Abstract
Background
Frailty is increasing in prevalence and poses a formidable challenge for clinicians. The cardiac surgery literature consists primarily of small single‐center studies with limited follow‐up, and the epidemiological features of frailty remain to be elucidated in long‐term follow‐up.
Methods and Results
We conducted a population‐based, retrospective, cohort study in Ontario, Canada, between 2008 and 2015. Frailty was defined using the Johns Hopkins Adjusted Clinical Groups frailty indicator (a multidimensional instrument validated for research using administrative data). The primary outcome was mortality. Mortality rates were calculated using the Kaplan‐Meier method. The hazard of death was assessed using a multivariable Cox proportional hazard model. Of 40 083 patients, 8803 (22%) were frail. At 4±2 years of follow‐up, age‐ and sex‐standardized mortality rate per 1000 person‐years was higher in frail (33; 95% confidence interval, 29–36) compared with nonfrail (22; 95% confidence interval, 19–24) patients. Frailty was associated with an increased risk of long‐term mortality (adjusted hazard ratio, 1.20; 95% confidence interval, 1.12–1.28) and greater differences in the survival of patients between 40 and 74 years of age than in those who were ≥85 years old.
Conclusions
Frailty was present in a large proportion of patients undergoing coronary artery bypass grafting and was independently associated with long‐term mortality. The adjusted risk of frailty‐related death was inversely proportional to age. Our findings highlight the need for more comprehensive preoperative risk stratification models to assist with optimal selection of operative candidates. In addition, we identified the <75 years age group as a potential target for comprehensive preoperative optimization programs, such as cardiac prehabilitation, nutritional augmentation, and psychosocial support.
Keywords: aging, coronary artery bypass graft surgery, mortality, prognosis, survival
Subject Categories: Cardiovascular Surgery, Revascularization, Mortality/Survival, Aging
Clinical Perspective
What Is New?
The prevalence of frailty was markedly higher in patients undergoing isolated coronary artery bypass grafting compared with those undergoing elective noncardiac surgery.
Frailty was associated with poor early and long‐term survival, especially in those between 40 and 74 years of age.
The adjusted long‐term frailty‐related mortality risk was inversely proportional to age.
What Are the Clinical Implications?
Frailty should be incorporated into preoperative risk stratification models to assist with optimal selection of operative candidates.
Effective preoperative interventions may improve outcomes, especially in younger patients.
Introduction
Frailty is an emerging concept in perioperative medicine but remains a poorly recognized and poorly investigated syndrome in patients undergoing cardiac surgery.1, 2, 3, 4 It is increasing in prevalence and presents a formidable challenge for clinicians who work to optimize these patients.5 Frailty is a common syndrome described as a diminished resiliency in response to stress as a result of decreased physiological reserve, increased burden of comorbidities, and altered multisystem homeostasis.6 Frailty increases susceptibility to adverse health outcomes and contributes to the difference between chronological and physiological age.7 It is associated with increased mortality, surgical site infections, length of hospital stay, increased healthcare expenditure, and readmission rates in patients presenting for a variety of major noncardiac surgeries.8, 9, 10, 11, 12 Frailty has also been reported as a risk factor for mortality, major adverse cardiac and cerebrovascular events, and increased length of hospital stay in cardiac surgical patients.1, 2, 13, 14, 15, 16 The cardiac surgery literature consists primarily of relatively small single‐center studies with limited follow‐up durations, and the epidemiological features of frailty remain to be elucidated in long‐term follow‐up. We investigated the prevalence of frailty and its association with long‐term mortality in patients who underwent primary isolated coronary artery bypass grafting (CABG) in Ontario, Canada, from 2008 to 2015.
Methods
Design and Study Population
We conducted a population‐based, retrospective, cohort study in Ontario, Canada, between October 1, 2008, and March 31, 2015. The Research Ethics Board of Sunnybrook Health Sciences (Toronto, ON, Canada) approved this study and waived the need for informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
This study included adult patients ≥40 years of age, who underwent primary isolated CABG in Ontario. We excluded patients who were non‐Ontario residents, who had a history of cardiac surgery, or who had missing information on age, sex, and left ventricular ejection fraction (EF; Figure 1). During the study period, Ontario was Canada's most populous province, with a publicly funded universal healthcare system that reimbursed all healthcare providers and services.
Figure 1.
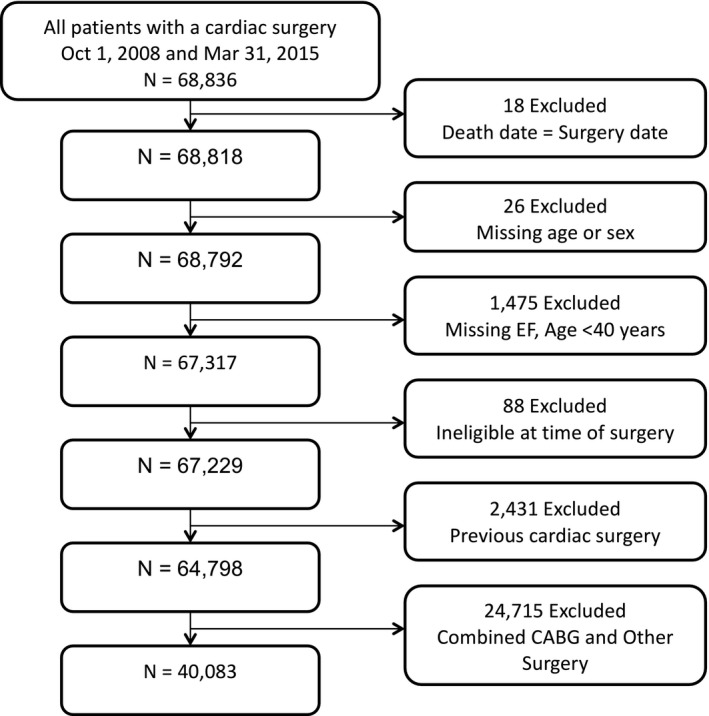
Cohort flowchart. CABG indicates coronary artery bypass grafting; EF, ejection fraction.
