve, transcatheter aortic valve implantation, valve dysfunction
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation
Clinical Perspective
What Is New?
Data on clinical outcomes and transcatheter aortic valve integrity after 5 years remain extremely scarce, and definitions used to date have been extremely heterogeneous.
This is the first systematic assessment of long‐term (8‐year) incidence of structural valve dysfunction after transcatheter aortic valve replacement.
This analysis defined, for the first time, the rates of bioprosthetic valve failure and structural valve dysfunction, defined according to newly standardized European Association of Percutaneous Cardiovascular Interventions/European Society of Cardiology/European Association for Cardio‐Thoracic Surgery criteria.
At 8 years after transcatheter aortic valve replacement, bioprosthetic valve failure and severe structural valve dysfunction occurred in only 4.5% and 2.4% of patients, respectively.
What Are the Clinical Implications?
It is key that standardized criteria will be applied when reporting transcatheter aortic valve performances at mid‐ to long‐term follow‐up.
If these favorable outcomes will be confirmed in larger series, transcatheter aortic valve replacement could be considered the preferred therapy for patients with severe aortic stenosis.
Introduction
Transcatheter aortic valve replacement (TAVR) is an established alternative for patients with severe aortic stenosis (AS).1 On the basis of the favorable outcomes of recent randomized clinical trials conducted in intermediate‐risk populations,2, 3 TAVR is progressively being offered to younger and lower‐risk patients. In this particular subset, life expectancy is expected to exceed that of initial candidates to TAVR (ie, elderly and very elderly patients at high or prohibitive surgical risk), which makes the question of long‐term prosthesis durability of crucial importance.
If transcatheter heart valve (THV) durability up to 5 years is already a well‐established reality, with low rates of structural valve dysfunction (SVD) demonstrated in large and methodologically rigorous studies,4, 5, 6, 7, 8 data on clinical outcomes and THV integrity after 5 years remain extremely scarce.9 An important challenge when reporting the rates of SVD (and comparing them with those of previous studies) deals with its definition. The few studies reporting on SVD after TAVR used heterogeneous criteria, rendering interstudy comparisons problematic.5, 6, 7, 8, 9, 10 To address this issue, the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Society of Cardiology (ESC), and the European Association for Cardio‐Thoracic Surgery (EACTS) recently introduced standardized criteria to define SVD of THV that aim at generating uniformity in data reporting of future studies assessing the long‐term durability of TAVR.11
On this background, the aim of this study was to report on the incidence of long‐term SVD and bioprosthetic valve failure (BVF), defined according to the new EAPCI‐ESC‐EACTS criteria, in a cohort of patients with TAVR who reached at least 5‐year follow‐up.
Methods
Additional data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Population
This study included 288 consecutive patients who underwent TAVR between June 4, 2007 to March 30, 2012 to allow for a follow‐up of at least 5 years. All patients received either the self‐expanding CoreValve (Medtronic Inc, Minneapolis, MN) or the balloon‐expandable Edwards‐SAPIEN XT (Edwards Lifesciences, Irvine, CA) THV. The device was delivered through either the transfemoral or the transsubclavian approach.
A multidisciplinary team, including cardiologists (D.G., W.D.), cardio‐thoracic surgeons (A.G.), anesthesiologists, geriatricians, and interventional cardiologists (M.B., C.S., C.T.), evaluated all available clinical and imaging data, and a consensus decision was obtained to determine individual eligibility for TAVR. The decision to perform TAVR was conditional on the presence of severe symptomatic AS with contraindications to or high risk for surgical aortic valve replacement, life expectancy of 1 year, and anatomical features suitable for intervention. All patients provided written informed consent for inclusion in the study. Screening investigations were performed in all patients before the procedure, with sizing of the THV performed by using multidetector computed tomography and an integration of echocardiography (transthoracic and/or transesophageal), angiography, and/or simultaneous aortography during balloon valvuloplasty,12 when multidetector computed tomography was not available. The local institutional review committee approved the study.
