Abstract
Natural products are used widely for preventing intimal hyperplasia (IH), a common cardiovascular disease. Four different cells initiate and progress IH, namely, vascular smooth muscle, adventitial and endothelial cells, and circulation or bone marrow-derived cells. Vascular smooth muscle cells (VSMCs) play a critical role in initiation and development of intimal thickening and formation of neointimal hyperplasia. In this review, we describe the different originating cells involved in vascular IH and emphasize the effect of different natural products on inhibiting abnormal cellular functions, such as VSMC proliferation and migration. We further present a classification for the different natural products like phenols, flavonoids, terpenes, and alkaloids that suppress VSMC growth. Abnormal VSMC physiology involves disturbance in MAPKs, PI3K/AKT, JAK-STAT, FAK, and NF-κB signal pathways. Most of the natural isolate studies have revealed G1/S phase of cell cycle arrest, decreased ROS production, induced cell apoptosis, restrained migration, and downregulated collagen deposition. It is necessary to screen optimal drugs from natural sources that preferentially inhibit VSMC rather than vascular endothelial cell growth to prevent early IH, restenosis following graft implantation, and atherosclerotic diseases.
1. Introduction
Intimal hyperplasia (IH) is a fibroproliferative disorder observed in vascular pathogenesis particularly in vessel anastomotic stenosis, atherosclerosis, blockage of vessel grafts, angioplasty, and in-stent restenosis [1]. IH is characterized by enhanced cell migration, proliferation, and differentiation that cause narrowing of the tunica intima. Several cells are associated with initiation and progression of IH, namely, vascular smooth muscle cells (VSMCs) [2], vascular adventitial cells [3], vascular endothelial cells (VEC) [4], and circulating bone marrow-derived cells [5]. These cells have different origins but may contribute to IH formation. For example, endothelial cells may undergo endothelial-to-mesenchymal transition (EndMT) acquiring a fibroproliferative mesenchymal phenotype whereas adventitia-derived stem cells may migrate to the intimal lesion site and differentiate into fibroblasts. VSMCs play a critical role in the initiation and development of intimal thickening and formation of neointimal hyperplasia [6, 7].
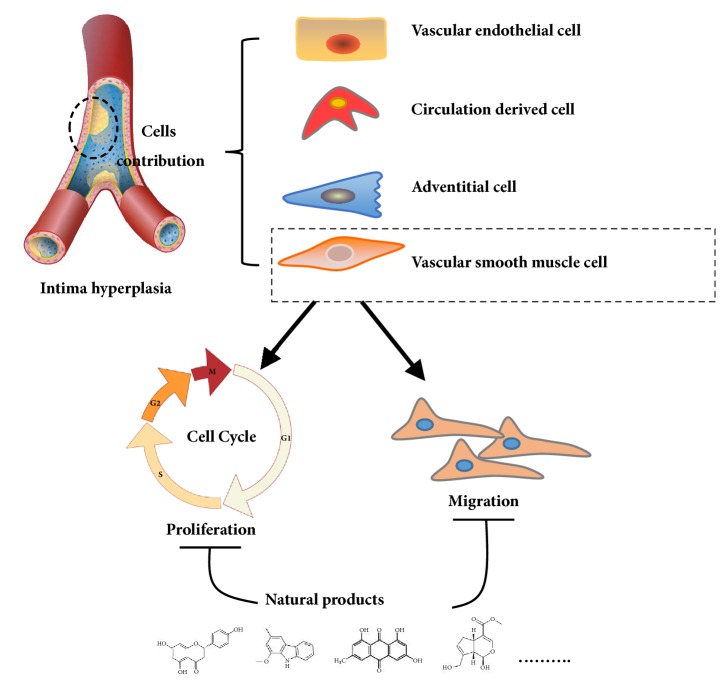
Many herbal medicines sourced from plants or foods have been used to prevent cardiovascular disease over the millennia. For example, green tea contains various flavanols that have antioxidative [8, 9], anti-inflammatory [10], antimicrobial [11], and hypolipidemic [9] effects. This pharmacological profile helps prevent atherosclerotic plaque formation caused by inflammation and oxidative stress. Red wine, another commonly enjoyed beverage, has long been believed to be rich in polyphenols [12], which act as powerful antioxidants. These assist in preventing lower density lipoprotein oxidation in heart disease and attenuating development of atherosclerotic disease in the hamster model [13, 14]. Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a typical polyphenol extracted from red wine, has been proven to inhibit proliferation of VSMCs in vitro [15]. Many natural compounds have been reported to be active and to have potential utility as clinical medicines. Tanshinone is an isolate from Salvia miltiorrhiza that has been used against cardiovascular disease in China [16]. Therefore, many active compounds with cosmopolitan distribution are being used as herbal medicines or foods, giving hope for screening for potential therapeutic agents against IH (Figure 1).
Figure 1.
Graphic abstract for different natural compounds for inhibiting vascular smooth muscle cells proliferation and migration.
Recent clinical studies have shown that rapamycin A, an VSMC inhibitor, prevents development of IH-induced vascular endothelial dysfunction [17]. This nonspecific cytotoxicity leads to stenosis and eventually to failure of vascular reconstruction after injury. Therefore, the ideal drug to prevent restenosis or IH is one that inhibits VSMC proliferation selectively while having minimal inhibitory effect on VEC proliferation.
2. Diverse Cells Involved in Vascular IH
As stated earlier, four different cell types are involved in the initiation and progression of IH. These are VSMCs, vascular adventitial cells, VECs, and circulating bone marrow-derived cells (Figure 2). VSMCs play a critical role in the initiation of intimal thickening and the formation neointimal hyperplasia. Physiologically VSMCs exist in two phenotypes, i.e., differentiated cells and proliferating cells, which are responsible for maintaining the homeostasis and function of vascular vessels [2, 6]. Stimulation by certain growth and inflammatory factors, such as platelet-derived growth factor, tumor necrosis factor-α (TNF-α), and thrombin, results in dedifferentiation into mature VSMCs [18, 19]. Mature and differentiated VSMCs exhibit loss of contractility and increased proliferation and expression of ECM protein and various cytokines. This phenomenon is responsible for intimal thickening leading to the neointimal hyperplasia formation that is observed in early-phase atherosclerosis.
Figure 2.
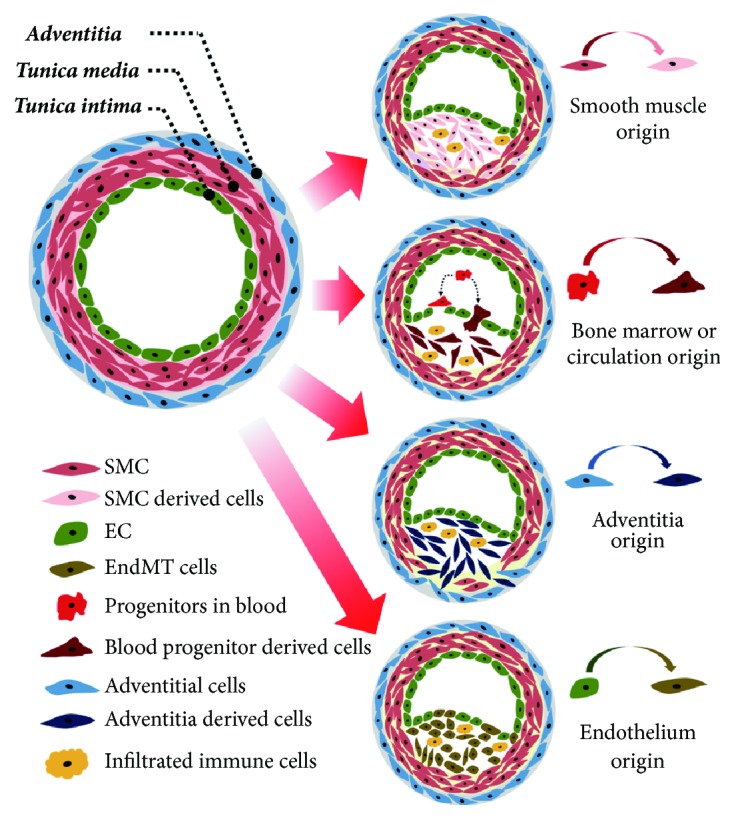
Four different cell origins contribute to blood vessel stenosis.
Endothelial-to-mesenchymal transition is a phenomenon where endothelial cells acquire a fibroproliferative mesenchymal phenotype through differential stimulation [20]. These transitioned endothelial cells mimic fibroblasts and have increased ECM production and migration capabilities. In vascular diseases, these transitioned endothelial cells can quickly migrate and differentiate into smooth muscle-like cells serving as a potential contributor to IH [21, 22]. EMT is reported to be modulated by shear stress in an ERK5-dependent manner, to contribute to neointimal hyperplasia, and to induce atherogenic differentiation [23]. In addition to adventitia-derived stem cells, circulating smooth muscle progenitor cells have also been implicated in the pathogenesis of neointimal hyperplasia [24] and in the recruitment of endothelial precursor cells after vascular trauma. The presence of bone marrow-derived cells in solid neointima or allograft lesions suggests their crucial involvement in lesion formation following vascular injury [5, 25]. Although various cells contribute to IH pathogenesis, smooth muscle cells are the main culprits in lesion formation. Therefore, therapeutic strategies that maintain VSMCs in a terminally differentiated state and inhibit their proliferation and migration can be useful in preventing neointimal hyperplasia.
3. Antiproliferation, Migration, and Cellular Functions of Abnormal VSMCs as a Target to Decrease Intimal Hyperplasia
VSMCs in the normal vascular tunica media express a range of smooth muscle cell markers including smooth muscle cell myosin heavy chain (MYH11), 22-kDa SMC lineage-restricted protein (SM22α/tagln), alpha smooth muscle actin (ACTA2), and smoothelin. VSMCs in vitro and in atherosclerosis undergo phenotypic switching with reduced expression of these markers, while increasing capacity for cell proliferation, migration, and secretion of various ECM proteins and cytokines. These phenotypic switches have long been considered of fundamental significance in IH progression.
Most studies investigating inhibition of VSMCs adopt drugs like rapamycin, sirolimus, or tacrolimus to induce VSMC apoptosis and cell cycle arrest at G1/S phase, suppress ROS production, inhibit VSMC migration, and downregulate collagen deposition. These approaches do not recover the mature VSMC immunophenotypes, but they do decrease neointimal formation and prevent stenosis following vascular injury. To investigate the anticellular function of drugs on VSMCs many models have been established in vitro and in vivo. For the in vitro experiments, inflammatory cytokines like TNF-α or some growth factors such as platelet-derived growth factor (PDGF) are used for inducing abnormal proliferation and migration of VSMCs. For the in vivo experiments, IH is usually induced using the vascular endothelial denudation model or carotid artery ligation injury.