Data Sources
The authors used the registry data of CorHealth Ontario and population‐level administrative healthcare databases with information on all Ontario residents available at the Institute for Clinical Evaluative Sciences. Individuals who underwent primary isolated CABG were identified from the CorHealth Ontario Cardiac Registry. The CorHealth Ontario Cardiac Registry captures data from all 19 hospitals that provide invasive cardiac procedures across the province. The registry contains detailed demographic, comorbidity, and procedural‐related information and has been validated through selected chart audits. In addition, EF and angiographic data in the CorHealth Ontario registry undergo core laboratory validation.17
Administrative databases were linked deterministically using encrypted identifiers that preserved patient confidentiality. The authors linked the CorHealth Ontario registry (date and type of cardiac procedures, comorbidities, EF, and angiographic data) with the Canadian Institute for Health Information's Discharge Abstract Database (comorbidities and hospital admissions) and Same Day Surgery database (comorbidities), Ontario Health Insurance Plan database (physician service claims), Registered Persons Database (ascertainment of vital statistics), and Canadian census. Although lacking in physiologic and laboratory measures, these administrative databases have been validated for many outcomes, exposures, and comorbidities.18, 19, 20, 21
Exposure
Assessments of frailty can be made with clinical scales,22 functional assessments,13, 23 and their combination,24 or by the presence of a constellation of frailty‐defining diagnoses.5, 8, 25 This last method uses the Johns Hopkins Adjusted Clinical Groups (ACG) frailty‐defining diagnoses indicator, which is an instrument based on 10 clusters of frailty‐defining diagnoses (ie, malnutrition, dementia, impaired vision, decubitus ulcer, incontinence of urine, loss of weight, poverty, barriers to access to care, difficulty in walking, and falls; Table S1). This binary indicator identifies frailty by the presence of ≥1 diagnostic clusters and was designed and validated for research of frailty‐related outcomes and resource use using administrative data.26, 27, 28, 29, 30 It has been used to study the prevalence, outcomes, and resource use in patients undergoing major elective noncardiac surgery5 and total joint arthroplasty in Ontario.8 Because of the proprietary nature of the ACG system, the authors are unable to provide the specific diagnostic codes used to define this indicator. In the present study, frailty was defined using comorbidity and health resource use data available within 5 years before the index CABG.
The ACG frailty indicator has been externally validated using the Vulnerable Elderly Scale. The findings of this validation study indicate that the ACG frailty indicator had moderate ability to discriminate between frail and nonfrail patients when compared with the Vulnerable Elderly Scale (area under the curve, 0.62).27 The lack of strength of correlation between the 2 instruments may reflect the lack of a gold standard for frailty assessment. To date, several frailty scales exist to capture related, but distinct, groups that form different dimensions of frailty.31 A direct comparison of 8 commonly used frailty instruments demonstrated limited agreement between these instruments.31 The ACG frailty indicator captures patients with multidimensional frailty at the population level and has been shown to accurately identify patients with limitations in activities of daily living.26
Covariates
Comorbidities were identified from the CorHealth Ontario registry and supplemented with data from the Discharge Abstract Database, the Same Day Surgery database, and the Ontario Health Insurance Plan database using International Classification of Diseases, Tenth Revision, Canada (ICD‐10‐CA) codes32 within 5 years before CABG using validated algorithms.18, 20, 33, 34, 35 We estimated socioeconomic status on the basis of patients’ neighborhood median income in the Canadian census and determined their residence (rural versus urban) using Statistics Canada definitions.36 Emergent procedural status was ascertained from the CorHealth Ontario registry and supplemented with Ontario Health Insurance Plan claims data, where the anesthesia provider identified the surgery as emergent under the American Society of Anesthesiologists physical status classification. Height, weight, and body mass index identified from the CorHealth Ontario registry were used to define morbid obesity (weight, >159 kg; or body mass index, ≥40 kg/m2). Preoperative EF was obtained from the CorHealth Ontario registry and classified as preserved if EF was ≥50% and reduced if EF was <50%. We then categorized heart failure (HF) status into 4 groups: HF with preserved EF, HF with reduce EF, preserved EF without HF, and reduced EF without HF.
Outcome
The primary outcome was death from any cause. We confirmed in‐hospital mortality using the Discharge Abstract Database and postdischarge mortality using the Registered Persons Database.
Statistical Analysis
L.Y.S. and A.B.E. had full access to all the data in the study and take responsibility for their integrity and for the data analysis. Continuous variables were expressed as mean (SD), and categorical variables were expressed as number (proportions). Mortality was assessed through March 31, 2016. Patients were censored when they lost possession of a valid Ontario health insurance number for 2 consecutive eligibility quarters (ie, have left the province of Ontario). Survival time was defined as date of index surgery until date of death or date of last follow‐up. Event rates in each group were calculated using the Kaplan‐Meier method and presented graphically, with the significance of differences in mortality between groups assessed using the log‐rank test. Age‐stratified mortality rates were standardized by sex, and pooled mortality rates were standardized by age and sex, using the 2011 Canadian population as the reference population. The relative hazard of death was assessed using Cox proportional hazard models with multivariable adjustment. To avoid redundant adjustment of risk factors that are already a part of the aggregate Johns Hopkins ACG frailty indicator, we did not control for medical comorbidities and instead adjusted only for age, sex, socioeconomic status, and case urgency status in our primary model. We then explored the association of frailty and long‐term mortality in subgroups stratified by age and sex by plotting adjusted Kaplan‐Meier curves within these subgroups.
The measure of association was hazard ratios (HRs) with 95% confidence intervals (CIs). Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC), with statistical significance defined by a 2‐sided P<0.05.