Data Collection and Definitions
Patients were enrolled in a prospective local Institutional TAVR registry at the Ferrarotto Hospital in Catania, Italy (REPLACE [Registry of Percutaneous Aortic Valve Replacement]),13 with clinical evaluations at 1 and 12 months and then yearly after TAVR. Transthoracic echocardiography was performed before hospital discharge and then at similar intervals at the implantation center. For patients located geographically far from our institution or unable to return to the implantation center for echocardiographic assessment, referring cardiologists performed the transthoracic echocardiogram and images were collected and analyzed at the implantation center. All clinical outcomes were defined according to the Valve Academic Research Consortium‐2.14
Echocardiography and THV‐Related Event Definitions
Aortic valve area was calculated with the continuity equation (velocity‐time integral method) from data derived before and after device implantation. Measurement of the left ventricular outflow tract for calculations of aortic valve area was performed with 2‐dimensional imaging in a zoomed‐up parasternal long‐axis view. SVD and BVF were defined according to the EAPCI‐ESC‐EACTS criteria, as follows. SVD was defined as hemodynamic changes in valve function, assessed by echocardiography, with or without evidence of morphological SVD (“isolated hemodynamic dysfunction”). Two degrees of hemodynamic SVD were defined: (1) Moderate SVD was defined as (a) mean gradient ≥20 and <40 mm Hg and/or ≥10 and <20 mm Hg change from baseline (before discharge or within 30 days of valve implantation) and/or (b) moderate new or worsening (>1+/4+) intraprosthetic aortic regurgitation. (2) Severe SVD was defined as (a) mean gradient ≥40 and/or ≥20 mm Hg change from baseline (before discharge or within 30 days of valve implantation) and/or (b) severe new or worsening (>2+/4+) intraprosthetic aortic regurgitation. BVF was defined as the composite of the following: (1) severe SVD at 30 days, 1 year, or yearly thereafter or at cardiac‐related interim visits; (2) repeated intervention for bioprosthetic valve dysfunction; and (3) valve‐related death or findings of bioprosthetic valve dysfunction at autopsy, likely related to death. Paravalvular regurgitation was graded as mild, moderate, or severe, according to Valve Academic Research Consortium‐2 criteria.
Statistical Analysis
Continuous variables are presented as mean±SD, whereas dichotomous parameters are presented as frequencies and percentages. Survival curves for the outcomes of interest were plotted according to the Kaplan‐Meier method (actuarial analysis). SVD and BVF were also reported as cumulative incidence function (actual analysis) to account for the competing risk of death. All estimates are presented with their 95% confidence interval (CI). All analyses were conducted in R (version 3.3.2) equipped with the “cmprsk” and “survival” packages.
Results
Population
A total of 288 patients with a mean age of 80.7±5.3 years were analyzed. Baseline demographic, clinical, and echocardiographic characteristics of the study population are summarized in Table 1. All patients had severe symptomatic AS (mean transaortic pressure gradients before the procedure, 53.3±15.9 mm Hg; mean aortic valve area, 0.5±0.3 cm2). The predicted 30‐day mortality, assessed by Society of Thoracic Surgery mortality score, was 8.1±5.1%. Most patients (n=195 [67.7%]) were in New York Heart Association functional class 3 or 4 before the procedure.
Table 1.
Baseline Characteristics
| Characteristics | Value (n=288) |
|---|---|
| Clinical parameters | |
| Age, y | 80.7±5.3 |
| BMI, kg/m2 | 26.7±5.3 |
| Female sex, n (%) | 168 (58.3) |
| Hypertension, n (%) | 248 (86.1) |
| Diabetes mellitus, n (%) | 76 (26.4) |
| Dyslipidemia, n (%) | 153 (53.1) |
| Prior acute heart failure, n (%) | 109 (37.8) |
| Prior myocardial infarction, n (%) | 53 (18.4) |
| Prior stroke, n (%) | 18 (6.3) |
| Prior TIA, n (%) | 21 (7.3) |
| Prior bypass graft surgery, n (%) | 31 (10.8) |
| Prior percutaneous coronary intervention, n (%) | 88 (30.6) |
| Peripheral vascular disease, n (%) | 17 (5.9) |
| Chronic obstructive pulmonary disease, n (%) | 102 (35.4) |
| Cirrhosis, n (%) | 8 (2.8) |
| Renal insufficiencya, n (%)* | 59 (20.5) |
| Atrial fibrillation, n (%) | 44 (15.3) |
| Prior pacemaker, n (%) | 28 (9.7) |
| NYHA class III and IV, n (%) | 195 (67.7) |
| STS score, % | 8.1±5.1 |
| Baseline echocardiographic parameters | |
| Left ventricular ejection fraction, % | 51.5±10.5 |
| Peak pressure gradient, mm Hg | 86.2±24.4 |
| Mean pressure gradient, mm Hg | 53.3±15.9 |
| Aortic valve area, cm2 | 0.5±0.3 |
Data are given as mean±SD unless otherwise indicated. BMI indicates body mass index; NYHA, New York Heart Association; STS, Society of Thoracic Surgery; and TIA, transient ischemic attack.
Glomerular filtration rate, <30 mL/min.