Dietary supplements and traditional herbal medicines are complementary medication approaches used in every society and are widely used for preventing IH in Asia and in other developed countries [26]. Many herbal drugs and foods have been verified as suppressing abnormal VSMC growth and inhibiting intima formation. The positive effects of the herbal medicines and plants depend on their active natural compounds including phenols, flavonoids, terpenes, and alkaloids. These natural products are involved in different signaling pathways that regulate abnormal VMSCs to attenuate IH.
4. Typical Signal Pathways Involved in Growth and Physiology of VSMCs in IH Disease
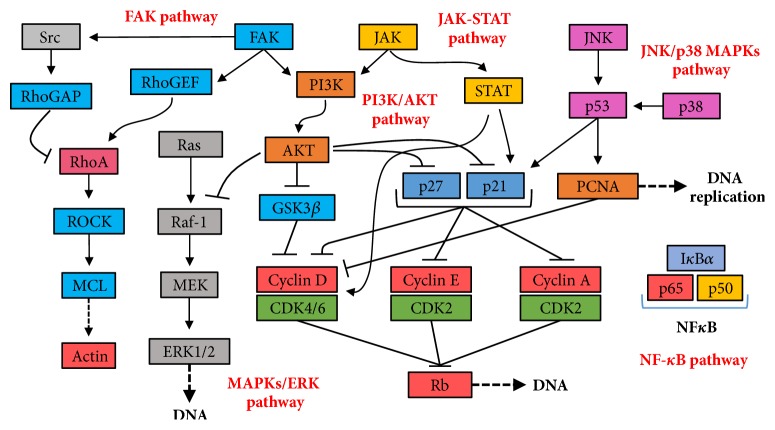
The six signaling pathways involved in most drug inhibitory VSMC studies (Figure 3) are mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPKs/ERK), phosphatidylinositol 3-kinases/Akt (PI3K/Akt), Janus kinase-signal transducer and activator of transcription (JAK-STAT), focal adhesion kinase (FAK), and nuclear factor kappa-light-chain-enhancer of activated B (NF-κB). MAPKs are involved in cell proliferation, differentiation, mitosis, cell survival, and apoptosis [27]. Three major families of MAPKs are extracellular signal-regulated kinase (ERK) [28], p38 kinase, and c-Jun N terminal kinase (JNK). These contribute to the two important signaling pathways, Ras/ERK-MAPK and JNK/p38-MAPK, which are involved in regulating VSMCs [29]. In antiproliferation studies of VSMCs, PI3K/Akt signaling pathway includes many key factors such as GSK3β, p21, and p27, which all inhibit cyclins and CDKs thereby interfering with cell cycle processes. GSK3β is one of the critical downstream molecules of the Akt signaling pathway involved in cell proliferation, metabolism, growth, and survival. It is reported that cyclin D is regulated by GSK3β [30] and that activation of GSK3β leads to exportation into cytoplasm for proteolysis, thus downregulating cyclin D1 expression [31]. The JAK-STAT signaling pathway transmits information from extracellular chemical signals to the nucleus resulting in DNA transcription and expression of genes involved in immunity, proliferation, differentiation, and apoptosis [32]. The downstream proteins in this pathway include cyclin D, p21, Bcl-2, and c-Myc, which are all directly involved in growth, apoptosis, and cell cycle progression in VSMC studies [33]. FAK is involved in cellular adhesion and migration [34]. FAK is typically located at structures known as focal adhesions, which are multiprotein structures including actin, filamin, and vinculin which link the ECM to the cytoplasmic cytoskeleton [35–37]. In addition, FAK interacts with PI3K and p53 [38, 39] and with the PI3K/Akt and MAPKs signaling pathways that are involved in cell cycle regulation. NF-κB controls many genes involved with inflammation which are crucial to progression of diseases including arthritis, asthma, and atherosclerosis [40, 41]. Inflammation also mediates abnormal movement and growth of VSMCs, while suppressing inflammation could attenuate neointimal hyperplasia significantly [42–45]. Therefore, different signaling pathways are involved in VSMC inhibition, which provides preferential protein targets for future drug screening.
Figure 3.
Key genes and pathways involved in restraining cell cycle and movements of VSMCs with natural products.
5. Different Natural Compounds Being Used for Preventing Neointimal Formation and Focus on VSMCs
5.1. Flavonoids Regulate Cell Cycle and Functions Inhibiting VSMCs Proliferation and Migration
Flavonoids are distributed throughout the plant kingdom and fulfill a diverse range of biological and pharmacological effects such as anti-inflammatory [46], antioxidant [47], antibacterial [48], antitumor [49], and antidiarrheal activities [50]. For treatment of cardiovascular disease, flavonoid studies have focused on reducing hypertension, risk of atherosclerosis, oxidative stress, and related signaling pathways in blood vessel cells, as well as modifying vascular inflammatory mechanisms [51, 52]. In this review, we described the chemical structure, category, source, and mechanism of action of some typical flavonoids that suppress VSMC function and inhibit IH (Table 1).
Table 1.
The structure, cells, category, source, and mechanism of typical flavonoid compounds on inhibiting VSMCs proliferation and migration.
| Compound name | Structure | Cells and animals | Category | Sources | Mechanism |
|---|---|---|---|---|---|
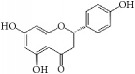
| (2S)-naringenin |
|
rASMCs | Flavonoid | Typha angustata | G0/G1 ↓; cyclins D1 ↓; cyclins E ↓; CDK2/4 ↓; PCNA ↓; pho of rb protein ↓ |
| Catechins |
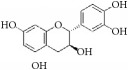
|
rASMCs and rat balloon injury | Flavonoid (Flavanols) | Green tea | TIMP-2 ↑, in vivo: TIMP-2 ↑ |
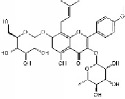
| Icariin |
|
hASMCs | Flavonoid (Prenylated flavonol glycoside) | Epimedium brevicornum | pERK1/2 ↓; G1/S ↓; PCNA ↓ |
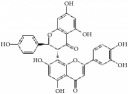
| Morelloflavone |
|
mVSMCs and mouse artery injury | Biflavonoid | Garcinia dulcis | FAK ↓; Src ↓; ERK ↓; RhoA ↓ |
| Puerarin |
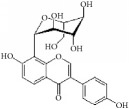
|
rASMCs and rat balloon injury | Isoflavone | Radix puerariae | ROS ↓; Nox ↓; PKC;PKCβ2 ↓; Rac1 ↓; p47phox ↓; p67phox ↓ |
| Kaempferol |
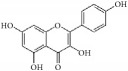
|
hpASMCs | Flavonoid | Widely (grapefruit, Ginkgo biloba) | miR-21 ↑; ROCK4/5/7 ↓ |
| Nobiletin |
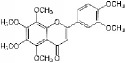
|
rASMCs and rat balloon injury | Flavonoid | Widely (citrus fruits) | ROS ↓; pERK1/2 ↓; NF-κB p65 ↓, in vivo: TNF-α ↓; IL-6 ↓ |
| Alpinetin |
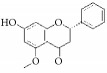
|
rASMCs | Flavonoid | Widely (Alpinia katsumadai, Amomum subulatum, and etc.) | LDH ↓; NO ↓ |
| Cyanidin-3-O-glucoside |
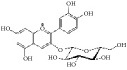
|
mASMCs | Flavonoid | Hibiscus sabdariffa | ROS ↓;NoxA1 ↓; pSTAT3 ↓ |
| Hesperetin |
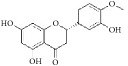
|
rpASMCs | Flavonoid | Widely (lemons and sweet oranges) | Block G1/S; cyclin D1 ↓; cyclin E ↓; CDK2/4 ↓; p38 ↓; p27 ↑; regulate AKT/GSK3β signaling pathway |
| Pinocembrin |
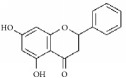
|
rAMSCs and rat aortic rings injury | Flavonoid | Propolis | ERK1/2 ↓; MLC2 ↓; AT1R ↓ |
| Glyceollins |
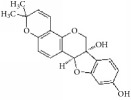
|
hASMCs | Isoflavone | Soybean | Arrest G1/S phase; CDK2 ↓; cyclin D1 ↓; p27kip1 ↑; p53 ↑; ROS ↓; pPDGFr-β ↓; phospholipase Cγ1↓; Akt ↓; ERK1/2 ↓ |
Nobiletin is widely distributed in citrus fruits and has been reported to inhibit VSMC proliferation and migration in vitro [44]. In addition, carotid balloon injured rats given nobiletin 25 mg/kg/day by gavage had significantly decreased neointimal hyperplasia via regulation of the ROS derived NF-κB pathway and decreased serum TNF-α and IL-6 concentrations [44]. Cyanidin-3-O-glucoside, an anthocyanin flavonoid, inhibited TNF-α-induced NoxA1 (a type of NADPH oxidase) and downregulated expression of both TNF-α and NoxA1 at transcriptional and translational levels [53]. (2S)-Naringenin, a typical flavonoid isolated from Typha angustata, inhibited PDGF-BB-induced proliferation of VSMCs via a G0/G1 arrest by suppressing cyclin D1/E and CDK 2/4 [54]. Hu and colleagues found that icariin reduced the amount of ox-LDL-induced proliferation of VSMCs through suppression of PCNA expression and inactivation of ERK1/2 [55]. Puerarin, isolated from Radix puerariae, exerted inhibitory effects on high glucose-induced VSMC proliferation via interfering with PKCβ2/Rac1-dependent ROS pathways, thus resulting in attenuation of neointimal formation [56]. Alpinetin is a well-known flavonoid isolated from a variety of plants such as Alpinia katsumadai, Amomum subulatum, and Scutellaria rivularis. It may have some protective effects on VSMCs as it decreases LDH leakage and inhibits production of NO in TNF-α-induced VSMC [57]. Hesperetin, a flavonoid, inhibits PDGFa-BB-induced pASMC proliferation via the AKT/GSK3β signaling pathway through upregulating p27 expression while suppressing cyclin D1/E, CDK2/4 and p38 [58]. Pinocembrin reduces the increased ERK1/2 phosphorylation that occurs in response to angiotensin II in both rat aortic rings ex vivo and VSMCs in vitro [59]. Glyceollins, which are isoflavonoids, inhibit PDGF-BB-induced hVSMC proliferation and migration by downregulating CDK2, cyclin D1, pPDGFr-β, phospholipase Cγ1, Akt, and ERK1/2 and interfering with ROS generation, while upregulating p27kip1 and p53 expression levels [60]. Morelloflavone is a biflavonoid, which has been found to block injury-induced neointimal hyperplasia via inhibition of VSMC migration and downregulation of FAK, Src, ERK and RhoA expression [61]. Some studies have demonstrated that a natural flavonoid, kaempferol, may induce miR-21. This results in downregulation of ROCK4, 5, and 7, which are critical for cytoskeletal organization and cell motility, leading to decreased cell migration [62]. Finally, green tea is beneficial for health due to its antioxidant, anticarcinogenic, anti-inflammatory, and antiradiation effects [63–65]. A large number of flavonoids, especially flavan-3-ols (“catechins”), inhibit IH in a rat balloon injury model through upregulation of TIMP-2 expression to modulate MMP activity [66]. From the above review, flavonoids are an important candidate compound type for screening natural drugs capable of inhibiting VSMC growth.