Sensitivity Analyses
We used generalized estimating equations to determine the adjusted association of patient‐level characteristics with long‐term mortality while accounting for clustering of patients within hospitals. In addition, we assessed whether the association of frailty and long‐term mortality would be altered by completeness of revascularization at the time of surgery. This was accomplished by adding completeness of revascularization to the original Cox proportional hazards model. Incomplete revascularization was defined as presence of ≥1 ungrafted vessels with ≥70% stenosis in the left anterior descending, circumflex, or right coronary artery territories. Finally, we repeated these sensitivity analyses in an expanded model exploring the association of frailty and long‐term mortality while adjusting for all comorbidities.
Results
Prevalence of Frailty in Patients Undergoing CABG
A total of 40 083 consecutive patients who underwent isolated CABG from 2008 to 2015 were included in the study (Figure 1). Of these patients, 8803 (22.0%) were frail. The prevalence of frailty was higher in older age groups. Specifically, 3562 (20.2%) of the 40 to 64 years age group, 3029 (21.7%) of the 65 to 74 years age group, 2018 (25.6%) of the 75 to 84 years age group, and 194 (31.5%) of the ≥85 years age group were frail. Table 1 summarizes the demographics and comorbidities of frail versus nonfrail patients. Frail patients were more likely to be women, to be >75 years of age, and to have rural places of residence, lower income status, hypertension, preserved EF, atrial fibrillation, remote and recent myocardial infarction, HF, cerebral and peripheral vascular disease, chronic pulmonary disease, diabetes mellitus, hypothyroidism, anemia, renal and liver disease, malignancies, and dementia.
Table 1.
Baseline Characteristics by Frailty Status
| Variable | Frail (n=8803) | Not Frail (n=31 280) | Total (N=40 083) | P Value |
|---|---|---|---|---|
| Age, mean±SD, y | 66.75±10.06 | 65.58±9.78 | 65.84±9.85 | <0.001 |
| 40–64 | 3562 (40.5) | 14 063 (45.0) | 17 625 (44.0) | <0.001 |
| 65–74 | 3029 (34.4) | 10 940 (35.0) | 13 969 (34.9) | |
| 75–84 | 2018 (22.9) | 5855 (18.7) | 7873 (19.6) | |
| ≥85 | 194 (2.2%) | 422 (1.3%) | 616 (1.5%) | |
| Female sex | 2661 (30.2) | 5587 (17.9) | 8248 (20.6) | <0.001 |
| Rural | 1481 (16.8) | 4650 (14.9) | 6131 (15.3) | <0.001 |
| Income quintile | ||||
| 1 (Lowest) | 1952 (22.2) | 5666 (18.1) | 7618 (19.0) | <0.001 |
| 2 | 1898 (21.6) | 6279 (20.1) | 8177 (20.4) | |
| 3 | 1674 (19.0) | 6401 (20.5) | 8075 (20.1) | |
| 4 | 1710 (19.4) | 6457 (20.6) | 8167 (20.4) | |
| 5 (Highest) | 1511 (17.2) | 6321 (20.2) | 7832 (19.5) | |
| Missing | 58 (0.7) | 156 (0.5) | 214 (0.5) | |
| Remote MI | 2698 (30.6) | 7657 (24.5) | 10 355 (25.8) | <0.001 |
| Recent MI | 5092 (57.8) | 13 123 (42.0) | 18 215 (45.4) | <0.001 |
| Previous PCI | 1475 (16.8) | 4812 (15.4) | 6287 (15.7) | 0.002 |
| Hypertension | 8045 (91.4) | 26 984 (86.3) | 35 029 (87.4) | <0.001 |
| Atrial fibrillation | 856 (9.7) | 1899 (6.1) | 2755 (6.9) | <0.001 |
| LVEF, % | ||||
| ≥50 | 19 746 (63.1) | 5237 (59.5) | 24 983 (62.3) | <0.001 |
| 35–49 | 7896 (25.2) | 2348 (26.7) | 10 244 (25.6) | |
| 20–34 | 3084 (9.9) | 1010 (11.5) | 4094 (10.2) | |
| <20 | 554 (1.8) | 208 (2.4) | 762 (1.9) | |
| HF status | ||||
| pEF, no HF | 4339 (49.3) | 17 892 (57.2) | 22 231 (55.5) | <0.001 |
| rEF, no HF | 2097 (23.8) | 8187 (26.2) | 10 284 (25.7) | |
| HFpEF | 898 (10.2) | 1854 (5.9) | 2752 (6.9) | |
| HFrEF | 1469 (16.7) | 3347 (10.7) | 4816 (12.0) | |
| Cerebral vascular disease | 1179 (13.4) | 2873 (9.2) | 4052 (10.1) | <0.001 |
| Peripheral vascular disease | 1448 (16.4) | 3681 (11.8) | 5129 (12.8) | <0.001 |
| COPD/asthma | 3050 (34.6) | 8089 (25.9) | 11 139 (27.8) | <0.001 |
| Diabetes mellitus | 4909 (55.8) | 13 999 (44.8) | 18 908 (47.2) | <0.001 |
| Morbid obesity | 3179 (36.1) | 8966 (28.7) | 12 145 (30.3) | <0.001 |
| Hypothyroidism | 156 (1.8) | 271 (0.9) | 427 (1.1) | <0.001 |
| Anemia | 626 (7.1) | 1019 (3.3) | 1645 (4.1) | <0.001 |
| Dialysis | 291 (3.3) | 552 (1.8) | 843 (2.1) | <0.001 |
| Chronic renal disease | 636 (7.2) | 1247 (4.0) | 1883 (4.7) | <0.001 |
| Liver disease | 94 (1.1) | 194 (0.6) | 288 (0.7) | <0.001 |
| Primary tumor | 468 (5.3) | 1397 (4.5) | 1865 (4.7) | <0.001 |
| Metastatic tumor | 52 (0.6) | 128 (0.4) | 180 (0.4) | 0.024 |
| Dementia | 104 (1.2) | 7 (0.0) | 111 (0.3) | <0.001 |
| Emergent surgery | 541 (6.1) | 2050 (6.6) | 2591 (6.5) | 0.17 |
Data are given as number (percentage) unless otherwise indicated. COPD indicates chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; pEF, preserved ejection fraction; rEF, reduced ejection fraction.