Procedural and 30‐Day Outcomes
Procedural data are shown in Table 2. Transfemoral access was used in 283 patients (98.3%); in 5 patients (1.7%) in whom the transfemoral approach was unfeasible, a transsubclavian access was used. The CoreValve prosthesis was implanted in 237 patients (82.3%), whereas the SAPIEN XT THV was used in 48 patients (16.7%). Balloon predilatation was performed in all patients. Valve Academic Research Consortium‐2–defined device success was obtained in 240 patients (83.3%). In 2 patients (0.7%), the THV was not deployed because of technical issues, and implantation of 2 THVs was required in 10 patients (3.5%). The 30‐day outcomes are listed in Table 3. Overall, 30‐day mortality was 9.0%. Disabling stroke and disabling bleeding were reported in 2.1% and 8.2% of patients, respectively. A permanent pacemaker was implanted in 16.3% of patients, in most cases because of permanent or intermittent third‐degree atrioventricular block.
Table 2.
Procedural Variables
| Variables | Value (n=288) |
|---|---|
| Approach | |
| Transfemoral | 283 (98.3) |
| Transsubclavian | 5 (1.7) |
| Device | |
| Medtronic CoreValve | 238 (82.6) |
| 26 mm | 132 (45.8) |
| 29 mm | 95 (33.0) |
| 31 mm | 11 (3.8) |
| Edwards SAPIEN XT | 48 (16.7) |
| 23 mm | 31 (10.8) |
| 26 mm | 17 (5.9) |
| Predilatation | 288 (100) |
| Postdilatation | 28 (9.7) |
| Two THVs implanted | 10 (3.5) |
| Aborted procedure | 2 (0.7) |
Data are given as number (percentage). THV indicates transcatheter heart valve.
Table 3.
The 30‐Day Clinical Outcomes
| Outcomes | Value (n=288) |
|---|---|
| Death | 26 (9.0) |
| Cardiovascular death | 15 (5.2) |
| Stroke/TIA | 12 (4.2) |
| Disabling stroke | 6 (2.1) |
| Nondisabling stroke | 1 (0.3) |
| TIA | 5 (1.7) |
| Life‐threatening bleeding | 18 (6.2) |
| Permanent PM | 47 (16.3) |
Data are given as number (percentage). PM indicates pacemaker; and TIA, transient ischemic attack.
Long‐Term Survival
Clinical follow‐up was available in all patients at a median follow‐up of 80.7 months. A total of 171 patients died at a median of 60.1 months (interquartile range, 51–72 months). Death was attributed to cardiovascular reasons in 60 patients (35%). Kaplan‐Meier survival estimates at 1, 4, and 8 years were 81.9%, 61.0%, and 29.8%, respectively (Figure 1).
Figure 1.
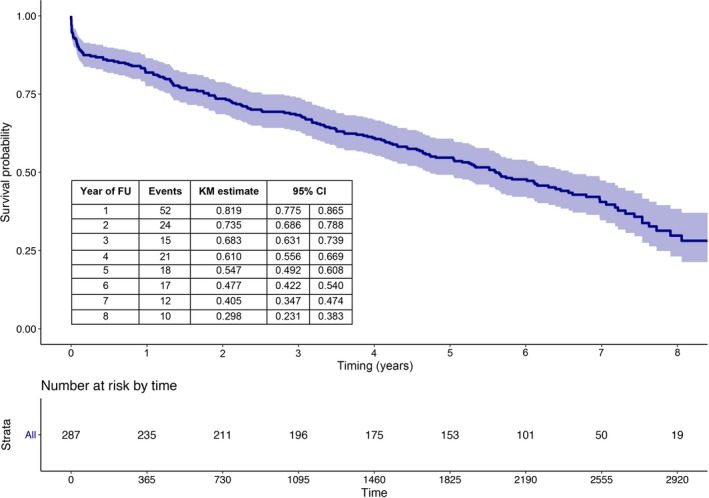
Kaplan‐Meier (KM) curve of survival from all‐cause death up to 8‐year follow‐up (FU). CI indicates confidence interval.
THV Durability
Among survivors at 5 years (n=158), the 6.7% of patients potentially suitable for transthoracic echocardiogram assessment were unavailable for follow‐up (Figure 2). Information on last available echocardiogram in the patients who died compared with the patients who were still alive at 5, 6, 7, and 8 years (sequentially) is reported in Table 4. Prosthesis performance during follow‐up is depicted in Figure 3. At discharge, mild and more than mild paravalvular regurgitation were observed in 147 patients (51.0%) and 38 patients (13.2%), respectively. Mean pressure gradients decreased from 53.3±15.9 mm Hg (pre‐TAVR) to 10.5±4.5 mm Hg (in‐hospital post‐TAVR) (P<0.001). Subsequently, there was a small, not significant, increase in the transaortic gradient throughout follow‐up (Figure 3).
Figure 2.