5.2. Polyphenols as an Antioxidants Restrain VSMC Proliferation and Migration to Attenuate IH
Polyphenols are distributed widely in vegetables and plants, green tea, black tea, and red wine. Recent studies have shown that they possess antioxidant, anti-inflammatory, and cardioprotective effects [67–69]. Some typical polyphenols prevent IH by restraining VSMC function including proliferation, migration, and fibrosis (Table 2). Salvianolic acid B is a typical polyphenol that is usually isolated from Salvia miltiorrhiza. It markedly reduces neointimal thickness by inducing neointimal cell apoptosis through upregulating p53 expression levels [70]. In another study, salvianolic acid B protected hAECs and neointimal formation through inhibition of LDL oxidation by reducing ROS generation [71]. Magnesium lithospermate B, a derivative of salvianolic acid B, prevented diabetic atherosclerosis via the Nrf2-ARE-NQO1 transcriptional pathway [72]. Magnolol (a phenol) is a powerful antioxidant that inhibited balloon injury-induced rabbit IH by downregulating MCP-1 expression [73]. In another work, magnolol inhibited VSMC migration via the cytoskeletal remodeling pathway through inhibition of β1-integrin expression, phosphorylation of FAK and MLC20, and activation of RhoA and Cdc42 [74]. Lithospermic acid, a polyphenol, arrested cell cycle progression at the G1 phase via downregulating expression of cyclin D1 and inhibiting ROS generation and ERK1/2 phosphorylation [75]. Moreover, lithospermic acid attenuated LPS-induced VSMC migration by inhibiting MMP-9 expression in a dose-dependent manner (25-100 μmol/L). Hispolon blocked balloon injury-induced neointimal hyperplasia via inhibition of VSMC proliferation. It also inhibited VSMC migration by lowering MMP-2/9 expression and increasing TIMP-1/2 expression through suppression of the FAK signaling pathway [76]. Lim and colleagues were of the view that obovatol blocked the cell cycle in G1 phase by downregulating expression of cyclins and CDKs, while selectively upregulating expression of p21Cip1, a well-known CDK inhibitor, both in vitro and in vivo [77].
Table 2.
The structure, cells, category, source, and mechanism of typical polyphenols compounds on inhibiting VSMCs proliferation and migration.
| Compound name | Structure | Cells and animals | Category | Sources | Mechanism |
|---|---|---|---|---|---|
| Salvianolic acid B |
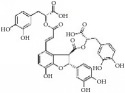
|
NeCs; HAECs and cholesterol-fed rabbits; rTASMCs and rats balloon injury |
Polyphenol | Salvia miltiorrhiza | (1) p53 ↑; NeCs apoptosis, (2) ROS ↓; LDL oxidation ↓; lipid deposition ↓, (3) PCNA ↓; NQO1 ↓; via Nrf2-ARE-NQO1 pathway |
| Caffeic acid phenethyl ester (CAPE) |
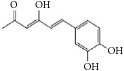
|
rASMCs | Polyphenol | Honeybee propolis | Blocking G0/1 to S phase; pp38 ↑;HiF1α ↑;HO-1 ↑ |
| Hispolon |
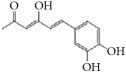
|
rTA-A10-VSMCs | Polyphenol | Phellinus linteus | MMP2 ↓;MMP9 ↓; TIMP-1 ↑;TIMP-2 ↑; pFAK ↓; pERK1/2 ↓;PI3K/AKT ↓ |
| [6]-shogaol |
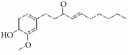
|
rASMCs | Phenols | Zingiber officinale | Inhibit DNA synthesis; activation of (Nrf2)/HO-1 pathway |
| Resveratrol |
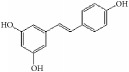
|
ncTASMCs; mASMCs |
Polyphenol | Widely (grapes, blueberries, raspberries, and etc.) | c-Src ↓, Rac1 ↓, cdc42 ↓, IRS-1 ↓, MEKK1 ↓, MEKK4 ↓; p-Src; pFAK ↓; pAKT ↓; pERK1/2 ↓ |
| Lithospermic acid |
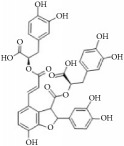
|
rTASMCs | Polyphenol | Salvia miltiorrhiza | ROS ↓; pERK1/2 ↓; cyclin D1 ↓; arresting cell cycle progression at the G1 phase; MMP9 ↓ |
| Magnolol |
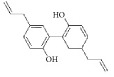
|
Cholesterol-fed rabbits; rVSMCs; rats balloon injury |
Polyphenol | Magnolia officinalis | (1) MCP-1 ↓, (2) Reduce collagen type I deposition; β1-integrin ↓;pFAK ↓;pMLC20 ↓; RhoA ↓;Cdc42 ↓ |
| Obovatol |
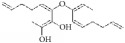
|
rASMCs; rats balloon injury | Biphenol | Magnolia obovata | Blocks the cell cycle in G1 phase; CDKs ↓;p21cip1 ↓ |
| Curcumin |
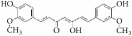
|
rTASMCs; rabbit artery injury; VECs; RAECs |
Phenols | Curcuma longa | (1) Inhibits PDGF Receptor Binding; PDGFr ↓; pERK1/2 ↓; pAkt ↓, (2) P-selectin ↓; E-selectin ↓; GPIIb/GPIIIa ↓, (3) MMP2 ↓; pRas ↓; MEK1/2 ↓; NF-κB p65 ↓, (4) Curcumin protects aortic endothelial cells; eNOS ↑; caveolin-1 ↓; |
Some studies have shown that curcumin (diarylheptanoid phenol) has potent antioxidant properties, which can be used for attenuating neointimal hyperplasia [78]. Curcumin has also been shown to inhibit PDGF-induced VSMC migration, proliferation, and collagen synthesis in a concentration-dependent manner [79], with a concentration range of 0.01 to 10 µmol/L inhibiting VSMC proliferation and migration. Curcumin-coated stents inhibited neointimal formation in the rabbit iliac artery stent model. Moreover, curcumin inhibited LPS-induced MMP-2 activity in rat VSMCs through Ras/MEK1/2 and NF-κB signaling [80].
Curcumin shows the ideal biological effects of inhibiting abnormal VSMC proliferation and migration without compromising VEC proliferation or delaying reendothelialization after blood vessel injury. Curcumin inhibited platelet adhesion to brain microvascular endothelial cells by decreasing expression of P-selectin, E-selectin, and GPIIb/GPIIIa in a concentration-dependent manner (30-240 μmol/L). Curcumin antagonized the detrimental effect of rapamycin on aortic endothelial cells in vitro, through upregulation of eNOS [81]. Hence, curcumin very selectively inhibited abnormal VSMC functions, such as PDGF-induced proliferation or migration, without impairing VECs. As a result, curcumin has been regarded as an ideal drug for attenuating atherosclerosis and restenosis. In summary, polyphenols exhibit beneficial and wide ranging biological effects relevant to prevention of IH. Polyphenols are worthy candidate compounds to be screened as natural drugs for inhibiting VSMCs.
5.3. Terpenes Suppress Abnormal VSMC Function against Neointimal Formation
Terpenes are proven cell cycle inhibitors for various cell types, especially tumor cells [82, 83]. Like similar compounds with active sites for regulating VSMC mitosis and DNA synthesis, terpenes lead cell proliferation and function arrest via cell cycle blockade or apoptosis induction (Table 3). Betulinic acid, a typical terpene, has been reported to inhibit growth and proliferation of VSMCs via arresting G1/S cell cycle in a dose-dependent manner [84]. A monoterpene, (S)-(-)-perillic acid, has been reported to decrease protein prenylation leading to DNA synthesis and inhibition of VSMCs [85]. A sesquiterpene lactone, parthenolide, arrested VSMC G0/G1 cell cycle via upregulating p21 and p27. It also increased IκBα expression and reduced Cox-2 expression in a time-dependent manner [86]. A special terpene, plumericin, arrested VSMCs in the G1/G0 phase of the cell cycle along with causing abrogated cyclin D1 expression, hindered Ser807/811-pRb protein [87], and blockade of STAT3 signaling via S-glutathionylation. Paclitaxel, a diterpenoid, has been used as an anticancer drug for decades and has been shown to prevent neointimal formation in oral administration studies [88]. Moreover, paclitaxel arrested VSMC G1/S phase by upregulating p21 and p53 in vitro [89]. Epothilone D is a paclitaxel-like microtubule-stabilizing agent that was isolated originally from the myxobacterium Sorangium cellulosum. It inhibits neointimal hyperplasia through blockade of VSMC CDK2 and pRb [90]. β-Elemene protected VECs from injury induced by H2O2 in vitro via downregulating MDA while upregulating T-AOC, SOD, GSH-Px, and CAT [91]. Meanwhile, β-elemene selectively inhibited VSMC proliferation/migration and inhibited neointimal formation in vivo following vascular injuries [91]. Recent studies have indicated that artemisinin effectively inhibited VSMC proliferation induced by TNF-α through apoptotic induction of the caspase pathway and cell cycle arrest [92, 93]. It also significantly inhibited neointimal formation in rat balloon injured carotid arteries. Therefore, terpenes are also notable candidate compounds for screening natural drugs capable of inhibiting VSMCs.
Table 3.