Thirty‐Day Mortality
At 30 days, 626 patients (1.6%) patients died, of whom 174 (27.8%) were frail. Table 2 summarizes the sex‐standardized mortality rates of frail and nonfrail patients, stratified by age. Frail patients had higher rates of 30‐day mortality than those who were not frail, across all but the ≥85 years age group.
Table 2.
Early and Long‐Term Mortality Rates After CABG, by Frailty Status
| Variable | Frail (n=8803) | Not Frail (n=31 280) | ||
|---|---|---|---|---|
| N (%) | SMR (95% CI)a | N (%) | SMR (95% CI)a | |
| 30‐d mortality | ||||
| Age, y | ||||
| 40–64 | 40 (0.5) | 0.3 (0.1–0.4) | 101 (0.3) | 0.2 (0.1–0.2) |
| 65–74 | 57 (0.6) | 0.1 (0.08–0.1) | 140 (0.4) | 0.07 (0.06–0.08) |
| 75–84 | 69 (0.8) | 0.1 (0.09–0.2) | 184 (0.6) | 0.1 (0.09–0.1) |
| ≥85 | 8 (0.09) | 0.04 (0.02–0.07) | 27 (0.09) | 0.08 (0.05–0.1) |
| Overall | 174 (2.0) | 0.5 (0.4–0.7) | 452 (1.4) | 0.4 (0.4–0.5) |
| Long‐term mortality | ||||
| Age, y | ||||
| 40–64 | 332 (3.8) | 13.9 (11.7–16.4) | 737 (2.4) | 7.1 (6.4–7.8) |
| 65–74 | 491 (5.6) | 6.7 (6.1–7.3) | 1037 (3.3) | 3.7 (3.5–3.9) |
| 75–84 | 540 (6.1) | 7.2 (6.6–7.9) | 1090 (3.5) | 5.0 (4.7–5.3) |
| ≥85 | 66 (0.7) | 4.2 (2.8–6.1) | 130 (0.4) | 4.3 (3.3–5.5) |
| Overall | 1429 (16.2) | 32.0 (29.2–35.1) | 2994 (9.6) | 20.0 (18.7–21.4) |
CABG indicates coronary artery bypass grafting; CI, confidence interval; SMR, standardized mortality rate.
Age‐stratified SMRs are standardized by sex. Overall SMRs are standardized by age and sex.
Long‐Term Mortality
The mean follow‐up period was 4±2 years, with a total follow‐up time of 58 081 087 person‐years. A total of 4423 patients (11.0%) died during long‐term follow‐up, of whom 1429 (32.3%) were frail. Age‐ and sex‐standardized long‐term mortality rate per 1000 person‐years was 33 (95% CI, 29–36) in frail patients and 22 (95% CI, 19–24) in nonfrail patients (Table 2). Adjusted Kaplan‐Meier survival curves illustrate lower probabilities of survival in patients who were frail at the time of surgery (Figure 2).
Figure 2.
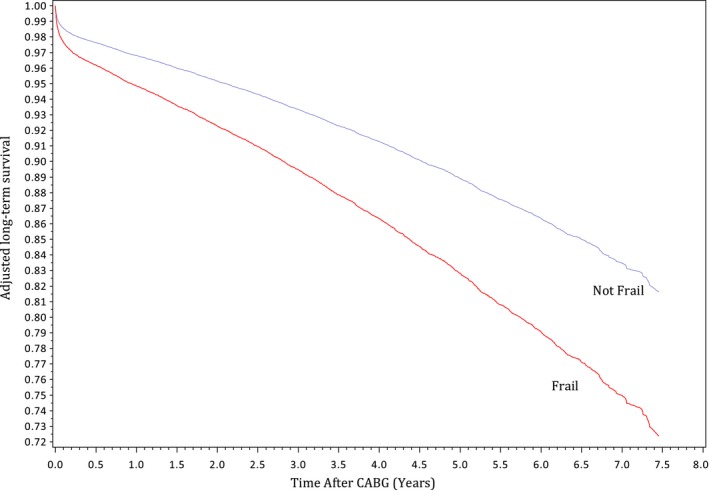
Adjusted estimated long‐term survival by frailty status. Curves were adjusted for age, sex, socioeconomic status, and case urgency status. CABG indicates coronary artery bypass grafting.
Table 3 illustrates the multivariable correlates of long‐term mortality. Frailty was correlated with an increased risk of mortality (adjusted HR, 1.63; 95% CI, 1.53–1.74). Other independent mortality correlates were age, socioeconomic status, and urgent case status. The association of frailty and long‐term mortality remained robust in the sensitivity analyses that accounted for clustering of patients within hospitals and completeness of revascularization. Incomplete revascularization was associated with a higher risk of long‐term mortality (adjusted HR, 1.28; 95% CI, 1.20–1.36). Furthermore, the association of frailty and long‐term mortality also remained robust in the sensitivity analysis that controlled for all comorbidities (Table S2). In this analysis, other independent mortality correlates were remote and recent myocardial infarction, hypertension, reduced EF, HF, cerebral and peripheral vascular disease, chronic pulmonary disease, diabetes mellitus, dialysis, chronic renal disease, anemia, malignancies, and dementia.
Table 3.