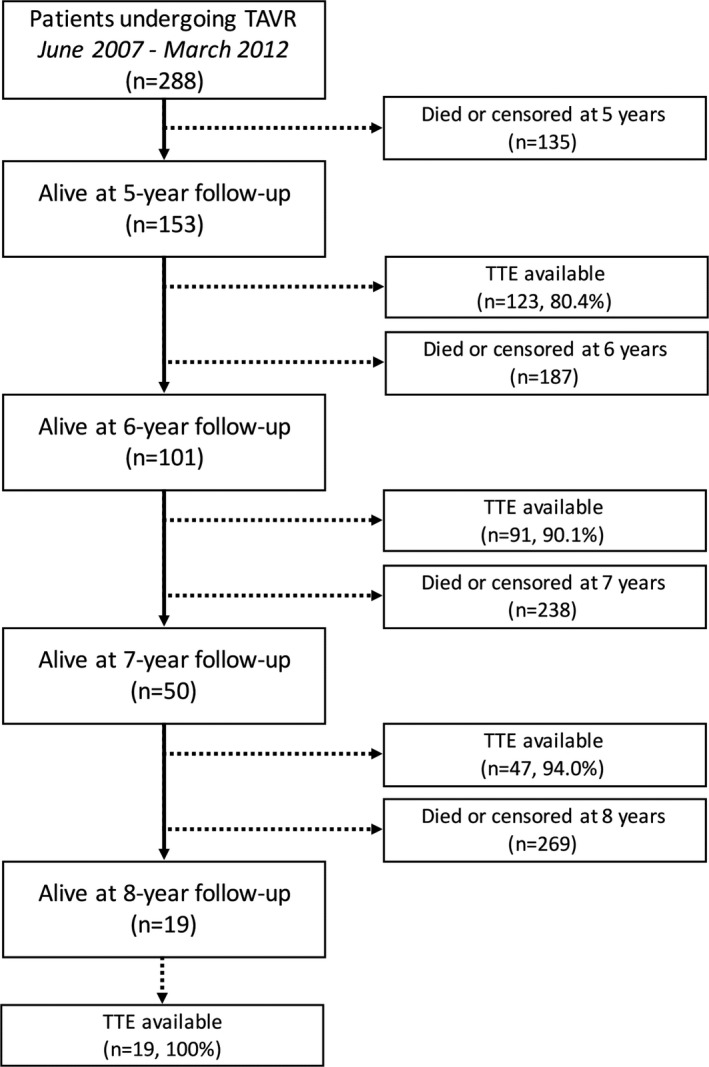
Flow diagram with the number of patients who died or had an echocardiographic follow‐up available at each year beyond 5 years. TAVR indicates transcatheter aortic valve replacement; and TTE, transthoracic echocardiogram.
Table 4.
Comparisons of Mean Gradient, SVD, and BVF Reported on Last Available Echocardiogram in the Patients Who Died vs Patients Who Were Still Alive at 5, 6, 7, and 8 Years (Sequentially)
| Years of Follow‐Up | No. of Patients | Gradient, Mean±SD | BVF | SVD | |||
|---|---|---|---|---|---|---|---|
| Alive | Dead | Alive | Dead | P Value | |||
| 5 | 123 | 130 | 11.9±9.1 | 11.6±6.1 | 0.715 | 7 | 4 |
| 6 | 91 | 147 | 10.9±5.3 | 11.9±6.8 | 0.287 | 7 | 4 |
| 7 | 47 | 159 | 12.3±7.2 | 12.0±6.7 | 0.824 | 7 | 4 |
| 8 | 19 | 169 | 12.0±6.6 | 14.5±8.6 | 0.141 | 9 | 5 |
BVF indicates bioprosthetic valve failure; and SVD, severe structural dysfunction.
Figure 3.
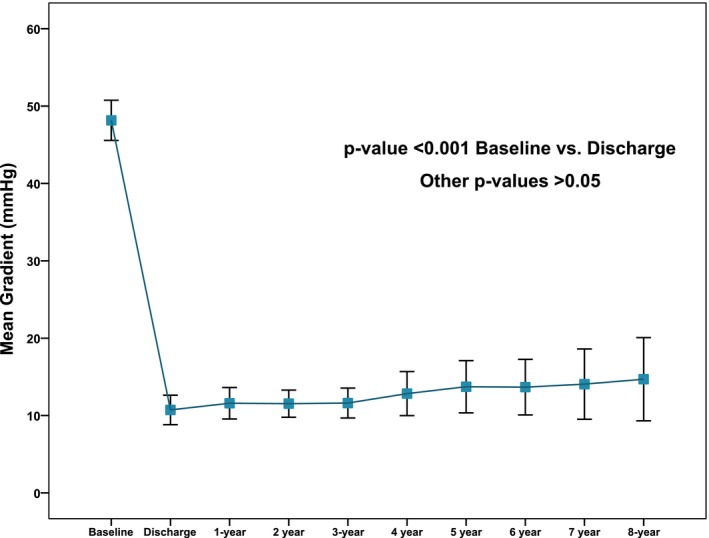
Time trends in transaortic mean gradient. All values refer to patients (n=19) with complete 8‐year follow‐up.