The structure, cells, category, source, and mechanism of terpenes on inhibiting VSMCs abnormal proliferation, migration, and functions.
| Compound name | Structure | Cells and animals | Category | Sources | Mechanism |
|---|---|---|---|---|---|
| Betulinic Acid |
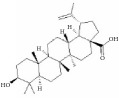
|
VSMCs | Terpene | Various plant sources widespread throughout the tropics | Inducing G1 Arrest and Apoptosis |
| Parthenolide |
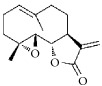
|
rVSMCs | Sesquiterpene lactone | Tanacetum parthenium | G0/G1 cell cycle arrest; p21 ↑; p27 ↑; IκBα ↑;Cox-2 ↓ |
| Plumericin |
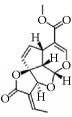
|
rAVSMCs | Iridoid (Terpene) | Himatanthus sucuuba | Block STAT3 signaling; arrest VSMCs in the G1/G0-phase; cyclin D1 ↓; pRb ↓ |
| Paclitaxel |
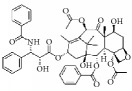
|
Rat balloon injury; hCASMCs (CC-2583) and VSMCs (CC-2571); rTASMCs and VECs | Diterpenoid | Taxus cuspidata | (1) prevent neointimal formation via oral administration, (2) arrest G1/S phase; p21 ↑; p53 ↑ |
| Epothilone D |
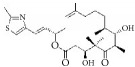
|
rTASMCs; carotid artery injury | Diterpenoid | Sorangium cellulosum | CDK2 ↓; pRb ↓ |
| β-elemene |
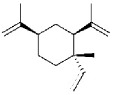
|
hUVECs and VSMCs (A7r5); rat balloon injury | Terpene | Curcuma wenyujin | Antioxidant; Casp 3/7/9 ↑; Migration ↓ |
| Artemisinin |
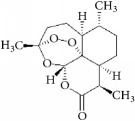
|
rVSMCs and rat balloon injury; rTASMCs | Sesquiterpene lactone | Artemisia annua | (1) arrest G0/G1 phase; cyclin D1/E ↓; CDK2/4 ↓; caspase 3/9 ↑; Bax ↑; Bcl-2 ↓, (2) PCNA ↓; caspase 3↑; Bax ↑; Bax/Bcl-2 ratio ↑ |
| (S)-(-)-Perillic acid |
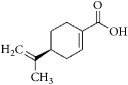
|
rASMCs | Monoterpene | Widely | Protein prenylation ↓ |
5.4. Alkaloids Exhibit Antiproliferation Biological Effect on VSMCs
Alkaloids are a group of naturally occurring chemical compounds that mostly contain basic nitrogen atoms. Alkaloids have diverse biological effects including those against tumors, hypertension, and pain. For vascular IH, some studies indicate that alkaloids hinder cell cycle progress, decrease ROS production, and inhibit VSMC migration (Table 4). A classic alkaloid, piperine, selectively inhibits VSMC proliferation with an IC50 of 11.8 μmol/L without influencing VEC growth [94]. Coptisine was isolated from Coptis chinensis and suppresses VSMC proliferation selectively at lower concentrations with a GI50 of 3.3 µmol/L (1.2 µg/mL) [95]. Vinpocetine, a potential derivative of vincamine, inhibits high glucose-induced proliferation of VSMCs by preventing ROS generation and affecting MAPK, PI3K/Akt, and NF-кB signaling, Wang, Wen, Peng, Li, Zhuang, Lu, Liu, Li, Li, and Xu [96]. Vinpocetine arrested G1/S phase of the cell cycle by downregulating cyclin D1 and pERK1/2. Alongside these effects, vinpocetine also inhibited VSMC migration and ROS production [97]. A quinazolinone alkaloid, halofuginone, selectively inhibited cell proliferation, ECM deposition, and type I collagen synthesis in VSMCs versus VECs, which attenuated injury-induced IH [98]. Carbazole or murrayafoline A inhibited PDGF-BB induced abnormal proliferation of VSMCs by downregulating cyclin D1/E, CDK2/4, and PCNA and phosphorylation of Rb [99]. Review of these recent studies on the effects of alkaloids provides hope for identification of useful drugs capable of inhibiting VSMC growth and preventing IH.
Table 4.
The structure, cells, category, source, and mechanism of alkaloids on inhibiting VSMCs abnormal proliferation, migration, and functions.
| Compound name | Structure | Cells and animals | Category | Sources | Mechanism |
|---|---|---|---|---|---|
| Piperine |
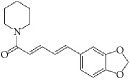
|
rASMCs | Alkaloid | Piper nigrum | Selectively inhibit VSMCs |
| Coptisine |
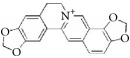
|
rVSMCs | Alkaloid | Coptis chinensis | Arrest G1/S phase |
| Vinpocetine |
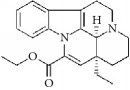
|
rVSMCs and rat balloon injury; rASMCs and mice carotid artery ligation injury | Alkaloid vincamine | Lesser periwinkle plants | (1) ROS ↓; apoptosis ↓; pAkt ↓; pJNK1/2 ↓; IκBα ↓; PCNA ↓; cyclin D ↓; Bcl-2 ↓, (2) Arrest G1/S phase; cyclin D1 ↓; p27Kip1 ↑; inhibit migration; pERK1/2 ↓; ROS ↓ |
| Halofuginone |
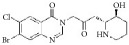
|
bASMCs | Quinazolinone alkaloid | Dichroa febrifuga | ECM synthesis and deposition ↓; Col I ↓ |
| Murrayafoline A |
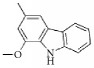
|
rASMCs | Carbazole alkaloid | Glycosmis stenocarpa Guillaumin | Arrest G1/S phase; cyclin D1/E ↓; CDK2/4 ↓; PCNA ↓; pRb ↓ |
5.5. Other Promising Natural Compounds for Preventing Intima Hyperplasia
As shown in Table 5, emodin is a typical anthraquinone compound beneficial for prevention of atherosclerosis due to its effects against inflammation, proliferation, and migration and its ability to induce apoptosis in VSMCs [100]. Moreover, emodin arrested growth and induced apoptosis and autophagy via enhanced ROS production and upregulation of p53 expression [101]. Emodin inhibited VSMC proliferation induced by angiotensin II through downregulation of PCNA and c-myc expression [102]. Moreover, emodin showed anti-inflammatory effects by inhibiting Hcy-induced CRP generation, a key inflammatory molecule in atherogenesis in VSMCs [103]. Emodin has also been shown to inhibit TNF-α-induced hASMC proliferation via caspase signaling and a mitochondrial-dependent apoptotic pathway by downregulating Bcl-2 and upregulating Bax expression [104]. Additionally, emodin reduced TNF-α induced migration of VSMCs by suppressing NF-κB activation and MMP2/9 expression levels [105]. Our recent study demonstrated that emodin efficiently and concentration-dependently (0.05 to 5 µmol/L) inhibited hVSMC proliferation more than hVEC proliferation in vitro, with less influence on reendothelialization of VECs in rat carotid artery balloon injury [106].
Table 5.
The structure, cells, category, source, and mechanism of promising compounds on suppressing VSMCs.
| Compound name | Structure | Cells and animals | Category | Sources | Mechanism |
|---|---|---|---|---|---|
| Bilirubin |
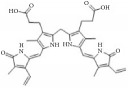
|
rVSMCs and mVSMCs; rat balloon injury | Ferric porphyrins | Heme | Inhibit MAPK signaling pathway; CDK2 ↓; Cyclin A/D1/E ↓; pRb ↓; YY1 ↓; p38 ↓ |
| capsaicin |
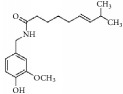
|
rASMCs | Capsaicinoids | Chili peppers | Inhibit DNA synthesis |
| Emodin |
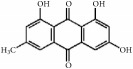
|
hUVSMCs; rTASMCs; hASMCs; rat balloon injury | Anthraquinone | Rheum officinale | (1) Arrest cell cycle, induce apoptosis and autophagy; ROS ↑; p53 ↑, (2) PCNA ↓; c-myc ↓, (3) CRP ↓;ROS ↓; pERK1/2 ↓; p38 ↓; PPARγ ↑, (4) Induce apoptosis; Bcl-2 ↓; Bax ↑, (5) MMP2/9 ↓; NF-κB activation ↓ |
| Rhein |
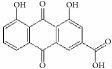
|
hASMCs | Anthraquinone | Rheum palmatum | Col I/III ↓; Wnt4/Dvl-1/β-catenin ↓; miR-126 ↑ |
| Ajoene |
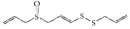
|
rASMCs | Organosulphur compound | Allium sativum | Inhibit protein prenylation and cholesterol biosynthesis |
| Gastrodin |
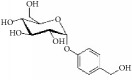
|
rASMCs, mice artery injury | Glucoside | Gastrodia elata B1 | Block S-phase; stabilised p27Kip1; PCNA ↓; pERK1/2 ↓; pp38 ↓; pAkt ↓; pGSK3β ↓ |
| Genipin |
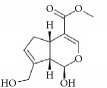
|
rTASMCs | Aglycon | Gardenia jasminoides | HO-1 ↑; pERK/MAPK ↓; pAkt ↓; ROS ↓ |
| Ginsenoside Rg1 |
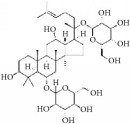
|
hASMCs; rat balloon injury | Steroid glycosides | Panax ginseng | (1) PCNA ↓; pERK2 ↓; c-fos ↓; MKP-1 ↑; (2) Arrest G1/S phase; GRKs ↓; PKC ↓; N-ras ↓; p21 ↑, (3) Cyclin D1 ↓; p53 ↑; p21WAF/CIP1 ↑; p27KIP1 ↑; inactivate PKB and ERK1/2 |
| Ostruthin |
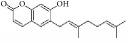
|
rTASMCs | Coumarins | Peucedanum ostruthium | Inhibit DNA synthesis |
| Lycopene |
|
Rabbit artery injury | Carotenoid | Widely (tomatoes, red carrots,) | TG ↓; TC ↓; LDL-C ↓; HDL-C ↑; SOD ↑; T-AOC ↑; MDA ↓; PCNA ↓; pERK1/2 ↓; Nox1 ↓; p22phox ↓; HMG-CoA ↓; ABCA1 ↑ |
| Methyl Protodioscin |
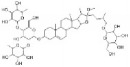
|
A7r5 VSMCs; rat balloon injury | Steroidal saponin | Dioscorea collettii | Arrest G1/S phase; ADAM15 ↓; MMP2/9 ↓; FAK ↓; ERK ↓; PI3K ↓; Akt ↓ |
| Tanshinone IIA |
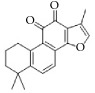
|
rASMCs; rat balloon injury | Phenolic acids | Salvia miltiorrhiza | Block cell cycle in G0/G1 phase; pERK1/2 ↓; c-fos ↓ |
| Sulforaphane |
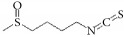
|
rASMCs; rat balloon injury | Organosulfur compounds | Widely (cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages) | p21 ↑; p53 ↑; CDK2 ↓; Cyclin E ↓; PCNA ↓ |
Methyl-protodioscin is a steroidal saponin that has been reported to inhibit neointimal formation by restraining VSMC proliferation and migration through downregulation of ADAM15, FAK, ERK, PI3K, Akt, and MMP-2/9 expression levels [107]. Salvia miltiorrhiza has been used to prevent cardiovascular diseases in traditional Chinese medicine over the millennia. Tanshinone-IIA is a principal active component of Salvia miltiorrhiza that suppresses abnormal VSMC proliferation by cell cycle arrest at G0/G1 phase and inhibits phosphorylation of ERK1/2 and c-fos expression [108]. It has been reported that ajoene (1-50 µnol/L) interfered with progression of the G1 phase in the cell cycle and restrained rat VSMC proliferation via inhibiting protein prenylation [109]. Gastrodin influenced the S phase entry of VSMCs and stabilized p27KIP1 expression. It also inhibited VSMC proliferation and attenuated neointimal hyperplasia by suppressing phosphorylation of ERK1/2, p38 MAPK, Akt, and GSK3β [110]. Genipin has been reported to inhibit TNF-α induced VSMC proliferation and migration in a dose-dependent manner by upregulating HO-1 expression, preventing ERK/MAPK and Akt phosphorylation, and additionally blocking generation of ROS [111]. Ginsenoside Rg1 is one of the main active components of Panax ginseng and is said to arrest G1/S phase in VSMCs by interfering with GRKs, PKC, and N-ras while upregulating p21 expression [112]. Vascular IH is significantly decreased when carotid artery balloon injured rats are intraperitoneally injected with ginsenoside Rg1 for 14 days [113]. Moreover, ginsenoside Rg1 significantly inhibited TNF-α-induced hASMC proliferation dose-dependently through downregulating cyclin D1, inactivating ERK1/2 and PKB, and upregulating expression of p53, p21WAF/CIP1, and p27KIP1 [114]. A coumarin called ostruthin is a major bioactive constituent of Peucedanum ostruthium and inhibited serum (10%)-induced VSMC proliferation in a dose-dependent manner [115].