Multivariable Predictors of Long‐Term Mortality After CABG
| Variable | Main Model | Cluster by Site | Completeness of Revascularization Added |
|---|---|---|---|
| Frailty | 1.63 (1.53–1.74) | 1.63 (1.53–1.74) | 1.63 (1.53–1.74) |
| Age group, y | |||
| 40–64 | Reference | Reference | Reference |
| 65–74 | 1.92 (1.77–2.07) | 1.9 (1.75–2.05) | 1.89 (1.75–2.05) |
| 75–84 | 3.73 (3.45–4.03) | 3.69 (3.41–3.99) | 3.63 (3.36–3.93) |
| ≥85 | 6.22 (5.34–7.25) | 6.05 (5.19–7.05) | 5.91 (5.06–6.9) |
| Sex (reference=male) | 1.04 (0.97–1.11) | 1.04 (0.97–1.11) | 1.04 (0.97–1.11) |
| Income quintile | |||
| 1 (Lowest) | 1.43 (1.3–1.57) | 1.41 (1.28–1.55) | 1.44 (1.31–1.59) |
| 2 | 1.27 (1.16–1.4) | 1.26 (1.14–1.39) | 1.27 (1.16–1.4) |
| 3 | 1.13 (1.03–1.25) | 1.13 (1.02–1.24) | 1.14 (1.04–1.26) |
| 4 | 1.12 (1.01–1.23) | 1.12 (1.01–1.23) | 1.12 (1.02–1.24) |
| 5 (Highest) | Reference | Reference | Reference |
| Missing | 1.42 (0.94–2.15) | 1.39 (0.92–2.1) | 1.41 (0.93–2.14) |
| Emergent surgery | 1.7 (1.54–1.87) | 1.7 (1.55–1.88) | 1.66 (1.51–1.83) |
| Incomplete revascularization | ··· | ··· | 1.28 (1.2–1.36) |
Data are given as hazard ratio (95% confidence interval). CABG indicates coronary artery bypass grafting.
Greater differences in long‐term mortality rates between frail and nonfrail patients were observed in the younger age groups (Table 2). The adjusted Kaplan‐Meier curves in Figure 3 demonstrated decreasing probability of survival with increasing age. Frailty contributed to greater differences in the survival of patients between 40 and 74 years of age and smaller differences in the long‐term survival of those who were ≥85 years old. When the relative hazard of death was plotted against age as a continuous variable (Figure 4), an inverse relationship was again illustrated, such that frailty posed a higher adjusted risk of mortality in younger patients and lower impact on older patients. When we explored the differences in survival by sex within each of the 4 age groups (Figure 5), we found higher probabilities of long‐term survival in men <75 years of age and in women ≥75 years of age.
Figure 3.
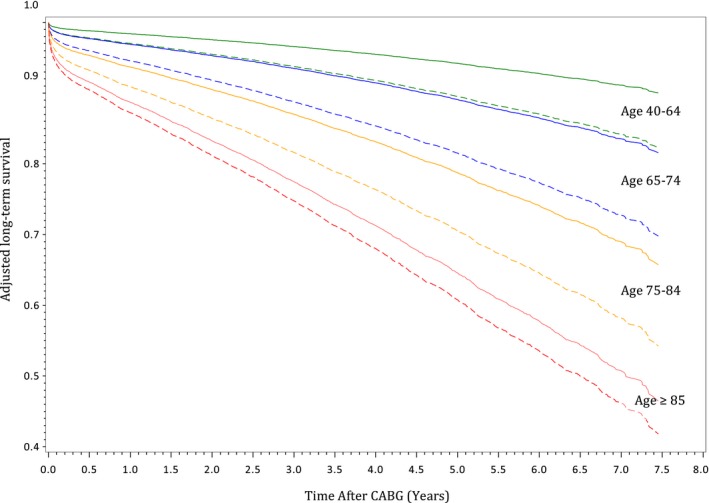
Adjusted estimated long‐term survival of frail and nonfrail patients, stratified by age group. Curves were adjusted for sex, socioeconomic status, and case urgency status. The solid lines represent estimated survival in nonfrail patients. The dotted lines represent estimated survival in frail patients. CABG indicates coronary artery bypass grafting.
Figure 4.
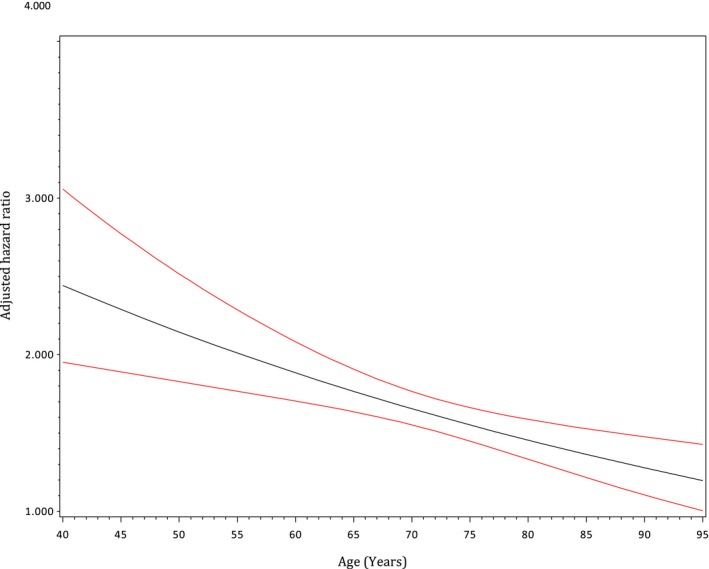
Age‐dependent adjusted relative hazard of long‐term mortality in frail vs nonfrail patients. The black line represents the hazard ratio (adjusted for all variables presented in Table 3, except for age). The red lines represent 95% confidence intervals.
Figure 5.
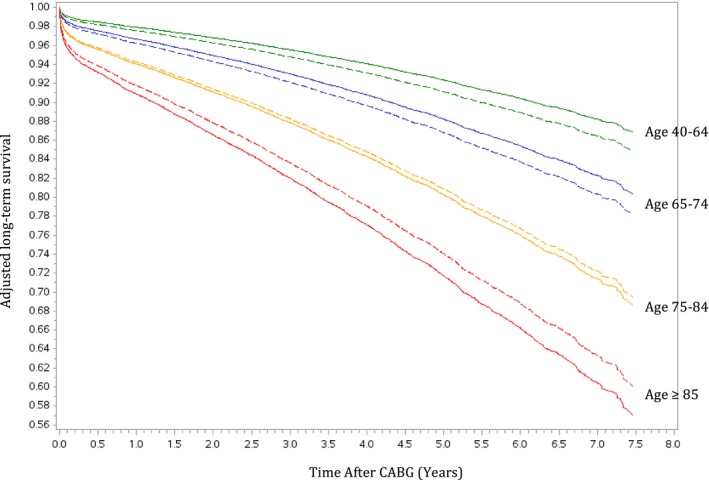
Adjusted estimated long‐term survival, stratified by age and sex. The solid lines represent estimated survival in men. The dotted lines represent estimated survival in women. CABG indicates coronary artery bypass grafting.