BVF was observed in a total of 11 patients (8‐year cumulative incidence function: 4.51%; 95% CI, 1.95%–8.76%) (Figures 4 and 5, left panels). Severe SVD was reported in 7 patients (8‐year cumulative incidence function: 2.39%; 95% CI, 0.77%–5.71%) (Figures 4 and 5, right panels). The survival rates free from BVF and severe SVD at 8 years are 95.4% (95% CI, 91.9%–98.9%) and 97.5% (95% CI, 0.95%–1.00%) (Figure 5). Moderate SVD was found in 13 patients (8‐year cumulative incidence function: 5.87%; 95% CI, 3.06%–9.96%). The 30‐day landmark analyses for BVF and severe SVD are shown in Figure 6.
Figure 4.
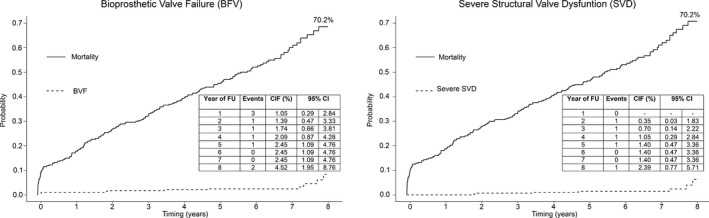
Cumulative incidence function (CIF) of bioprosthesis valve failure (BVF) and severe structural valve dysfunction (SVD) up to 8‐year follow‐up (FU). CI indicates confidence interval.
Figure 5.
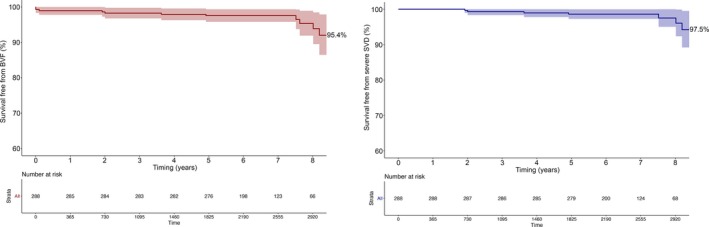
Kaplan‐Meier curve reporting freedom from bioprosthetic valve failure (BVF) and severe structural valve dysfunction (SVD).
Figure 6.
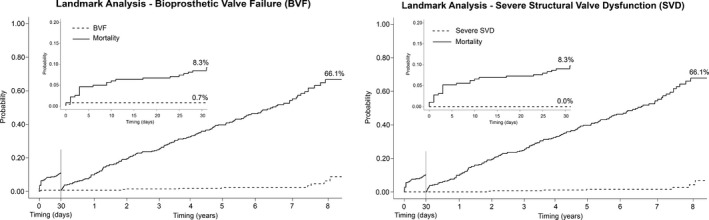
Landmark analyses of cumulative incidence function (CIF) of bioprosthesis valve failure (BVF) and severe structural valve dysfunction (SVD) during the first 30 days after the procedure (left side of each graph) and from 30 days to 8 years (right side of each graph). CI indicates confidence interval.
Among the 11 patients meeting criteria for BVF (Table 5), 4 had valve‐related deaths: 1 patient had aortic annulus rupture after SAPIEN XT implantation (death at day 1), 1 patient died because of left main occlusion after CoreValve deployment (intraprocedural death), and the last 2 patients died at days 37 and 2784 because of acute heart failure (low CoreValve implantation causing severe paravalvular regurgitation). The remaining 7 patients had severe SVD. Of the latter (Figure 7), 4 had severe restenosis, 1 had mixed severe stenosis and regurgitation, and 1 experienced valve endocarditis at 2 years, causing severe intraprosthetic aortic regurgitation. Two cases (1 severe restenosis and 1 severe intraprosthetic regurgitation) were effectively treated with redo TAVR, and the other 2 patients were asymptomatic and, therefore, left untreated. Severe SVD caused symptoms in 3 patients, whereas all cases of moderate SVD were found in asymptomatic patients. No other cases of late THV deterioration were observed. No cases of valve thrombosis or late valve embolization were reported.
Table 5.