Most foods contain various biologically active constituents that act to prevent and cure neointimal hyperplasia by inhibiting abnormal VSMC proliferation and migration. A well-known carotenoid, lycopene, is abundant in tomatoes and its products and has been reported to inhibit neointimal hyperplasia in a rabbit restenosis model. It does this by regulation of blood lipid concentrations and suppression of oxidative stress [116]. Sulforaphane, an organosulfur compound, mostly found in cruciferous vegetables significantly inhibited PDGF-BB-induced VSMC proliferation by upregulating p21 and p53 expression, while CDK2, cyclin E, and PCNA expression was suppressed [117].
6. Selective Inhibition of VSMCs versus VECs Shows Significant
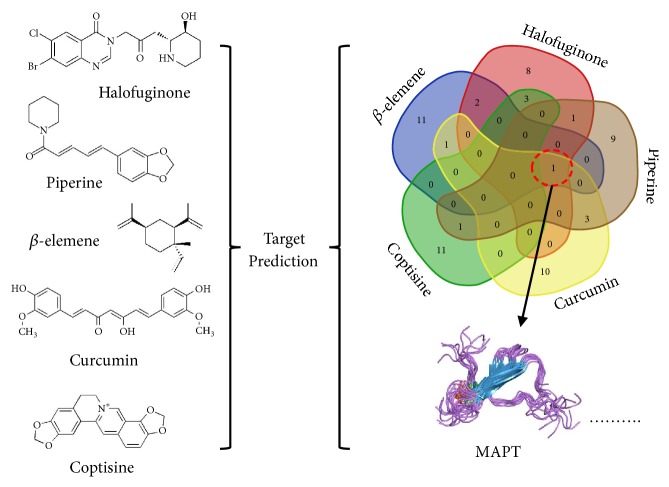
Although many natural products inhibit VSMC function, most anti-smooth muscle proliferation drugs such as rapamycin (in-stent coating) also inhibit VEC proliferation and delay reendothelialization. This nonspecific cytotoxicity leads to restenosis and final graft or stent implantation failure. When screening for selective natural drugs that inhibit smooth muscle cell proliferation and migration, it is necessary to combine computer-aided design, bioinformatics, and a high-throughput screening platform. In this review, we selected certain drugs including chemosynthetic (idarubicin) and some natural (β-elemene, coptisine, halofuginone, piperine, and curcumin) compounds that possess specificity for suppressing proliferation of VSMCs over VECs. The chemical structure of the natural compounds has no typical similarity and cannot be analyzed using structural-activity relationships of molecular-protein binding sites. However, an online tool “Swiss Target Prediction” was used to predict potential targets of these compounds [118]. Most of the predicted targets of these drugs were membrane receptors, enzymes, kinases, proteases, or transporter proteins (Table 6). The analyses showed that microtubule-associated protein TAU (MAPT) is the most frequent protein target among them (Figure 4). This stabilizes microtubules and influences transportation of cellular secretory proteins. Moreover, MAPT has been reported to accelerate cancer cell growth [119], while its inactivation through gene knockdown suppressed cell proliferation [120]. Therefore, it is speculated that the diverse affinity of a natural drug to different functional protein targets may be one of the key factors for different selectivity profiles on VSMCs or VECs. Common targets like MAPT could be used as one of the important indicators in screening selective inhibitory drugs in future studies.
Table 6.
The selected potential targets of the compounds.
| Idarubicin | Halofuginone | Piperine | β -elemene | Curcumin | Coptisine | |
|---|---|---|---|---|---|---|
| Seq | Predicted target names (most related top 15) | |||||
| 1 | MAPT | BCHE | MAOA | MAPT | MAPT | CHRM4 |
| 2 | MBNL1 | ACHE | MAOB | TDP1 | TLR9 | CHRM1 |
| 3 | MBNL2 | MAPK8 | SIGMAR1 | CXCR3 | TDP1 | CHRM2 |
| 4 | MBNL3 | MAPK9 | MBNL1 | SLC6A2 | Unknown | CHRM5 |
| 5 | MMP2 | MAPK10 | MBNL2 | SLC6A3 | MBNL1 | CHRM3 |
| 6 | MMP9 | MAPK11 | MBNL3 | LDLR | MBNL2 | BCHE |
| 7 | APP | MAPK14 | MAPT | VLDLR | MBNL3 | ADRA2A |
| 8 | SNCA | HTR1A | DRD2 | LRP8 | GLO1 | CYP2D6 |
| 9 | APLP2 | HTR1B | DRD3 | HSD11B1 | AKT1 | ADRA2B |
| 10 | SNCG | MAPT | HDAC3 | BACE1 | AKT2 | ADRA2C |
| 11 | SNCB | HTR2A | HDAC1 | HSD11B1L | AKT3 | ACHE |
| 12 | TDP1 | DRD2 | HDAC2 | BACE2 | HSD17B3 | HTR2A |
| 13 | EGFR | DRD1 | DYRK1A | HTR1A | HSD17B12 | HTR2C |
| 14 | ERBB2 | OPRM1 | HDAC6 | HTR1D | CRYZ | HTR2B |
| 15 | ERBB3 | OPRD1 | CTSL1 | HTR1B | APP | SIGMAR1 |
Figure 4.
The compounds potential target: MAPT which is a common target.
7. Conclusion
This review highlighted the originating four cells that may contribute to IH and then focused on VSMCs due to their involvement in intima formation as a consequence of abnormal proliferation, migration, and physiology. It further summarized typical signaling pathways such as MAPKs, PI3K/Akt, JAK-STAT, FAK, and NF-κB and their involvement in the abnormal activities of VSMCs. Based on these the above cell origins and pathways, we organized and classified different natural isolates including phenols, flavonoids, terpenes, and alkaloids that have suppressing effects on VSMCs. In addition, many natural drugs not only induce apoptosis and arrest cell cycle in VSMCs, but also impair VECs leading to vascular restenosis and failure of blood vessel remodeling. Thus, it is crucial to screen desirable drugs from natural sources that preferentially inhibit VSMCs versus VECs to prevent IH in the early stages, restenosis following graft implantation, and even atherosclerotic diseases.
Acknowledgments
This work was supported by National Key R&D Plan (2018YFA0108700, 2016YFA0101100), National Natural Science Foundation of China (81873471, 11602181, 30371414, 30571839, 30872540, 31330029, 81170214, 81270297, 11602181), the Fundamental Research Funds for the Central Universities, South-Central University for Nationalities (Grant Number: CZQ18019), the China Postdoctoral Science Foundation (Grant Number: 2018M630867), the Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (Grant Number: CQKLBST-2018-009 and CQKLBST-2018-006), and the Fundamental Research Funds for the Central Universities (WUT: 2016IVB063, 2018IB005). The authors thank Dr. Peng Zhu for help in organizing and writing the manuscript.