Discussion
This population‐based study found a high prevalence of frailty in patients undergoing CABG and higher early and long‐term mortality rates in these patients. Three main findings were derived from this study. (1) The burden of frailty was markedly higher in patients undergoing CABG (22%) compared with patients undergoing major noncardiac surgery (3%) during a similar time period in Ontario.5 (2) Frail patients had lower probabilities of early and long‐term survival compared with their nonfrail counterparts. This difference in survival was more evident in the younger age groups (<75 years). (3) The adjusted long‐term frailty‐related mortality risk was inversely proportional to age.
Burden of Frailty in Patients Undergoing CABG
Frailty is a syndrome prevalent in the geriatric population that does not have a universally accepted definition. The different measures of frailty include physical phenotype,37 clinical functional assessments,22 and functional assessments in combination with disability or laboratory values; these have all been studied in the general population.7 The prevalence of frailty assessed by physical phenotype is 9.9% and 13.6% when using a broader definition in adults >64 years of age.7 In comparison, a study of 152 cardiac surgery patients using 3 different frailty scales reported a prevalence of 20% to 46%, depending on the method chosen to define frailty.14 When 11 different frailty measures were compared in a similarly sized cardiac surgery cohort, a prevalence of 4.8% to 47% was reported.38 These diverse ranges likely reflect the diversity of frailty instruments used, the poor correlation between instruments, and the differences in the prevalence of frailty by age and surgery type. The present study used the Johns Hopkins ACG frailty‐defining diagnoses indicator and found that the prevalence of frailty was 22% in patients undergoing CABG ≥40 years of age, which is in keeping with the ranges described for other cardiac surgery cohorts. It is, however, markedly higher than the 3.1% prevalence found in a contemporary Ontario noncardiac surgery cohort (2002–2012) aged ≥65 years using the same ACG indicator.5 This higher prevalence of frailty in cardiac surgery patients most likely reflects the higher proportion of medically complex patients presenting for cardiac versus noncardiac surgery. Frail patients are more likely to experience procedural failure, complications, and worsening frailty after the hospitalization39 that is common after CABG. Our findings highlight the need for comprehensive risk stratification tools to optimize the patient selection process and to facilitate patient‐centered discussions on treatment options.
Frailty and Perioperative Risk Stratification
Clinicians often struggle to quantify the perioperative risk of morbidity and mortality in elderly patients, because tools such as the Society of Thoracic Surgeons risk and the European System for Cardiac Operation Risk Evaluation were not designed to comprehensively assess the complex interaction between comorbidities and biological versus physiological age. The European System for Cardiac Operation Risk Evaluation II has been shown to overestimate mortality in isolated patients undergoing CABG,40 whereas the Society of Thoracic Surgeons score underestimates it.13 However, the incorporation of a frailty score into such risk models has been shown to improve model discrimination.14, 23, 24 The high burden of frailty in patients undergoing CABG accentuates the need for further research to validate these more inclusive risk scores in larger population‐based cohorts and for prospective trials guided by these new scores to determine whether alternative revascularization strategies (eg, percutaneous coronary intervention) and/or comprehensive preoperative nutritional, psychological support, and physical conditioning programs would improve outcomes in frail patients.41, 42, 43
Frailty and Mortality
To our knowledge, this is the largest cohort study to describe the long‐term outcomes of frailty in patients undergoing CABG surgery. Frailty has been studied in the cardiac surgery literature using a variety of definitions. A Veteran Affairs report on 11 815 patients undergoing CABG described an intermediate‐term mortality HR of 2.4 (95% CI, 1.6–3.7) at 31 to 210 days for patients who had totally dependent functional status and an HR of 1.5 (95% CI, 1.1–1.9) for partially dependent individuals.44 The second largest frail‐related study in cardiac surgery patients was based on a single center cohort of 3826 patients.1 This study found frailty to be associated with an increased risk of mortality in hospital (adjusted odds ratio, 1.8; 95% CI, 1.1–3.0) and at 2 years (adjusted HR, 1.5; 95% CI, 1.1–2.2). We found an HR of 1.6 (95% CI, 1.5–1.7) associated with frailty at longer follow‐up of 4±2 years. Our effect size is similar to those published in the literature, and our findings suggest that although frailty had a larger impact on short‐term mortality,1 its adverse impact remained robust in long‐term follow‐up. In addition, the wide range of published prevalence and frailty‐related mortality risk is likely because of the differences in frailty instruments used. A systematic review of 8 studies directly comparing 9 different frailty instruments used in cardiac surgery cohorts noted that the multicomponent instruments provided better mortality risk prediction, despite poorer discrimination than their abbreviated single‐domain counterparts.3 The Johns Hopkins ACG frailty indicator is a comprehensive tool that provides a multidimensional snapshot of a patient's physical status and is able to predict the risk of long‐term mortality in this cohort of patients undergoing CABG.
Our finding of the modifying effect of age in the association between frailty and mortality corroborated other contemporary population‐based reports in Ontario.5 This frailty‐age interaction has otherwise not been well defined in the literature. Frailty has traditionally been described as a syndrome of elderly people.45 In our study, the presence of frailty‐defining diagnoses was a stronger predictor of mortality in younger patients. Our findings highlight the importance of concepts of “physiological” versus “biological” aging in the prognosis of patients. This finding may also reflect the limited life expectancy regardless of frailty status in those ≥85 years of age and/or possibly the limited discriminative ability of the John's Hopkins frailty indicator in this advanced age group because of the existence of many comorbidities in this age group that overlap with components of this frailty indicator. Further studies are needed to explore the validity of different frailty instruments in a variety of age groups.