Details of Patients Experiencing Severe BVF
| Patient no. | Age, y | THV | BVF Explanation | Timing of BVF, d | BVF Management and Comments | Vital Status | Timing to Death/Follow‐Up, d |
|---|---|---|---|---|---|---|---|
| 1 | 75 | CV (26 mm) | Severe SVD (severe stenosis) | 2991 | Successful redo TAVR (CV, 26 mm) | Alive | 3535 |
| 2 | 81 | CV (31 mm) | Left main occlusion | 0 | Unsuccessful PCI | Died | 0 |
| 3 | 80 | SXT (26 mm) | Aortic annulus rupture | 1 | None | Died | 1 |
| 4 | 73 | CV (26 mm) | Severe PVR and MR (low implantation)a | 37 | Unsuccessful THV snaring and repositiona | Died | 37 |
| 5 | 79 | CV (26 mm) | Severe SVD (severe stenosis) | 2926 | None (asymptomatic) | Alive | 3051 |
| 6 | 82 | CV (26 mm) | Severe SVD (new severe intraprosthetic AR because of infective endocarditis) | 701 | Successful redo TAVR (CV, 26 mm) | Alive | 2520 |
| 7 | 82 | SXT (26 mm) | Severe SVD (mixed severe stenosis and intraprosthetic AR) | 1325 | None (asymptomatic) | Died | 1731 |
| 8 | 81 | CV (29 mm) | Severe SVD (severe stenosis) | 1795 | Unsuccessful OAT (asymptomatic) | Died | 2014 |
| 9 | 83 | CV (29 mm) | Severe SVD (new moderate intraprosthetic AR) | 731 | None (asymptomatic) | Died | 2068 |
| 10 | 81 | CV (29 mm) | Severe SVD (severe stenosis) | 2744 | None (asymptomatic) | Alive | 2969 |
| 11 | 87 | CV (26 mm) | Severe PVR (low implantation) | 2744 | None (patient refused redo TAVR) | Died | 2784 |
AR indicates aortic regurgitation; BVF, bioprosthesis valve failure; CV, CoreValve; MR, mitral regurgitation; OAT, oral anticoagulant therapy; PCI, percutaneous coronary intervention; PVR, paravalvular regurgitation; SVD, structural valve dysfunction; SXT, SAPIEN XT; TAVR, transcatheter aortic valve replacement; and THV, transcatheter heart valve.
The THV deployed low into the left ventricle caused severe PVR and impingement of the anterior mitral leaflet, leading to severe MR. Repositioning of the index THV using the “snaring technique” was attempted instead of implantation of a second THV.
Figure 7.
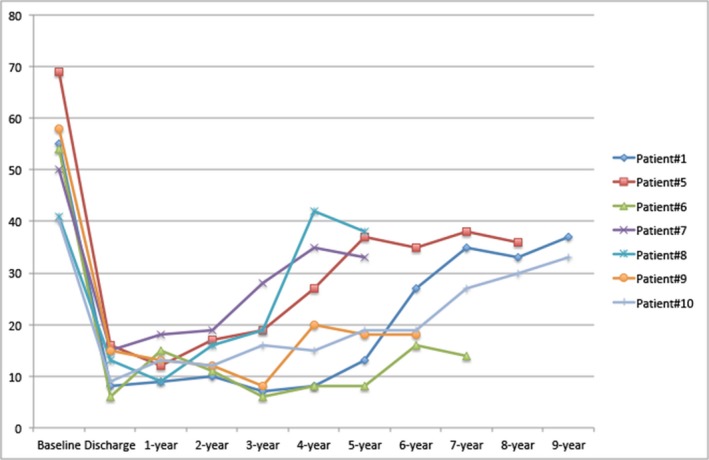
Changes in transvalvular gradient over time are seen in a selected group of patients (n=7) diagnosed as having severe structural valve dysfunction.
Discussion
TAVR is now considered as a valuable alternative to conventional aortic valve replacement in high‐ and intermediate‐risk patients affected by severe AS. Although many studies have confirmed the durability of TAVR up to 5 years, there is still a paucity of data on the incidence of structural deterioration and valve failure at longer‐term follow‐up. This analysis adds to the current knowledge of long‐term durability and structural integrity of THVs with the following observations: (1) in an elderly population (mean age, 80 years), we showed acceptable long‐term outcomes, with an 8‐year survival rate of 29.8%; (2) at 8 years, BVF, severe SVD, and moderate SVD, according to the new EAPCI/ESC/EACTS definitions, occurred in only 4.5%, 2.4%, and 5.9% of patients, respectively; (3) and reintervention with redo TAVR was performed successfully in 2 patients (the remaining patients with severe SVD and no symptoms did not need any additional invasive treatment).