Abbreviations
- IH:
Intimal hyperplasia
- EndMT:
Endothelial-to-mesenchymal transition
- rASMCs:
Rat aortic smooth muscle cells
- rTASMCs:
Rat thoracic aortic smooth muscle cells
- VSMCs:
Vascular smooth muscle cells
- CA:
Carotid artery
- RAECs:
Rat aortic endothelial cells
- HAECs:
Human aortic endothelial cells
- VECs:
Vascular endothelial cells
- hUVECs:
Human umbilical vein endothelial cells
- hUVSMCs:
Human umbilical vein smooth muscle cells
- NeCs:
Neointimal cells
- rTA-A10-VSMCs:
Rat thoracic aorta A10 vascular smooth muscle cells
- ncTASMCs:
Newborn calf thoracic aorta smooth muscle cells
- mASMCs:
Mice aortic smooth muscle cells
- hPASMCs:
Human pulmonary artery smooth muscle cells
- rPASMCs:
Rat pulmonary artery smooth muscle cells
- hCASMCs:
Human coronary artery smooth muscle cells
- bASMCs:
Bovine aortic smooth muscle cells
- MYH11:
Smooth muscle cell myosin heavy chain
- SM22α/tagln:
SMC lineage-restricted protein
- ACTA2:
Alpha smooth muscle actin
- ECM:
Extracellular matrix
- TNF-α:
Tumor necrosis factor-α
- PDGF:
Platelet-derived growth factor
- ERK:
Extracellular signal-regulated kinase
- MMP:
Matrix metalloproteinase
- MAPK:
Mitogen-activated protein kinase
- JNK:
c-Jun N terminal kinase
- PCNA:
Proliferating cell nuclear antigen
- PI3K:
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- AKT:
Serine/threonine kinase 1
- CDK:
Cyclin-dependent kinase
- JAK:
Janus kinase
- STAT:
Signal transducer and activator of transcription protein
- FAK:
Focal adhesion kinase
- NF-κB:
Nuclear factor kappa B
- LDL:
Low-density lipoprotein
- ROS:
Reactive oxygen specie
- IL-1β:
Interleukin 1-β
- LPS:
Lipopolysaccharide
- Nox:
NADPH oxidase
- TIMP:
Tissue inhibitors of metalloproteinase
- NOS:
Nitric oxide synthase
- IC50:
Half maximal inhibitory concentration
- miR-21:
MicroRNA-21
- NO:
Nitric oxide
- LDH:
Lactate dehydrogenase
- eNOS:
Nitric oxide synthase
- pPDGFr-β:
β-type platelet-derived growth factor receptor
- ROCK:
Rho-associated protein kinase
- Rb:
Retinoblastoma tumor suppressor protein family.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Kang Xu, Mohanad Kh Al-ani, and Xin Pan designed the project, performed the experiments, collected the data, and wrote the manuscript. Qingjia Chi analyzed the data and wrote and revised the manuscript. Nianguo Dong and Xuefeng Qiu designed the project, gave financial support, and wrote and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Subbotin V. M. Analysis of arterial intimal hyperplasia: Review and hypothesis. Theoretical Biology and Medical Modelling. 2007;4, article no. 41 doi: 10.1186/1742-4682-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett M. R., Sinha S., Owens G. K. Vascular smooth muscle cells in atherosclerosis. Circulation Research. 2016;118(4):692–702. doi: 10.1161/circresaha.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y., Xu Q. Adventitial biology: differentiation and function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(7):1523–1529. doi: 10.1161/atvbaha.110.221176. [DOI] [PubMed] [Google Scholar]
- 4.Kipshidze N., Dangas G., Tsapenko M., et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. Journal of the American College of Cardiology. 2004;44(4):733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 5.Skowasch D., Jabs A., Andrié R., Dinkelbach S., Lüderitz B., Bauriedel G. Presence of bone-marrow- and neural-crest-derived cells in intimal hyperplasia at the time of clinical in-stent restenosis. Cardiovascular Research. 2003;60(3):684–691. doi: 10.1016/j.cardiores.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Michel J.-B., Li Z., Lacolley P. Smooth muscle cells and vascular diseases. Cardiovascular Research. 2012;95(2):135–137. doi: 10.1093/cvr/cvs172. [DOI] [PubMed] [Google Scholar]
- 7.Lacolley P., Regnault V., Nicoletti A., Li Z., Michel J.-B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovascular Research. 2012;95(2):194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 8.Lee Ching H., Lin Ruey H., Liu S. H., Lin-Shiau S. Y. Mutual interactions among ingredients of betel quid in inducing genotoxicity on Chinese hamster ovary cells. Mutation Research - Genetic Toxicology. 1996;367(2):99–104. doi: 10.1016/0165-1218(95)00081-X. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino K., Hara Y., Sano M., Tomita I. Antioxidative effects of black tea theaflavins and thearubigin on lipid peroxidation of liver homogenates induced by tert-butyl hydroperoxide. Biological & Pharmaceutical Bulletin. 1994;17(1):146–149. doi: 10.1248/bpb.17.146. [DOI] [PubMed] [Google Scholar]
- 10.Sano M., Suzuki M., Miyase T., Yoshino K., Maeda-Yamamoto M. Novel antiallergic catechin derivatives isolated from oolong tea. Journal of Agricultural and Food Chemistry. 1999;47(5):1906–1910. doi: 10.1021/jf981114l. [DOI] [PubMed] [Google Scholar]
- 11.Koga K., Hisamura M., Kanetaka T., Yoshino K., Matsuo Y., Tanaka T. Proanthocyanidin Oligomers Isolated from Salacia reticulata leaves potently Inhibit Pancreatic Lipase Activity. Journal of Food Science. 2013;78(1):H105–H111. doi: 10.1111/1750-3841.12001. [DOI] [PubMed] [Google Scholar]
- 12.Sovak M. Grape extract, resveratrol, and its analogs: A review. Journal of Medicinal Food. 2001;4(2):93–105. doi: 10.1089/109662001300341752. [DOI] [PubMed] [Google Scholar]
- 13.Luximon-Ramma A., Neergheen V. S., Bahorun T., et al. Assessment of the polyphenolic composition of the organic extracts of Mauritian black teas: A potential contributor to their antioxidant functions. BioFactors. 2006;27(1-4):79–91. doi: 10.1002/biof.5520270108. [DOI] [PubMed] [Google Scholar]
- 14.Goszcz K., Deakin S. J., Duthie G. G., Stewart D., Leslie S. J., Megson I. L. Antioxidants in cardiovascular therapy: panacea or false hope? Frontiers in Cardiovascular Medicine. 2015;2, article 29:1–22. doi: 10.3389/fcvm.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araim O., Ballantyne J., Waterhouse A. L., Sumpio B. E. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. Journal of Vascular Surgery. 2002;35(6):1226–1232. doi: 10.1067/mva.2002.124358. [DOI] [PubMed] [Google Scholar]
- 16.Du J.-R., Li X., Zhang R., Qian Z.-M. Tanshinone inhibits intimal hyperplasia in the ligated carotid artery in mice. Journal of Ethnopharmacology. 2005;98(3):319–322. doi: 10.1016/j.jep.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Li F., Shang X., Du X., Chen S. Rapamycin Treatment Attenuates Angiotensin II -induced Abdominal Aortic Aneurysm Formation via VSMC Phenotypic Modulation and Down-regulation of ERK1/2 Activity. Current Medical Science. 2018;38(1):93–100. doi: 10.1007/s11596-018-1851-z. [DOI] [PubMed] [Google Scholar]
- 18.Lindqvist A., Nilsson B.-O., Ekblad E., Hellstrand P. Platelet-derived growth factor receptors expressed in response to injury of differentiated vascular smooth muscle in vitro: Effects on ca2+ and growth signals. Acta Physiologica Scandinavica. 2001;173(2):175–184. doi: 10.1046/j.1365-201X.2001.00873.x. [DOI] [PubMed] [Google Scholar]
- 19.Cao L., Pan D., Li D., et al. Relation between anti-atherosclerotic effects of IRAK4 and modulation of vascular smooth muscle cell phenotype in diabetic rats. American Journal of Translational Research. 2016;8(2):899–910. [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Duffhues G., García de Vinuesa A., ten Dijke P. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Developmental Dynamics. 2018;247(3):492–508. doi: 10.1002/dvdy.24589. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T., Carrier E. J., Talati M. H., et al. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2018;314(1):L118–L126. doi: 10.1152/ajplung.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li A., Peng W., Xia X., Li R., Wang Y., Wei D. Endothelial-to-Mesenchymal Transition: A Potential Mechanism for Atherosclerosis Plaque Progression and Destabilization. DNA and Cell Biology. 2017;36(11):883–891. doi: 10.1089/dna.2017.3779. [DOI] [PubMed] [Google Scholar]
- 23.Moonen J. A., Lee E. S., Schmidt M., et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovascular Research. 2015;108(3):377–386. doi: 10.1093/cvr/cvv175. [DOI] [PubMed] [Google Scholar]
- 24.Saiura A., Sata M., Hirata Y., Nagai R., Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nature Medicine. 2001;7(4):382–383. doi: 10.1038/86394. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K., Sata M., Natori T., et al. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. The FASEB Journal. 2008;22(2):428–436. doi: 10.1096/fj.06-6884com. [DOI] [PubMed] [Google Scholar]
- 26.Li P., Pan C., Sheu M., et al. Deep Sea Water Prevents Balloon Angioplasty-Induced Hyperplasia through MMP-2: An In Vitro and In Vivo Study. PLoS ONE. 2014;9(5):p. e96927. doi: 10.1371/journal.pone.0096927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson G., Robinson F., Gibson T. B., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews. 2001;22(2):153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 28.Asati V., Mahapatra D. K., Bharti S. K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. European Journal of Medicinal Chemistry. 2016;109:314–341. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Zhong J. RAS and downstream RAF-MEK and PI3K-AKT signaling in neuronal development, function and dysfunction. biological chemistry. 2016;397(3):215–222. doi: 10.1515/hsz-2015-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romorini L., Garate X., Neiman G., et al. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Scientific Reports. 2016;6(1) doi: 10.1038/srep35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimura T., Kakuda S., Ochiai Y., Kuwahara Y., Takai Y., Fukumoto M. Targeting the AKT/GSK3β/Cyclin D1/Cdk4 Survival Signaling Pathway for Eradication of Tumor Radioresistance Acquired by Fractionated Radiotherapy. International Journal of Radiation Oncology • Biology • Physics. 2011;80(2):540–548. doi: 10.1016/j.ijrobp.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 32.Kiu H., Nicholson S. E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30(2):88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fish E. N., Platanias L. C. Interferon receptor signaling in malignancy: A network of cellular pathways defining biological outcomes. Molecular Cancer Research. 2014;12(12):1691–1703. doi: 10.1158/1541-7786.MCR-14-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan J.-L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 35.Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons. J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(11):5192–5196. doi: 10.1016/0168-9525(92)90249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwasaki H., Yoshimoto T., Sugiyama T., Hirata Y. Activation of cell adhesion kinase β by mechanical stretch in vascular smooth muscle cells. Endocrinology. 2003;144(6):2304–2310. doi: 10.1210/en.2002-220939. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler A. P., Ridley A. J. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Experimental Cell Research. 2004;301(1):43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Angers-Loustau A., Côté J.-F., Charest A., et al. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. The Journal of Cell Biology. 1999;144(5):1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaller M. D. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. Journal of Cell Science. 2010;123(7):1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 40.Huang W.-C., Sala-Newby G. B., Susana A., Johnson J. L., Newby A. C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042507.e42507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieb W., Gona P., Larson M. G., et al. Biomarkers of the osteoprotegerin pathway: clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(9):1849–1854. doi: 10.1161/atvbaha.109.199661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues-Diez R. R., Garcia-Redondo A. B., Orejudo M., et al. The C-terminal module IV of connective tissue growth factor, through EGFR/Nox1 signaling, activates the NF-κB pathway and proinflammatory factors in vascular smooth muscle cells. Antioxidants & Redox Signaling. 2015;22(1):29–47. doi: 10.1089/ars.2013.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren J., Wang Q., Morgan S., et al. Protein Kinase C-δ (PKCδ) Regulates Proinflammatory Chemokine Expression through Cytosolic Interaction with the NF-κB Subunit p65 in Vascular Smooth Muscle Cells. The Journal of Biological Chemistry. 2014;289(13):9013–9026. doi: 10.1074/jbc.M113.515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan S., Tang Q., Liu W., Zhu R., Li B. Nobiletin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in a rat carotid artery injury model. Drug Development Research. 2014;75(8):489–496. doi: 10.1002/ddr.21230. [DOI] [PubMed] [Google Scholar]
- 45.Shah P. K. Inflammation, neointimal hyperplasia, and restenosis: As the leukocytes roll, the arteries thicken. Circulation. 2003;107(17):2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto Y., Gaynor R. B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. The Journal of Clinical Investigation. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cazarolli L. H., Zanatta L., Alberton E. H., et al. Flavonoids: prospective drug candidates. Mini-Reviews in Medicinal Chemistry. 2008;8(13):1429–1440. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 48.Cushnie T. P. T., Lamb A. J. Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents. 2011;38(2):99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 49.de Sousa R. R., Queiroz K. C., Souza A. C., et al. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. Journal of Enzyme Inhibition and Medicinal Chemistry. 2007;22(4):439–444. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 50.Schuier M., Sies H., Illek B., Fischer H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. Journal of Nutrition. 2005;135(10):2320–2325. doi: 10.1093/jn/135.10.2320. [DOI] [PubMed] [Google Scholar]
- 51.Siasos G., Tousoulis D., Tsigkou V., et al. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Current Medicinal Chemistry. 2013;20(21):2641–2660. doi: 10.2174/0929867311320210003. [DOI] [PubMed] [Google Scholar]
- 52.Van Dam R. M., Naidoo N., Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Current Opinion in Lipidology. 2013;24(1):25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 53.Luo X., Fang S., Xiao Y., et al. Cyanidin-3-glucoside suppresses TNF-α-induced cell proliferation through the repression of Nox activator 1 in mouse vascular smooth muscle cells: involvement of the STAT3 signaling. Molecular and Cellular Biochemistry. 2012;362(1-2):211–218. doi: 10.1007/s11010-011-1144-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee J. J., Yi H., Kim I. S., et al. (2S)-naringenin from Typha angustata inhibits vascular smooth muscle cell proliferation via a G0/G1 arrest. Journal of Ethnopharmacology. 2012;139(3):873–878. doi: 10.1016/j.jep.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 55.Hu Y., Liu K., Yan M., Zhang Y., Wang Y., Ren L. Icariin inhibits oxidized low-density lipoprotein-induced proliferation of vascular smooth muscle cells by suppressing activation of extracellular signal-regulated kinase 1/2 and expression of proliferating cell nuclear antigen. Molecular Medicine Reports. 2016;13(3):2899–2903. doi: 10.3892/mmr.2016.4813. [DOI] [PubMed] [Google Scholar]
- 56.Zhu L.-H., Wang L., Wang D., et al. Puerarin attenuates high-glucose and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radical Biology & Medicine. 2010;48(4):471–482. doi: 10.1016/j.freeradbiomed.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Li Y.-J., Du G.-H. Effects of alpinetin on rat vascular smooth muscle cells. Journal of Asian Natural Products Research. 2004;6(2):87–92. doi: 10.1080/1028602031000135558. [DOI] [PubMed] [Google Scholar]
- 58.Wei L., Deng W., Cheng Z., et al. Effects of hesperetin on platelet-derived growth factor-BB-induced pulmonary artery smooth muscle cell proliferation. Molecular Medicine Reports. 2016;13(1):955–960. doi: 10.3892/mmr.2015.4625. [DOI] [PubMed] [Google Scholar]
- 59.Li L., Pang X.-B., Chen B.-N., et al. Pinocembrin inhibits angiotensin II-induced vasoconstriction via suppression of the increase of [Ca2+]i and ERK1/2 activation through blocking AT1R in the rat aorta. Biochemical and Biophysical Research Communications. 2013;435(1):69–75. doi: 10.1016/j.bbrc.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 60.Kim H. J., Cha B.-Y., Choi B., Lim J. S., Woo J.-T., Kim J.-S. Glyceollins inhibit platelet-derived growth factor-mediated human arterial smooth muscle cell proliferation and migration. British Journal of Nutrition. 2012;107(1):24–35. doi: 10.1017/S0007114511002571. [DOI] [PubMed] [Google Scholar]
- 61.Pinkaew D., Cho S. G., Hui D. Y., et al. Morelloflavone blocks injury-induced neointimal formation by inhibiting vascular smooth muscle cell migration. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790(1):31–39. doi: 10.1016/j.bbagen.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K., Kim S., Moh S. H., Kang H. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression. Molecular and Cellular Biochemistry. 2015;407(1-2):143–149. doi: 10.1007/s11010-015-2464-5. [DOI] [PubMed] [Google Scholar]
- 63.Xu J., Xu Z., Zheng W. A review of the antiviral role of green tea catechins. Molecules. 2017;22(8) doi: 10.3390/molecules22081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haghighatdoost F., Nobakht M. Gh B. F., Hariri M. Effect of Green Tea on Plasma Adiponectin Levels: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Journal of the American College of Nutrition. 2017;36(7):541–548. doi: 10.1080/07315724.2017.1333470. [DOI] [PubMed] [Google Scholar]
- 65.Wang D., Gao Q., Wang T., et al. Green tea infusion protects against alcoholic liver injury by attenuating inflammation and regulating the PI3K/Akt/eNOS pathway in C57BL/6 mice. Food & Function. 2017;8(9):3165–3177. doi: 10.1039/C7FO00791D. [DOI] [PubMed] [Google Scholar]
- 66.Cheng X. W., Kuzuya M., Sasaki T., et al. Green tea catechins inhibit neointimal hyperplasia in a rat carotid arterial injury model by TIMP-2 overexpression. Cardiovascular Research. 2004;62(3):594–602. doi: 10.1016/j.cardiores.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 67.Natsume M. Polyphenols: Inflammation. Current Pharmaceutical Design. 2018;24(2):191–202. doi: 10.2174/1381612823666171109104141. [DOI] [PubMed] [Google Scholar]
- 68.Oliviero F., Scanu A., Zamudio-Cuevas Y., Punzi L., Spinella P. Anti-inflammatory effects of polyphenols in arthritis. Journal of the Science of Food and Agriculture. 2018;98(5):1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- 69.Sarubbo F., Esteban S., Miralles A., Moranta D. Effects of resveratrol and other polyphenols on SIRT1: Relevance to brain function during aging. Current Neuropharmacology. 2018;16(2):126–136. doi: 10.2174/1570159X15666170703113212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hung H. H., Chen Y. L., Lin S. J., et al. A salvianolic acid B-rich fraction of Salvia miltiorrhiza induces neointimal cell apoptosis in rabbit angioplasty model. Histology and Histopathology. 2001;16(1):175–183. doi: 10.14670/HH-16.175. [DOI] [PubMed] [Google Scholar]
- 71.Yang T.-L., Lin F.-Y., Chen Y.-H., et al. Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. Journal of the Science of Food and Agriculture. 2011;91(1):134–141. doi: 10.1002/jsfa.4163. [DOI] [PubMed] [Google Scholar]
- 72.Hur K. Y., Kim S. H., Choi M.-A., et al. Protective effects of magnesium lithospermate B against diabetic atherosclerosis via Nrf2-ARE-NQO1 transcriptional pathway. Atherosclerosis. 2010;211(1):69–76. doi: 10.1016/j.atherosclerosis.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y.-L., Lin K.-F., Shiao M.-S., Chen Y.-T., Hong C.-Y., Lin S.-J. Magnolol, a potent antioxidant from Magnolia officinalis, attenuates intimal thickening and MCP-1 expression after balloon injury of the aorta in cholesterol-fed rabbits. Basic Research in Cardiology. 2001;96(4):353–363. doi: 10.1007/s003950170043. [DOI] [PubMed] [Google Scholar]
- 74.Karki R., Kim S. B., Kim D. W. Magnolol inhibits migration of vascular smooth muscle cells via cytoskeletal remodeling pathway to attenuate neointima formation. Experimental Cell Research. 2013;319(20):3238–3250. doi: 10.1016/j.yexcr.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., Wang W.-Y., Wang Y.-P. Inhibitory effects of lithospermic acid on proliferation and migration of rat vascular smooth muscle cells. Acta Pharmacologica Sinica. 2009;30(9):1245–1252. doi: 10.1038/aps.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chien Y.-C., Huang G.-J., Cheng H.-C., Wu C.-H., Sheu M.-J. Hispolon attenuates balloon-injured neointimal formation and modulates vascular smooth muscle cell migration via AKT and ERK phosphorylation. Journal of Natural Products. 2012;75(9):1524–1533. doi: 10.1021/np3002145. [DOI] [PubMed] [Google Scholar]
- 77.Lim Y., Kwon J.-S., Kim D.-W., et al. Obovatol from Magnolia obovata inhibits vascular smooth muscle cell proliferation and intimal hyperplasia by inducing p21Cip1. Atherosclerosis. 2010;210(2):372–380. doi: 10.1016/j.atherosclerosis.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 78.Kunnumakkara A. B., Bordoloi D., Padmavathi G., et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology. 2016;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang X., Thomas D. P., Zhang X., et al. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(1):85–90. doi: 10.1161/01.atv.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Y., Feng J., Li J., Fan Z. Curcumin prevents lipopolysaccharide-induced matrix metalloproteinase-2 activity via the Ras/MEK1/2 signaling pathway in rat vascular smooth muscle cells. Molecular Medicine Reports. 2017;16(4):4315–4319. doi: 10.3892/mmr.2017.7037. [DOI] [PubMed] [Google Scholar]