Consistent with other reports,1, 13 sex was not an independent predictor of long‐term mortality in our pooled analysis. However, our adjusted Kaplan‐Meier plots (Figure 5) demonstrated a higher probability of long‐term survival in men <75 years of age and in women ≥75 years of age. Further studies are needed to explore the sex differences in the prognosis of patients undergoing CABG and means to improve outcomes in women and men.
Weaknesses and Strengths
This study has several limitations. First, the prevalence and outcomes of frailty are representative of perioperative practices in Ontario. Similar research needs to be conducted in other settings to confirm the generalizability of our findings. However, during the study period, 38% of the Canadian population46 resided in Ontario, and our universal healthcare system allowed for unbiased representation from diverse demographic groups. Second, our data sources lacked some relevant detailed physiologic measures of physical and nutritional frailty, such as the 5‐minute walk test, grip strength, albumin,47 liver function, creatinine, and brain natriuretic peptide.24 The inability to measure, and thereby adjust for, differences in such characteristics could have explained, in part, the differences in mortality rates observed in this study. Despite this shortcoming, our capture of covariate information was based on validated algorithms within high‐quality administrative databases, which were complemented by detailed patient‐level data from the robust CorHealth Ontario provincial registry. Our CABG cohort was well defined using valid procedural codes that were cross validated with the CorHealth Ontario registry records,48 and our ascertainment of mortality was based on province‐wide vital statistics data, ensuring complete follow‐up for all patients. Third, the binary Johns Hopkins ACG frailty‐defining diagnoses indicator did not allow for outcome assessment at different levels of frailty. However, this indicator is also an externally validated multidimensional method for characterizing frailty that has been used in high‐impact population‐based studies of frailty‐related outcomes using a similar set of Ontario administrative databases.5, 8 Finally, cohort studies are by nature subjected to residual confounding. However, our study was the largest to date to describe the long‐term outcomes of frail patients. In addition, the inclusion of candidates undergoing a single low‐risk cardiac procedure allowed for the examination of the frailty‐mortality relationship without the interfering effect of surgical complexity.
Conclusions
Frailty was present in a disproportionate number of patients undergoing CABG compared with major noncardiac surgery. Frailty was independently associated with long‐term mortality. More important, the adjusted risk of frailty‐related death was inversely proportional to age, such that frailty was a stronger predictor of long‐term mortality in those <75 years of age. Our findings highlight the need for more comprehensive preoperative risk stratification models to assist with optimal selection of operative candidates. In addition, the subgroup of younger patients who are frail may benefit most from preoperative optimization programs, such as cardiac prehabilitation, nutritional augmentation, and psychosocial support.41, 42, 43
Sources of Funding
We acknowledge support from an operating grant from the University of Ottawa Department of Anesthesiology and Pain Medicine (grant 4566). Tu is supported by a Canada Research Chair in Health Services Research and a Career Investigator Award from the Heart and Stroke Foundation of Ontario. The funders do not have a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; nor in the preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. Johns Hopkins ACG Frailty‐Defining Diagnosis Indicators
Table S2. Multivariable Predictors of Long‐Term Mortality Post CABG in the Expanded Model
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The authors acknowledge that the clinical registry data used in this analysis are from participating hospitals through CorHealth Ontario, which serves as an advisory body to the MOHLTC, is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access, and equity in the delivery of the continuum of adult cardiac, vascular, and stroke services in Ontario, Canada. The authors also acknowledge the use of data compiled and provided by the Canadian Institute for Health Information. These data sets were linked using unique encoded identifiers and analyzed at the ICES. The analyses, conclusions, opinions, and statements expressed in the article are those of the authors, and do not necessarily reflect those of the previously described agencies.
(J Am Heart Assoc. 2018;7:e009882 DOI: 10.1161/JAHA.118.009882.)
References
- 1. Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. [DOI] [PubMed] [Google Scholar]
- 2. Sepehri A, Beggs T, Hassan A, Rigatto C, Shaw‐Daigle C, Tangri N, Arora RC. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–3117. [DOI] [PubMed] [Google Scholar]
- 3. Kim D, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016;165:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furukawa H, Tanemoto K. Frailty in cardiothoracic surgery: systematic review of the literature. Gen Thorac Cardiovasc Surg. 2015;63:425–433. [DOI] [PubMed] [Google Scholar]
- 5. McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1‐year postoperative mortality following major elective noncardiac surgery: a population‐based cohort study. JAMA Surg. 2016;151:538–545. [DOI] [PubMed] [Google Scholar]
- 6. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 7. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. [DOI] [PubMed] [Google Scholar]
- 8. McIsaac DI, Beaule PE, Bryson GL, Van Walraven C. The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty: a population‐based cohort study. Bone Joint J. 2016;98‐B:799–805. [DOI] [PubMed] [Google Scholar]
- 9. Nieman CL, Pitman KT, Tufaro AP, Eisele DW, Frick KD, Gourin CG. The effect of frailty on short‐term outcomes after head and neck cancer surgery. Laryngoscope. 2018;128:102–110. [DOI] [PubMed] [Google Scholar]
- 10. Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183:40–46. [DOI] [PubMed] [Google Scholar]
- 11. Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27:904–908. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Pederson JL, Churchill TA, Wagg AS, Holroyd‐Leduc JM, Alagiakrishnan K, Padwal RS, Khadaroo RG. Impact of frailty on outcomes after discharge in older surgical patients: a prospective cohort study. Can Med Assoc J. 2018;190:E184–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 14. Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, Morin JF, Langlois Y, Ohayon SM, Monette J, Boivin JF, Shahian DM, Bergman H. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. [DOI] [PubMed] [Google Scholar]
- 15. Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single‐center experience. JACC Cardiovasc Interv. 2012;5:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sundermann S, Dademasch A, Rastan A, Praetorius J, Rodriguez H, Walther T, Mohr FW, Falk V. One‐year follow‐up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;13:119–123; discussion 123. [DOI] [PubMed] [Google Scholar]
- 17. Tu JV, Ko DT, Guo H, Richards JA, Walton N, Natarajan MK, Wijeysundera HC, So D, Latter DA, Feindel CM, Kingsbury K, Cohen EA; Cardiac Care Network of Ontario's Variations in Revascularization Practice in Ontario Working Group . Determinants of variations in coronary revascularization practices. CMAJ. 2012;184:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tu K, Campbell NRC, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 19. Juurlink D, Preya C, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study: ICES Investigative Report. Toronto, ON, Canada: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 20. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. [DOI] [PubMed] [Google Scholar]
- 22. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Arenaza DP, Pepper J, Lees B, Rubinstein F, Nugara F, Roughton M, Jasinski M, Bazzino O, Flather M. Preoperative 6‐minute walk test adds prognostic information to Euroscore in patients undergoing aortic valve replacement. Heart. 2010;96:113–117. [DOI] [PubMed] [Google Scholar]
- 24. Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high‐risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. [DOI] [PubMed] [Google Scholar]
- 25. Chimukangara M, Helm MC, Frelich MJ, Bosler ME, Rein LE, Szabo A, Gould JC. A 5‐item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc. 2017;31:2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abrams C, Lieberman R, Weiner J. Development and evaluation of the Johns Hopkins University risk adjustment models for Medicare+ choice plan payment. 2003. Available at: https://www.hopkinsacg.org/document/development-and-evaluation-of-the-johns-hopkins-university-risk-adjustment-models-for-medicarechoice-plan-payment/. Accessed June 24, 2018.