As the duration of implanted THVs increases, valve durability and dysfunction become more crucial issues.15 No significant increases in mean THV gradient or cases of structural valve deterioration were reported during the 5‐year follow‐up of the PARTNER (Placement of Aortic Transcatheter Valves) I trial.7
Three studies have also reported outcomes after TAVR with either the Edwards SAPIEN or CoreValve bioprosthesis up to 5 years5, 6, 8: 2 of them5, 6 did not suggest any major concerns about durability of THVs, with stable transprosthetic gradients over time and a rate of SVD of 3.4% and 4.2%, respectively, using different definitions. More recently, Muratori and colleagues performed a systematic echocardiographic study of 96 patients undergoing TAVR with the SAPIEN XT who reached 5‐year follow‐up.8 SVD, defined as leaflet thickening ≥3 mm, presence of calcification, and abnormal leaflet motion, was reported in 29 patients (30%). However, SVD was associated with severe stenosis only in 1 patient and with mild stenosis in 7 patients.8
A key limitation of the previously described studies is the lack of uniform and standardized criteria to define SVD. Recently, a joint consensus statement from the EAPCI, ESC, and EACTS has introduced standardized definitions of SVD and BVF in assessing long‐term durability of transcatheter and surgical aortic bioprosthetic valves.11 The scope of the new definition is to introduce consistency in the reporting of long‐term durability of both THV and surgical bioprostheses, with focus not only on survival or reoperation, but also on the clinical implications of SVD. The present study is the first reporting on long‐term THV performance using such definitions. We were able to obtain >5‐year echocardiographic assessment in >90% of patients, and these data confer strength to the present analysis. At 8 years, the incidence of BVF was 4.5%: THV‐related death was definite in 4 patients. Remaining cases of BVF were caused by severe SVD, which was reported in a total of 7 patients. Restenosis, with or without concomitant regurgitation, was the most common cause of SVD, being reported in 5 cases as a consequence of calcification and tissue ingrowth. Isolated new intraprosthetic regurgitation was found in 2 patients as a consequence of reduced leaflet mobility (leaflet calcification) or endocarditis.
Surgical aortic bioprostheses have shown 10‐year freedom from valvular failure in the range of 60% to 90%. Reported rates of SVD requiring reoperation range widely from 6% to 47% by 12 to 20 years after surgical implantation.16, 17, 18, 19 At 5 years, freedom from structural failure is generally >95%, and although early failure requiring reoperation or leading to mortality has been reported, freedom from reoperation at 5 years is also generally >95%.20, 21
Limitations
The present analysis has some limitations, including the relatively small sample size and its single‐center nature. Also, follow‐up on echocardiographic data was performed in a “survival cohort,” with death possibly exerting a competing risk that may have biased our results. To limit this effect, we reported both actuarial and actual estimates, as recommended by the EAPCI/ESC/EACTS consensus statement.
Conclusions
In an aged population of patients with symptomatic severe AS treated with first‐generation bioprostheses, TAVR was associated with a survival rate of 29.8% but low rates of BVF and SVD at 8 years.
Disclosures
Barbanti is a consultant for Edwards Lifesciences. Corrado Tamburino has received speaker honoraria from Medtronic Inc. All other authors have no conflicts of interest to declare.
(J Am Heart Assoc. 2018;7:e008440 DOI: 10.1161/JAHA.117.008440.)
References
- 1. Barbanti M, Webb JG, Gilard M, Capodanno D, Tamburino C. Transcatheter aortic valve implantation in 2017: state of the art. EuroIntervention. 2017;13:AA11–AA21. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 3. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, Szeto WY, Greason KL, Kereiakes D, Ailawadi G, Whisenant BK, Devireddy C, Leipsic J, Hahn RT, Pibarot P, Weissman NJ, Jaber WA, Cohen DJ, Suri R, Tuzcu EM, Svensson LG, Webb JG, Moses JW, Mack MJ, Miller DC, Smith CR, Alu MC, Parvataneni R, D'Agostino RB, Leon MB. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 4. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators . Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 5. Toggweiler S, Humphries KH, Lee M, Binder RK, Moss RR, Freeman M, Ye J, Cheung A, Wood DA, Webb JG. 5‐Year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:413–419. [DOI] [PubMed] [Google Scholar]
- 6. Barbanti M, Petronio AS, Ettori F, Latib A, Bedogni F, De Marco F, Poli A, Boschetti C, De Carlo M, Fiorina C, Colombo A, Brambilla N, Bruschi G, Martina P, Pandolfi C, Giannini C, Curello S, Sgroi C, Gulino S, Patanè M, Ohno Y, Tamburino C, Attizzani GF, Immè S, Gentili A, Tamburino C. 5‐Year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Interv. 2015;8:1084–1091. [DOI] [PubMed] [Google Scholar]