- 81.Guo N., Chen F., Zhou J., et al. Curcumin attenuates rapamycin-induced cell injury of vascular endothelial cells. Journal of Cardiovascular Pharmacology. 2015;66(4):338–346. doi: 10.1097/FJC.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 82.Islam M. T. Diterpenes and Their Derivatives as Potential Anticancer Agents. Phytotherapy Research. 2017;31(5):691–712. doi: 10.1002/ptr.5800. [DOI] [PubMed] [Google Scholar]
- 83.Kiyama R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. European Journal of Pharmacology. 2017;815:405–415. doi: 10.1016/j.ejphar.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 84.Vadivelu R. K., Yeap S. K., Ali A. M., Hamid M., Alitheen N. B. Betulinic Acid inhibits growth of cultured vascular smooth muscle cells in vitro by inducing g(1) arrest and apoptosis. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7. doi: 10.1155/2012/251362.251362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferri N., Arnaboldi L., Orlandi A., et al. Effect of S(-) perillic acid on protein prenylation and arterial smooth muscle cell proliferation. Biochemical Pharmacology. 2001;62(12):1637–1645. doi: 10.1016/S0006-2952(01)00808-5. [DOI] [PubMed] [Google Scholar]
- 86.Weng S.-X., Sui M.-H., Chen S., et al. Parthenolide inhibits proliferation of vascular smooth muscle cells through induction of G0/G1 phase cell cycle arrest. Journal of Zhejiang University SCIENCE B. 2009;10(7):528–535. doi: 10.1631/jzus.B0820351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heiss E. H., Liu R., Waltenberger B., et al. Plumericin inhibits proliferation of vascular smooth muscle cells by blocking STAT3 signaling via S-glutathionylation. Scientific Reports. 2016;6(1) doi: 10.1038/srep20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D., Kwon J. S., Kim Y. G., et al. Novel Oral Formulation of Paclitaxel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model. Circulation. 2004;109(12):1558–1563. doi: 10.1161/01.CIR.0000124063.74526.BE. [DOI] [PubMed] [Google Scholar]
- 89.Blagosklonny M. V., Darzynkiewicz Z., Halicka H. D., et al. Paclitaxel induces primary and postmitotic G1 arrest in human arterial smooth muscle cells. Cell Cycle. 2004;3(8):1050–1056. [PubMed] [Google Scholar]
- 90.Kim T.-J., Lim Y., Kim D.-W., et al. Epothilone D, a microtubule-stabilizing compound, inhibits neointimal hyperplasia after rat carotid artery injury by cell cycle arrest via regulation of G1-checkpoint proteins. Vascular Pharmacology. 2007;47(4):229–237. doi: 10.1016/j.vph.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Wu L., Wang G., Tang S., Long G., Yin T. Protection of Endothelial Cells, Inhibition of Neointimal Hyperplasia by β-elemene in an Injured Artery. Cardiovascular Drugs and Therapy. 2011;25(3):233–242. doi: 10.1007/s10557-011-6305-9. [DOI] [PubMed] [Google Scholar]
- 92.Cao Q., Jiang Y., Shi J., et al. Artemisinin inhibits tumour necrosis factor- Clinical and Experimental Pharmacology and Physiology. 2015;42(5):502–509. doi: 10.1111/1440-1681.12375. [DOI] [PubMed] [Google Scholar]
- 93.Wang H.-Y., Huang R.-P., Han P., et al. The effects of artemisinin on the proliferation and apoptosis of vascular smooth muscle cells of rats. Cell Biochemistry & Function. 2014;32(2):201–208. doi: 10.1002/cbf.2995. [DOI] [PubMed] [Google Scholar]
- 94.Mair C. E., Liu R., Atanasov A. G., et al. Piperine Congeners as Inhibitors of Vascular Smooth Muscle Cell Proliferation. Planta Medica. 2015;81(12-13):1065–1074. doi: 10.1055/s-0035-1546165. [DOI] [PubMed] [Google Scholar]
- 95.Tanabe H., Suzuki H., Nagatsu A., Mizukami H., Ogihara Y., Inoue M. Selective inhibition of vascular smooth muscle cell proliferation by coptisine isolated from Coptis rhizoma, one of the crude drugs composing Kampo medicines Unsei-in. Phytomedicine. 2006;13(5):334–342. doi: 10.1016/j.phymed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Wang K., Wen L., Peng W., et al. Vinpocetine Attenuates Neointimal Hyperplasia in Diabetic Rat Carotid Arteries after Balloon Injury. PLoS ONE. 2014;9(5):p. e96894. doi: 10.1371/journal.pone.0096894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai Y., Knight W. E., Guo S., Li J.-D., Knight P. A., Yan C. Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration. The Journal of Pharmacology and Experimental Therapeutics. 2012;343(2):479–488. doi: 10.1124/jpet.112.195446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagler A., Miao H.-Q., Aingorn H., Pines M., Genina O., Vlodavsky I. Inhibition of collagen synthesis, smooth muscle cell proliferation, and injury-induced intimal hyperplasia by halofuginone. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(1):194–202. doi: 10.1161/01.ATV.17.1.194. [DOI] [PubMed] [Google Scholar]
- 99.Han J., Kim Y., Jung S., et al. Murrayafoline A Induces a G. The Korean Journal of Physiology & Pharmacology. 2015;19(5):421–426. doi: 10.4196/kjpp.2015.19.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dong X., Fu J., Yin X., et al. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytotherapy Research. 2016;30(8):1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X., Zou Y., Sun A., et al. Emodin induces growth arrest and death of human vascular smooth muscle cells through reactive oxygen species and p53. Journal of Cardiovascular Pharmacology. 2007;49(5):253–260. doi: 10.1097/FJC.0b013e318033dfb3. [DOI] [PubMed] [Google Scholar]
- 102.Wang S., Liu Y., Fan F., Yan J., Wang X., Chen J. Inhibitory effects of emodin on the proliferation of cultured rat vascular smooth muscle cell-induced by angiotensin II. Phytotherapy Research. 2008;22(2):247–251. doi: 10.1002/ptr.2301. [DOI] [PubMed] [Google Scholar]
- 103.Pang X., Liu J., Li Y., Zhao J., Zhang X., Sen U. Emodin Inhibits Homocysteine-Induced C-Reactive Protein Generation in Vascular Smooth Muscle Cells by Regulating PPARγ Expression and ROS-ERK1/2/p38 Signal Pathway. PLoS ONE. 2015;10(7):p. e0131295. doi: 10.1371/journal.pone.0131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heo S., Yun H., Park W., Park S. Emodin inhibits TNF-α-induced human aortic smooth-muscle cell proliferation via caspase- and mitochondrial-dependent apoptosis. Journal of Cellular Biochemistry. 2008;105(1):70–80. doi: 10.1002/jcb.21805. [DOI] [PubMed] [Google Scholar]
- 105.Meng L., Yan D., Xu W., Ma J., Chen B., Feng H. Emodin inhibits tumor necrosis factor-alpha-induced migration and inflammatory responses in rat aortic smooth muscle cells. International Journal of Molecular Medicine. 2012;29(6):999–1006. doi: 10.3892/ijmm.2012.940. [DOI] [PubMed] [Google Scholar]
- 106.Xu K., Al-ani M. K., Wang C., et al. Emodin as a selective proliferative inhibitor of vascular smooth muscle cells versus endothelial cells suppress arterial intima formation. Life Sciences. 2018;207:9–14. doi: 10.1016/j.lfs.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 107.Chung Y., Pan C., Wang C. C., et al. Methyl Protodioscin, a Steroidal Saponin, Inhibits Neointima Formation in Vitro and in Vivo. Journal of Natural Products. 2016;79(6):1635–1644. doi: 10.1021/acs.jnatprod.6b00217. [DOI] [PubMed] [Google Scholar]
- 108.Li X., Du J.-R., Yu Y., Bai B., Zheng X.-Y. Tanshinone IIA inhibits smooth muscle proliferation and intimal hyperplasia in the rat carotid balloon-injured model through inhibition of MAPK signaling pathway. Journal of Ethnopharmacology. 2010;129(2):273–279. doi: 10.1016/j.jep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 109.Ferri N., Yokoyama K., Sadilek M., et al. Ajoene, a garlic compound, inhibits protein prenylation and arterial smooth muscle cell proliferation. British Journal of Pharmacology. 2003;138(5):811–818. doi: 10.1038/sj.bjp.0705126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu L., Guan H., Cui C., et al. Gastrodin inhibits cell proliferation in vascular smooth muscle cells and attenuates neointima formation in vivo. International Journal of Molecular Medicine. 2012;30(5):1034–1040. doi: 10.3892/ijmm.2012.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang F., Jiang R., Zhu X., Zhang X., Zhan Z. Genipin Inhibits TNF-α-Induced Vascular Smooth Muscle Cell Proliferation and Migration via Induction of HO-1. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0074826.e74826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma Z., Gao Y., Wang Y., Tan H., Xiao C., Wang S. Ginsenoside Rg1 inhibits proliferation of vascular smooth muscle cells stimulated by tumor necrosis factor-α1. Acta Pharmacologica Sinica. 2006;27(8):1000–1006. doi: 10.1111/j.1745-7254.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 113.Gao Y., Deng J., Yu X.-F., Yang D.-L., Gong Q.-H., Huang X.-N. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2. Journal of Ethnopharmacology. 2011;138(2):472–478. doi: 10.1016/j.jep.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 114.Zhang H.-S., Wang S.-Q. Ginsenoside Rg1 inhibits tumor necrosis factor-α (TNF-α)- induced human arterial smooth muscle cells (HASMCs) proliferation. Journal of Cellular Biochemistry. 2006;98(6):1471–1481. doi: 10.1002/jcb.20799. [DOI] [PubMed] [Google Scholar]
- 115.Joa H., Vogl S., Atanasov A. G., et al. Identification of ostruthin from peucedanum ostruthium rhizomes as an inhibitor of vascular smooth muscle cell proliferation. Journal of Natural Products. 2011;74(6):1513–1516. doi: 10.1021/np200072a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mao M., Lei H., Liu Q., et al. Lycopene inhibits neointimal hyperplasia through regulating lipid metabolism and suppressing oxidative stress. Molecular Medicine Reports. 2014;10(1):262–268. doi: 10.3892/mmr.2014.2186. [DOI] [PubMed] [Google Scholar]
- 117.Yoo S.-H., Lim Y., Kim S.-J., et al. Sulforaphane inhibits PDGF-induced proliferation of rat aortic vascular smooth muscle cell by up-regulation of p53 leading to G1/S cell cycle arrest. Vascular Pharmacology. 2013;59(1-2):44–51. doi: 10.1016/j.vph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Gfeller D., Grosdidier A., Wirth M., Daina A., Michielin O., Zoete V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Research. 2014;42(1):W32–W38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamauchi A., Kobayashi A., Oikiri H., Yokoyama Y. Functional role of the Tau protein in epithelial ovarian cancer cells. Reproductive Medicine and Biology. 2017;16(2):143–151. doi: 10.1002/rmb2.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang J., Yu Y., Liu W., Li Z., Wei Z., Jiang R. Microtubule-associated protein tau is associated with the resistance to docetaxel in prostate cancer cell lines. Research and Reports in Urology. 2017;9:71–77. doi: 10.2147/RRU.S118966. [DOI] [PMC free article] [PubMed] [Google Scholar]