- 27. Sternberg SA, Bentur N, Abrams C, Spalter T, Karpati T, Lemberger J, Heymann AD. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18:e392–e397. [PubMed] [Google Scholar]
- 28. Weiner JP, Abrams C. The Johns Hopkins Adjusted Clinical Groups Technical Reference Guide, version 9.0. Baltimore, MD, USA: Johns Hopkins University Press; 2009.
- 29. Bronskill SE, Carter MW, Costa AP, Esensoy AV, Gill SS, Gruneir A, Henry DA, Hirdes JP, Jaakkimainen RL, Poss JW, Wodchis WP. Aging in Ontario: An ICES Chartbook of Health Services Use by Older Adults—A Technical Report. 2010. Available at: https://www.ices.on.ca/Publications/Atlases-and-Reports/2010/Aging-in-Ontario. Accessed June 24, 2018. [Google Scholar]
- 30. Bronskill SE, Camacho CX, Gruneir A, Ho MM. Health system use by frail Ontario seniors: an in‐depth examination of four vulnerable cohorts. Toronto: Institute for Clinical Evaluative Sciences (ICES). 2011. http://www.ices.on.ca/file/ICES-AgingReport-2011.pdf.
- 31. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all‐cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. [DOI] [PubMed] [Google Scholar]
- 32. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 33. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 34. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying patients with physician‐diagnosed asthma in health administrative databases. Can Respir J. 2009;16:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6:388–394. [DOI] [PubMed] [Google Scholar]
- 36. du Plessis V, Beshiri R, Bollman RD, Clemeson H. Definitions of “Rural”: Agriculture and Rural Working Paper Series, No. 61. Ottawa, ON, Canada: Statistics Canada; 2002. [Google Scholar]
- 37. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 38. Marshall L, Griffin R, Mundy J. Frailty assessment to predict short term outcomes after cardiac surgery. Asian Cardiovasc Thorac Ann. 2016;24:546–554. [DOI] [PubMed] [Google Scholar]
- 39. Graham MM, Simpson CS. Aging well in an era of high‐tech cardiovascular care. Can J Cardiol. 2017;33:961–962. [DOI] [PubMed] [Google Scholar]
- 40. Guida P, Mastro F, Scrascia G, Whitlock R, Paparella D. Performance of the European System for Cardiac Operative Risk Evaluation II: a meta‐analysis of 22 studies involving 145,592 cardiac surgery procedures. J Thorac Cardiovasc Surg. 2014;148:3049–3057.e1. [DOI] [PubMed] [Google Scholar]
- 41. Furze G, Dumville JC, Miles JNV, Irvine K, Thompson DR, Lewin RJP. “Prehabilitation” prior to CABG surgery improves physical functioning and depression. Int J Cardiol. 2009;132:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stammers AN, Kehler DS, Afilalo J, Avery LJ, Bagshaw SM, Grocott HP, Légaré J‐F, Logsetty S, Metge C, Nguyen T, Rockwood K, Sareen J, Sawatzky J‐A, Tangri N, Giacomantonio N, Hassan A, Duhamel TA, Arora RC. Protocol for the PREHAB study—Pre‐operative Rehabilitation for reduction of Hospitalization After coronary Bypass and valvular surgery: a randomised controlled trial. BMJ Open. 2015;5:e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marmelo F, Rocha V, Goncalves D. The impact of prehabilitation on post‐surgical complications in patients undergoing non‐urgent cardiovascular surgical intervention: systematic review and meta‐analysis. Eur J Prev Cardiol. 2018;25:404–417. [DOI] [PubMed] [Google Scholar]
- 44. Gardner SC, Grunwald GK, Rumsfeld JS, Mackenzie T, Gao D, Perlin JB, McDonald G, Shroyer AL. Risk factors for intermediate‐term survival after coronary artery bypass grafting. Ann Thorac Surg. 2001;72:2033–2037. [DOI] [PubMed] [Google Scholar]
- 45. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen‐Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. [DOI] [PubMed] [Google Scholar]
- 46. Statistics Canada . Population by year, by province, by territory. 2017; Retrieved February 24, 2018, from http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm.
- 47. Rapp‐Kesek D, Stahle E, Karlsson TT. Body mass index and albumin in the preoperative evaluation of cardiac surgery patients. Clin Nutr. 2004;23:1398–1404. [DOI] [PubMed] [Google Scholar]
- 48. Lee DS, Stitt A, Wang X, Yu JS, Gurevich Y, Kingsbury KJ, Austin PC, Tu JV. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Johns Hopkins ACG Frailty‐Defining Diagnosis Indicators
Table S2. Multivariable Predictors of Long‐Term Mortality Post CABG in the Expanded Model