- 7. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG; PARTNER 1 trial investigators . 5‐Year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 8. Muratori M, Fusini L, Tamborini G, Gripari P, Ghulam Ali S, Mapelli M, Fabbiocchi F, Trabattoni P, Roberto M, Agrifoglio M, Alamanni F, Bartorelli AL, Pepi M. Five‐year echocardiographic follow‐up after TAVI: structural and functional changes of a balloon‐expandable prosthetic aortic valve. Eur Heart J Cardiovasc Imaging. 2017. Available at: https://academic.oup.com/ehjcimaging/advance-article-abstract/doi/10.1093/ehjci/jex046/3095867?redirectedFrom=fulltext. Accessed February 15, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Arsalan M, Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol. 2016;13:360–367. [DOI] [PubMed] [Google Scholar]
- 10. Del Trigo M, Muñoz‐Garcia AJ, Wijeysundera HC, Nombela‐Franco L, Cheema AN, Gutierrez E, Serra V, Kefer J, Amat‐Santos IJ, Benitez LM, Mewa J, Jiménez‐Quevedo P, Alnasser S, Garcia Del Blanco B, Dager A, Abdul‐Jawad Altisent O, Puri R, Campelo‐Parada F, Dahou A, Paradis JM, Dumont E, Pibarot P, Rodés‐Cabau J. Incidence, timing, and predictors of valve hemodynamic deterioration after transcatheter aortic valve replacement: multicenter registry. J Am Coll Cardiol. 2016;67:644–655. [DOI] [PubMed] [Google Scholar]
- 11. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Tamburino C, Piazza N, Hancock J, Mehilli J, Byrne RA, Baumbach A, Kappetein AP, Windecker S, Bax J, Haude M. Standardised definitions of structural deterioration and valve failure in assessing long‐term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017;38:3382–3390. DOI: 10.1093/eurheartj/ehx303. [DOI] [PubMed] [Google Scholar]
- 12. Barbanti M, Sgroi C, Immè S, Aruta P, Deste W, Gulino S, Cannata S, Giarratana A, Bottari V, Giannazzo D, Garretto V, Patanè M, Benvenuto E, Capodanno D, Tamburino C. Usefulness of contrast injection during balloon aortic valvuloplasty before transcatheter aortic valve replacement: a pilot study. EuroIntervention. 2014;10:241–247. [DOI] [PubMed] [Google Scholar]
- 13. Barbanti M, Gulino S, Capranzano P, Immè S, Sgroi C, Tamburino C, Ohno Y, Attizzani GF, Patanè M, Sicuso R, Pilato G, Di Landro A, Todaro D, Di Simone E, Picci A, Giannetto G, Costa G, Deste W, Giannazzo D, Grasso C, Capodanno D, Tamburino C. Acute kidney injury with the renalguard system in patients undergoing transcatheter aortic valve replacement: the PROTECT‐TAVI Trial (PROphylactic effecT of furosEmide‐induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter Aortic Valve Implantation). JACC Cardiovasc Interv. 2015;8:1595–1604. [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB; Valve Academic Research Consortium‐2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 15. Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N, Latib A, Sinning JM, Won‐Keun K, Bleiziffer S, Bedogni F, Kapadia S, Tchetche D, Rodés‐Cabau J, Fiorina C, Nombela‐Franco L, De Marco F, de Jaegere PP, Chakravarty T, Vaquerizo B, Colombo A, Svensson L, Lange R, Nickenig G, Möllmann H, Walther T, Della Rosa F, Elhmidi Y, Dvir D, Brambilla N, Immè S, Sgroi C, Gulino S, Todaro D, Pilato G, Petronio AS, Tamburino C. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9:e003930. [DOI] [PubMed] [Google Scholar]
- 16. Biglioli P, Spampinato N, Cannata A, Musumeci A, Parolari A, Gagliardi C, Alamanni F. Long‐term outcomes of the Carpentier‐Edwards pericardial valve prosthesis in the aortic position: effect of patient age. J Heart Valve Dis. 2004;13(suppl 1):S49–S51. [PubMed] [Google Scholar]
- 17. Neville PH, Aupart MR, Diemont FF, Sirinelli AL, Lemoine EM, Marchand MA. Carpentier‐Edwards pericardial bioprosthesis in aortic or mitral position: a 12‐year experience. Ann Thorac Surg. 1998;66:S143–S147. [DOI] [PubMed] [Google Scholar]
- 18. Borger MA, Ivanov J, Armstrong S, Christie‐Hrybinsky D, Feindel CM, David TE. Twenty‐year results of the Hancock II bioprosthesis. J Heart Valve Dis. 2006;15:49–55; discussion 55–56 [PubMed] [Google Scholar]
- 19. Myken PS, Bech‐Hansen O. A 20‐year experience of 1712 patients with the Biocor porcine bioprosthesis. J Thorac Cardiovasc Surg. 2009;137:76–81. [DOI] [PubMed] [Google Scholar]
- 20. Fleisher AG, Lafaro RJ, Moggio RA. Immediate structural valve deterioration of 27‐mm Carpentier‐Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2004;77:1443–1445. [DOI] [PubMed] [Google Scholar]
- 21. Izutani H, Shibukawa T, Kawamoto J, Mochiduki S, Nishikawa D. Early aortic bioprosthetic valve deterioration in an octogenarian. Ann Thorac Surg. 2008;86:1369–1371. [DOI] [PubMed] [Google Scholar]


